Aller au contenu | Navigation | Accès directs | Connexion
- Bibliothèques
- Orientation & Insertion
- Nos unités de recherche
- Nos marchés publics
- Faculté Arts & Sciences Humaines (ASH) Voir le site
- Faculté Centre d'Études Supérieures de la Renaissance Voir le site
- Faculté Droit Economie & Sciences Sociales Voir le site
- Faculté IAE Voir le site
- IUT IUT de Blois Voir le site
- IUT IUT de Tours Voir le site
- Faculté Lettres & Langues Voir le site
- Faculté Médecine Voir le site
- Faculté Odontologie Voir le site
- Faculté Pharmacie Voir le site
- Ecole Polytech Tours Voir le site
- Faculté Sciences & Techniques Voir le site
- GIS Collegium Santé Voir le site

M. Philippe Rosset
Coordonnées, discipline(s).
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article
Pericyte-Like Progenitors Show High Immaturity and Engraftment Potential as Compared with Mesenchymal Stem Cells
Affiliations Stromalab Unité Mixte de Recherche (UMR) Université Paul Sabatier/Centre National de la Recherche Scientifique (CNRS) 5273, U1031 Institut national de la santé et de la recherche médicale (Inserm), Etablissement Français du Sang-Pyrénées-Méditerranée, Toulouse, France, EA3855, Université François Rabelais, Tours, France
Affiliations Inserm UMR957, Physiopathologie de la Résorption Osseuse et Thérapie des Tumeurs Osseuses Primitives, Université de Nantes, Nantes, France, Centre Hospitalier Universitaire Trousseau, Tours, France
Affiliation Inserm UMR957, Physiopathologie de la Résorption Osseuse et Thérapie des Tumeurs Osseuses Primitives, Université de Nantes, Nantes, France
Affiliation Stromalab Unité Mixte de Recherche (UMR) Université Paul Sabatier/Centre National de la Recherche Scientifique (CNRS) 5273, U1031 Institut national de la santé et de la recherche médicale (Inserm), Etablissement Français du Sang-Pyrénées-Méditerranée, Toulouse, France
* E-mail: [email protected]
- Amina Bouacida,
- Philippe Rosset,
- Valérie Trichet,
- Fabien Guilloton,
- Nicolas Espagnolle,
- Thomas Cordonier,
- Dominique Heymann,
- Pierre Layrolle,
- Luc Sensébé,
- Frédéric Deschaseaux

- Published: November 7, 2012
- https://doi.org/10.1371/journal.pone.0048648
- Reader Comments
Mesenchymal stem cells (MSCs) and pericyte progenitors (PPs) are both perivascular cells with similar multipotential properties regardless of tissue of origin. We compared the phenotype and function of the 2 cell types derived from the same bone-marrow samples but expanded in their respective media – pericyte conditions (endothelial cell growth medium 2 [EGM-2]) for PPs and standard medium (mesenchymal stem cell medium [MSM]) for MSCs. After 3 weeks of culture, whatever the expansion medium, all cells showed similar characteristics (MSC markers and adipo-osteo-chondroblastic differentiation potential), although neuronal potential was greater in EGM-2– than MSM-cultured cells. As compared with MSM-cultured MSCs, EGM-2–cultured PPs showed higher expression of the pericyte-specific antigen 3G5 than α-smooth muscle actin. In addition, EGM-2–cultured PPs showed an immature phenotype, with upregulation of stemness OCT4 and SOX2 proteins and downregulation of markers of osteoblastic, chondroblastic, adipocytic and vascular smooth muscle lineages. Despite having less effective in vitro immunosuppression capacities than standard MSCs, EGM-2–cultured PPs had higher engraftment potentials when combined with biomaterials heterotopically-transplanted in Nude mice. Furthermore, these engrafted cells generated more collagen matrix and were preferentially perivascular or lined trabeculae as compared with MSM-cultured MSCs. In conclusion, EGM-2–cultured PPs are highly immature cells with increased plasticity and engraftment potential.
Citation: Bouacida A, Rosset P, Trichet V, Guilloton F, Espagnolle N, Cordonier T, et al. (2012) Pericyte-Like Progenitors Show High Immaturity and Engraftment Potential as Compared with Mesenchymal Stem Cells. PLoS ONE 7(11): e48648. https://doi.org/10.1371/journal.pone.0048648
Editor: Giovanni Camussi, University of Torino, Italy
Received: June 19, 2012; Accepted: September 27, 2012; Published: November 7, 2012
Copyright: © 2012 Bouacida et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Funding: This work was supported by the Agence Nationale de la Recherche Technologies pour la Santé et l’Autonomie, ATOS project N° 024-03 (2007–2010) and the European Commission Seventh Framework Programme (FP7/2007–2013) (grant no. 241879), through the REBORNE project. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: The authors have declared that no competing interests exist.
Introduction
Mesenchymal stem cells (MSCs) (also called multipotent mesenchymal stromal cells) are tissue-resident multipotential cells that give rise to bone cells, adipocytes, smooth muscle (SM) cells and hematopoietic-supportive stromal cells [1] , [2] , [3] , [4] . They have been identified in several tissues, including bone marrow (BM), dental pulp, adipose tissue and umbilical cord blood. Such cells are selected by their capacity to adhere to plastic culture flasks and expand through fibroblastic colonies (colony formation unit fibroblasts [CFU-fs]) within media containing at least 10% fetal bovine serum (FBS) [5] . However, this ex vivo expansion protocol hides their true nature, which explains the growing number of works seeking to describe them directly in vivo in their native forms.
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pone.0048648.t001
Native BM-MSCs express surface markers such as STRO-1, CD49a, CD73, CD146 and CD271, which led researchers to observe them mainly at the abluminal position of vessels [5] , [6] , [7] , [8] , [9] . Indeed, non-hematopoietic CD146+ cells, capable of self-renewal and supporting hematopoiesis, were identified as perivascular cells [8] . However, pericytes are also defined as populations of cells, including all perivascular cells, with similar morphologic capacity (multi-branched cells) to directly interact with endothelial cells and have common markers and properties of vascular smooth muscle cells (VSMCs; induction of contractive acto-myosine proteins) [6] . Pericytes are also multipotent cells capable of generating adipocytes, osteoblasts and chondrocytes [7] , [8] . They can be characterized by nestin, aminopeptidase N (CD13), 3G5 antigen and NG2, all also described in BM-MSCs [9] , [10] , [11] , [12] , [13] . Hence, whatever their origin, BM or adipose tissue, MSCs were recently classified as pericytic cells, even if all pericytes are not MSCs [11] . Because of the close similarities with pericytes, MSCs could be at the forefront of organogenesis and tissue regeneration. For instance, recent data in mice showed that cells located at the pericytic position and at the front of invading vessels could form bone during endochondral mechanisms of skeletogenesis or after injury [14] . However, no studies have compared pericytes and MSCs from the same BM samples.
Here, we investigated the similarities between and MSC behavior and properties in MSCs and pericytic cells cultured in standard MSC medium (MSM) or endothelial cell growth medium 2 (EGM-2) for pericyte expansion, respectively. Cultured BM-derived pericytic cells showed progenitor/stem cell properties, with higher plasticity and immaturity state than standard MSCs.
MSC phenotype of cells cultured with endothelial cell growth medium 2 (EGM-2) and standard medium for MSCs (MSM). ( A , B ) Photonic microscopy of morphology of cultured cells, and ( C ) quantification by flow cytometry of expression of membrane molecules characterizing bone-marrow MSCs with antibodies against CD markers conjugated with FITC or phycoerythrine. ( D ) Alizarin-red, Nile-red and COL2a1 staining for osteoblastic, chondroblastic and adipocytic potential, respectively.
https://doi.org/10.1371/journal.pone.0048648.g001
Materials and Methods
We collected adult human BM samples from healthy volunteers undergoing orthopedic surgery. The study followed the ethical guidelines of the University Hospital of Tours (Tours, France) and was approved by the ethics committee Comité de protection des personnes (Tours - Région Centre [Ouest-1]). Patients gave their written informed consent for use of samples. For each donor, BM mononuclear cells were plated in 1) MSM, consisting of minimum essential medium supplemented with 10% FBS (Stem-Cell Technologies, Vancouver, BC, Canada) and 1% penicillin/streptomycin (Invitrogen, Paisley, UK); or 2) EGM-2 (PromoCell GmbH, Heidelberg, Germany). On day 10, cells at 80% confluence were split and expanded (passage 1). We used cells at passage 1 or 2 from at least 3 different donors for all studies. Peripheral blood mononuclear cells (PBMCs) were obtained from informed consent donors following ethical guidelines of Etablissement Français du Sang Pyrénées-Méditerranée (Toulouse, France).
MSC Multipotential Assay
MSC multipotentiality was determined after cell differentiation into osteoblasts, chondroblasts, or adipocytic cells as previously described [15] , [16] . Osteoblastic and chondroblastic differentiation was revealed by staining with Alizarin red (Sigma-Aldrich, St. Louis, MO, USA) and anti-COL2a1 (clone 6B3, Millipore, Billerica, MA), respectively, and adipocytes by staining with Nile-red oil solution (Sigma-Aldrich). Undifferentiated cells cultured in expansion medium were a negative control. Revealing neuronal potential was as previously described [17] , [18] . EGM-2– or MSM-cultured cells were cultured in neuronal induction medium, DMEM F12 with L-glutamine, 1% insulin-transferrine-selenium, and 1% penicillin–streptomycin (Invitrogen). We added Forskolin (40 µg/mL), nerve growth factor (50 ng/mL), NT3 (70 ng/mL), NT4 (100 ng/mL), and fibroblast growth factor 2 (125 ng/µL) (Euromedex, Souffelweyersheim, France). The culture medium was replaced every 3 to 4 days. Negative control cells were maintained without any induction medium. After neuronal differentiation, neurosphere-like colonies were obtained from EGM-2 conditions only. These spheres were dissociated into single cells by use of trypsin and resuspended in differentiation media (for osteogenesis, adipogenesis and neurogenesis). For all induction cultures, cells were maintained for 9 or 13 days before harvesting for RNA extraction or fixation with 4% paraformaldehyde for immunofluorescence.
Photonic microscopy of cells cultured under MSM ( A ) or EGM-2 conditions ( B ) with neuron-inducing medium on days 6 and 13, and fluorescence microscopy of human microtubule-associated protein 2 (hMAP2). After 2 weeks of neurogenic culture, only MSM culture greatly decreased MSC cell number ( A ), whereas EGM-2–derived cells still survived and aggregated, forming floating neurosphere-like structures expressing MAP2 protein ( B ). ( C ) Neurosphere-like structures underwent induction of neuronal, adipocytic and osteoblastic differentiation by culture in appropriate media after their dissociation. Sphere-derived cells re-adhered and Nile-red and Alizarin-red staining showed adipocytes and osteoblastic cells, respectively. Neurogenic culture induced the expression of neuron-specific markers p75, MAP2, AP2α and Nestin. Nuclei were stained with DAPI.
https://doi.org/10.1371/journal.pone.0048648.g002
Quantification of Bipotential CFU-fs
Sorted cell subsets were seeded at 4 to 6 cell concentrations into 96-well plates (12–24 wells/dilution). At day 10, using an inverted phase microscope colonies of more than 50 cells (CFU-fs) were counted. Then, CFU-fs from wells were induced to differentiate into adipocytes or osteoblasts as described. The frequency of responding cells at any one time was calculated from the proportion of negative wells by use of the Poisson formula.
Assessment of Functional Potential of Pericytic Cells
Selected cells were cultured with Matrigel (Becton Dickinson, Franklin Lakes, NJ, USA) overnight before examination under a photonic microscope. Cultured cells from both conditions were co-cultured with human umbilical vein endothelial cells (HUVECs; Promocell) for 5 days, then stained with anti-von Willebrand Factor (vWF) (Dako, Trappes, France) and anti-αSM actin antibodies.
Assessment of pericyte phenotype by pericyte-specific 3G5 antibody ( A , B ) and function by seeding onto matrigel ( C , D , E ) in cells cultured with EGM-2 ( A, C ) or MSM ( B, D ). Cultured human umbilical vein endothelial cells (HUVECs) were incubated with anti-von Willebrand factor (vWF) antibody revealing Weibel-Palade bodies ( E1 ). EGM-2–cultured cells were seeded onto HUVECs and incubated for 5 days and labeled with ( E2 ) anti-α-smooth muscle (SM)-actin and anti-vWF factor antibodies. ( F ) Proportion of EGM-2– or MSM-cultured cells (from the same bone-marrow [BM] sample) expressing αSM actin and 3G5 antigen (3G5 Ag) before or after priming into the opposite medium (MSM or EGM-2, respectively).
https://doi.org/10.1371/journal.pone.0048648.g003
Flow Cytometry Studies
Both cell types were examined by flow cytometry. Cells were washed in phosphate buffered saline/human serum albumin solution, and the following primary antibodies conjugated to phytoerythrin (PE) or fluorescein isothiocyanate (FITC) were added at the appropriate concentrations: stem cell factor receptor (CD117), CMH-II, and CD105 (Diaclone, Besançon, France); mouse monoclonal antibody (Mab) anti-human CD34, CD45, and CD146 (Millipore); Mabs anti-human CD133 (Miltenyi Biotec), anti-human vascular endothelial growth factor-type 2 receptor (anti-VEGF-R2), CD49a, CD49b, CD166, CD73, CD44, and Tie2 (Becton Dickinson); and rabbit polyclonal anti-human CD144 (VE-cadherin) (Valbiotech, Paris, France). Antibodies of the same isotype were used as negative controls (Diaclone, Immunotech, Becton Dickinson and Millipore). Cells were passed through the Becton Dickinson FACSort (Mountain View, CA) equipped with the Cellquest software program. The fluorescence histogram for each monoclonal antibody was displayed with that of the corresponding control antibody.
( A ) Quantitative RT-PCR of relative mRNA expression of markers of chondrogenesis ( COL2a1 , SOX9 , SOX6 , DLX2 ), adipogenesis ( PPARγ2 , ADIPOQ , LEP ), osteogenesis ( ALPL , PTHR1 , RUNX2 , DLX5 , OSC , OPG , RANKL ), and vascular smooth muscle (VSM) ( EMX2 , CNN3 , ELN , MYOC ). Expression was relative to that of GAPDH and depicted as fold change relative to that for pericyte progenitors (PPs). Data are mean±SEM from 6 experiments. *p<0.01; **p<0.001 compared with PPs. ( B ) Western blot analysis of protein level of RUNX2 in 3 different BM productions (BM1, BM2, and BM3) of bone-marrow MSCs and PPs. β-actin was a positive internal control.
https://doi.org/10.1371/journal.pone.0048648.g004
After expansion, EGM-2– or MSM-cultured cells were cultured in chamber slides, and confluent cells were stained with 3G5 antibody (produced from the ATCC cell line and purified by RD-Biotech, Besançon, France) or by anti-αSM actin (clone 1A4, Sigma-Aldrich), and then at 37°C for 45 min with FITC-labeled goat anti-mouse or donkey anti-rabbit secondary antibody (Invitrogen). After neuronal differention, cells were incubated with the antibodies anti-Nestin, -AP2α, -p75, -MAP2, -SOX10, -SLUG and anti-NeuN (see supporting information). Positive cells were counted and compared to total cell counts for percentage positive cells.
Real-time RT-PCR
Total RNA was extracted from differentiated and undifferentiated cells and purified with use of the RNAeasy purification kit (Qiagen, Courtaboeuf, France). Reverse transcription involved the Takara Kit (St. Germain en Laye, France) in a final volume containing 1 µg RNA. The reagents in 20 µL PCR included 1 X SYBR GREEN mix (Biorad, Marnes-la-Coquette, France) and 25 ng cDNA. The primers are in Table 1 . All real-time PCR reactions were performed in duplicate. mRNA expression was normalized to that of GAPDH.
Quantitative RT-PCR ( A ) and western blot ( B ) analysis of the mRNA and protein expression, respectively, of stemness specific markers OCT4A, SOX2 and quantitative RT-PCR analysis of NANOG mRNA expression. Data are mean±SD from 3 experiments. *p<0.01; **p<0.001 compared with PPs. Protein extracts of cells from 3 different BM cultures (BM1, BM2, BM3) of PPs vs MSCs were tested. β-actin was a positive internal control. ND: not determined. MEL1: protein extract of human embryonic stem cell line MEL1 as positive control.
https://doi.org/10.1371/journal.pone.0048648.g005
Western Blot Analysis
Proteins were extracted, separated by 10% Tris-glycine on SDS-PAGE, and transferred to nitrocellulose membrane (Amersham Biosciences, Saclay, France), then incubated with anti-OCT4, anti-SOX2 and anti-RUNX2 antibodies (Abcam), 1 µg/mL, then peroxidase-conjugated mouse anti-rabbit IgG antibody (Sigma-Aldrich) and visualized by enhanced chemiluminescence (Thermo Fisher, Courtaboeuf, France). β-actin was an internal control (Sigma-Aldrich).
EGM-2–derived PPs or MSM-derived MSCs were seeded into biphasic calcium phosphate (BCP) biomaterial discs and inserted in the back of mice ( A ). Several BCP discs were not loaded with cells as negative controls ( B ). After 8 weeks, all discs were removed and evaluated for collagen matrix deposition within transplants and human cell engraftment. Vessels and bone collagen were observed after hematoxylin (left) or Masson’s trichrome (right) staining, respectively. Dotted black lines indicate the outer border of discs. Red arrows show vessels, and blue arrows show bone matrix. ( C ) BCP discs loaded with EGM-2–cultured PPs show trabeculae lined with cells engulfed within matrix on Masson’s trichrome staining (green fibers).
https://doi.org/10.1371/journal.pone.0048648.g006
Animal Study and Histology
All animal study procedures were performed in accordance with a protocol approved by the local committee for animal care and ethics (French ethics committee CEEA.PdL.06 and local veterinary services (license n°C44015)). Macroporous biphasic calcium phosphate (BCP) ceramic discs, 8 mm in diameter and 3 mm thick (Biomatlante SA, Vigneux de Bretagne, France), were composed of 20 hydroxyapatite and 80 wt.% β-tricalcium phosphate and sterilized by gamma irradiation (total porosity: 70% to 75%; macroporosity: 400 µm). All experiments involved a minimum of 5 animals per group. In total, 200 µL medium containing 7.5×10 5 human MSCs were seeded onto BCP discs, and after their absorption, 1 mL medium was added (experiments were repeated with MSCs from 3 different donors). The cells were cultured in the tested media for 7 days in low attachment plates, and medium was refreshed twice weekly. Five samples were used for each condition.
Alu sequences were checked in cells from microsections of BCP transplants seeded with PPs cultured with EGM-2 ( A–C ) or MSCs with MSM ( D ). ( A ) Human cells were depicted by red staining in nuclei (arrowheads). ( B ) and ( C ) enlargement of the squared area in ( A ). Human cells lined BCP trabeculae, formed fibrous tissue, or were at the abluminal position of vessels ( B, C ). ( D ) Very few cells of human origin were detected with seeding of MSM-cultured MSCs. ( E ) Quantification of human vs host cells involved counting the number of Alu+ nuclei (*p<0.001) in more than 10 fields per transplant and 3 transplants.
https://doi.org/10.1371/journal.pone.0048648.g007
Cultured BCP discs were subcutaneously implanted in the backs of NMRI nude male mice (Elevage Roger Janvier, Le Genest Saint Isle, France). A 1-cm-long incision was made on each side of the mouse back, and blunt dissection was used to separate the skin from subcutaneous connective tissue to form pockets into which discs were inserted. The mice were killed after 8 weeks by lethal intracardiac injection of thiopental, and the implants were retrieved. The explants were processed according to standard operating procedures for non-decalcified histology. Samples were decalcified in EDTA, dehydrated and embedded into paraffin; 100-µm-thick cross sections were obtained by use of a diamond-saw microtome (Leica SP 1600, Leica Microsystems, Nanterre, France), stained with hematoxylin or Masson’s trichrome and analyzed by light microscopy.
The human origin of cells within the transplants was confirmed by in situ hybridization of human-specific genomic repetitive sequence as described [19] . A locked nucleic acid (LNA) probe was designed within the human Alu-Sb2 subfamily consensus sequence (Genbank no. HSU14570) with the advanced online design software of Exiqon (Vedbaek, Denmark). The LNA-Alu probe was labeled with Digoxigenin (DIG) at the 5′ and 3′ ends. Deparaffinized and rehydrated sections were treated with 1 mg/ml pepsin in 0.1 N HCl at 37°C for 30 min. After a wash with PBS, 70 nM Alu-LNA probe was added to tissue sections in 2X SSC (300 mM sodium chloride, 30 mM trisodium citrate, pH 7.4), 50% deionized formamide and 10% dextran sulfate. Denaturation of DNA was carried out at 95°C for 5 min, followed by hybridization at 37°C for 2 hr. After 2X SSC 50% deionized formamide washes, the hybridization of LNA-Alu probe was revealed with horseradish peroxydase-conjugated anti-DIG antibody conjugated (1/50e; Roche Diagnostic France) with use of an AEC staining kit (Sigma Aldrich). Nuclei were counterstained with hematoxylin and sections were dehydrated and mounted with Faramount medium (Dako). Sections of human osteosarcoma in muscle of nude mouse were positive and negative controls during in situ hybridization with the LNA-Alu probe.
Statistical Analysis
Data are expressed as mean ± SEM and were analyzed by nonparametric Kruskal-Wallis test. Two-sided P<0.05 was considered statistically significant.
EGM-2-derived Cells are Highly Plastic Progenitors
We split BM mononuclear cells from donors and cultured them in in vitro medium adapted for pericytes, EGM-2 [20] , and compared results to culture in medium for MSCs, MSM. Culture in EGM-2 produced CFU-fs. EGM-2- and MSM-cultured cells did not differ in lag phase before colony appearance, proliferation or CFU-f number, size or number of bipotential CFU-f (adipocyte and osteoblastic potentials) (data not shown). As well, EGM-2– and MSM-cultured cells did not differ in MSC phenotypic characterization, including morphologic features, membrane molecules or ability to generate osteoblasts, chondroblasts and adipocytes ( Figure 1 ). However, when EGM-2-derived cells were cultured under neurogenic conditions, cells aggregated to form MAP2+ neurosphere-like structures, which were never observed with standard MSCs ( Figure 2 ). After their dissociation, the cells were assessed for adipocyte and osteo-chondroblastic multipotentiality. We observed lipid-droplet–containing cells or mineralization, although with low efficiency. In addition, after prolonged neurogenic induction, we observed neuronal cells. Of note, in contrast to standard MSCs, EGM-2–cultured MSCs showed a significant increase in commitment toward a neuronal lineage ( Figure 2 & Figure S1 ).
EGM-2 Culture Consistently Produced a Pericyte Phenotype
We assessed the perivascular/pericytic phenotype and function of cells in our in vitro conditions. We characterized pericytes with anti-αSM actin and -3G5 antibodies, 2 markers used for pericyte characterization [6] . 3G5 staining was greater for EGM-2– than MSM-cultured MSCs ( Figure 3A, B ). Because pericytes can themselves (and without endothelial cells) form interconnected networks between them when seeded on basement membrane proteins found in Matrigel [21] , [22] , we tested this potential after overnight incubation. As compared with MSM-cultured MSCs, only EGM-2–cultured cells organized into pericyte-like networks ( Figure 3C, D ). As well, when seeded with HUVECs, EGM-2–cultured cells were organized near HUVECs as αSM actin+ cells recovering von Willebrand factor+ endothelial cells and forming structures resembling tubes ( Figure 3E ). Therefore, EGM-2-cultured cells exhibited a pericyte phenotype and function that was more marked than with MSM-cultured MSCs. Surprisingly, almost all MSM-cultured but only a few EGM-2–cultured PPs were positive for αSM actin.
We wondered whether the media were capable of modulating 3G5 antigen and αSM actin expression, so we cultured cells in MSM until confluence and shifted them to EGM-2 and vice versa ( Figure 3F ). Whatever the combination after culture in MSM, all cells remained 3G5 dim but αSM actin+. Therefore, the standard MSC phenotype persisted in MSCs, whatever the culture conditions used, as compared with EGM-2 cells, which showed modified expression of 3G5 antigen or αSM actin in MSM culture and rendered them phenotypically indistinguishable from standard MSCs. Because the αSM actin molecule is a differentiation-stage–dependent marker of VSMC lineage, EGM-2-cultured PPs might be phenotypically more immature than standard MSCs [6] . Therefore, regarding these data EGM-2 derived cells were hereafter referred to as pericyte progenitors (PPs).
EGM-2-cultured PPs are more Immature than are MSM-cultured MSCs
Classical MSCs express αSM actin and several lineage markers because of their differentiation potential [23] . In addition to αSM actin for the VSMC lineage, osteoblastic, chondroblastic and adipocytic lineages are revealed by the expression of the molecules RUNX2, SOX6 and PPARγ, respectively [23] . The mRNA expression of all genes characterizing chondroblasts ( COL2A1 , SOX9 , SOX6 and DLX2 ), adipocytes ( AdipoQ , LEP ), osteoblasts ( ALPL , PTHR1 , RUNX2 , DLX5 and OSC ), and VSM ( EMX2 , CNN3 , ELN , MYOC ) was lower in EGM-2– than MSM-cultured cells, but the mRNA level of PPARγ2 was comparable ( Figure 4A ).
RUNX2 (a master gene of skeletogenesis) is expressed in BM-MSCs [1] . Hence, it typifies BM-MSCs as skeletal stem cells (i.e., cells generating all BM mesenchymal lineages). We found RUNX2 protein level decreased in EGM-2-cultured PPs, which confirmed the RT-PCR mRNA results ( Figure 4B ).
We focused on the molecules NANOG, OCT4 and SOX2 that are specific to immaturity/stemness characterizing embryonic and induced pluripotent stem cells (ES and iPS, respectively) [24] . OCT4 has several isoforms, mainly OCT4A and OCT4B, and only OCT4A contributes to the stemness properties found in ES and iPS cells [25] . The mRNA expression of OCT4A and SOX2 was greater (2- and 20-fold increase, respectively) in EGM-2– than MSM-cultured cells ( Figure 5A ), but the 2 cells types did not differ in NANOG expression. The protein level of OCT4A was consistently found in EGM-2 culture but was not or was barely detected in MSM-cultured MSCs; the protein expression of SOX2 was detected only in EGM-2–cultured PPs ( Figure 5B ). Furthermore, upregulated OCT4A and SOX2 expression was associated with decreased RUNX2 expression in EGM-2–cultured PPs, which was opposite to that in MSM-cultured cells. Therefore, with the increased protein expression of stemness markers and concomitant decrease in lineage markers, EGM-2-cultured PPs were phenotypically more immature than MSM-cultured MSCs.
EGM-2-cultured PPs Show Strong Engraftment Potential
We investigated engraftment capacity in vivo in nude mice. BM cells were cultured in EGM-2 or MSM and then seeded into bone substitutes (BCP discs). After adherence, biomaterials were inserted in mice for 8 weeks. Collagen matrix inside the biomaterials was greater for discs seeded with EGM-2– than MSM-cultured cells ( Figure 6 ). Furthermore, discs seeded with EGM-2–cultured PPs showed cells lining trabecules and engulfed within extracellular matrix as compared with discs seeded with MSM-cultured MSCs ( Figure 6C ). In seeking the human origin of cells with Alu sequences, we observed numerous human cells with a mesenchymal appearance only within EGM-2–derived transplants ( Figure 7A ). In contrast to EGM-2–cultured PPs, few MSM-cultured MSCs of human origin were detected (48.1%±6 vs. 1.8±0.7 human to host cells, respectively, p<0.001) ( Figure 7D and E ). We found 3 types of localizations in EGM-2–derived transplants: the perivascular position, within the fibrous matrix and along BCP trabeculae (most). Interestingly, despite their strong engraftment potential, PPs had significantly lower effective immunosuppression properties than MSCs in vitro ( Figure S2 ).
The abluminal position seems to be the preferential niche for several types of multipotential cells such as MSCs and PPs, whatever the tissue of origin. This position allows them to maximize all of their properties (i.e., organogenesis, tissue renewal and regeneration post-injury). MSCs and PPs share numerous properties and are phenotypically similar. Here, we aimed to compare these 2 types of cells from human BM. In vitro -derived PPs cultured in EGM-2 showed more plasticity, stronger expression of stemness markers (immaturity) and more engraftment potential than classically cultured MSCs. Notably, at 8 weeks post-transplantation in mice, EGM-2–derived PPs showed strong capacity for collagen matrix formation and were found mainly in perivascular and trabecular niches.
Despite the strong engrafment potential of PPs in mice, PPs showed in vitro immunosuppression but at a lower extent than for MSCs. The reactivity of PPs with immune cells was previously not investigated and deserves further study.
Phenotypes of BM-derived PPs and MSCs are similar except in 3G5 and αSM actin expression. 3G5 is used to characterize or select pericytes [6] , [13] . Despite any comparison between BM MSCs and BM PPs, 3G5 was also found expressed by MSCs, although in their native form [26] , [27] . 3G5 was found in cultured MSCs, but its expression seems to be downregulated with standard culture [27] . This finding is in agreement with our results showing that standard culture conditions (MSM) irreversibly decreased 3G5 antigen expression. Furthermore, such decrease was followed by induced expression of αSM actin protein.
αSM actin is also widely used as a pericyte and MSC marker whatever the tissue of origin [28] . Our data showed few cells positive for αSM actin when expanded in EGM-2. This discrepancy can be explained by the immaturity of EGM-2 derived cells. Indeed, in VSM differentiation processes, αSM actin is an early-differentiation stage marker, which implies that more immature cells do not express it [29] , [30] . This suggestion was confirmed recently by several reports showing that pericyte precursors from iPS or perivascular progenitor cells do not express αSM actin [31] , [32] . However, the αSM actin gene promoter contains the CArG box, a cis-element regulating its expression, as well as those of the VSMC lineage (e.g., SM22α and CD49a) [33] . The CarG element specifically binds the serum response factor, which is stimulated by serum [34] . Because serum is in much higher content in standard medium for MSC expansion (10% FBS) than in EGM-2 (2% FBS), standard culture conditions are thus more likely to induce αSM actin expression than EGM-2 medium. Nevertheless, despite the absence of αSM actin expression, EGM-2-derived PPs could give rise to cells with pericyte capacities because they can cover endothelial cells and form a network in Matrigel, even in the absence of endothelial cells [21] , [22] . Interestingly, these pericytes became αSM actin+ when interacting closely with HUVECs in co-cultures, which suggests their induction from endothelial cells as previously described [35] . Therefore, despite the reduced αSM actin expression (mechanisms that remain to be clarified), EGM-2–cultured PPs showed pericyte properties.
The reduced αSM actin expression in PPs suggested that they were highly immature. This hypothesis was confirmed by the low expression of a panel of lineage markers concomitant with the upregulated expression of stemness markers. Indeed, OCT4 and SOX2 proteins were consistently expressed with EGM-2 culture, and their expression was opposite to that of the osteoprogenitor marker RUNX2. This finding agrees with OCT4 and SOX2 being stemness proteins allowing self-renewal of ES cells, with greatly decreased expression when cells commit to a lineage [36] . The finding is also in line with a recent report demonstrating that early-passage MSCs, or MSCs with p21 knocked out, showed upregulated expression of OCT4, SOX2 and NANOG to the detriment of tissue-specific markers and that knock-down of OCT4 or NANOG enhanced the expression of the tissue markers [37] . The authors used 16.6% FBS and hypoxic (1% O 2 ) conditions for expansion of MSCs. Therefore, the culture conditions are crucial for the stem-cell potential of MSCs. Here, we showed that pericyte-specific culture conditions generated PPs with more immature features that standard MSCs.
Despite the well-known plasticity of standard MSCs, the immature state of EGM-2–cultured PPs was associated with greater differentiation potential because they yielded reliable neuronal differentiation, whereas most MSM-cultured MSCs did not survive after induction. Interestingly, after induction, EGM-2-expanded PPs formed aggregates resembling neurospheres consisting of cells capable of generating osteoblasts, adipocytes and neuronal cells. Cells forming neurospheres can be stem-cell–like cells [38] . This phenomenon was previously reported for PPs from post-natal rat aorta and perivascular progenitor cells from human adult vena saphena, which further demonstrates a similar phenotype to the PPs we describe [32] , [39] .
Therefore, as compared with standard culture conditions for MSCs, culture with EGM-2 selects PPs with broader potentiality, probably because of their immature state. However, we cannot exclude that BM contains several types of non-hematopoietic multipotential cells and in vitro conditions could select one type for their expansion. Nor can we exclude that highly immature non-hematopoietic multipotential cells can be expanded better under EGM-2 than standard culture conditions or, in other words, standard conditions may allow for more restrictive stem-cell potentials, probably because of proteins in serum. Indeed, we showed the irreversible gain in αSM-actin expression when cells were cultured under standard conditions ( Figure 3F ).
In conclusion, EGM-2 can be used for expansion of highly immature PPs with strong differentiation and engraftment capacities. These conditions can also allow for studying BM adult stem-cell biology and may improve in vitro protocols for clinical use. However, several questions remaining to be solved include the origin of EGM-2-derived PPs, their true stemness states, their complete differentiation capacities and the molecular mechanisms yielding such properties.
Supporting Information
Neuron differentiation potential of cells cultured in endothelial growth medium 2 (EGM-2) and mesenchymal stem cell medium (MSM). ( A ) Quantitative RT-PCR analysis of mRNA level of neuronal markers MAP2 , β3Tubulin or β3T , MEST , GRIA3 and ( B ) in situ immunofluorescence ( Table ) of SLUG, NeuN, MAP2, and SOX10 levels. Differentiation potentials are at the bottom of the panel. For neuronal differentiation characterization, cells were fixed and permeabilized after neuronal induction, then incubated at 37°C for 1 h with the primary antibody mouse anti-human Nestin (hNestin) clone 196908, anti-SLUG or anti-SOX10 (R&D Systems, Lille, France); mouse anti-hNeuN (Sigma-Aldrich); mouse anti-human microtubule-associated protein 2 (hMAP2) clone M-9942 (Sigma-Aldrich); rabbit anti-hP75 (nerve growth factor receptor [NGFR]) (ab-8878, Abcam, Paris); rabbit anti-hAP2α (Abcam, ab108311) and then at 37°C for 45 min with FITC-labeled goat anti-mouse or donkey anti-rabbit secondary antibody (Invitrogen). Positive cells were counted and compared to total cell counts for percentage positive cells.
https://doi.org/10.1371/journal.pone.0048648.s001
Immunosuppression potential assay of PPs and MSCs. For in vitro immunosuppression assay 50×10 3 cells cultured in EGM-2 or MSM were co-cultured with 100×10 3 CD3+ T cells isolated from PBMCs by use of a T-cell purification kit (Miltenyi Biotec, Bergish Gladbach, Germany). We used 3 donors for MSCs or PPs and 3 donors for PBMCs. Before the co-cultures, CD3+ cells were labelled with 5-(and-6)-carboxyfluorescein diacetate, succinimidyl ester (CFSE, Invitrogen) as fluorescent cell-tracing reagent. T cells were then activated with CD3+CD28+ microbeads (Miltenyi Biotec). After 5 days, all cells were recovered, and T cells were stained with anti-CD3 and anti-CD45 antibodies (Miltenyi Biotec). The proportion of cycling CD3+CD45+ T cells was quantified by fluorescence decrease of CFSE as compared with both uncycling cells (non-stimulated cells) and stimulated T cells cultured without MSCs or PPs. Analysis involved use of the Cyan™ flow cytometer (Beckman Coulter, Villepinte, France) and Kaluza™ software. The percentage of CD3+ T lymphocytes proliferating during the co-cultures (LyT + MSCs or LyT + PPs) were calculated. Stimulated T lymphocytes without MSCs or PPs (LyT) were used as control cells, for 100% of cycling cells. Data are mean ± SEM from 3 experiments.
https://doi.org/10.1371/journal.pone.0048648.s002
Author Contributions
Conceived and designed the experiments: AB VT TC PL FD. Performed the experiments: AB VT TC FD FG NE. Analyzed the data: AB VT TC PL FD. Contributed reagents/materials/analysis tools: PR DH PL LS FD. Wrote the paper: VT FD.
- View Article
- Google Scholar
- 31. Dar A, Domev H, Ben-Yosef O, Tzukerman M, Zeevi-Levin N, et al. (2012) Multipotent Vasculogenic Pericytes from Human Pluripotent Stem Cells Promote Recovery of Murine Ischemic Limb. Circulation.
- 37. Tsai CC, Su PF, Huang YF, Yew TL, Hung SC (2012) Oct4 and Nanog Directly Regulate Dnmt1 to Maintain Self-Renewal and Undifferentiated State in Mesenchymal Stem Cells. Mol Cell.
Philippe Rosset
Areas of expertise.
Many experts have expertise treating multiple conditions. MediFind uses a variety of data sources to determine what conditions a expert treats and their level of experience.
- Adult Soft Tissue Sarcoma
- Hip Replacement
- Septic Arthritis
- Infectious Arthritis
- Kienbock's Disease
- Knee Replacement
- Liposarcoma
- Myxoid Liposarcoma
- Osteoarthritis
- Osteolysis Syndrome Recessive
- Osteomyelitis
- Osteonecrosis
- Osteosarcoma
- Solitary Fibrous Tumor
About Philippe Rosset
Philippe Rosset practices in Tours, France. Rosset is highly rated in 3 conditions, according to our data. His top areas of expertise are Adult Soft Tissue Sarcoma, Septic Arthritis, Bone Tumor, Hip Replacement, and Osteotomy.
His clinical research consists of co-authoring 96 peer reviewed articles and participating in 7 clinical trials in the past 15 years.
Background & Education
Clinical research.
Clinical research consists of overseeing clinical studies of patients undergoing new treatments and therapies, and publishing articles in peer reviewed medical journals. Experts who actively participate in clinical research are generally at the forefront of the fields and aware of the most up-to-date advances in treatments for their patients.
7 Clinical Trials
96 total publications.
- Case report
- Open access
- Published: 17 December 2018
Acute Tibial osteomyelitis caused by intraosseous access during initial resuscitation: a case report and literature review
- Thomas Chalopin 1 , 2 ,
- Adrien Lemaignen 1 , 2 ,
- Antoine Guillon 3 ,
- Arnaud Geffray 4 ,
- Gaelle Derot 4 ,
- Olivier Bahuaud 1 , 2 ,
- Charles Agout 5 ,
- Philippe Rosset 5 ,
- Claire Castellier 6 ,
- Gonzague De Pinieux 6 ,
- Anne-Sophie Valentin 7 ,
- Louis Bernard 1 , 2 ,
- Frederic Bastides ORCID: orcid.org/0000-0001-6335-172X 1 , 2 , 8 &
Centre De Référence Des Infections Ostéo-Articulaires Du Grand-Ouest (CRIOGO) Study Team
BMC Infectious Diseases volume 18 , Article number: 665 ( 2018 ) Cite this article
6262 Accesses
8 Citations
1 Altmetric
Metrics details
Intra-osseous (IO) access is recommended in cases of pre-hospital emergency or resuscitation when intravascular (IV) route is difficult or impossible. Despite recent improvement in IO devices and increasing indications, it remains rarely used in practice. Various complications have been reported but are uncommon.
Case presentation
We report a case of massive acute tibial osteomyelitis in an adult male three months after an IO catheter insertion for emergency drug infusion. We review the literature on association between IO access and acute osteomyelitis in children and adults.
Conclusions
Emergency-care givers and radiologists should be informed about this infrequent complication in order to make early diagnosis and initiate adequate antibiotic therapy.
Peer Review reports
Intraosseous (IO) access is considered as an effective route in adults requiring emergency administration of fluids or medication for initial resuscitation [ 1 ]. IO infusion is an important and safe alternative to the intravenous route in cases of difficult vascular access due to obesity, edema, or exhausted venous access in special populations (e.g drug-addicts). Despite improvements in IO devices and prehospital management of cardio-pulmonary resuscitation, IO infusion is not commonly used in clinical practice [ 2 , 3 ]. Complications after IO access are uncommon. However, extravasation, air embolism or skin abscesses have been reported. Osteomyelitis occurs in less than 1% [ 4 , 5 ] with a very small number of cases reported, to the best of our knowledge [ 6 , 7 , 8 , 9 , 10 ]. We report an unusual case of massive acute tibial osteomyelitis in an adult, three months after an IO infusion used in an initial resuscitation at home. We proposed to review the cases published in the English language literature with paying attention particularly to risk factors, diagnosis, treatment and outcome, to contribute to the description and management of this condition.
A forty-year-old psychotic and intravenous-drug-addicted Caucasian male was cared by prehospital service for coma due to drugs overdose. In this emergency situation, without any intravenous access available, an IO device (EZ-IO™; Teleflex Medical, Research Triangle Park, NC, USA.) was promptly inserted by the emergency medical technician (EMS) on scene in the upper portion of the left tibia to administer therapeutics and initiate mechanical ventilation. Hospitalization in the intensive care unit of the University Hospital of Tours (France) with close monitoring lasted three days. Several attempts to establish another IV catheter were unsuccessful and no central catheter was used as the need for infusion was expected to be short due to a rapid clinical improvement. The IO catheter was removed at Day 1, with report of local inflammation around the insertion site. An erysipelas was diagnosed. Treatment with oral amoxicillin-clavulanic acid (1gx3/day) was introduced. The patient reported psychiatric problems with schizophrenia, multiple intravenous-drug intoxications with coma, and regular cocaine and heroin use. He left the hospital against medical advice three days after IO device removal.
Three months later, he asked for a consultation in the same hospital because of fever and bone pain in the left leg and was hospitalized again. Other complaints were chills and inability to walk normally and to bear weight on his left leg. Redness, warmth, point tenderness and swelling on the site of the IO access were present (Fig. 1 a, arrow). He was afebrile, without hemodynamical instability. Laboratory results were only significant for leukocytosis at 12.4.10 9 /L and C reactive protein at 51.2 mg/l. Blood cultures were negative. Routine radiographs revealed an ill-defined osteolysis of the metaphysis and the epiphysis with a condensed area and blurred periosteal appositions (Fig. 1 b). The magnetic resonance showed an important marrow edema with T1-weighted hyposignal (Fig. 1 c) and fat-saturated-T2-weighted hypersignal (Fig. 1 d) extending in the left tibia, measuring twenty-one centimeters. Soft tissues were infiltrated. No abscess was visualized but the radiologist could not achieve gadolinium injection because IV access was lacking. MR imaging was compatible with the diagnosis of osteomyelitis.
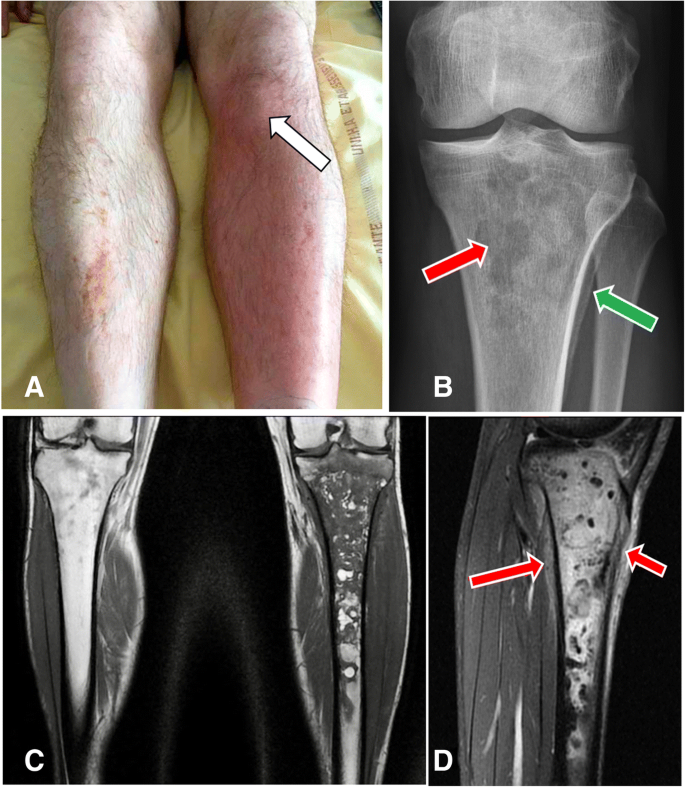
a Image of the left tibia, well-defined area with redness, warmth and swelling over his left proximal tibia corresponding to the site of the intraosseous injection (arrow). The contralateral leg is normal. b X-rays of left proximal tibia in front view with multiple ill-defined lytic lesion in the metaphysis and the epiphysis and geographic pattern (red arrow) and also proximal tibial blurred periosteal appositions (green arrow). c Coronal T1-weighted section with massive epiphyso-metaphyseal hypoT1 intra-medullary bone edema extended to the diaphysis up to the middle third of the leg. d Sagittal fat-sat T2–weighted section with significant hyperT2 infiltration of soft tissues (arrows)
An open biopsy of the left tibia revealed Gram positive cocci with focal signs of acute osteomyelitis, bone remodeling and marrow fibrosis containing polymorphic inflammatory infiltrate comprising neutrophils, foamy macrophages, lymphocytes and plasma cells. Treatment with piperacillin-tazobactam and vancomycin was initiated. Culture from the surgical site grew methicillin-susceptible Staphylococcus aureus (MSSA). An oral switch with levofloxacin 750 mg/day and rifampin 900 mg/day for six weeks was introduced. A favorable outcome was noted eighteen months later.
IO infusion provides access to a non-collapsible venous complex, enabling administration of resuscitation drug therapy to start treatment of shock, cardiac arrest or in severe trauma. In adults, the European Resuscitation Council Guidelines for Resuscitation established in 2015 that IO route is required in emergency situations whenever peripheral access cannot be achieved: it can be used for infusion, drug administration and blood samples [ 11 ]. Two prospective trials in children and adults, consolidated by several studies, documented that IO access was safe and effective for initial resuscitation cases [ 12 , 13 , 14 , 15 , 16 ].
In practice, IO-access is rarely used despite many advents in IO insertion devices making the procedure easier and faster [ 3 , 17 ]. In 2012 in France, 29% of practitioners (intensivists, EMS, anesthetists) have used an IO kit in real-life. Concerning training, 55% of them have been trained to IO procedure (83% of EMS and 33% of intensivists) [ 18 ]. Currently, several IO insertion devices are approved by the Food & Drug Administration (FDA) [ 19 ]. Different insertion sites have been evaluated with three possible locations: the proximal tibia, the distal tibia and the proximal humerus [ 20 ]. The most frequently used is the proximal tibia, located 2 cm below the tibial tuberosity and 1 to 2 cm medial in the middle of the flat bone surface as in our patient [ 21 ].
The procedure of IO access must be performed under strictly sterile conditions. Even if multiple studies proved IO access to be safe and effective compared to IV route, various complications have been reported [ 1 , 22 , 23 ]. Two studies concluded that fluid extravasation is the most common complication (12%) following by skin abscesses, cellulitis or embolism [ 4 , 5 ].
Acute osteomyelitis is an inflammatory process in bone and bone marrow, most often caused by pyogenic bacteria, with different pathogenesis, either haematogenously-acquired, or associated with peripheral vascular disease like diabetes, or with contiguous-focus infection as in our case. Today, the spectrum of osteomyelitis is changing. It occurs most frequently from contiguous-focus spread after an open fracture, reconstructive surgery, or with a direct inoculation from trauma as IO access. Therefore, we performed an extensive literature search of databases for articles published up to 1997, to correctly report the osteomyelitis incidence with new IO devices. We found only two cases in the literature reporting an acute osteomyelitis in an adult after IO infusion, other cases occurred in children where IO access is more frequent (Table 1 ). In published studies with a large cohort, osteomyelitis occurs in less than 1% of patients (children and adults) and thus is the most unusual late complication reported due to direct inoculation. In a retrospective, online questionnaire-based investigational study, in 1.802 cases of IO use, the rate of osteomyelitis is 0.4%, corresponding to seven patients [ 23 ]. This rate is very close to those reported by Rosetti et al. , (0.6%) or Leidel et al. , (0.6%) [ 5 , 12 ]. Published data suggest that osteomyelitis is considered as a serious late complication to IO infusion, with a very low rate, with clinical and bacteriological heterogeneity. This is the first case of IO-access acute osteomyelitis reported in our institution with the use of around 40 IO-devices each year.
Our case demonstrated a massive delayed osteomyelitis in a comorbid drug-addict patient who is predisposed to cutaneous infection, and with a frequent indolent course reported in osteomyelitis secondary to a contiguous focus of infection. Even if erysipelas was initially suspected and treated, treatment was not taken entirely and osteomyelitis was rapidly evoked three months later after clinical examination and X-Ray. This data emphasizes that complete and early treatment of the erysipelas might prevent the development of subsequent osteomyelitis in this situation. Rapid management is required to avoid more severe complications such as chronic bone and articular dislocation. The key for successful management is early diagnosis, based on clinical examination, imaging procedures including conventional radiographs and MRI, bone sampling (open biopsy) for microbiological and pathological examination to enable targeted and long-lasting antibiotic strategy. Staphylococcus aureus is definitely the most frequent pathogen responsible for osteomyelitis in any age group, mainly methicillin-susceptible strains (MSSA), and it is responsible for up to 70–90% of confirmed cases [ 24 , 25 ]. This infection requires prompt antibiotic therapy, initially IV and then switch to oral antibiotics. However, no consensus was found regarding the duration of antibiotic treatment, but some authors suggest the possibility of reducing the IV antibiotic duration to a few days and “step-down” to an oral antibiotic with targeted oral therapy [ 26 , 27 ] for at least six weeks. In the future, development of novel and more effective strategies, such as the local delivery of antibiotics, biofilm disruptors or immunotherapy could improve therapeutic outcomes.
In conclusion, intraosseous infusion is an effective alternative if IV access is not readily attainable for adults requiring urgent parenteral access for initial resuscitation. We reported here a potentially limb threatening acute tibial contiguous-focus osteomyelitis three months after intraosseous catheter insertion. One has to keep in mind that adverse events with low incidence rate can be easily underestimated. Thus, we believe that it is important to continue to report long-term infectious complications of this unusual infusion procedure as they are difficult to capture by conventional clinical trials.
Petitpas F, Guenezan J, Vendeuvre T, et al. Use of intra-osseous access in adults: a systematic review. Crit Care. 2016;20:102.
Article CAS Google Scholar
Shavit I, Hoffmann Y, Galbraith R, et al. Comparison of two mechanical intraosseous infusion devices: a pilot, randomized crossover trial. Resuscitation. 2009;80:1029–33.
Article Google Scholar
Santos D, Carron PN, Yersin B, et al. EZ-IO(®) intraosseous device implementation in a pre-hospital emergency service: a prospective study and review of the literature. Resuscitation. 2013;84(4):440–5.
Buck ML, Barbara SW, Jefferson MS. Intraosseous drug administration in children and adults during cardiopulmonary resuscitation. Ann Pharmacother. 2007;41:1679–86.
Rosetti VA, Thompson BM, Miller J, et al. Intraosseous infusion: an alternative route of pediatric intravascular access. Ann Emerg Med. 1985;14:885–8.
Dogan A, Irmak H, Harman M, et al. Tibial osteomyelitis following intraosseous infusion: a case report. Acta Orthop Traumatol Turc. 2004;38(5):357–60.
PubMed Google Scholar
Henson N, Payan J, Terk M. Tibial subacute osteomyelitis with intraosseous abscess: an unusual complication of intraosseous infusion. Skelet Radiol. 2011;40:239–42.
Rosovsky M, FitzPatrick M, Goldfarb C, et al. Bilateral osteomyelitis due to intraosseous infusion: case report and review of the English-language literature. Pediatr Radiol. 1994;24:72–3.
Stoll E, Golej J, Burda G, et al. Osteomyelitis at the injection site of adrenalin through an intraosseous needle in a 3-month-old infant. Resuscitation. 2002;53:315–8.
Yee D, Deolankar R, Marcantoni J, et al. Tibial osteomyelitis following pre-hospital intraosseous access. Clin Pract Cases Emerg Med. 2017;1(4):391–4.
Soar J, Nolan JP, Bottiger BW, et al. European resuscitation council guidelines for resuscitation 2015. Resuscitation. 2015;95:100–47.
Leidel BA, Kirchhoff C, Bogner V, et al. Comparison of intraosseous versus central venous vascular access in adults under resuscitation in the emergency department with inaccessible peripheral veins. Resuscitation. 2012;83(1):40–5.
Lewis P, Wright C. Saving the critically injured trauma patient: a retrospective analysis of 1000 uses of intraosseous access. Emerg Med J. 2015;32(6):463–7.
Hoskins SL, do Nascimento P Jr, Lima RM, et al. Pharmacokinetics of intraosseous and central venous drug delivery during cardiopulmonary resuscitation. Resuscitation. 2012;83:107–12.
Banerjee S, Singhi SC, Singh S, et al. The intraosseous route is a suitable alternative to intravenous route for fluid resuscitation in severely dehydrated children. Indian Pediatr. 1994;31:1511–20.
CAS PubMed Google Scholar
Brickman KR, Krupp K, Rega P, et al. Typing and screening of blood from intraosseous access. Ann Emerg Med. 1992;21:414–7.
Weiser G, Hoffmann Y, Galbraith R, Shavit I. Current advances in intraosseous infusion - a systematic review. Resuscitation. 2012;83:20–6
Abbal B, Perbet S, Pereira B, et al. Azurea group. Use of the intraosseous access in adult patients in France in 2012. Annfar. 2014-02:006.
Committee ECC. Subcommittees and task forces of the American Heart Association. 2005 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2005;112:IV1–203.
Anson J. Vascular access in resuscitation: is there a role for the intraosseous route? Anesthesiology. 2014;120(4):1015–31.
Polat O, Oguz A, Eneyli M, et al. Applied anatomy for Tibial intraosseous access in adults: a Radioanatomical study. Clin Anat. 2017;00:00–0.
Luck RP, Haines C, Mull CC. Intraosseous access. J Emerg Med. 2010;39(4):468–75.
Hallas P, Brabrand M, Folkestad L. Complication with intraosseous access: inquiry of Scandinavian users’ experiences. Westjem. 2013;1(12000).
Thomsen I, Creech CB. Advances in the diagnosis and management of pediatric osteomyelitis. Curr Infect Dis Rep. 2011;13:451–60.
Lew DP, Waldvogel FA. Osteomyelitis. Lancet. 2004;364(9431):369–79.
Peltola H, Pääkkönen M, Kallio P, et al. Osteomyelitis-septic arthritis (OM-SA) study group. Prospective, randomized trial of 10 days versus 30 days of antimicrobial treatment, including a short-term course of parenteral therapy, for childhood septic arthritis. Clin Infect Dis. 2009;48:1201–10.
Mitchell C, David W, Anderson E. At al. Osteomyelitis: recent advances in pathophysiology and therapeutic strategies. Westjem. 2013;14(5):440–43.
Download references
Acknowledgements
Not Applicable.
Availability of data and materials
Author information, authors and affiliations.
Department of Internal Medicine and Infectious Diseases, University Hospital of Tours, Hospital Bretonneau, Tours, France
Thomas Chalopin, Adrien Lemaignen, Olivier Bahuaud, Louis Bernard & Frederic Bastides
François Rabelais University, Tours, France
Department of Intensive Care Unit, University Hospital of Tours, Tours, France
Antoine Guillon
Department of Medical Imaging, University Hospital of Tours, Tours, France
Arnaud Geffray & Gaelle Derot
Department of Orthopedic Surgery, University Hospital of Tours, Tours, France
Charles Agout & Philippe Rosset
Department of Anatomopathology, University Hospital of Tours, Tours, France
Claire Castellier & Gonzague De Pinieux
Bacteriological Laboratory, University Hospital of Tours, Tours, France
Anne-Sophie Valentin
2 boulevard Tonnellé, 37044, Tours, Cedex 9, France
Frederic Bastides
You can also search for this author in PubMed Google Scholar
Contributions
TC and FB collected the data and wrote the manuscript; AL, AG, AG, CA, PR, OB, AL, FB and LB treated the patient and approved the manuscript; GDP, CC provided and analyzed tissue sections; ASV, GD, AG contributed essential tools. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Frederic Bastides .
Ethics declarations
Ethics approval and consent to participate, consent for publication.
Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images. The consent form is held by the authors’ institution and is available for review.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Chalopin, T., Lemaignen, A., Guillon, A. et al. Acute Tibial osteomyelitis caused by intraosseous access during initial resuscitation: a case report and literature review. BMC Infect Dis 18 , 665 (2018). https://doi.org/10.1186/s12879-018-3577-8
Download citation
Received : 25 April 2018
Accepted : 03 December 2018
Published : 17 December 2018
DOI : https://doi.org/10.1186/s12879-018-3577-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Acute osteomyelitis
- Intra-osseous access
- Resuscitation
- Staphylococcus aureus
- Antibiotics

BMC Infectious Diseases
ISSN: 1471-2334
- Submission enquiries: [email protected]
- General enquiries: [email protected]
FILIPE ROSSET
FILIPE ROSSET
Filipe Rosset is a Brazilian guitarist and composer whose guitar mastery and creative talent have won recognition and admiration both in his country and internationally. His musical career is marked by a unique ability to innovate in music, transcending sonic and creative barriers.
It is interesting to know that Filipe Rosset is releasing his first eBook "Future Sounds with the Hexatonic Minor Scale Vol.1", which offers an innovative perspective on guitar phrasing. This book promises to be a significant contribution to the understanding and exploration of the minor hexatonic scale.
FUTURE SOUND S
ONLINE LESSONS
Free fillet lower leg flap for coverage after hemipelvectomy or hip disarticulation
Affiliations.
- 1 Département de chirurgie orthopédique 2, centre hospitalo-universitaire Tours - faculté de medecine, université de Tours, 37000 Tours, France. Electronic address: [email protected].
- 2 Département de chirurgie orthopédique 2, centre hospitalo-universitaire Tours - faculté de medecine, université de Tours, 37000 Tours, France; Inserm UN UMR 1238, PhyOs, bone sarcomas and remodeling of calcified tissues, faculté de médecine de Nantes, 44000 Nantes, France.
- 3 Département de chirurgie oncologique, centre Léon-Bérard, 28, rue Laënnec, 69008 Lyon, France.
- 4 Département de chirurgie orthopédique et reconstructive, centre hospitalo-universitaire Cochin - Port Royal, 27, rue du Faubourg Saint-Jacque, 75014 Paris, France.
- 5 Département de chirurgie plastique et reconstructive, centre hospitalo-universitaire de Tours - faculté de médecine, université de Tours, 37000 Tours, France.
- PMID: 30595412
- DOI: 10.1016/j.otsr.2018.10.018
Introduction: Tumor resection is the gold standard treatment for soft tissue and bone sarcomas. In the pelvis, this may require a hemipelvectomy that can compromise primary skin closure. Flaps are essential in this context; however the vascularization of potential pedicled flaps may have been removed during tumor excision. Using healthy tissue from the amputated limb as a free flap is an excellent coverage option.
Hypothesis: The free fillet flap from an amputated lower limb is a simple and reliable coverage technique after hemipelvectomy or hip disarticulation.
Material and methods: Seven patients were operated on at three specialty centers: six transpelvic amputations (external hemipelvectomy) and one hip disarticulation. In three cases, the flap consisted of the superficial posterior compartment of the calf area and in the three other cases, the lower leg compartments with the fibula and its intact periosteum. Complications were documented.
Results: Clear resection margins were achieved in all patients. The mean follow-up at the final visit was 13 months (range, 6.5 to 21 months). Six patients had complications but only one resulted in loss of the flap. Four patients were able to be fitted with a hip prosthesis.
Discussion: The free fillet flap from an amputated lower limb is a reliable coverage technique (86%) after hemipelvectomy or hip disarticulation. In the 16 cases previously reported in the literature, there were no wound-healing failures. Local flaps are often too fragile with insufficient muscular padding. This free flap is the preferred first-line technique as it spares other potential free flaps in case of failure without increasing the morbidity of a procedure that is already extensive. This coverage technique should be one the options considered after external hemipelvectomy.
Level of evidence: IV, retrospective study.
Keywords: Fillet flap; Free leg flap; Hemipelvectomy; Hip disarticulation; Spare part concept; Transpelvic amputation.
Copyright © 2018 Elsevier Masson SAS. All rights reserved.
- Arthroplasty, Replacement, Hip / methods*
- Bone Neoplasms / surgery*
- Disarticulation / methods*
- Free Tissue Flaps*
- Hemipelvectomy / methods*
- Middle Aged
- Osteosarcoma / surgery*
- Plastic Surgery Procedures / methods*
- Retrospective Studies
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Wiley-Blackwell Online Open

Sarcomas in patients over 90: Natural history and treatment—A nationwide study over 6 years
Clémence basse.
1 Centre Léon Bérard, University Claude Bernard Lyon I, Lyon, France
Antoine Italiano
2 Department of Medical Oncology, Institut Bergonié, Bordeaux, France
Nicolas Penel
3 Department of Medical Oncology, Centre Oscar Lambret, CHRU, Lille, France
Olivier Mir
4 Department of Orthopedic and Traumatology Surgery, University Hospital, Lille, France
Claire Chemin
Maud toulmonde, florence duffaud.
5 Department of Medical Oncology, Timone University Hospital, Marseille, France
Axel Le Cesne
6 Department of Medicine and Surgery, Gustave Roussy Cancer Campus, Paris, France
Christine Chevreau
7 Medical Oncology, University Institute of Cancerology, Toulouse, France
Carlos Maynou
Philippe anract.
8 Orthopaedic Department, Cochin University Hospital, Paris, France
François Gouin
9 Department of Orthopedic Surgery, Nantes University Hospital, Nantes, France
10 Department of Oncology, Institut de Cancérologie de Lorraine, Vandoeuvre‐Lès‐Nancy, France
Nelly Firmin
11 Department of Medicine, Val d'Aurelle Institute, Montpellier, France
Jean‐Emmanuel Kurtz
12 Medical Oncology & Orthopedy Department, Strasbourg University Hospital, Rennes, France
Pierre Kerbrat
13 Centre Eugène Marquis, Medical Oncology, Rennes, France
Sophie Piperno‐Neumann
14 Department of Medical Oncology, Institut Curie, Paris, France
François Bertucci
15 Département of Medical Oncology, Institut Paoli‐Calmettes, Marseille, France
Philippe Rosset
16 Department of Orthopedic and Traumatology Surgery, Tours University Hospital, Tours, France
Nicolas Isambert
17 Centre Georges‐Francois Leclerc, Dijon, France
Emmanuelle Bompas
18 Medical Oncology Department, René Gauducheau, Saint‐Herblain, France
Pascale Dubray‐Longeras
19 Centre Jean Perrin, Clermont‐Ferrand, France
Fabrice Fiorenza
20 Department of Orthopedics Surgery and Traumatology, Limoges University Hospital, Limoges, France
Christine Le Maignan
21 Department of Dermatology and INSERM Unité 976, Saint Louis University Hospital, Paris, France
Loïc Chaigneau
22 Department of Medical Oncology, Jean Minjoz University Hospital, Besançon, France
Antoine Thyss
23 Department of Medical Oncology, Centre Antoine Lacassagne, Nice, France
Olivier Bouché
24 Institut Jean Godinot & Reims University Hospital, Reims, France
Jean‐Christophe Eymard
Corinne delcambre lair.
25 Department of Medicine, François‐Baclesse Institute, Caen, France
Julien Adam
Marie karanian, céleste lebbé, aurélien dupré, pierre meeus, mehdi brahmi, armelle dufresne, françoise ducimetière, isabelle ray‐coquard, jean‐yves blay.
Soft tissue sarcomas (STS) are rare tumors accounting for less than 1% of human cancers. While the highest incidence of sarcomas is observed in elderly, this population is often excluded or poorly represented in clinical trials. The present study reports on clinicopathological presentation, and outcome of sarcoma patients over 90 recorded in the Netsarc.org French national database. NETSARC ( netsarc.org ) is a network of 26 reference sarcoma centers with specialized multidisciplinary tumor board (MDTB), funded by the French National Cancer Institute to improve the outcome of sarcoma patients. Since 2010, presentation to an MDTB, second pathological review, and collection of sarcoma patient characteristics and follow‐up are collected in a database Information of patients registered from January 1, 2010, to December 31, 2016, in NETSARC were collected, analyzed and compared to the younger population. Patients with sarcomas aged >90 have almost exclusively sarcomas with complex genomics (92.0% vs . 66.3%), are less frequently metastatic (5.3% vs . 14·7%) at diagnosis, have more often superficial tumors (39.8% vs . 14.7%), as well as limbs and head and neck sites (75.2% vs . 38.7%) (all p < 0.001). Optimal diagnostic procedures and surgery were less frequently performed in patients over 90 ( p < 0.001). These patients were less frequently operated in NETSARC centers, as compared to those of younger age groups including aged 80–90. However, local relapse‐free survival, metastatic relapse‐free survival and relapse‐free survival were not significantly different from those of younger patients, in the whole cohort, as well as in the subgroup of operated patients. As expected overall survival was worse in patients over 90 ( p < 0.001). Patients over 90 who were not operated had worse overall survival than younger patients (9.9 vs . 27.3 months, p < 0.001). Patients with STS diagnosed after 90 have distinct clinicopathological features, but comparable relapse‐free survival, unless clinical practice guidelines recommendations are not applied. Standard management should be proposed to these patients if oncogeriatric status allows.
Short abstract
What's new?
While the highest incidence of soft‐tissue sarcoma (STS) is observed in the elderly, this population is often excluded or poorly represented in clinical trials. Therefore, little is known about the characteristics, treatment, and outcomes of STS in these patients. In this study, the authors analyzed numerous clinical characteristics of patients with sarcoma diagnosed at age 91 or older. They conclude that standard STS management and clinical practice guidelines should be followed for these patients if possible.
Abbreviations
Introduction.
Soft tissue sarcomas (STS) are rare tumors accounting for less than 1% of human cancers, with a yearly incidence close to 5.9/100,000. 1 , 2 , 3 , 4 , 5 Death due to tumor is frequent in this group of diseases. 5 While the highest incidence of sarcomas is observed in patients aged between 75 and 84 years with a rate of 16%, 1 , 5 elderly patients are often excluded or very poorly represented in clinical trials. 6 Therefore, little is known about characteristics, treatment and outcomes concerning patients aged over 65, in particular for those over 90 years with STS. 6 , 7
Sarcomas represent a heterogeneous group with over 80 different pathological subtypes. 8 Sarcoma with “simple genomics” include those with specific translocations, well‐differentiated liposarcomas with 12q amplicon and GIST with tyrosine kinase mutation accounting for 12–20% of cases each; those with “complex genomic” profiles (e.g., undifferentiated pleomorphic sarcomas [UPS], leiomyosarcomas [LMS] and myxofibrosarcomas [MFS]) account for 50% of all STS. 9 , 10 , 11
The objective of this work was to analyze the presentation and outcome of sarcoma patients over 90 years, a group of patients unreported in the literature, with the aim of providing data which could guide the management of the general elderly patient population with sarcomas.
Patients and Methods
Netsarc network.
NETSARC is the French reference network for the management of soft tissue and visceral sarcomas, collecting clinical, centrally reviewed pathology, therapeutics and outcome of all sarcoma patients in 26 centers. The registry was approved in October 2009 by the INCa (Institut National du Cancer) and the competent authorities (CNIL) in 2010. 5 These databases have been approved by the French Ethics Committee and Agency in charge of noninterventional trials: Comité consultatif sur le traitement de l'information en matière de recherche dans le domaine de la Santé (CCTIRS: number of approval 09.594) and Commission Nationale Informatique et Liberté (CNIL: number of approval 909,510). In the present work, we used information of patients registered from January 1, 2010, to December 2016. Mean follow‐up of this series is 17 months. Patient's data (gender, histology, grade, depth, size, localization, treatment, relapse and survival) were collected from the NETSARC database ( https://netsarc.org ).
Statistical analysis
Collected data were analyzed using IBM SPSS Statistics version 20 (IBM, Paris, France). Chi‐square and Fisher exact tests were performed to analyze the data sets of the different age groups. Survival was plotted according to the Kaplan–Meier method, compared to the log‐rank test. Cox model was used for multivariate analysis of prognostic factors.
A total of 12,835 incident patients with sarcomas, with 113 (0.9%) patients aged >90 were registered in the national NETSARC sarcoma database between January 1, 2010, and December 31, 2016. Patients’ characteristics are described in Table Table1. 1 . Sarcomas with complex genomics were overrepresented in patients over 90 when compared to patients under 90 (92.0% vs . 66.3%, p < 0.001). Undifferentiated pleomorphic sarcoma (UPS), LMS and MFS were the most represented histological subtypes in patients over 90 (Table (Table1). 1 ). Limb and head and neck sites, and superficial locations were overrepresented in patients over 90 ( p ≤ 0.001). Male patients were overrepresented in patients aged >90 while representing close to 25% of French citizen aged >90.
Patient characteristics
Italics refer to molecular subtypes of sarcomas.
( https://www.insee.fr/fr/statistiques/1892086?sommaire=1912926 ), male patients represented 45% of the patients in this age group. Patients >90 were also less frequently metastatic at initial diagnosis ( p = 0·009), even when considering only patients who had a CT scan (data not shown). Prior history of cancers and radiotherapy were no more frequent in patients aged over 90 vs . younger patients (Table (Table1). 1 ). Genetic predisposition was observed in none of the 113 patients over 90 vs . 123 reported in the remaining population.
Patient management
The adherence to ESMO clinical practice guidelines (2,3) was then analyzed (Table (Table2). 2 ). While biopsy rate was similar, patients aged over 90 had less frequently appropriate pretreatment imaging than patients under 90 (56.6% vs . 75.1%, p ≤ 0.001). This was true also when comparing patients aged >90 to the group of 60–80 or 80–90 (Table (Table3 3 ).
Disease management in patients aged above and under 90
Sarcoma patient characteristics and management across age groups
Bold indicates significant p value ( p < 0.05).
Surgery was less frequently performed in patients over 90 years (60.2% vs . 78.3%, p ≤ 0.001); they also had less frequently neoadjuvant treatment. This did not result in significant differences in terms of resection margins at first or at second surgery (Table (Table2). 2 ). Resection rates (Table (Table2) 2 ) and final result of the surgical removal (Table (Table3) 3 ) were also not significantly different when comparing only patients for whom the R criterion was documented.
Patients over 90 as compared to other age groups
Table Table3 3 presents an analytic description of histologies, clinical presentations and management within different age subgroups. As expected, histological subtypes were extremely different across age groups, in particular across the extremes. It is interesting to note that differences were also observed in the three elder groups, on histologies, depth, grade and metastasis. As compared to the 80–90 years group, histotypes, depth, metastasis at diagnosis and gender of patient with sarcoma aged >90 were different (Table (Table3). 3 ). Regarding patient management, compliance to CPGs was the lowest in patients aged >90, together with the final quality of surgery (lowest R0 rate, highest R2 rate), with fewer patients operated in reference centers. Overall a significant trend of decrease of compliance to guidelines, management in reference centers and final quality of surgery over age groups was observed, from the children and adolescent/young adults to the older age group (Table (Table3 3 ).
Relapse and survival
Local progression‐free survival (PFS), metastatic PFS and PFS were not significantly different in the two populations (Figs. (Figs.1 1 a – 1 c ). Relapse‐free survival of patients aged over 90 vs . those aged 90 or under was also similar when the analysis was conducted only with operated patients (Fig. (Fig.1 1 d ). No difference was observed either for local relapse‐free survival not for metastatic relapse‐free survival for operated patients (data not shown). As expected, overall survival was worse in patients >90 (Fig. (Fig.1 1 e ). Importantly, the overall survival of patients who were not operated was significantly worse in patients aged over 90 vs . younger patients (9.9 vs . 27.1 months, p < 0.001) with no patient alive at 3 years in the older group vs . 40% in the younger one (Fig. (Fig.1 1 f ). Using Cox model, with grade, size, depth, site, gender, presentation to a multidisciplinary team (MDT) prior to treatment 5 and surgery in expert center 12 and age >90 as tested variables, age >90 was not identified as an independent prognostic factor for either relapse‐free survival or PFS (data not shown).

Progression, relapse and survival in sarcoma patients aged over or under 90. ( a ) Local progression‐free survival of patients aged over 90 (green) and younger (blue). ( b ) Metastatic progression‐free survival of patients aged over 90 (green) and younger (blue). ( c ) Progression‐free survival of patients aged over 90 (green) and younger (blue). ( d ) Relapse‐free survival of operated patients aged over 90 (green) and younger (blue). ( e ) Overall survival of patients aged over 90 (green) and younger (blue). ( f ) Overall survival of nonoperated patients aged over 90 (green) and younger (blue). [Color figure can be viewed at wileyonlinelibrary.com ]
This analysis of a 6‐year nationwide registry of incident sarcoma patients includes over 12,000 patients with 113 (0.9%) patients aged >90. It identifies for the first time‐specific pathological and clinical characteristics of sarcomas diagnosed in patients over 90: these are almost exclusively sarcomas with complex genomics, more frequently superficial tumors, from limb sites or head and neck sites. Despite an obvious difference in life duration, sarcoma patients aged over 90 do not have a higher frequency of previous radiotherapy, previous cancers, and none genetic predisposing condition. Patients aged over 90 are also less frequently metastatic at initial presentation than the younger sarcoma patient population and relative to the sex ratio at this age in the French population, more frequently males. These clinicopathological and clinical specificities of sarcomas occurring at an older age are unexpected and had not been previously recognized to our knowledge. It is interesting to observe that these characteristics are not equally shared by the groups aged 60–80 or 80–90, but quite characteristic of this age group. Sarcomas occurring in higher age may result in more from accumulation of external oncogenic events over the lifetime; limb and head and neck sites may suggest exposure to the sun as risk factors to be tested. It is particularly notable that less than 3% of sarcoma in this population was translocation‐related sarcomas.
Less adequate management is offered to patients with STS over 90, in particular regarding pretreatment imaging and surgery. Of note, oncogeriatric assessment 13 is not part of the NETSARC data set, and it is likely that coexisting conditions have largely contributed to these differences with younger patients. However, when patients over 90 are treated with surgery according to clinical practice guidelines (within NETSARC, the ESMO guidelines are used as reference), 2 , 3 relapse‐free survival and PFS remain similar to that of the younger population in univariate and also multivariate analysis where classical prognostic factors are introduced. 2 , 3 , 5 This points to the need not to undertreat patients, including those aged over 90, and to adopt the classical CPGs for sarcoma management in patients at all age when oncogeriatric status allows. As expected, the overall survival of sarcoma patients aged over 90 is shorter than that of younger patients. Median overall survival is 25 months in this population of patients over 90 (whose median age is 92), an outcome which must be compared to the 50 months life expectancy for the general French population at the age of 90. 14
To the best of our knowledge, this is the first series reporting on the different biology and natural history of patients with sarcomas occurring at a very high age. Sarcoma occurring after 90 has a specific biology and natural history, but not intrinsically a worse cancer‐specific prognosis. If fit according to geriatric assessment, this patient population, should be treated according to the general CPGs for sarcomas. It is reasonable to infer a similar statement for younger geriatric sarcoma patients.
Acknowledgements
The authors would like to thank the teams and leaders of the French National Cancer Institute (INCa) for the continuous support to the project.
Conflict of interests: Dr JEK reports travel support and honoraria from Pharmamar, travel support and advisory board for Astra‐Zeneca, travel support and advisory board for Tesaro, and advisory board for Clovis; Dr OM has acted as consultant for Amgen, Astra‐Zeneca, Bayer, Bristol‐Myers Squibb, Blueprint medicines, Eli‐Lilly, Merck, Novartis, Pfizer, Roche, Janssen, Servier, and Vifor Pharma. All other co‐authors report no competing interests or conflict of interests.

IMAGES
VIDEO
COMMENTS
Philippe ROSSET | Cited by 2,913 | of University of Tours, Tours (UFR) | Read 213 publications | Contact Philippe ROSSET
M. Philippe Rosset. Coordonnées. Mail [email protected]. Discipline(s) Chirurgie orthopédique et traumatologique. Pratique Inscription Offre de formation Santé Sport Culture Contacts Plans d'accès ...
Ph ROSSET | Cited by 630 | of University of Tours, Tours (UFR) | Read 31 publications | Contact Ph ROSSET
Philippe Rosset, Louis Bernard and. Leslie Grammatico-Guillon [Opens in a new window] ... Unit of Clinical Epidemiology (EpiDcliC), Teaching Hospital of Tours, Tours, France Department of Infectious Diseases, Teaching Hospital of Tours, Tours, France Medical School, University of Tours, Tours, France. Emeline Laurent Affiliation:
Bone fracture healing: cell therapy in delayed unions and nonunions. 2015 Jan;70:93-101. doi: 10.1016/j.bone.2014.07.033. , Philippe Rosset 2 , Daniel Lozano 3 , Julien Stanovici 2 Christian Ermthaller 4 Florian Gerbhard. 25093266.
Enrique Gómez-Barrena 1 , Philippe Rosset 2 , Florian Gebhard 3 , Philippe Hernigou 4 , ... Université François-Rabelais de Tours, CHU de Tours, Tours, France. 3 Department of Traumatology, Hand-, Plastic-, and Reconstructive Surgery, Center of Surgery, University of Ulm, Ulm, Germany.
Philippe Rosset Affiliation: Service of Orthopedic Surgery, University Hospital, Tours, France. Louis Bernard ... Leslie Grammatico-Guillon, MD, PhD, Department of Medical Information and Public Health, EE1 EES, University Hospital, Tours, France ([email protected]). Article Supplementary materials Metrics Article contents. Abstract ...
Philippe Rosset, Valérie Trichet, Fabien Guilloton, Nicolas Espagnolle, Thomas Cordonier, Dominique Heymann, Pierre Layrolle, ... The study followed the ethical guidelines of the University Hospital of Tours (Tours, France) and was approved by the ethics committee Comité de protection des personnes (Tours - Région Centre [Ouest-1]). Patients ...
Multifactorial aetiology defines non-unions, with a biological and a mechanical distortion of the timeline of bone healing.Research on new advances to increase osteogenesis and promote non-union healing is strongly directed towards new forms of cell products.Basic science and research on non-union treatments is needed to compile preclinical data on new treatments.Bone marrow concentration and ...
Philippe Rosset practices in Tours, France... Many experts have expertise treating multiple conditions. MediFind uses a variety of data sources to determine what conditions a expert treats and their level of experience.
Be´atrice Marie7, Christophe Delfour8,Se´bastien Aubert9, Philippe Rosset10, Anne de Muret1, ... Pathologie, CHU Tours, Hopital Trousseau, Avenue de la Re´pub-lique, Tours 37044, France.
Christine Vignon, 1 , 2 Christelle Debeissat, 3 Marie-Thérèse Georget, 3 Didier Bouscary, 4 Emmanuel Gyan, 1 , 2 , 5 Philippe Rosset, 6 and Olivier Herault 1 , 2 , 3 , * Zoran Ivanovic, Editor. Author ... Tours, France). Cells were centrifuged, seeded in flasks at a density of 5×10 3 per cm 2 in MEM alpha culture medium supplemented ...
Charles Agout & Philippe Rosset. Department of Anatomopathology, University Hospital of Tours, Tours, France. ... Tours, Cedex 9, France. Frederic Bastides. Authors. Thomas Chalopin. View author publications. You can also search for this author in PubMed Google ...
Revision total hip arthroplasty in the setting of a large proximal femoral deficiency or a peri-prosthetic fracture remains a challenging problem. We describe the development, surgical technique and the use of cementless revision stems with distal inter-locking screws to provide immediate stability of the femoral implant.
During the study period, 42 105 patients were hospitalised for VO in France involving 60 878 hospital stays. The mean VO incidence was 7.8/100 000 over the study period, increasing from 6.1/100 000 in 2010 to 11.3/100 000 in 2019. The mean age was 64.8 years old and the sex ratio was 1.56.
Filipe Rosset is a Brazilian guitarist and composer whose guitar mastery and creative talent have won recognition and admiration both in his country and internationally. His musical career is marked by a unique ability to innovate in music, transcending sonic and creative barriers. It is interesting to know that Filipe Rosset is releasing his ...
Grammatico-Guillon, Leslie Perreau, Caroline Miliani, Katiuska L'Heriteau, Francois Rosset, Philippe Bernard, Louis Lepelletier, Didier Rusch, Emmanuel and Astagneau, Pascal 2017. Association of Partial Hip Replacement With Higher Risk of Infection and Mortality in France. Infection Control & Hospital Epidemiology, Vol. 38, Issue. 1, p. 123.
Steven Roulet 1 , Louis-Romée Le Nail 2 , Gualter Vaz 3 , Antoine Babinet 4 , Valérie Dumaine 4 , Aurélie Sallot 5 , Philippe Rosset 2 Affiliations 1 Département de chirurgie orthopédique 2, centre hospitalo-universitaire Tours - faculté de medecine, université de Tours, 37000 Tours, France.
The fastest and most reliable way to see the best sights of Moscow. Red Square, St Basil's Cathedral, Sparrow Hills, the Bolshoi Theatre, Christ the Saviour Cathedral, Tverskaya Street, Kremlin and other popular attractions. BOOK NOW. For all. 3,5 - 4 h.
Get the chance to chat with locals and learn about their lives. Get a more intimate experience of the city on a small-group tour. This is an ideal tour for first-time visitors to Moscow. Book My Tour Learn More. Very popular. 2 Hours. Iconic metro stations, The world's deepest metro station, walking. From € 38.
Patients' characteristics are described in Table 1. Sarcomas with complex genomics were overrepresented in patients over 90 when compared to patients under 90 (92.0% vs. 66.3%, p < 0.001). Undifferentiated pleomorphic sarcoma (UPS), LMS and MFS were the most represented histological subtypes in patients over 90 (Table 1 ).
🎧 Wear headphones for the best experience.In this video, we will walk along the famous tourist routes of Moscow, take a walk along the renovated embankments...
🎧 Wear headphones for the best experience.In this video, we will walk through the beautiful streets of old Moscow, as well as visit some new districs.Moscow...