
- Introduction
- Palp/Percus
- Auscultation

Palpation/Percussion
Thoracic expansion:.
- Is used to evaluate the symmetry and extent of thoracic movement during inspiration.
- Is usually symmetrical and is at least 2.5 centimeters between full expiration and full inspiration.
- Can be symmetrically diminished in ankylosing spondylitis .
- Can be unilaterally diminished in chronic fibrotic lung disease , extensive lobar pneumonia, large pleural effusions, bronchial obstruction and other disease states.
Percussion:
Percussion is the act of tapping on a surface, thereby setting the underlying structures in motion, creating a sound and palpable vibration. Percussion is used to determine whether underlying structures are fluid-filled, gas-filled, or solid. Percussion:
- Penetrates 5 - 6 centimeters into the chest cavity.
- May be impeded by a very thick chest wall.
- Produces a low-pitched, resonant note of high amplitude over normal gas-filled lungs.
- Produces a dull, short note whenever fluid or solid tissue replaces air filled lung (for example lobar pneumonia or mass) or when there is fluid in the pleural space (for example serous fluid, blood or pus).
- Produces a hyperresonant note over hyperinflated lungs (e.g. COPD ).
- Produces a tympanitic note over no lung tissue (e.g. pneumothorax ).
Diaphragmatic excursion:
- Can be evaluated via percussion.
- Is 4-6 centimeters between full inspiration and full expiration.
- May be abnormal with hyperinflation , atelectasis , the presence of a pleural effusion , diaphragmatic paralysis, or at times with intra-abdominal pathology.

- Report problem with article
- View revision history
Citation, DOI, disclosures and article data
At the time the article was created Craig Hacking had no recorded disclosures.
At the time the article was last revised Craig Hacking had the following disclosures:
- Philips Australia, Paid speaker at Philips Spectral CT events (ongoing)
These were assessed during peer review and were determined to not be relevant to the changes that were made.
- Diaphragm fluoroscopy
The fluoroscopic sniff test , also known as diaphragm fluoroscopy , is a quick and easy real time fluoroscopic assessment of diaphragmatic motor function (excursion). It is used most often to confirm absence of muscular contraction of the diaphragm during inspiration in patients with phrenic nerve palsy or breathing difficulties following stroke . Chest radiograph demonstrating a newly elevated hemidiaphragm often precedes a sniff test.
In critically unwell patients who can not attend the fluoroscopy unit in the radiology department, bedside US assessment can be used to demonstrate appropriate diaphragmatic movement with normal respiration and when asked to sniff (see case 5).
The following technique is suggested:
ask the patient to practice sniffing before the study
with the patient either standing (preferred) or supine, perform frontal fluoroscopy of the diaphragm at rest, breathing quietly through an open mouth
ask the patient to take a few quick short breaths in with a closed mouth ('sniffs') causing rapid inspiration
occasionally, repeating (3) in the lateral projection is required to evaluate the posterior hemidiaphragms
In normal diaphragmatic motion:
the diaphragm contracts during inspiration: moves downwards
the diaphragm relaxes during expiration: moves upwards
both hemidiaphragms move together
in healthy patients 1-2.5 cm of excursion is normal in quiet breathing 2
3.6-9.2 cm of excursion is normal in deep breathing 2
up to 9 cm can be seen in young or athletic individuals in deep inspiration 2
excursion in women is slightly less than men 2
In abnormal diaphragmatic motion:
the affected hemidiaphragm does not move downwards during inspiration
paradoxical motion can occur
Interpretation
Absence of diaphragmatic movement confirms phrenic nerve palsy in the appropriate clinical setting. A mass anywhere along the course of the phrenic nerve requires further workup, usually with neck and chest CT. A hilar mass due to lung cancer is the most common finding on CT and a classic exam case.
Normal diaphragmatic excursion can also be impaired in patients with:
previous diaphragmatic trauma or surgery
neuromuscular disorders
previous stroke
- 1. Nason LK, Walker CM, McNeeley MF et-al. Imaging of the diaphragm: anatomy and function. Radiographics. 2012;32 (2): E51-70. doi:10.1148/rg.322115127 - Pubmed citation
- 2. Boussuges A, Gole Y, Blanc P. Diaphragmatic motion studied by m-mode ultrasonography: methods, reproducibility, and normal values. Chest. 2009;135 (2): 391-400. doi:10.1378/chest.08-1541 - Pubmed citation
- Nason L, Walker C, McNeeley M, Burivong W, Fligner C, Godwin J. Imaging of the Diaphragm: Anatomy and Function. RadioGraphics. 2012;32(2):E51-70. doi:10.1148/rg.322115127 - Pubmed
Incoming Links
- Diaphragmatic paralysis
- Phrenic nerve palsy
- Ultrasound diaphragmatic sniff test
- Left hilar mass causing phrenic nerve palsy
- Large right diaphragmatic hernia
- Hemidiaphragmatic paralysis
- Abnormal sniff test
- Normal sniff test
- Phrenic nerve palsy with positive sniff test
Promoted articles (advertising)
ADVERTISEMENT: Supporters see fewer/no ads
By Section:
- Artificial Intelligence
- Classifications
- Imaging Technology
- Interventional Radiology
- Radiography
- Central Nervous System
- Gastrointestinal
- Gynaecology
- Haematology
- Head & Neck
- Hepatobiliary
- Interventional
- Musculoskeletal
- Paediatrics
- Not Applicable
Radiopaedia.org
- Feature Sponsor
- Expert advisers

- Open access
- Published: 22 October 2021
Diaphragmatic excursion is correlated with the improvement in exercise tolerance after pulmonary rehabilitation in patients with chronic obstructive pulmonary disease
- Masashi Shiraishi ORCID: orcid.org/0000-0001-5410-1331 1 , 2 ,
- Yuji Higashimoto 1 ,
- Ryuji Sugiya 1 ,
- Hiroki Mizusawa 1 ,
- Yu Takeda 1 ,
- Shuhei Fujita 1 ,
- Osamu Nishiyama 2 ,
- Shintarou Kudo 3 ,
- Tamotsu Kimura 1 ,
- Yasutaka Chiba 4 ,
- Kanji Fukuda 1 ,
- Yuji Tohda 2 &
- Hisako Matsumoto 2
Respiratory Research volume 22 , Article number: 271 ( 2021 ) Cite this article
4447 Accesses
8 Citations
4 Altmetric
Metrics details
In patients with chronic obstructive pulmonary disease (COPD), the maximum level of diaphragm excursion (DE max ) is correlated with dynamic lung hyperinflation and exercise tolerance. This study aimed to elucidate the utility of DE max to predict the improvement in exercise tolerance after pulmonary rehabilitation (PR) in patients with COPD.
This was a prospective cohort study. Of the 62 patients with stable COPD who participated in the outpatient PR programme from April 2018 to February 2021, 50 completed the programme. Six-minute walk distance (6MWD) was performed to evaluate exercise tolerance, and ultrasonography was performed to measure DE max . Responders to PR in exercise capacity were defined as patients who demonstrated an increase of > 30 m in 6MWD. The receiver operating characteristic (ROC) curve was used to determine the cut-off point of DE max to predict responses to PR.
Baseline levels of forced expiratory volume in 1 s, 6MWD, maximum inspiratory pressure, DE max and quadriceps muscle strength were significantly higher, and peak dyspnoea of modified Borg (mBorg) scale score was lower in responders (n = 30) than in non-responders (n = 20) to PR (p < 0.01). In multivariate analysis, DE max was significantly correlated with an increase of > 30 m in 6MWD. The area under the ROC curve of DE max to predict responders was 0.915, with a sensitivity and specificity of 83% and 95%, respectively, at a cut-off value of 44.9 mm of DE max .
DE max could adequately predict the improvement in exercise tolerance after PR in patients with COPD.
Chronic obstructive pulmonary disease (COPD) is a progressive disease characterised by minimally reversible airflow limitation [ 1 ]. The main feature of COPD is the inability of patients to cope with their activities of daily life due to shortness of breath. Although the pathophysiological mechanisms involved in the development of dyspnoea and poor exercise tolerance in patients with COPD are complex, dynamic lung hyperinflation (DLH) plays a central role [ 2 ] by increasing ventilatory workload and decreasing the pressure-generating capacity of the inspiratory muscles.
Pulmonary rehabilitation (PR) is a non-pharmacological intervention and has been reported to improve dyspnoea, exercise capacity and quality of life of patients with COPD [ 3 ]. Owing to a body of evidence, PR is now established as the standard of care for patients with COPD [ 4 ]. However, not all patients with COPD benefit from PR to the same extent. Therefore, identifying patients who are likely to achieve maximum benefit from the PR programme is crucial. So far, several studies have shown that severe airflow limitation or poor exercise tolerance at baseline may predict a better response to PR [ 5 , 6 ], but another study has reported inconsistent findings [ 7 ]. Furthermore, one study reported that patients with severe dyspnoea did not respond well to PR and patients with milder dyspnoea responded well [ 8 ].
Considering the role of DLH in the development of dyspnoea and poor exercise tolerance in patients with COPD, objective measures that reflect the degree of DLH may help in identifying good responders to PR. Previously, we reported that there was an association between increased dyspnoea due to DLH on exercise and decreased exercise capacity in patients with COPD and reduced mobility of the diaphragm, which was assessed by the maximum level of diaphragm excursion (DE max ) using ultrasonography [ 9 ]. Other research groups reported the utility of ultrasonographic assessment of diaphragmatic mobility in COPD in understanding its association with 6-min walk distance (6MWD), dyspnoea [ 10 ] and increased mortality [ 11 ].
However, there have been no reports on the association between diaphragmatic mobility and the effect of PR to improve exercise tolerance. The primary aim of this study is to clarify the role of DE max to predict the improvement in exercise tolerance after PR in patients with COPD.
Materials and methods
Study design and subjects.
This was a single-centre, observational, prospective cohort study. The study included 62 patients with clinically stable COPD who visited the Department of Respiratory Medicine and Allergology, Kindai University Hospital, between April 2018 and February 2021. The exclusion criteria included unstable medical conditions that could cause or contribute to breathlessness, such as metabolic, cardiovascular or other respiratory diseases, or any other disorders that could interfere with exercise testing, such as neuromuscular diseases or musculoskeletal problems. This study was approved by the Ethics Committee of Kindai University School of Medicine. Written informed consent was obtained from all participants.
Measurements
All participants underwent ultrasonography (Xario 200, Toshiba, Tokyo, Japan) for the assessment of their DE max . Using the liver as an acoustic window (Fig. 1 A), a convex 3.5 MHz probe was used to measure the excursions of the right hemidiaphragm according to the techniques mentioned in previous studies [ 9 , 12 , 13 ]. The M-mode cursor was rotated and placed on the axis of diaphragmatic displacement on the stored image, and displacement measurements were performed. Measurements were performed during each of the three deep breaths, and DE max was measured (Fig. 1 B). The maximum value obtained for the three deep breaths was used. 6MWD was performed to evaluate walking capacity according to the American Thoracic Society (ATS)/European Respiratory Society (ERS) statement [ 14 , 15 , 16 ]. All participants performed the 6MWD test before and after the PR programme, and the magnitude of their perceived breathlessness and their leg fatigue was rated using a 1–10-point Borg scale. Responders to PR in exercise capacity were defined as those who demonstrated more than 30 m increase in 6MWD after the PR programme, which was the definition of minimal clinically important difference (MCID) for 6MWD [ 17 ].
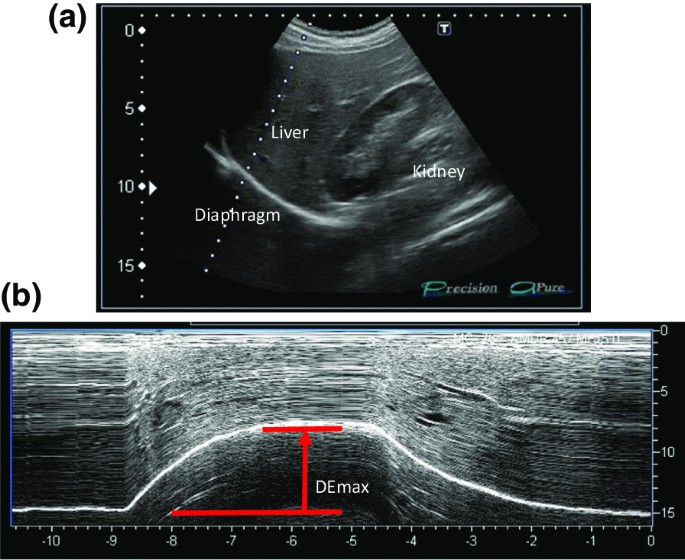
Representative image of the right diaphragm. The probe was positioned below the right costal margin between the midclavicular and anterior axillary lines. A Two-dimensional ultrasonographic image of the right hemidiaphragm (B-mode). Diaphragmatic movements were recorded in M-mode during deep breathing (DE max ) ( B )
Spirometry (CHESTAC-800, Chest, Tokyo, Japan) was performed following the 2005 ATS/ERS recommendations [ 18 ] for measuring forced vital capacity (FVC), forced expiratory volume in 1 s (FEV 1 ) and inspiratory capacity. Respiratory muscle strength was assessed by measuring the maximum inspiratory pressure (PI max ) generated against an occluded airway at residual volume [ 19 ] (SP-370, Fukuda Denshi, Tokyo, Japan). A hand-held dynamometer (μTasF-1, Anima Corp., Tokyo) was used to measure quadriceps muscle strength (QMS). The impact of COPD on health status was assessed using the COPD assessment test (CAT), a patient-completed questionnaire on eight items, namely, cough, phlegm, chest tightness, breathlessness, limited activities, confidence leaving home, sleeplessness and energy. The scores for each of the items range from 0 to 5 points, resulting in a CAT total score ranging from 0 to 40 points [ 20 ], and MCID of CAT is 2 points [ 21 ]. In all patients with COPD, emphysema was evaluated by computed tomography of the chest. A SYNAPSE VINCENT volume analyser (FUJIFILM Medical, Tokyo, Japan) was used to measure the low attenuation area (%LAA).
Rehabilitation programme
The outpatient PR programme was conducted twice a week for 12 weeks (24 sessions), including aerobic exercise training (ergometer and walking exercise) at 60–70% of peak workload for 20–40 min and upper- and lower-limb muscle strength training for 10–20 min.
Sample size
The sample size was estimated using R software. The analysis based on 6MWD data from the PR programme revealed that 40 subjects were required if the expected area under the curve (AUC) below the receiver operating characteristic (ROC) curve was 0.80, the power was 90%, and the significance level was 0.01. Furthermore, we anticipated a dropout from the PR programme. Thus, we set the sample size to 50 participants.
Statistical analysis
Responders and non-responders were compared using t -test, the Wilcoxon rank-sum test or χ 2 test, as appropriate. The paired t -test or the Wilcoxon signed-rank test was used to evaluate the changes in the parameters before and after the PR programme. The Pearson correlation coefficient was used to analyse the relationship between changes in 6MWD and independent variables because changes in 6MWD were normally distributed. Additionally, multivariate logistic regression models were used to assess the ability of variables to predict a response to PR. The ROC curve method was used to assess the ability of DE max to predict a response to PR. All statistical analyses were performed using the JMP software programme (JMP®, Version 14; SAS Institute Inc., Cary, NC, USA).
Out of the 62 patients included in the study, 50 completed the PR programme (Fig. 2 ). Two patients dropped out because of severe exacerbation of COPD, and 10 patients discontinued the PR owing to the coronavirus pandemic. Table 1 presents the baseline characteristics of the participants. After the PR programme, scores for CAT, 6MWD, peak dyspnoea and leg fatigue of the modified Borg (mBorg) scale, and QMS improved significantly (Table 2 ). Thirty patients showed an increase of > 30 m in 6MWD after PR (responders: 60%), and 20 patients (40%) were defined as non-responders. Baseline levels of %FEV 1 , 6MWD, PI max , DE max and QMS were significantly higher and those of CAT score and peak dyspnoea of mBorg scale were significantly lower in responders than in non-responders (Table 1 ). Changes in 6MWD were significantly correlated with baseline levels of CAT, %FEV 1 , peak dyspnoea of mBorg scale, PI max , DE max (Fig. 3 ) and QMS and marginally correlated with baseline levels of 6MWD (Table 3 ).
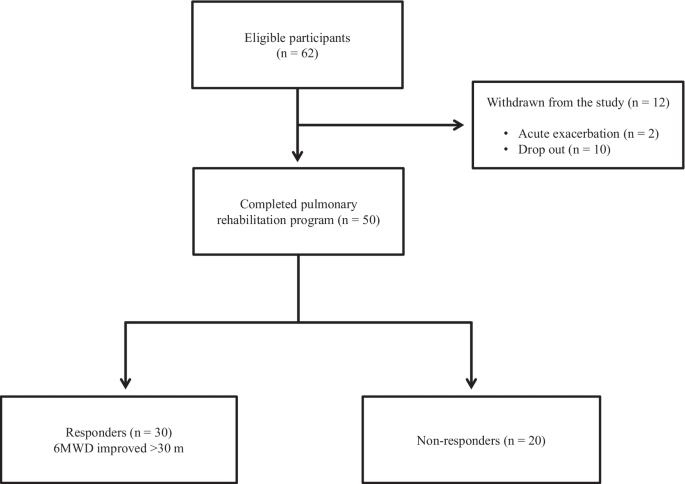
Study flow diagram. COPD chronic obstructive pulmonary disease, PR pulmonary rehabilitation, 6MWD 6-min walk distance
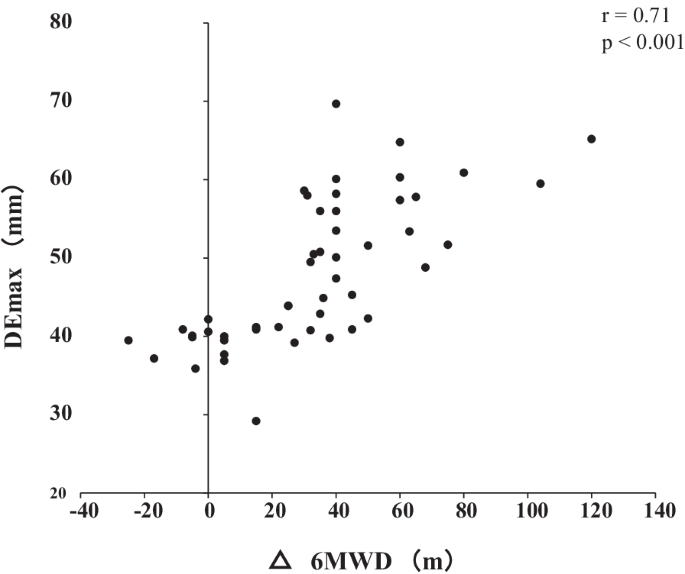
Relationship between DE max and the changes in 6MWD after pulmonary rehabilitation. Changes in 6MWD were significantly positively correlated with DE max (r = 0.72; p < 0.001). DE max maximum diaphragmatic excursion, 6MWD 6-min walk distance
In multivariate analysis, DE max alone significantly contributed to the prediction of responders (Table 4 , Model 1). When using PI max instead of DE max because PI max and DE max showed a strong association (r = 0.73), both PI max and %FEV 1 contributed to the prediction (Table 4 , Model 2). The area under the ROC curve of DE max to predict the responders was 0.915, with a sensitivity of 83% and a specificity of 95% at a cut-off value of 44.9 mm of DE max (Fig. 4 ). The significance of DE max in the predictability of responders remained even when the analysis was confined to severe patients (%FEV 1 < 50%, n = 23; AUC = 0.88, sensitivity = 70% and specificity = 100% at a cut-off value of 44.9 mm).
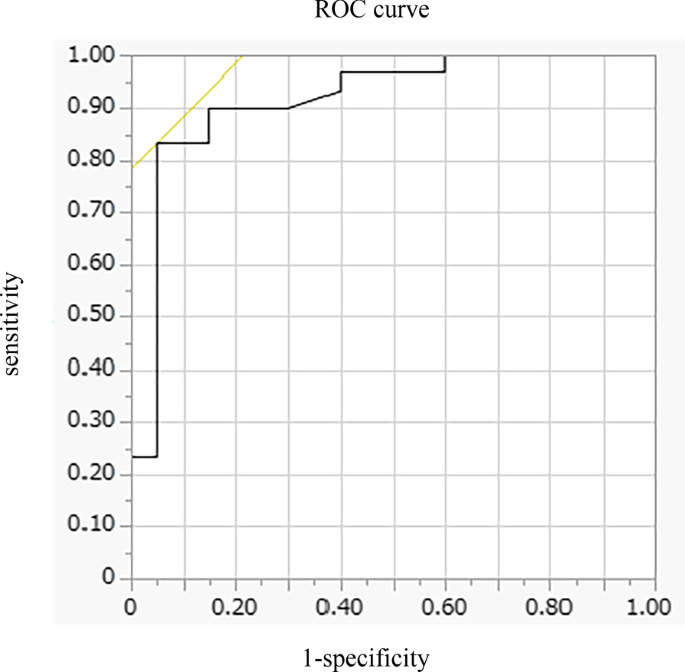
Receiver operating characteristic (ROC) curve for baseline DE max in relation to the response to pulmonary rehabilitation. ROC curve estimates the ability of DE max to predict a clinically important improvement in 6MWD (> 30 m) after pulmonary rehabilitation (AUC = 0.915, sensitivity = 83% and specificity = 95% at a cut-off point of 44.9 mm of DE max ). AUC area under the curve, 6MWD 6-min walk distance, DE max maximum diaphragmatic excursion
This is the first study to demonstrate the utility of DE max to predict the responsiveness of patients with COPD to 12-week PR. In this study, multivariate analysis revealed that greater baseline DE max was the only factor that predicted the responsiveness to PR, independent of baseline %FEV 1 . Additionally, the model using DE max had better prediction performance than that using PI max . The AUC of DE max to predict the 30 m or more improvement in 6MWD after the PR was 0.915, with a sensitivity of 83% and a specificity of 95% at 44.9 mm.
PR is beneficial to patients with chronic respiratory disease, including COPD [ 3 ], and generally improves exercise performance, health-related quality of life and dyspnoea [ 22 ], which was confirmed in this study. Ideally, PR was proven to be effective in all patients, but the response to PR varies considerably between individual patients [ 8 , 23 , 24 , 25 ]. Indeed, in this study, the improvement in 6MWD was less than that in MCID in 40% of the patients regardless of the degree of severity of COPD. Therefore, identifying predictors of a response is crucial in ensuring better PR efficacy and personalisation of PR programmes for patients with COPD.
In this study, the baseline values of %FEV 1 , PI max , DE max , QMS and 6MWD were positively associated with Δ6MWD in univariate analysis, suggesting that a better baseline condition was associated with a higher proportion of patients who achieved MCID after PR. These findings are consistent with those of previous studies that showed that patients with higher levels of %FEV 1 or FEV 1 /VC achieved greater improvement in 6MWD after PR [ 7 , 26 , 27 ] and a study in which patients with milder mMRC scores could achieve MCID of 6MWD after PR [ 8 ], but not for those with worst mMRC score, although others studies showed contradictory results [ 5 , 6 , 28 , 29 , 30 ] or found no significant baseline characteristics to predict a response to PR [ 31 ]. The discrepancy between the findings cannot be fully explained, but it might be due to the differences in the studied population and strength or length of PR. In this study, the mean %FEV 1 of the participants was 56.0%, which was relatively higher than that of other studies (mean %FEV 1 of 40–50% in most studies) [ 5 , 6 , 28 ], despite similar inclusion criteria throughout the studies, i.e., not limited to severe COPD in most studies. Thus, no ceiling effect with a PR programme that included high-intensity load exercise training for 20–40 min was observed in our population.
In this study, an important finding is that greater DE max at baseline was the only factor that predicted the responders in 6MWD after PR. In addition, the model using DE max had better prediction performance than that using PI max . The high predictability of DE max may be because of its strong association with DLH and dyspnoea during exercise, as reported previously [ 9 ]. DLH is involved in the development of dyspnoea, and both are important factors to determine the improvement in 6MWD in patients with COPD. Therefore, DE max that reflects the degree of DLH and dyspnoea during exercise was superior to other physiological indices to predict responders.
Furthermore, the virtuous cycle observed in our PR programme that included high-intensity load exercise training might be a result of the improvement in ventilation pattern. Improving the ventilation pattern would be easier with greater DE max , as shown in studies of mechanically ventilated patients [ 32 ], which may have reduced dyspnoea during exercise after 12 weeks of PR and improved exercise tolerance. Exercise therapy is a central component of PR, which significantly reduces blood lactate levels during exercise, reduces minute ventilation and improves exercise tolerance [ 33 ]. The high-intensity load exercise training, which is performed at 60–80% of the maximum oxygen uptake, has a higher physiological effect than low exercise load. Patients with greater DE max may be able to perform higher load training, which resulted in effective PR.
Diaphragm ultrasonography has been widely and successfully used to identify diaphragmatic dysfunction by showing its association with 6MWD, dyspnoea [ 10 ], extubation failure in mechanically ventilated patients [ 32 ], and increased mortality [ 11 ]. Recently, Lewinska and Shahnazzaryan proposed its use in pulmonary physiotherapy of patients with COPD [ 34 ]. In most previous studies, diaphragm ultrasonography was used to assess DE max , i.e., the measurement of the excursion of the right hemidiaphragm, as used in this study, and diaphragm thickness that assessed the length and thickness of the zone of apposition of the diaphragm against the rib cage [ 35 , 36 ]. However, it is difficult to measure diaphragm thickness in patients with severe COPD because the length of the zone of apposition is shorter in patients with COPD than that in control subjects [ 37 ], whereas it is easy to measure DE max, which shows high intra- and inter-observer reliability [ 38 ]. Bhatt et al. showed that improvement in 6MWD was associated with that in DE max during forced expiration when the effectiveness of pursed lips breathing was assessed in the PR of patients with COPD [ 39 ]. Corbellini et al. demonstrated greater improvement in DE max during inspiration after PR, which was associated with an increase in the inspiratory capacity [ 40 ]. The normal and cut-off values of DE max during normal respiration, forced respiration, and voluntary sniffing have been described for each gender [ 38 ]. Thus, DE max would be a useful and reliable measure for incorporation into the PR assessment. Furthermore, in clinical settings, this objective measure of DE max has additional advantages as it requires minimum effort in patients and can be applied to the PR programme at home if portable ultrasonography is used. However, the assessment of DE max has a limitation. The procedures pertaining to positioning of patients, breathing patterns, and the selected hemidiaphragm are not standardised at present, which may hamper the routine use of DE max at this moment. Standardisation of these parameters would further facilitate the use of DE max in clinical settings and for research purpose.
There are some limitations to this study. This was a single-centre study involving a relatively small number of participants, and their baseline condition might have been relatively preserved. Nonetheless, 46% of the participants showed FEV 1 < 50%, and the utility of DE max was also observed in these patients with severe airflow limitation. Furthermore, in this study, few patients discontinued the PR programme, except for patients who discontinued during the coronavirus pandemic, which indicates that there was no severe mismatch between the PR programme and the patients’ ability to successfully complete this programme. As another limitation, we did not evaluate any malnutrition factors, which could be an important determinant of diaphragmatic mobility. Nonetheless, DE max was a stronger predictor of the effectiveness of PR than other parameters, including QMS or lung function using multivariate analysis. Further studies with a large number of patients are required, and the utility of DE max should be examined in patients with the most severe form of COPD with a low-intensity load exercise programme.
In conclusion, DE max , which is a reliable and easy to perform measurement, could adequately predict the improvement in exercise tolerance after PR in patients with COPD. Assessment of DE max could aid in making medical decisions associated with therapeutic strategies.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
Chronic obstructive pulmonary disease
Dynamic lung hyperinflation
- Pulmonary rehabilitation
6-Min walk distance
Minimal clinically important difference
Forced vital capacity
Forced expiratory volume in 1 s
Maximum inspiratory pressure
Quadriceps muscle strength
COPD assessment test
Low attenuation area
Area under the curve
Receiver operating characteristic
Modified Borg
Global initiative for chronic obstructive lung disease (gold). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: 2020 report. . https://goldcopd.org/gold-reports/ last accessed: 20 Jan 2020.
Gagnon P, Guenette JA, Langer D, Laviolette L, Mainguy V, Maltais F, Ribeiro F, Saey D. Pathogenesis of hyperinflation in chronic obstructive pulmonary disease. Int J COPD. 2014;9:187–201.
Google Scholar
Spruit MA, Singh SJ, Garvey C, ZuWallack R, Nici L, Rochester C, Hill K, Holland AE, Lareau SC, Man WD, et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188:e13-64.
Article PubMed Google Scholar
Dong J, Li Z, Luo L, Xie H. Efficacy of pulmonary rehabilitation in improving the quality of life for patients with chronic obstructive pulmonary disease: evidence based on nineteen randomized controlled trials. Int J Surg. 2020;73:78–86.
Boutou AK, Tanner RJ, Lord VM, Hogg L, Nolan J, Jefford H, Corner EJ, Falzon C, Lee C, Garrod R, et al. An evaluation of factors associated with completion and benefit from pulmonary rehabilitation in COPD. BMJ Open Respir Res. 2014;1:e000051.
Article PubMed PubMed Central Google Scholar
Costi S, Crisafulli E, Trianni L, Beghe B, Faverzani S, Scopelliti G, Chetta A, Clini E. Baseline exercise tolerance and perceived dyspnea to identify the ideal candidate to pulmonary rehabilitation: a risk chart in COPD patients. Int J Chron Obstruct Pulmon Dis. 2019;14:3017–23.
van Ranst D, Otten H, Meijer JW, van’t Hul AJ. Outcome of pulmonary rehabilitation in COPD patients with severely impaired health status. Int J Chron Obstruct Pulmon Dis. 2011;6:647–57.
Garrod R, Marshall J, Barley E, Jones PW. Predictors of success and failure in pulmonary rehabilitation. Eur Respir J. 2006;27:788–94.
Article CAS PubMed Google Scholar
Shiraishi M, Higashimoto Y, Sugiya R, Mizusawa H, Takeda Y, Fujita S, Nishiyama O, Kudo S, Kimura T, Chiba Y, et al. Diaphragmatic excursion correlates with exercise capacity and dynamic hyperinflation in COPD patients. ERJ Open Res 2020, 6.
Paulin E, Yamaguti WPS, Chammas MC, Shibao S, Stelmach R, Cukier A, Carvalho CRF. Influence of diaphragmatic mobility on exercise tolerance and dyspnea in patients with COPD. Respir Med. 2007;101:2113–8.
Yamaguti WPdS, Paulin E, Salge JM, Chammas MC, Cukier A, de Carvalho CRF. Diaphragmatic dysfunction and mortality in patients with COPD. J Bras Pneumol. 2009;35:1174–81.
Boussuges A, Gole Y, Blanc P. Diaphragmatic motion studied by m-mode ultrasonography: methods, reproducibility, and normal values. Chest. 2009;135:391–400.
Testa A, Soldati G, Giannuzzi R, Berardi S, Portale G, Gentiloni Silveri N. Ultrasound M-Mode assessment of diaphragmatic kinetics by anterior transverse scanning in healthy subjects. Ultrasound Med Biol. 2011;37:44–52.
Laboratories ATSCoPSfCPF. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–7.
Article Google Scholar
Holland AE, Spruit MA, Troosters T, Puhan MA, Pepin V, Saey D, McCormack MC, Carlin BW, Sciurba FC, Pitta F, et al. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J. 2014;44:1428–46.
Singh SJ, Puhan MA, Andrianopoulos V, Hernandes NA, Mitchell KE, Hill CJ, Lee AL, Camillo CA, Troosters T, Spruit MA, et al. An official systematic review of the European Respiratory Society/American Thoracic Society: measurement properties of field walking tests in chronic respiratory disease. Eur Respir J. 2014;44:1447–78.
Polkey MI, Spruit MA, Edwards LD, Watkins ML, Pinto-Plata V, Vestbo J, Calverley PMA, Tal-Singer R, Agustí A, Bakke PS, et al. Six-minute-walk test in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187:382–6.
Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–38.
Lisboa C, Munoz V, Beroiza T, Leiva A, Cruz E. Inspiratory muscle training in chronic airflow limitation: comparison of two different training loads with a threshold device. Eur Respir J. 1994;7:1266–74.
Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34:648–54.
Kon SSC, Canavan JL, Jones SE, Nolan CM, Clark AL, Dickson MJ, Haselden BM, Polkey MI, Man WDC. Minimum clinically important difference for the COPD Assessment Test: a prospective analysis. Lancet Respir Med. 2014;2:195–203.
Lacasse Y, Goldstein R, Lasserson TJ, Martin S. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2006:CD003793.
Spruit MA, Gosselink R, Troosters T, Kasran A, Van Vliet M, Decramer M. Low-grade systemic inflammation and the response to exercise training in patients with advanced COPD. Chest. 2005;128:3183–90.
de Torres JP, Pinto-Plata V, Ingenito E, Bagley P, Gray A, Berger R, Celli B. Power of outcome measurements to detect clinically significant changes in pulmonary rehabilitation of patients with COPD. Chest. 2002;121:1092–8.
Troosters T, Gosselink R, Decramer M. Exercise training in COPD: how to distinguish responders from nonresponders. J Cardiopulm Rehabil. 2001;21:10–7.
Vagaggini B, Costa F, Antonelli S, De Simone C, De Cusatis G, Martino F, Santerini S, Paggiaro P. Clinical predictors of the efficacy of a pulmonary rehabilitation programme in patients with COPD. Respir Med. 2009;103:1224–30.
Scott AS, Baltzan MA, Fox J, Wolkove N. Success in pulmonary rehabilitation in patients with chronic obstructive pulmonary disease. Can Respir J. 2010;17:219–23.
Crisafulli E, Gorgone P, Vagaggini B, Pagani M, Rossi G, Costa F, Guarriello V, Paggiaro P, Chetta A, de Blasio F, et al. Efficacy of standard rehabilitation in COPD outpatients with comorbidities. Eur Respir J. 2010;36:1042–8.
Zanini A, Chetta A, Gumiero F, Della Patrona S, Casale S, Zampogna E, Aiello M, Spanevello A. Six-minute walking distance improvement after pulmonary rehabilitation is associated with baseline lung function in complex COPD patients: a retrospective study. Biomed Res Int. 2013;2013:1–6.
Ragaselvi S, Janmeja AK, Aggarwal D, Sidana A, Sood P. Predictors of response to pulmonary rehabilitation in stable chronic obstructive pulmonary disease patients: a prospective cohort study. J Postgrad Med. 2019;65:101–6.
CAS PubMed PubMed Central Google Scholar
Selzler A-M, Simmonds L, Rodgers WM, Wong EYL, Stickland MK. Pulmonary rehabilitation in chronic obstructive pulmonary disease: predictors of program completion and success. COPD J Chronic Obstr Pulm Dis. 2012;9:538–45.
Li C, Li X, Han H, Cui H, Wang G, Wang Z. Diaphragmatic ultrasonography for predicting ventilator weaning: a meta-analysis. Medicine (Baltimore). 2018;97:e10968.
Rabinovich RA, Ardite E, Troosters T, Carbo N, Alonso J, Gonzalezde Suso JM, Vilaro J, Barbera JA, Polo MF, Argiles JM, et al. Reduced muscle redox capacity after endurance training in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164:1114–8.
Lewinska A, Shahnazaryan K. The use of diaphragm ultrasonography in pulmonary physiotherapy of COPD patients: a literature review. J Clin Med 2020; 9.
Gibson GJ, Whitelaw W, Siafakas N, Supinski GS, Fitting JW, Bellemare F, Loring SH, Troyer AD, Grassino AE. ATS/ERS Statement on respiratory muscle testing. Am J Respir Crit Care Med. 2002;166:518–624.
Summerhill EM, El-Sameed YA, Glidden TJ, McCool FD. Monitoring recovery from diaphragm paralysis with ultrasound. Chest. 2008;133:737–43.
McKenzie DK, Butler JE, Gandevia SC. Respiratory muscle function and activation in chronic obstructive pulmonary disease. J Appl Physiol. 2009;107:621–9.
Laveneziana P, Albuquerque A, Aliverti A, Babb T, Barreiro E, Dres M, Dubé BP, Fauroux B, Gea J, Guenette JA, et al. ERS statement on respiratory muscle testing at rest and during exercise. Eur Respir J 2019; 53.
Bhatt SP, Luqman-Arafath TK, Gupta AK, Mohan A, Stoltzfus JC, Dey T, Nanda S, Guleria R. Volitional pursed lips breathing in patients with stable chronic obstructive pulmonary disease improves exercise capacity. Chron Respir Dis. 2013;10:5–10.
Corbellini C, Boussuges A, Villafane JH, Zocchi L. Diaphragmatic mobility loss in subjects with moderate to very severe COPD may improve after in-patient pulmonary rehabilitation. Respir Care. 2018;63:1271–80.
Download references
Acknowledgements
Not applicable.
This work was supported by Grants-in-Aid for Scientific Research (21K11325).
Author information
Authors and affiliations.
Department of Rehabilitation Medicine, Kindai University School of Medicine, 377-2 Onohigashi, Osakasayama, Osaka, 5898511, Japan
Masashi Shiraishi, Yuji Higashimoto, Ryuji Sugiya, Hiroki Mizusawa, Yu Takeda, Shuhei Fujita, Tamotsu Kimura & Kanji Fukuda
Department of Respiratory Medicine and Allergology, Kindai University School of Medicine, Osaka, Japan
Masashi Shiraishi, Osamu Nishiyama, Yuji Tohda & Hisako Matsumoto
Inclusive Medical Science Research Institute, Morinomiya University of Medical Sciences, Osaka, Japan
Shintarou Kudo
Division of Biostatistics, Clinical Research Center, Kindai University School of Medicine, Osaka, Japan
Yasutaka Chiba
You can also search for this author in PubMed Google Scholar
Contributions
MS, YH, and YC made substantial contributions to the conception and design of the work. MS, YH, and RS made substantial contributions to the data acquisition. MS and HM made substantial contributions to the analysis. All of the listed authors designed the study and were involved in the interpretation of the data. MS and HM drafted the work. YH, MS, TK, YC, ON, KS, KF, YT, and HM revised the report critically for important intellectual content. All authors approved the final version to be published and agreed to be accountable for all aspects of the work. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Masashi Shiraishi .
Ethics declarations
Ethics approval and consent to participate.
This study was approved by the Ethics Committee of Kindai University School of Medicine (31-086). Written informed consent was obtained from all participants.
Consent for publication
If the manuscript is accepted, we approve it for publication in Respiratory Research.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Shiraishi, M., Higashimoto, Y., Sugiya, R. et al. Diaphragmatic excursion is correlated with the improvement in exercise tolerance after pulmonary rehabilitation in patients with chronic obstructive pulmonary disease. Respir Res 22 , 271 (2021). https://doi.org/10.1186/s12931-021-01870-1
Download citation
Received : 09 July 2021
Accepted : 15 October 2021
Published : 22 October 2021
DOI : https://doi.org/10.1186/s12931-021-01870-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Diaphragmatic excursion
- Six-minute walk distance (6MWD)
Respiratory Research
ISSN: 1465-993X
- General enquiries: [email protected]
- Open access
- Published: 16 November 2021
The role of diaphragmatic ultrasound as a predictor of successful extubation from mechanical ventilation in respiratory intensive care unit
- Randa Salah Eldin Mohamed 1 ,
- Abeer Salah Eldin Mohamed 1 ,
- Waleed Fouad Fathalah 2 ,
- Mohamed Farouk Mohamed 1 &
- Ahmed Aelgharib Ahmed 3
The Egyptian Journal of Bronchology volume 15 , Article number: 51 ( 2021 ) Cite this article
2857 Accesses
2 Citations
Metrics details
A Correction to this article was published on 14 December 2021
This article has been updated
The diaphragm muscle whose dysfunction may be very common in patients undergoing mechanical ventilation (Ferrari G, De Filippi G, Elia F, Panero F, Volpicelli G, Aprà F. Crit Ultrasound J 6:8, 2014). Aim: To evaluate real-time ultrasound in the evaluation of diaphragmatic thickening, thickening fraction, and/or excursion to predict extubation outcomes. We aimed to compare these parameters with other traditional weaning measures is a fundamental.
Out of 80 included patients, 20 (25%) have failed extubation. Diaphragmatic thickening (DT), thickening fraction (DTF), and/or excursion (DE) were significantly higher in the successful group compared to those who failed extubation ( p < 0.05). Cutoff values of diaphragmatic measures associated with successful extubation (during tidal breathing) were ≥ 17 mm for DE; ≥ 2.1 cm for DT inspiration; ≥ 15.5 mm for DT expiration, functional residual capacity (FRC); and ≥ 32.82% for DTF %, giving 68%, 95%, 62%, and 90% sensitivity, respectively, and 65%, 100%, 100%, and 75% specificity, respectively. Cutoff values of diaphragmatic parameters associated with successful extubation (during deep breathing) were > 28.5 mm DT Insp, total lung capacity (TLC); >22.5mm DT Exp (RV); >37 DTF %; and > 31 mm DE, giving 100%, 73%, 97%, and 75% sensitivity and 65%, 75%, 100%, and 55% specificity, respectively. Rapid shallow breathing index (RSBI) had 47% sensitivity but 90% specificity.
Ultrasound evaluation of diaphragmatic parameters could be a good predictor of weaning in patients who passed the T-tube.
The diaphragm is an important respiratory muscle, and dysfunction is very common in patients receiving mechanical ventilation. Diaphragm fatigue occurs even in patients who successfully pass the spontaneous breathing test (SBT) [ 1 ]. Interrupting ventilation too early can lead to increased cardiovascular and respiratory pressure (CO2) retention and hypoxemia with up to 25% of patients requiring reinstitution of ventilator support. Unnecessary delays in liberation from mechanical ventilation also can be deleterious. Complications such as ventilator-associated pneumonia and ventilator-induced diaphragm atrophy can be seen with short periods of mechanical ventilation, thereby prolonging mechanical ventilation [ 2 ]. As SBT monitoring is insensitive to detect early signs of load-capacity imbalance [ 3 ], the evaluation of the diaphragmatic thickening fraction (DTF) may be also helpful to assess diaphragmatic function and its contribution to respiratory workload [ 1 ]. Ultrasound can be used to detect the deflection of the diaphragm, which helps to identify patients with diaphragm dysfunction [ 4 ].
This prospective study was carried out on 40 patients who are mechanically ventilated due to pulmonary disease, 40 patients on mechanical ventilation due to non-pulmonary disease at respiratory ICU, and 40 chronic obstructive pulmonary disease (COPD) patients from an outpatient clinic serving as controls at Embaba Chest Hospital, Cairo, Egypt, during a period from January 2018 to November 2019. Written informed consent was obtained from all patients prior to enrollment according to approval at the local committee of Beni-suef University Hospital. Patients on mechanical ventilation due to pulmonary disease (pneumonia, COPD, bronchial asthma, bronchiectasis …. etc.) and non-pulmonary disease (pulmonary edema, myocardial infarction, etc.) were included in this study. Patients with pneumothorax, pleural effusion, neuromuscular diseases, and suspicious diaphragmatic paralysis (raised copula in chest X-ray); patients with pleurodesis; and patients who presented with stridor (due to upper airway involvement due to mechanical ventilation in last 6 months) were excluded from this study.
Study design
Patients were assessed by the following: Acute Physiology and Chronic Health Evaluation II (APACHE II) score, Charlson comorbidity index (CCI), and diaphragm ultrasound. M-mode ultrasound was used to assess diaphragmatic excursion, and movement B-mode ultrasound was used to assess diaphragmatic thickness. Once patients were stable and both ventilator and biochemical parameters were accepted for weaning, T-tube was attempted for 2 h. Patients who passed the SBT on T-tube were included in data analysis and followed up for 48h after extubation where they received oxygen through Venturi mask or nasal oxygen and followed up for 48 h after extubation. Successful extubation was defined as maintenance of spontaneous breathing for > 48 h following extubation. Extubation failure was defined as the inability to maintain spontaneous breathing for at least 48 h, without any ventilatory support. All patients were studied with the head of the bed elevated between 20 and 40°. Diaphragmatic thickness (DT) was measured using a 7–10-MHz linear ultrasound probe set to B-mode. The right hemidiaphragm was imaged at the zone of apposition of the diaphragm and rib cage in the midaxillary line between the 8th and 10th intercostal spaces. The DT was measured at end expiration and end inspiration. The percent change in DT between end expiration and end inspiration (DTF %) was calculated as (DT end inspiration − DT end expiration/DT end expiration) × 100 [ 5 ].
Diaphragmatic excursion (DE)
The convex probe is placed in the right subcostal region parallel to the intercostal space to measure the range of the diaphragmatic movement using the M-mode method with the cursor crossing the diaphragm to assess the highest and lowest points as an indicator for the diaphragmatic mobility range [ 6 , 7 ]. The maneuver was repeated at least three times and the average measurement is taken. Measurement of diaphragmatic thickness and excursion was recorded during tidal breathing and deep breathing (Fig. 1 ).
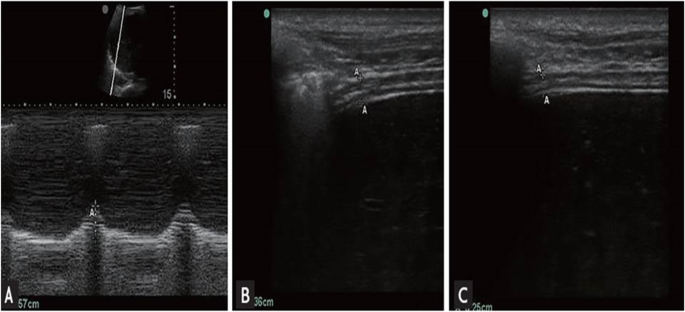
M-mode of diaphragm excursion ( A ) and B-mode of diaphragm thickness ( B , inspiration; C , expiration)
Criteria of weaning
The criteria of weaning are 1- positive end-expiratory pressure (PEEP) ≤ 5 cm H2O 2- Fraction of inspired oxygen (FiO2) < 0.5 3- Respiratory rate (RR) < 30 breaths/min 4- rapid shallow breathing index < 105, and PaO2/FiO2 > 200.
Criteria for failure
The criteria for failure are change in mental status, onset of discomfort, diaphoresis, respiratory rate > 35 breaths/min, and hemodynamic instability (heart rate > 140, systolic blood pressure >180) [ 8 ]. Patients were divided into two groups: group A included 40 patients on mechanical ventilation due to pulmonary diseases to compare parameters of weaning to diaphragmatic thickness and excursion during tidal breathing and deep breathing. Group B includes 40 patients on mechanical ventilation due to non-pulmonary diseases to compare parameters of weaning to diaphragmatic thickness and excursion during tidal breathing and deep breathing.
The collected data was revised, coded, tabulated, and introduced to a PC using the Statistical Package for Social Science (SPSS 17). Data was presented and suitable analysis was done according to the type of data obtained for each parameter. The distributions of quantitative variables were tested for normality. Quantitative data were described using mean and standard deviation for normally distributed data while abnormally distributed data was expressed using the median. For normally distributed data, comparisons between both groups were done using an independent t -test, while abnormally distributed data was assessed using the Mann-Whitney test. A receiver operator characteristic curve (ROC curve) was used to find out the best cutoff value and the validity of a certain variable. Agreement of the different predictive values of the outcome was used and was expressed in sensitivity, specificity, positive predictive value, and negative predictive value.
During the study period (Fig. 2 ), we evaluated 162 patients ready for weaning. Forty chronic obstructive pulmonary disease (COPD) patients (stable) served as a control group. Forty-two patients were excluded, 10 of which had pleural effusion, 4 patients had pneumothorax, 10 patients had diaphragmatic paralysis, and 18 patients were non-cooperative. Eighty patients (on T-tube) undergoing SBT were divided into two groups: group A included 40 patients (non-pulmonary-related cause) and had their diagnosis as follows: 24 (60%) had congestive heart failure, 4 (10%) had diabetes mellitus, 4 (10%) had sepsis other than pneumonia, 2 (5%) had epilepsy, 2 (5%) had embolic hemiplegia, and 4 (10%) had chronic renal failure. Out of group A patients, 9 patients (11.25%) had failed weaning of which 4 patients needed reintubation and 5 patients needed non-invasive positive ventilation of which 2 patients were reintubated and 3 patients died. Group B included 40 patients (pulmonary-related cause) and had their diagnosis as follows: 21 (53%) had COPD, 8 (20%) had asthma, 5 (13%) had bronchiectasis, 5 (13%) had pneumonia, and 1 (3%) had viral influenza H1N1. Out of group B patients, 11 patients (13.75%) had failed weaning, of which 6 patients needed reintubation and 5 patients needed non-invasive positive ventilation of which 3 patients were reintubated and 2 patients died. Regarding ultrasound diaphragmatic parameters (during tidal breathing) (Table 1 ), DT Insp mm, DT Expt (FRC) mm, DTF %, and DE in centimeters were significantly higher [24 mm (23.25–26) vs.18 mm (17–19.15), p < 0.001; 17 mm (15–18) vs.14 mm (12.3–15), p < 0.001; 44.41% (35.07–67.12) vs. 30.38% (23.34–38.07), p < 0.001; 1.95 cm (1.53–2.75) vs. 1.66 cm (1.09–1.94), p <0.003]. Regarding ultrasound diaphragmatic parameters during deep breathing (Table 1 ), DT Insp (TLC) mm, DT Exp (RV) mm, DTF %, and DE in cm were significantly higher [36.5 mm (33–39.75) vs. 26 mm (23.25–29.75), p < 0.001; 25 mm (22–27) vs. 20.5 mm (18–22.75), p < 0.001; 50% (43.05–58.2) vs. 25% (23.8–26.99), p < 0.001; 3.6 cm (3–5.4) vs. 2.95 cm (1.73–4.05), p < 0. 0.01] respectively in the successfully extubated group compared to the failed one (Table 1 ). AUC was used to assess the accuracy of diaphragmatic parameters in predicting failed extubation (during tidal breathing) (Table 2 ) (Fig. 3 ). A cutoff value of DT Exp (FRC) > 15.5mm was associated with successful extubation with 62% sensitivity and 100% specificity, a cutoff value of DTF % > 32.82 was associated with successful extubation with 90% sensitivity and 75% specificity, a cutoff value of DE > 1.7 cm was associated with successful extubation with 68% sensitivity and 65% specificity, and the optimum cutoff value of DT Insp > 21 mm was associated with successful extubation with 95% sensitivity and 100% specificity (Table 2 ) (Fig. 3 ). A cutoff value (during deep breathing) of DT Exp (RV) > 22.5 was associated with successful extubation with 73% sensitivity and 75% specificity, a cutoff value of DE > 3.1 was associated with successful extubation with 75% sensitivity and 55% specificity, a cutoff value of DT Insp (TLC) > 28.5mm was associated with successful extubation with 100% sensitivity and 65% specificity, and the optimum cutoff value of > 37 DTF % was associated with successful extubation with 97% sensitivity and 100% specificity but AUC 100% (Table 2 ) (Fig. 4 ). Among the traditional weaning parameter (RSBI, minute ventilation, RR, and PaO2/FiO2), PaO2/FiO2 was significantly more in the successful extubation group than the failed one [206 (197.3–211.8) vs. 190 (185–199.8), p < 0.0.001] (Table 1 ) (Fig. 5 ). All DT parameters were significantly higher in the COPD group than in failed weaning in the pulmonary group (B) (Table 3 ).
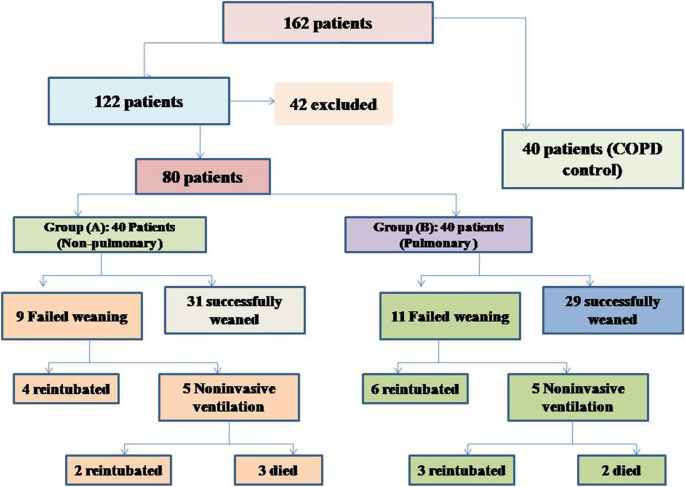
Flow chart showing criteria of patients’ selection and follow-up through the study
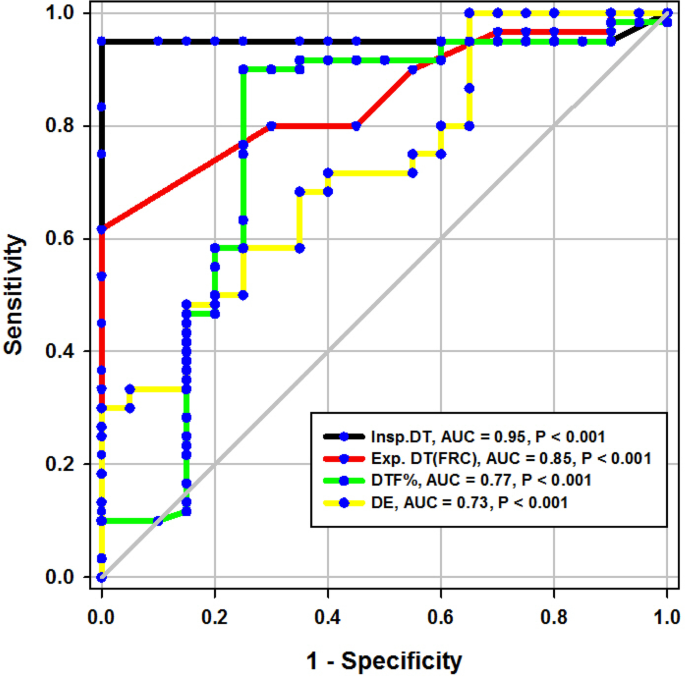
ROC curve of diaphragmatic parameters during tidal breathing
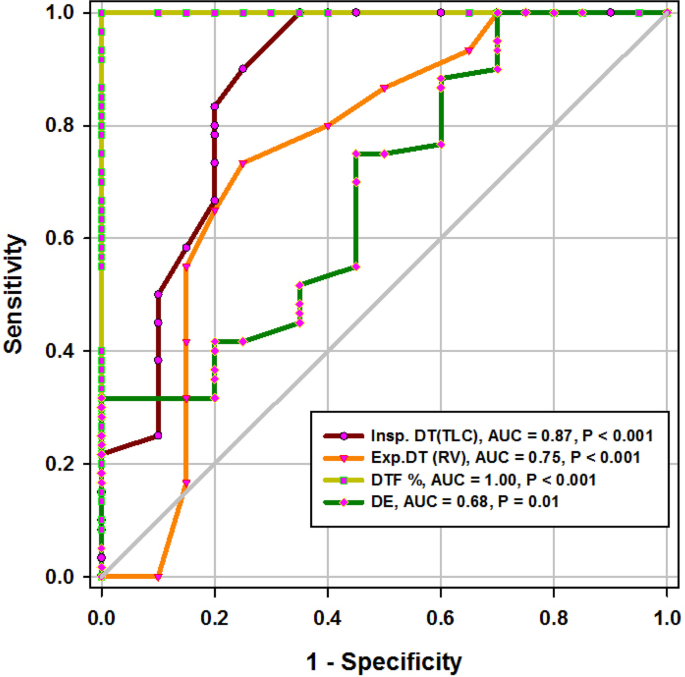
ROC curve of diaphragmatic parameters during deep breathing
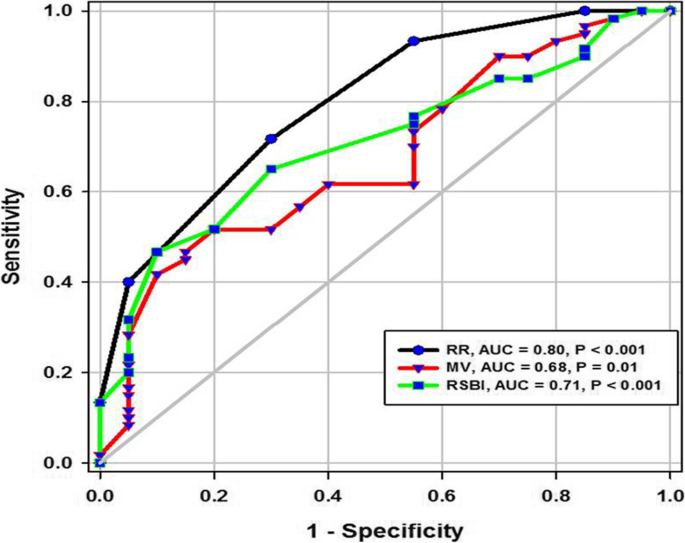
ROC curve of RR, MV, and RSBI in the prediction of successful weaning
The diaphragm is the main respiratory muscle, which plays an important role in the respiratory movement, and its dysfunction predisposes to prolonged duration of mechanical ventilation and respiratory complications. Sonographic evaluation has recently started to become popular in the intensive care unit (ICU) for assessing diaphragmatic function [ 9 ]. In comparing the control COPD cases with others who suffered from MV with failed weaning experience, regarding US parameters during tidal breathing, both of inspiratory, expiratory DT, DE, and DTF % were significantly higher in the COPD group (control) than in the failed weaning group (B) ( p < 0.001). Furthermore, during deep breathing techniques, all DT parameters were significantly higher in the COPD group than in the weaning failure group ( p < 0.001). In our knowledge, this is the first study that compared pulmonary diseases and COPD as regards the diaphragmatic ultrasound parameter (Table 3 ).
Diaphragmatic thickness during tidal breathing (Fig. 3 )
In the present study, DT at end inspiration in the successful group was 24 mm (23.25–26), versus failed group 18 mm (17–19.15), p < 0.001, with a cutoff point > 21mm, 95% sensitivity, 100% specificity, 100% PPV, 99% NPV, and an AUC 95% (Tables 1 and 2 ). Similarly, Farghaly and Hasan [ 3 ] found DT at end inspiration in a successful group was 24 mm (22–28), versus failed group 18 mm (15–20), with a cutoff point ≥ 21 mm, 77.5% sensitivity, 86.6% specificity, and an AUC of 83.1%. In the present study, DT (FRC) at end expiration in a successful group was 17 mm (15–18), versus failed group 14 mm (12.3–15), p = 0.001, with a cutoff point >15.5%, 62% sensitivity, 100% specificity, 100% PPV, 92% NPV, and an AUC 85% (Tables 1 and 2 ) (Fig. 3 ). Similarly, Farghaly and Hasan found that DT at end expiration in a successful group was 16 mm (11.2–18.7), versus failed group 11 mm (10–15), with a cutoff point ≥ 10.5 mm, 80% sensitivity, 50% specificity, and an AUC 68.8% [ 3 ]. In the present study, DTF% in a successful group was 44.41% (35.07–67.12), versus failed group 30.38% (23.34–38.07), with a cutoff point > 32.82%, 90% sensitivity, 75% specificity, 44% PPV, 97% NPV, and an AUC 77% (Tables 1 and 2 ) (Fig. 3 ). This result is consistent with studies by Farghally and Hasan [ 3 ] and Dinino et al. [ 10 ] which demonstrated that DTFs with a cutoff point more than 34 and 30, respectively, were associated with weaning success and better ICU outcomes. In contrast with Umbrello et al. [ 4 ], who observed patients after major elective surgery and first weaning failure, they reported that a cutoff point of DTF more than 20% was associated with weaning success, and this may be explained by the absence of surgical patients in this study. In the present study, DE in a successful group is 1.9 cm (1.53–2.75), versus failed group 1.66 cm (1.09–1.94), p = 0.001, with a cutoff point > 1.7 cm, 68% sensitivity, 65% specificity, 30% PPV, 90% NPV, and an AUC 0.73 (Tables 1 and 2 ) (Fig. 3 ). This result is consistent with the studies done by Matamis et al. [ 9 ] and Palkar et al. [ 11 ] who confirmed that DE at a cutoff point of more than 1.65 cm and 1.64 cm, respectively, was associated with weaning success and better ICU outcomes. Also, Gursel et al. [ 12 ] reported that tidal diaphragmatic excursion using standard ultrasound devices (SD) is 1.76 ± 0.69 cm (0.58–3.30) and using pocket-sized ultrasound devices (PSDs) 1.62 ± 0.70 cm (0.50–3.00). In the present study, the AUC of the DT Insp (95) was more than that of DTF (77), while AUC of DT Exp (FRC) (85) was more than that of DTF (77). In contrast, Farghaly and Hasan stated that AUC of DT (83.1) at end inspiration was more than DT (68.8) at the end expiration and AUC of DT (68.8) at the end expiration was less than DTF (70. 8). Also, it was found that AUC of DT (61) at the end expiration was less than that of DTF (79) alone [ 3 ]. In the present study, the DE was less (68%) sensitive than that DT Insp (95%), and the specificity of DT Insp (100%) was more than that of DE (30%) (Table 2 ). Similarly, Farghaly and Hasan observed that diaphragm excursion should not be used in the assessment of diaphragmatic contractile activity, whereas diaphragm thickening is a good indicator of respiratory effort [ 3 ]. Also, Umbrello et al. observed that during pressure support ventilation, diaphragm thickening was more accurate than diaphragm excursion and suggested that the use of diaphragm excursion is of little help during PSV and should not be used in the assessment of diaphragmatic contractile activity [ 4 ]. In contrast, Hayat et al. [ 13 ] reported that diaphragmatic excursion is a good method for predicting the weaning outcome.
Diaphragmatic thickness during deep breathing
In the current study, diaphragm thickness at TLC in a successful group was 36 mm (33–39.75), versus failed group 26 mm (23.25–29.75) with a cutoff point 28.5, 100% sensitivity, 65% specificity, 39% PPV, 100% NPV, and an AUC 0.87, while diaphragm thickness at RV in the successful group was 25 mm (22–27), versus failed group 20.5 mm (18–22.75) with a cutoff point 22.5 mm, 73% sensitivity, 75% specificity, 39% PPV, 93% NPV, and an AUC 0.75 (Tables 1 and 2 ) (Fig. 4 ). Similarly, Ferrari et al. stated that diaphragm thickness (DT) at TLC in a successful group was 38 mm (29–45), versus failed group 30 mm (20–40) [ 1 ], while DT at RV in a succeeded group was 25 mm (19–28), versus failed group 24 mm (17–30). Moreover, Gursel et al. found that the maximal inspiratory thickness was SD 47 ± 16mm (23–68) and PSDs 45 ± 12mm (24–91). In contrast, Pirompanich and Romsaiyut noted that DT at TLC in a succeeded group was 35 ± 13 and 38 mm (IQR 29–45), versus failed group 31 ± 13 mm and 30 mm (IQR 20–40) [ 12 ], while diaphragm thickness at RV in a successful group was 22 ± 09 mm and 25 mm (IQR 19–28), versus failed group 25 ± 11 mm and 24 mm (IQR 17–30).There were higher values about RV in the failed group more than the successful group, and these variables can be explained by different causes for mechanical ventilation as well as different ventilation periods and different ethnic groups which may affect the thickness of the diaphragm. In the present study, DTF in a successful group was 50% (43.05–58.20), versus failed group 25% (23.80–26.99), with a cutoff point of 37%, 97% sensitivity, 100% specificity, 97% PPV, 100% NPV, and an AUC 1 (Tables 1 and 2 ) (Fig. 4 ). These results are consistent with studies done by Ferrari et al. [ 1 ] which demonstrated that DTFs of more than 36% were associated with weaning success and better ICU outcomes. Our study found that DE in a successful group was 3.6 cm (3–5.4), versus failed group 2.95 cm (1.73–4.05), with a cutoff point DE 3.1 cm, 75% sensitivity, 55% specificity, 27% PPV, 91% NPV, and an AUC 0.68 (Tables 1 and 2 ) (Fig. 4 ). Similarly, Carrie et al. found that DE in the successful group was 4.1 ±2. 1cm, versus failed group 3 ± 1.8cm with a cutoff point DE 2.7cm [ 14 ]. Also, Gursel et al. found in their study DE (±SD) was 2.97 ± 1.18cm (1.33–5.40) and PSDs 2.67 ± 0.90cm (1.30–4.70) [ 12 ]. Moreover, Lerolle et al. reported that DE less than 2.5 cm was a predictor of weaning failure, in post-cardiac patients connected to mechanical ventilation [ 15 ]. In the present study, the DTF was more specific and sensitive with a higher AUC (100%, 97%, 1) than DE (55%, 75%, 0.91) (Table 2 ) (Fig. 4 ). This result is consistent with the studies by Samanta et al. [ 16 ] and Ferrari et al. [ 1 ] who reported that the DTF is more accurate than DE in the prediction of successful weaning. In the present study, DT Insp (TLC) is more sensitive and specific (100%, 65%) than DE (75%, 55%). The AUC of DT Insp (TLC) was more than that of DT Exp (RV) (0.87 and 0.75, respectively). The AUC of DTF was more than the AUC of DT Insp (TLC) (100 and 87, respectively) (Table 2 ) (Fig. 4 ). In contrast, Farghaly and Hasan observed that the AUC of DT at end inspiration was more than DT at end expiration (83.1 and 68. 8, respectively) [ 3 ]. Also, Di Nino et al. observed that the AUC for DT end expiration was less than that for DTF% alone (0.79 and 0.61, respectively) [ 10 ]. However, they determined DT, DTF, and DE during tidal breathing, while in the current study, DT, DTF, and DE were assessed during tidal and deep breathing. In the present study, the AUC of DTF during deep breathing was more than DT Insp during tidal breathing (100 and 95, respectively), while the AUC of DT Insp was more than DT Insp (TLC) (95 and 87, respectively) (Table 2 ). In the present study, the RSBI in the successful group was 58 (52–63) breath/min/L, versus failed group 46 (41–51) breath/min/L, p < 0.005, and a cutoff value for RSBI was 35.5 b/min with 47% sensitivity, 90% specificity, 51% PPV, 188% NPV, and the AUC of 71% in predicting extubation failure (Tables 1 and 2 ) (Fig. 5 ). Similarly, Farghaly and Hasan observed that the RSBI in a successful group was 51.5 (44–79), versus failed group 50 (40–65), p <0.005 [ 3 ]. Also, Pirompanich and Romsaiyut found that the average RSBI in a successful group was 54. 3 ± 22.8, versus failed group 47.7 ± 14.8, p < 0.012 [ 14 ]. In contrast, Ferrari et al. observed that the RSBI in a successful group was 70 (57–83), versus failed group 120 (110–148), p < 0.0001 [ 1 ]. This variation can be explained by different causes for mechanical ventilation as well as different ventilation periods, which may affect the outcome of the weaning process. During tidal breathing, the specificity of RSBI was less than DT at insp and DT Exp (FRC) at end expiration (90, 100, and 100). But the specificity of RSBI is more than DTF and DE (90, 75, and 65). But the AUC of RSBI is less than DT Insp, DT Exp (FRC), DTF, DE, TLC, RV, and DTF (71, 95, 85, 77, 73, 87, 75, and 100, respectively). The AUC of RSBI during forced expiration and inspiration is more than DE (71 and 68, respectively) (Table 2 ) (Figs. 3 , 4 , and 5 ). Similarly, DiNino et al. reported that the diaphragmatic thickness and diaphragmatic thickness fraction are more accurate than RSBI, for predicting successful weaning [ 10 ]. Also, Pirompanich and Romsaiyut observed that integration of DTF (right) (AUC 95%) and RSBI (AUC 70%) are more accurate than RSBI (AUC 70%), for foretelling of successful extubation [ 17 ]. Similarly, Farghaly and Hasan reported that the diaphragm thickness, DTF, and DE during tidal breathing are more accurate than RSBI [ 3 ]. They recommended to consider the use of these parameters with RSBI to improve weaning outcome. In addition, Hayat et al. reported that the DE during tidal breathing is more accurate than RSBI, but they did not use DT and DTF in the comparison [ 13 ]. Ramakrishnan and Siddiqui reported that the diaphragmatic excursion is probably better in predicting extubation success than RSBI [ 18 ].
Fate of the studied patients
In the present study, as regards group A, the number of patients with successful weaning was 31 (77.5%) versus 9 (22.5%) of weaning failure, while in group B, the number of patients with successful weaning was 29 (72.5%) versus 11 (27.5%) of weaning failure. This is consistent with Esteban et al. [ 8 ], 27%. This is in contrast with Ferrari et al. [ 1 ] who reported a 63% failure rate. This variation can be explained by different causes for mechanical ventilation as well as different ventilation periods before starting the weaning process, which may affect the outcome of the weaning process.
Study limitations
The measurements of the diaphragm were not supplemented with direct measurements (such as the maximal expiratory pressure, maximal inspiratory pressure, and transdiaphragmatic pressure). This study was done in the respiratory care unit, and there were no surgical treated patients. While the (reference) thickness of the diaphragm in many diseases, e.g., COPD, pneumonia, and DM, is still unknown, the golden standard of measuring the diaphragmatic strength is phrenic nerve stimulation, and comparing it with sonographic findings was not done in this study. This study did not target a certain chest disease in its assessment of the diaphragm. The right hemidiaphragm was used in the diaphragmatic assessment being easier in imaging than the left hemidiaphragm which is often impeded by intestinal and gastric gas.
Conclusions
Ultrasound of the diaphragm is a simple, easy, non-invasive, and inexpensive method useful to evaluate the thickness of the diaphragm in the zone of apposition. Assessment of DT, DTF by diaphragm ultrasound in B-mode, and DE in M-mode represents a new weaning index with highly accurate results in comparison to the other traditional indices as RSBI, so they can be used as predictive parameters to assess the weaning process outcome.
Ultrasound of the diaphragm is a simple, easy, non-invasive, and inexpensive method useful to evaluate the diaphragmatic muscle. Parameters like diaphragmatic thickness and diaphragmatic excursion can be recorded by real-time ultrasound and could have many clinical reflections. The diaphragmatic thickness fraction during deep breathing could be a good foreteller of weaning from mechanical ventilation.
What this paper contributes to our knowledge
Assessment of diaphragmatic thickness, by diaphragmatic ultrasound in B-mode and diaphragmatic excursion in M-mode, can be used as predictive parameters to assess the weaning process outcome in patients on mechanical ventilation.
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Change history
14 december 2021.
A Correction to this paper has been published: https://doi.org/10.1186/s43168-021-00103-9
Abbreviations
Acute Physiology and Chronic Health Evaluation II
Brightness mode
Charlson comorbidity index
Chronic obstructive lung disease
Control mechanical ventilation
Computerized tomography
Diaphragmatic excursion
Diabetes mellitus
Diaphragmatic thickness
Diaphragmatic thickness at the end expiration
Diaphragmatic thickness fraction
Diaphragmatic thickness at end inspiration
- Diaphragmatic ultrasound
Functional residual capacity
Focus thoracic ultrasound
Hypertension
Intensive care unit
Ischemic heart disease
Interquartile range
Inferior vena cava
Lung ultrasound
Motion mode
Magnetic resonance imaging
Mechanical ventilation
Non-invasive ventilation
Negative predictive value
Prolonged mechanical ventilation
Positive predictive value
Pocket-sized ultrasound devices
Pressure support ventilation
Transdiaphragmatic pressure
Rapid shallow breathing index
Residual volume
Spontaneous breathing
Standard ultrasound devices
Standard deviation
Spatial pulse length
Diaphragm thickness at functional residual capacity
Total lung capacity
Thoracic ultrasound
Ventilator-induced diaphragmatic dysfunction
Tidal volume
Two-dimensional
Three-dimensional
Abdel Rahman DA, Saber S, El-Maghraby A (2020) Diaphragm and lung ultrasound indices in prediction of outcome of weaning from mechanical ventilation in pediatric intensive care unit. Indian J Pediatr 87(6):413–420. https://doi.org/10.1007/s12098-019-03177-y
Boussuges A, Gole Y, Blanc P (2009) Diaphragmatic motion studied by m-mode ultrasonography: methods, reproducibility, and normal values. Chest 135(2):391–400. https://doi.org/10.1378/chest.08-1541
Article PubMed Google Scholar
Carrié C, Bonnardel E, Vally R, et al (2016) Vital capacity impairment due to neuromuscular disease and its correlation with diaphragmatic ultrasound: a preliminary study. Ultrasound Med Biol 42(1):143–149. https://doi.org/10.1016/j.ultrasmedbio.2015.09.020 .
DiNino E, Gartman EJ, Sethi JM, McCool FD (2014) Diaphragm ultrasound as a predictor of successful extubation from mechanical ventilation. Thorax 69(5):423–427. http://dx.doi.org/10.1136/thoraxjnl-2013-204111
Esteban A, Alía I, Tobin MJ, et al (1999) Effect of spontaneous breathing trial duration on outcome of attempts to discontinue mechanical ventilation. Spanish Lung Failure Collaborative Group. Am J Respir Crit Care Med 159(2):512–518. https://doi.org/10.1164/ajrccm.159.2.9803106
Article CAS PubMed Google Scholar
Farghaly S, Hasan AA (2017) Diaphragm ultrasound as a new method to predict extubation outcome in mechanically ventilated patients. Aust Crit Care 30(1):37–43. https://doi.org/10.1016/j.aucc.2016.03.004
Ferrari G, De Filippi G, Elia F, Panero F, Volpicelli G, Aprà F (2014) Diaphragm ultrasound as a new index of discontinuation from mechanical ventilation. Crit Ultrasound J 7;6(1):8. https://doi.org/10.1186/2036-7902-6-8
Grosu HB, Lee YI, Lee J, et al (2012) Diaphragm muscle thinning in patients who are mechanically ventilated. Chest 142(6):1455–1460. https://doi.org/10.1378/chest.11-1638
Gursel G, Inci K, Alasgarova Z (2018) Can diaphragm dysfunction be reliably evaluated with pocket-sized ultrasound devices in intensive care unit? Crit Care Res Pract 1;2018:5192647. https://doi.org/10.1155/2018/5192647
Hayat A, Khan A, Khalil A, Asghar A (2017) Diaphragmatic excursion: Does it predict successful weaning from mechanical ventilation? J Coll Physicians Surg Pak 27(12):743-746. PMID: 29185398
PubMed Google Scholar
Lerolle N, Guérot E, Dimassi S, et al (2009) Ultrasonographic diagnostic criterion for severe diaphragmatic dysfunction after cardiac surgery. Chest 135(2):401–407. https://doi.org/10.1378/chest.08-1531
Matamis D, Soilemezi E, Tsagourias M, et al (2013) Sonographic evaluation of the diaphragm in critically ill patients. Technique and clinical applications. Intensive Care Med 39(5):801–810. https://doi.org/10.1007/s00134-013-2823-1
Palkar A, Narasimhan M, Greenberg H, et al (2018) Diaphragm excursion-time index: a new parameter using ultrasonography to predict extubation outcome. Chest 153(5):1213–1220. https://doi.org/10.1016/j.chest.2018.01.007
Pirompanich P, Pirompanich S (2018) Use of diaphragm thickening fraction combined with rapid shallow breathing index for predicting success of weaning from mechanical ventilator in medical patients. J Intensive Care 6:6. https://doi.org/10.1186/s40560-018-0277-9
Ramakrishnan P, Siddiqui S (2018) Extubation success can be better predicted by diaphragmatic excursion using ultrasound compared to rapid shallow breathing index. Indian J Anaesth 62(10):814–815. https://doi.org/10.4103/ija.IJA_428_18
Samanta S, Singh RK, Baronia AK, et al (2017) Diaphragm thickening fraction to predict weaning-a prospective exploratory study. J Intensive Care 13;5:62. https://doi.org/10.1186/s40560-017-0258-4
Umbrello M, Formenti P (2016) Ultrasonographic assessment of diaphragm function in critically ill subjects. Respir Care 61(4):542–555. https://doi.org/10.4187/respcare.04412
Vivier E, Mekontso Dessap A, Dimassi S, et al (2012) Diaphragm ultrasonography to estimate the work of breathing during non-invasive ventilation. Intensive Care Med 38(5):796–803. https://doi.org/10.1007/s00134-012-2547-7
Download references
Acknowledgements
We would like to express our great gratitude to the National Institute Chest Hospital for their great support.
Author information
Authors and affiliations.
Department of Pulmonology, Beni Suef University Faculty of Medicine, Beni Suef, Egypt
Randa Salah Eldin Mohamed, Abeer Salah Eldin Mohamed & Mohamed Farouk Mohamed
Department of Endemic Medicine and Hepatology, Cairo University Kasr Alainy Faculty of Medicine, Cairo, Egypt
Waleed Fouad Fathalah
National Institute of Chest Hospital, 2 street Talat harb, Giza, Egypt
Ahmed Aelgharib Ahmed
You can also search for this author in PubMed Google Scholar
Contributions
AA collected the patient’s data and wrote the initial manuscript, WF did the ultrasonic assessment of the diaphragm and revised the manuscript, MF performed workup and sample analysis, ASM performed the computations and verified the analytical methods, and RSM revised the manuscript. MF, ASM, and RSM were major contributors in writing the manuscript, supervised, and reviewed the data collection and statistical analysis. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Ahmed Aelgharib Ahmed .
Ethics declarations
Ethics approval and consent to participate.
This study was approved by the ethics committee of Beni-suef University, Faculty of Medicine, with approval number: FMBSUREC/05012020/Ahmed. The subject participant provided written consent.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The author group has been updated and the original article [1] has been corrected.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Mohamed, R.S.E., Mohamed, A.S.E., Fathalah, W.F. et al. The role of diaphragmatic ultrasound as a predictor of successful extubation from mechanical ventilation in respiratory intensive care unit. Egypt J Bronchol 15 , 51 (2021). https://doi.org/10.1186/s43168-021-00095-6
Download citation
Received : 14 April 2021
Accepted : 07 October 2021
Published : 16 November 2021
DOI : https://doi.org/10.1186/s43168-021-00095-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Diaphragmatic thickening
- Thickening fraction
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 08 December 2020
Changes in diaphragmatic excursion and lung compliance during gynaecologic surgery: open laparotomy versus laparoscopy—a prospective observational study
- Kyungmi Kim 1 ,
- Kyoung-Sun Kim 1 ,
- A. Rom Jeon 1 ,
- Jong-Yeon Park 1 &
- Woo-Jong Choi 1
Scientific Reports volume 10 , Article number: 21458 ( 2020 ) Cite this article
1546 Accesses
6 Citations
Metrics details
- Medical research
- Risk factors
This study compared the effects of open versus laparoscopic radical hysterectomy on intraoperative diaphragmatic excursion and lung compliance. We enrolled 20 women per group; Group O’s members underwent open radical hysterectomy, while Group L’s members underwent laparoscopic radical hysterectomy. Diaphragmatic excursion was measured by assessing tidal ventilation using M-mode ultrasonography before intubation (T0), after intubation with mechanical ventilation (T1), 90 min after incision (T2), and at the end of the operation with recovery of muscle relaxation (T3). Peak inspiratory pressure and static lung compliance were measured using an anaesthesia machine combined with a ventilator. Diaphragmatic excursion was significantly lower in Group L than in Group O at T2 (5.3 ± 1.7 mm vs. 7.7 ± 2.0 mm, P < 0.001) and T3 (8.4 ± 1.9 vs. 10.4 ± 2.4, P = 0.011). Impaired diaphragmatic excursion at T3 (< 10 mm under mechanical ventilation) occurred in 15 patients (83.3%) in Group L and seven (38.9%) in Group O (P = 0.006). Changes over time in peak inspiratory pressure and static lung compliance differed significantly between the two groups (P < 0.001 each). Laparoscopic radical hysterectomy decreased diaphragmatic excursion and static lung compliance significantly more than open radical hysterectomy.
Korean clinical trial number: Korean Clinical Trials Registry (KCT0004477) (Date of registration: November 18 2019) ( https://cris.nih.go.kr/cris/search/search_result_st01_en.jsp?seq=14963<ype=&rtype= ).

Similar content being viewed by others

Changes in respiratory mechanics of artificial pneumothorax two-lung ventilation in video-assisted thoracoscopic esophagectomy in prone position
Yoshinori Tanigawa, Kimihide Nakamura, … Yoshiro Sakaguchi
Physiological benefits of lung recruitment in the semi-lateral position after laparoscopic surgery: a randomized controlled study
Eun Jung Oh, Eun Ji Lee, … Jeong-Jin Min

Electric impedance tomography and protective mechanical ventilation in elective robotic-assisted laparoscopy surgery with steep Trendelenburg position: a randomized controlled study
Pasquale Buonanno, Annachiara Marra, … Maria Vargas
Introduction
Laparoscopic surgery is generally preferred to open abdominal surgery because the former is associated with a lower incidence of pulmonary complications and a shorter hospital stay 1 , 2 . However, we previously reported that laparoscopic radical hysterectomy was associated with the development of impaired diaphragmatic excursion at the end of the operation 3 . Our previous results suggested that laparoscopic radical hysterectomy aggravated physiological changes in pulmonary parameters and worsened diaphragmatic excursions. Laparoscopic radical hysterectomy requires the steep Trendelenburg position and pneumoperitoneum, which results in cephalic displacement of the diaphragm and a reduction in diaphragmatic movement. Moreover, numerous reports have emphasised applying positive end-expiratory pressure (PEEP) and recruitment manoeuvres during laparoscopic surgery because of the Trendelenburg position and pneumoperitoneum 4 , 5 , 6 , 7 , 8 .
This implies that the use of laparoscopic surgery cannot guarantee zero incidences of postoperative pulmonary complications. We then encountered the difficult situation of having to decide what kind of surgery is the better option for patient safety when underlying diseases (e.g., morbid obesity, chronic obstructive pulmonary disease, and interstitial lung disease) are present that are associated with a high risk of postoperative pulmonary complications.
The present study therefore compared the impact of the type of gynaecological surgery, open versus laparoscopic radical hysterectomy, on diaphragmatic excursion and lung compliance.
The demographic characteristics of the participants are shown in Table 1 . There were no significant differences between the patients who underwent open and laparoscopic radical hysterectomy.
Despite the changes in diaphragmatic excursions not having any significant group-by-time interactions (P = 0.079), as shown in Fig. 1 , the mean diaphragmatic excursions were significantly lower in patients who underwent laparoscopic radical hysterectomy at T2 (5.3 ± 1.7 mm vs 7.7 ± 2.0 mm, P < 0.001) and T3 (8.4 ± 1.9 mm vs 10.4 ± 2.4 mm, P = 0.011). Impaired diaphragmatic excursion at T3, defined as diaphragmatic excursion < 10 mm under mechanical ventilation, occurred in 15 (83.3%) patients who underwent laparoscopic radical hysterectomy and seven (38.9%) who underwent open radical hysterectomy (P = 0.006). The intra-observer correlation coefficient of measuring the diaphragmatic excursion was 0.991 (95% confidence interval 0.987–0.993, P < 0.001).
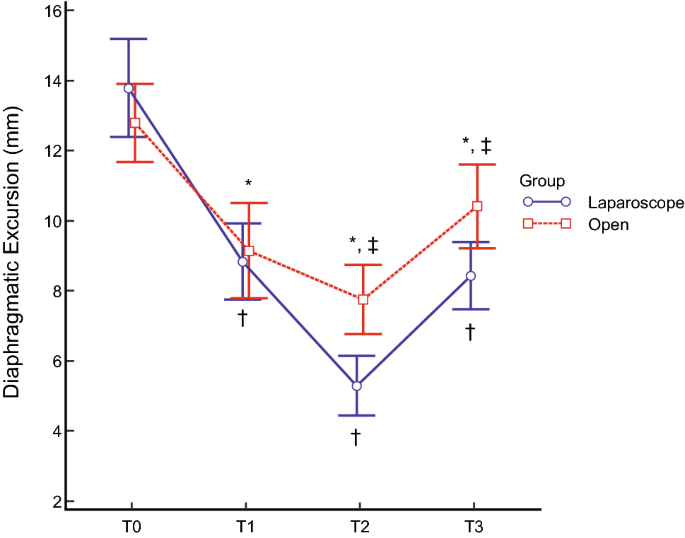
Diaphragmatic excursions at each surgical time point. Diaphragmatic movement decreased after anaesthetic induction and gradually decreased during the operation in both groups. Diaphragmatic excursions were significantly lower in patients undergoing laparoscopic (blue line) relative to those undergoing open (red line) surgery at T2 (P < 0.001) and T3 (P = 0.011). *P < 0.05 compared with T0 in patients who underwent open radical hysterectomy. † P < 0.05 compared with T0 in patients who underwent laparoscopic radical hysterectomy. ‡ Significant difference between the two groups. T0 = before intubation; T1 = after intubation; T2 = 90 min after the incision; T3 = at the end of the operation with recovery of muscle relaxation.
Table 2 shows the arterial blood gas analysis and pulmonary variables during the operation. Peak inspiratory pressure (PIP) was increased at T2 and slightly decreased at T3 in both groups, with the changes in PIP over time differing significantly between the two groups (P < 0.001). Plateau pressure, dynamic lung compliance and static lung compliance were significantly changed over time between the two groups (each P < 0.001). As shown in Fig. 2 , the static lung compliance decreased at T2 and recovered at T3, with the changes in static lung compliance over time differing significantly between these two groups (P < 0.001).
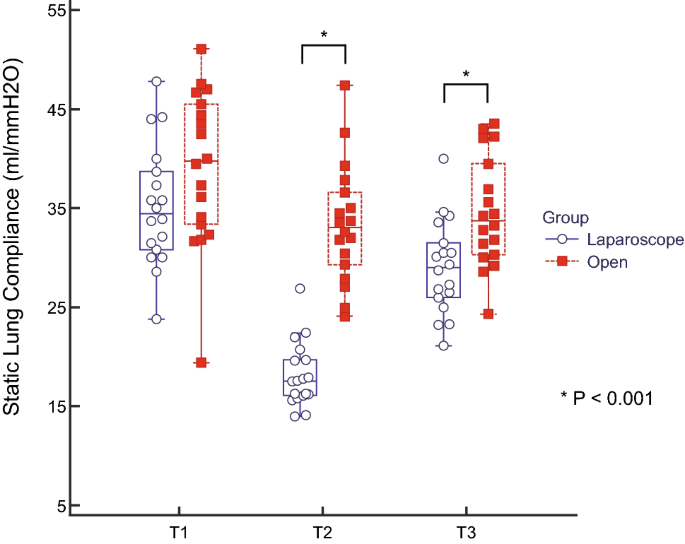
Static lung compliance during each type of surgery. Box-and-whisker plots of static lung compliance in patients who underwent laparoscopic (blue box) and open (red box) radical hysterectomy. Static lung compliance in patients who underwent laparoscopic surgery was reduced significantly during the operation and was significantly lower than in the open-surgery group at the end of the operation (P < 0.001). *The two groups differed significantly. T1 = after intubation; T2 = 90 min after the incision; T3 = at the end of the operation with recovery of muscle relaxation.
Six (33.3%) patients who underwent open and seven (38.9%) who underwent laparoscopic radical hysterectomy had abnormal findings on chest X-ray within 15 postoperative days (P = 0.729). There was no patient with higher than grade II postoperative pulmonary complications.
The present study showed that intraoperative diaphragmatic excursion and static lung compliance were reduced more in patients who underwent laparoscopic than in those who underwent open radical hysterectomy. Moreover, impaired diaphragmatic excursion at the end of surgery occurred more frequently in patients who underwent laparoscopic than in those who underwent open radical hysterectomy.
Our previous study showed that laparoscopic radical hysterectomy reduced diaphragmatic excursion and lung compliance not only during the operation but also at the end of the surgery after neuromuscular reversal 3 . In addition, the present study found that laparoscopic radical hysterectomy worsened diaphragmatic excursion and lung compliance more than open radical hysterectomy did. Laparoscopic radical hysterectomy starts with pneumoperitoneum and requires the steep Trendelenburg position. Pneumoperitoneum leads to the cephalic displacement of the diaphragm, increments in the peak and plateau airway pressures, and a decrease in lung compliance. The steep Trendelenburg position aggravates these pulmonary mechanics during the operation. Laparoscopic radical hysterectomy, therefore, reduces diaphragmatic movement and lung compliance 7 , 9 , 10 , 11 . A comparison of intraoperative lung compliance between patients undergoing open and laparoscopic cholecystectomy showed that the reduction in compliance was significantly greater in the patients who underwent laparoscopic cholecystectomy 12 . Similarly, the present study showed that laparoscopic radical hysterectomy worsened pulmonary mechanics, with reduced lung compliance followed by decreased diaphragmatic excursion.
Open radical hysterectomy also reduces the diaphragmatic excursion and lung compliance, although to a lesser extent than laparoscopic radical hysterectomy, suggesting that general anaesthesia affects both diaphragmatic excursion and lung compliance. General anaesthesia has been reported to alter the location and movement of the diaphragm because it contains a muscle relaxant and is administered in a supine position under mechanical ventilation, reducing lung volume due to atelectasis and airway collapse 13 , 14 . In line with previous reports, our results showed that diaphragmatic excursion was lower at T1 than at T0 in both groups.
Despite the recovery of muscle relaxation in both groups (train-of-four [TOF] ratio > 0.9), diaphragmatic excursion at T3 did not return to the value at T0. Diaphragmatic movement has previously been shown to be significantly lower postoperatively than preoperatively 15 . Moreover, postoperative atelectasis was found to persist for 24 h after laparoscopic surgery and for 48 h after open surgery 16 . Similarly, our results showed that diaphragmatic excursion was impaired at T3, though muscle relaxation recovered, with a TOF ratio > 0.9. Although the exact mechanism of impaired diaphragmatic excursion has not been determined, the impairment might be caused by a reduction in lung volume during the operation and the anatomical peculiarity of the diaphragm, which has a C-shaped fibrous structure. Further studies are required to evaluate the mechanisms responsible for reduced diaphragmatic excursion after an operation.
Ultrasound has been reported to be reliable in assessing diaphragmatic excursion qualitatively and quantitatively 17 , 18 , 19 , 20 . Sonographic evaluation of the diaphragm might be a feasible method of assessing diaphragmatic function 19 . Ultrasound examinations can be performed numerous times since they expose neither the patient nor the surgeon to hazardous chemicals or radiation; they require only a few minutes to perform and are more precise in diagnosing diaphragmatic dysfunction than fluoroscopy 18 , 21 . Ultrasound assessment of diaphragmatic function during the intraoperative and immediate postoperative periods might become routine for evaluating patients at high risk of postoperative pulmonary complications. In addition to being useful in assessing diaphragmatic kinetics 22 , our results highlight the importance of ultrasound in evaluating the diaphragm when assessing the respiratory function.
The present study had several limitations. This observational study was performed at a single centre, with diaphragmatic excursions in all patients measured by a single examiner. The intra-observer correlation coefficient was determined after measuring diaphragmatic excursions though the inter-observer correlation coefficient cannot be confirmed. Moreover, the patients were not randomised to either laparoscopic or open radical hysterectomy. However, there were not significant differences in baseline characteristics between the two groups.
Given that laparoscopic abdominal surgery aggravates atelectasis formation and results in decreased lung compliance, the application of PEEP and recruitment manoeuvres during surgery could increase oxygenation and improve lung mechanics 4 , 5 , 6 , 7 , 8 . However, there was a multicentre observational study in which around 20% of patients did not receive PEEP during routine anaesthetic practice 23 . Applying PEEP may sometimes be regarded as an optional manoeuvre. Our study was designed to evaluate diaphragmatic movement only during the operation. We therefore did not assess the impact of PEEP or recruitment manoeuvres on diaphragmatic excursion, highlighting the need for further studies.
Finally, although postoperative chest X-rays were obtained within 15 days, diaphragmatic excursion on all postoperative days could not be assessed. The results of postoperative X-rays did not differ significantly between the two groups.
Even though our results cannot be generalised to all patients who undergo radical hysterectomy, they may be helpful when deciding on the type of surgery to perform for patients who have underlying lung diseases that are associated with postoperative pulmonary complications and require point-of-care during the perioperative period. Further studies are required to determine the time taken to recover from diaphragmatic impairment and to assess long-term postoperative outcomes.
In conclusions, laparoscopic radical hysterectomy decreases diaphragmatic excursion and lung compliance significantly more than open radical hysterectomy. The former procedure requires the Trendelenburg position and pneumoperitoneum. Furthermore, impaired diaphragmatic excursion at the end of surgery was more frequent in patients who underwent laparoscopic radical hysterectomy than in those who underwent open radical hysterectomy.
All included patients provided written informed consent. The study protocol was approved by the Institutional Ethics Committee of the Asan Medical Center (AMC IRB 2019-0761, Seoul, Korea), and the study was registered in the Korean Clinical Trials Registry (KCT0004477). This study enrolled 20 adult patients (American Society of Anesthesiologists physical status I–II) who prospectively underwent elective open radical hysterectomy. The control group consisted of 20 patients who had previously undergone laparoscopic radical hysterectomy 3 . Patients were excluded if they had chronic obstructive pulmonary disease, respiratory dysfunction, or a body mass index > 30 kg/m 2 . Two patients who underwent open radical hysterectomy were withdrawn from this study: one with a poor echo window due to the operation field, and the other with upper airway obstruction during induction of anaesthesia. In addition, two control patients who underwent laparoscopic radical hysterectomy were withdrawn, one due to conversion to laparotomy, and the other because there was a poor echo window. Figure 3 shows that this study was performed according to the STROBE guidelines.
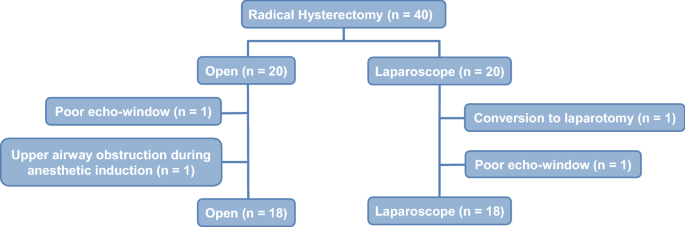
Flow diagram of patients who underwent radical hysterectomy. Forty patients were enrolled. Two patients were withdrawn from each group. Data from 36 patients were analysed.
Clinical data
Baseline characteristics were recorded, including patient age, body mass index, and history of systemic disease (e.g., hypertension and diabetes). Operative characteristics were recorded, including the length of the operation, the volume of administered fluids, and the results of intraoperative arterial blood gas analysis. Pulmonary mechanics, including PIP, plateau pressure, dynamic lung compliance and static lung compliance, were acquired from the anaesthesia machine (Primus Ⓡ , Dragger, Lubeck, Germany) combined with a ventilator. The definition of postoperative pulmonary complications was the same as that used in a previous study by Dindo et al., which was reported in Annals of Surgery 24 . The electronic medical records and chest X-rays within 15 days after the surgery of study patients were using to check the postoperative pulmonary complications.
Anaesthesia and mechanical ventilation
All anaesthesia procedures conformed to the standards for anaesthesia at our institution. Anaesthesia was induced via the administration of pentothal sodium (5 mg/kg), rocuronium (0.6 mg/kg), and remifentanil (effective site concentration, 2.0–5.0 ng/ml). Anaesthesia was maintained with desflurane (1.0 minimum alveolar concentration), continuous infusion of remifentanil (effective site concentration, 1.0–3.0 ng/ml), and rocuronium (0.3 mg/kg/h). At the end of the operation, muscle relaxation was reversed via the administration of sugammadex (2.0 mg/kg), with recovery defined as a TOF peripheral nerve stimulation ratio > 0.9. Patient vital signs monitored during surgery included heart rate, peripheral oxygen saturation, electrocardiograph, continuous arterial blood pressure, bispectral index, TOF ratio, and end-tidal carbon dioxide concentration. Mechanical ventilation was maintained with a volume-controlled mode of 50% of inspired oxygen fraction. Ventilator settings included a target tidal volume of 8 ml/kg of ideal body weight without PEEP and a respiratory rate of 10–14 cycles/min based on the end-tidal carbon dioxide concentration.
Measurement of diaphragmatic excursion
Diaphragmatic excursion was measured twice at each time point by a single well-trained expert (K.K.) using a 5–2 MHz convex transducer and an Edge II ultrasound machine (SonoSite, Inc., Bothell, WA), as described previously 3 . The magnitude of the diaphragmatic excursion at each time point was defined as the average of the two measurements. Time points for the measurement of diaphragmatic excursion included before intubation with mechanical mask ventilation in a supine position (T0, bispectral index < 60, TOF ratio > 0.9) with a tidal volume of 8 ml/kg of ideal body weight and a respiratory rate of 12 cycles/min; after intubation with mechanical ventilation (T1, bispectral index < 60, TOF ratio = 0, supine position); 90 min after incision (T2, Trendelenburg position); and at the end of the operation with recovery of muscle relaxation in a supine position (T3, bispectral index < 60, TOF ratio > 0.9) under mechanical ventilation. Diaphragmatic impairment was defined an excursion of diaphragm was < 10 mm under mechanical ventilation 25 , 26 , 27 .
Statistical analysis and sample size calculation
A pilot study showed that the mean between-group difference in diaphragmatic excursion at T3 was 2.0 ± 2.2 mm. At an α of 0.05 and a power of 0.8, and assuming a 10% dropout rate, 20 patients per group were required.
Baseline characteristics and perioperative variables were compared between the two groups. Continuous variables are expressed as mean ± standard deviation and were compared using Student’s t-tests, whereas categorical variables are expressed as counts and percentages and were compared using the χ 2 and Fisher’s exact tests. Serial changes in diaphragmatic excursion and pulmonary variables in the two groups were compared by repeated measures of the two-way ANOVA followed by a Bonferroni correction. Data were managed and statistical analyses were performed using IBM SPSS Statistics 21.0 software (IBM, Armonk, NY).
Data availability
All data generated or analysed during this study are available from the corresponding author upon reasonable request.
Koc, A., Inan, G., Bozkirli, F., Coskun, D. & Tunc, L. The evaluation of pulmonary function and blood gas analysis in patients submitted to laparoscopic versus open nephrectomy. Int. Braz. J. Urol. 41 , 1202–1208 (2015).
Article Google Scholar
Staehr-Rye, A. K. et al. Minimal impairment in pulmonary function following laparoscopic surgery. Acta Anaesthesiol. Scand. 58 , 198–205 (2014).
Article CAS Google Scholar
Kim, K. et al. Changes of diaphragmatic excursion and lung compliance during major laparoscopic pelvic surgery: a prospective observational study. PLoS ONE 13 , e0207841 (2018).
Cinnella, G. et al. Effects of recruitment maneuver and positive end-expiratory pressure on respiratory mechanics and transpulmonary pressure during laparoscopic surgery. Anesthesiology 118 , 114–122 (2013).
Zhou, Z. F. et al. Effects of intraoperative PEEP on postoperative pulmonary complications in patients undergoing robot-assisted laparoscopic radical resection for bladder cancer or prostate cancer: study protocol for a randomized controlled trial. Trials 20 , 304 (2019).
Futier, E. et al. Intraoperative recruitment maneuver reverses detrimental pneumoperitoneum-induced respiratory effects in healthy weight and obese patients undergoing laparoscopy. Anesthesiology 113 , 1310–1319 (2010).
Cakmakkaya, O. S., Kaya, G., Altintas, F., Hayirlioglu, M. & Ekici, B. Restoration of pulmonary compliance after laparoscopic surgery using a simple alveolar recruitment maneuver. J. Clin. Anesth. 21 , 422–426 (2009).
Lee, H. J., Kim, K. S., Jeong, J. S., Shim, J. C. & Cho, E. S. Optimal positive end-expiratory pressure during robot-assisted laparoscopic radical prostatectomy. Korean J. Anesthesiol. 65 , 244–250 (2013).
Manner, T., Aantaa, R. & Alanen, M. Lung compliance during laparoscopic surgery in paediatric patients. Paediatr. Anaesth. 8 , 25–29 (1998).
Oikkonen, M. & Tallgren, M. Changes in respiratory compliance at laparoscopy: measurements using side stream spirometry. Can. J. Anaesth. 42 , 495–497 (1995).
Normando, V. M., Brito, M. V., de Araujo Junior, F. A. & Albuquerque, B. C. Effects of pneumoperitoneum on the amplitude of diaphragmatic excursion in pigs. J. Bras. Pneumol. 32 , 16–22 (2006).
Volpino, P., Cangemi, V., D’Andrea, N., Cangemi, B. & Piat, G. Hemodynamic and pulmonary changes during and after laparoscopic cholecystectomy. A comparison with traditional surgery. Surg. Endosc. 12 , 119–123 (1998).
Grieco, D. L. et al. Lung volumes, respiratory mechanics and dynamic strain during general anaesthesia. Br. J. Anaesth. 121 , 1156–1165 (2018).
Magnusson, L. & Spahn, D. R. New concepts of atelectasis during general anaesthesia. Br. J. Anaesth. 91 , 61–72 (2003).
Kim, S. H. et al. An evaluation of diaphragmatic movement by M-mode sonography as a predictor of pulmonary dysfunction after upper abdominal surgery. Anesth. Analg. 110 , 1349–1354 (2010).
Eichenberger, A. et al. Morbid obesity and postoperative pulmonary atelectasis: an underestimated problem. Anesth. Analg. 95 , 1788–1792 (2002).
Houston, J. G. et al. Ultrasonic evaluation of movement of the diaphragm after acute cerebral infarction. J. Neurol. Neurosurg. Psychiatry 58 , 738–741 (1995).
Ayoub, J. et al. Non-invasive quantification of diaphragm kinetics using m-mode sonography. Can. J. Anaesth. 44 , 739–744 (1997).
Holtzhausen, S., Unger, M., Lupton-Smith, A. & Hanekom, S. An investigation into the use of ultrasound as a surrogate measure of diaphragm function. Heart Lung 47 , 418–424 (2018).
Lerolle, N. et al. Ultrasonographic diagnostic criterion for severe diaphragmatic dysfunction after cardiac surgery. Chest 135 , 401–407 (2009).
Testa, A. et al. Ultrasound M-mode assessment of diaphragmatic kinetics by anterior transverse scanning in healthy subjects. Ultrasound Med. Biol. 37 , 44–52 (2011).
Haji, K. et al. Interpreting diaphragmatic movement with bedside imaging, review article. J. Crit. Care 34 , 56–65 (2016).
Jaber, S. et al. A multicentre observational study of intra-operative ventilatory management during general anaesthesia: tidal volumes and relation to body weight. Anaesthesia 67 , 999–1008 (2012).
Dindo, D., Demartines, N. & Clavien, P. A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 240 , 205–213 (2004).
Boussuges, A., Gole, Y. & Blanc, P. Diaphragmatic motion studied by m-mode ultrasonography: methods, reproducibility, and normal values. Chest 135 , 391–400 (2009).
Kim, W. Y., Suh, H. J., Hong, S. B., Koh, Y. & Lim, C. M. Diaphragm dysfunction assessed by ultrasonography: influence on weaning from mechanical ventilation. Crit. Care Med. 39 , 2627–2630 (2011).
DiNino, E., Gartman, E. J., Sethi, J. M. & McCool, F. D. Diaphragm ultrasound as a predictor of successful extubation from mechanical ventilation. Thorax 69 , 423–427 (2014).
Download references
Author information
Authors and affiliations.
Laboratory for Cardiovascular Dynamics, Department of Anesthesiology and Pain Medicine, Asan Medical Center, University of Ulsan College of Medicine, 88, Olympic-ro 43-gil, Songpa-gu, Seoul, 05505, Republic of Korea
Kyungmi Kim, Kyoung-Sun Kim, A. Rom Jeon, Jong-Yeon Park & Woo-Jong Choi
You can also search for this author in PubMed Google Scholar
Contributions
K.K: Conceptualization, Methodology, Software, Formal analysis, Data curation, Writing—original draft preparation, Writing—review and editing, Visualization. K.-S.K: Methodology, Software, Data curation. A.R.J: Software, Resources, Visualization. J.-Y.P: Conceptualization, Validation, Investigation, Resources, Writing—review and editing, Supervision. W.-J.C: Conceptualization, Methodology, Validation, Formal analysis, Writing—original draft preparation, Writing—review and editing, Project administration.
Corresponding author
Correspondence to Woo-Jong Choi .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Kim, K., Kim, KS., Jeon, A.R. et al. Changes in diaphragmatic excursion and lung compliance during gynaecologic surgery: open laparotomy versus laparoscopy—a prospective observational study. Sci Rep 10 , 21458 (2020). https://doi.org/10.1038/s41598-020-78375-2
Download citation
Received : 29 May 2020
Accepted : 17 November 2020
Published : 08 December 2020
DOI : https://doi.org/10.1038/s41598-020-78375-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
The effects of robot-assisted laparoscopic surgery with trendelenburg position on short-term postoperative respiratory diaphragmatic function.
BMC Anesthesiology (2024)
Effects of dynamic individualized PEEP guided by driving pressure in laparoscopic surgery on postoperative atelectasis in elderly patients: a prospective randomized controlled trial
- Jing-yan Lin
BMC Anesthesiology (2022)
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Diaphragmatic excursion is correlated with the improvement in exercise tolerance after pulmonary rehabilitation in patients with chronic obstructive pulmonary disease
Affiliations.
- 1 Department of Rehabilitation Medicine, Kindai University School of Medicine, 377-2 Onohigashi, Osakasayama, Osaka, 5898511, Japan. [email protected].
- 2 Department of Respiratory Medicine and Allergology, Kindai University School of Medicine, Osaka, Japan. [email protected].
- 3 Department of Rehabilitation Medicine, Kindai University School of Medicine, 377-2 Onohigashi, Osakasayama, Osaka, 5898511, Japan.
- 4 Department of Respiratory Medicine and Allergology, Kindai University School of Medicine, Osaka, Japan.
- 5 Inclusive Medical Science Research Institute, Morinomiya University of Medical Sciences, Osaka, Japan.
- 6 Division of Biostatistics, Clinical Research Center, Kindai University School of Medicine, Osaka, Japan.
- PMID: 34686189
- PMCID: PMC8532083
- DOI: 10.1186/s12931-021-01870-1
Background: In patients with chronic obstructive pulmonary disease (COPD), the maximum level of diaphragm excursion (DE max ) is correlated with dynamic lung hyperinflation and exercise tolerance. This study aimed to elucidate the utility of DE max to predict the improvement in exercise tolerance after pulmonary rehabilitation (PR) in patients with COPD.
Methods: This was a prospective cohort study. Of the 62 patients with stable COPD who participated in the outpatient PR programme from April 2018 to February 2021, 50 completed the programme. Six-minute walk distance (6MWD) was performed to evaluate exercise tolerance, and ultrasonography was performed to measure DE max . Responders to PR in exercise capacity were defined as patients who demonstrated an increase of > 30 m in 6MWD. The receiver operating characteristic (ROC) curve was used to determine the cut-off point of DE max to predict responses to PR.
Results: Baseline levels of forced expiratory volume in 1 s, 6MWD, maximum inspiratory pressure, DE max and quadriceps muscle strength were significantly higher, and peak dyspnoea of modified Borg (mBorg) scale score was lower in responders (n = 30) than in non-responders (n = 20) to PR (p < 0.01). In multivariate analysis, DE max was significantly correlated with an increase of > 30 m in 6MWD. The area under the ROC curve of DE max to predict responders was 0.915, with a sensitivity and specificity of 83% and 95%, respectively, at a cut-off value of 44.9 mm of DE max .
Conclusion: DE max could adequately predict the improvement in exercise tolerance after PR in patients with COPD.
Keywords: COPD; Diaphragmatic excursion; Pulmonary rehabilitation; Six-minute walk distance (6MWD).
© 2021. The Author(s).
Publication types
- Observational Study
- Aged, 80 and over
- Clinical Decision-Making
- Diaphragm / diagnostic imaging
- Diaphragm / physiopathology*
- Exercise Therapy*
- Exercise Tolerance*
- Lung / physiopathology*
- Predictive Value of Tests
- Prospective Studies
- Pulmonary Disease, Chronic Obstructive / diagnosis
- Pulmonary Disease, Chronic Obstructive / physiopathology
- Pulmonary Disease, Chronic Obstructive / rehabilitation*
- Recovery of Function
- Resistance Training
- Time Factors
- Treatment Outcome
- Ultrasonography
Grants and funding
- 21K11(325)/science and technology innovative research team in higher educational institutions of hunan province
- Research article
- Open access
- Published: 27 January 2023
Clinical values of diaphragmatic movement in patients with chronic obstructive pulmonary disease
- Taehwa Kim 1 , 2 na1 ,
- Sungchul Huh 3 na1 ,
- Jae Heun Chung 1 , 2 ,
- Yun Seong Kim 1 , 2 ,
- Ra Yu Yun 3 , 4 ,
- Onyu Park 5 &
- Seung Eun Lee ORCID: orcid.org/0000-0002-4266-7722 1 , 2
BMC Pulmonary Medicine volume 23 , Article number: 33 ( 2023 ) Cite this article
2113 Accesses
1 Citations
1 Altmetric
Metrics details
The limitation of activity due to dyspnea in chronic obstructive pulmonary disease (COPD) patients is affected by diaphragmatic dysfunction and reduced lung function. This study aimed to analyze the association between diaphragm function variables and forced expiratory volume in the first second (FEV1) and to estimate the clinical significance of diaphragm function in the correlation between COPD severity and lung function.
This prospective, single-center, cross-sectional observational study enrolled 60 COPD patients in a respiratory outpatient clinic. Data for baseline characteristics and the dyspnea scale were collected. Participants underwent a pulmonary function test (PFT), a 6-minute walk test (6MWT), and diaphragm function by ultrasonography.
The right excursion at forced breathing showed the most significant correlation with FEV1 ( r = 0.370, p = 0.004). The cutoff value was 6.7 cm of the right diaphragmatic excursion at forced breathing to identify the FEV1 above 50% group. In the group with a right diaphragmatic excursion at forced breathing < 6.7 cm, modified Medical Research Council (mMRC), St. George's Respiratory Questionnaire and the total distance of 6MWT showed no difference between groups with FEV1 under and above 50% ( p > 0.05). In the group with ≥ 6.7 cm, mMRC and the total distance of 6MWT showed a significant difference between FEV1 under and above 50% ( p = 0.014, 456.7 ± 69.7 m vs. 513.9 ± 60.3 m, p = 0.018, respectively).
The right diaphragmatic forced excursion was closely related to FEV1, and analysis according to the right diaphragmatic forced excursion-based cut-off value showed a significant difference between both groups. When the diaphragm function was maintained, there was a lot of difference in the 6MWT’s factors according to the FEV1 value. Our data suggest that diaphragmatic function should be performed when interpreting PFT.
Peer Review reports
Introduction
The most common complaint in respiratory diseases regardless of the disease type is dyspnea [ 1 ]. COPD is characterized by worsening dyspnea during movement [ 2 ]. COPD restricts various activities of daily living due to shortness of breath, leading to poor quality of life and increased mortality and morbidity [ 3 ]. There are many causes of dyspnea; however, for patients with stable COPD, a major contributor is the weakening of the respiratory muscles, excluding conditions such as acute infectious diseases [ 4 ].
The diaphragm is the main respiratory muscle, particularly the inspiratory muscles. The weakness of the diaphragm in COPD has been extensively studied. Some studies have reported a significant reduction in diaphragmatic excursion in patients with COPD [ 5 , 6 , – 7 ]. Lung hyperinflation-associated shortening of the diaphragm has traditionally been considered a major cause of diaphragmatic weakness [ 8 ]. Also, there were previous studies about diaphragmatic thickness. Diaphragmatic thickness was a factor related to weaning and prognosis in patients under mechanical ventilation [ 9 , 10 ]. Recently, several studies have reported the clinical value of diaphragm ultrasonography according to COPD severity, and even compared to traditional methods, the diagnostic value of ultrasonography has proven to be reliable and useful [ 11 ]. Ultrasonography is also commonly used in medical facilities because it can be carried out anywhere, has no associated radiation risk, and can be used to adequately visualize the structure of the diaphragm [ 12 ].
Furthermore, 6MWT is an important tool for assessing exercise capacity and functional status in patients with COPD. Diaphragmatic weakness can impair physical performance, especially the 6MWT [ 13 , 14 ]. A previous study reported that pulmonary function was significantly correlated with the 6MWT in patients with severe and very severe COPD [ 15 ]. The relationship between 6MWT and PFT is a matter of connecting and understanding the respiratory muscles. PFT is used to measure the volume and flow rate of the lungs, and 6MWT is an important test for evaluating the exercise capacity and functional status of patients.
When we summarize the above, PFT correlates with 6MWT in COPD patients [ 15 ]. 6MWT can evaluate physical performance of COPD patients. Physical performance can also reflect diaphragmatic weakness [ 13 , 14 ]. Therefore, PFT correlates with 6MWT, 6MWT reflects physical performance, and physical performance was associated with diaphragmatic weakness. This relationship of PFT and diaphragmatic weakness can be expressed as follows for the patient. If the pulmonary function expressed by PFT is good, or if case which the power and strength of the respiratory muscles are good when PFT remains the same, breathing is more stable. Therefore, understanding the physiological principles of the respiratory muscle performance that establish the relationship these and compensate for this is important for managing the patient’s condition. Through this study, a review of the correlation between the PFT reflecting the 6MWT and diaphragm ultrasound features of respiratory muscle may be helpful to understand the physiological principles of patients with COPD.
Thus, this study aimed to analyze diaphragm movement characteristics using ultrasonography in patients with COPD and clarify its association with pulmonary function.
Study design and methods
Study design and participants.
This single-center, prospective, cross-sectional observational study recruited participants from a tertiary hospital outpatient respiratory clinic between April 2020 and April 2021. The inclusion criteria were: 1) patients 18 years old or older diagnosed with COPD by a pulmonologist; COPD diagnostic criterion was a post-bronchodilator FEV1/forced vital capacity (FVC) ratio < 0.70 based on the Global Initiative for Chronic Obstructive Lung Disease (GOLD), 2) patients who could maintain the required posture for diaphragm function measurement by ultrasonography and stable breathing during the examination such as 6MWT. Patients unable to cooperate with the examination and unstable patients requiring immediate medical intervention were excluded. Patients with interstitial lung disease featured on chest computed tomography (CT) that could affect diaphragm movement were also excluded.
Sixty-nine patients were enrolled, six of whom with combined interstitial lung disease on CT were excluded. Two patients were lost to follow-up, and one died before all examinations were completed. Finally, 60 patients completed all examinations for the study protocol and were included in the analysis.
All patients provided informed consent before participating in the study. Each patient’s clinical information was collected from four domains: pulmonary function, exercise capacity, body composition, and diaphragm function. Pulmonary function was evaluated through spirometry, MIP, and maximal expiratory pressure (MEP). Exercise capacity and body composition were assessed using the 6MWT and bioelectrical impedance analysis (BIA). Diaphragm dysfunction is defined as loss of muscle contractility [ 16 ]. To evaluated diaphragm dysfunction, we was assessed using ultrasonography in both the M-mode and B-mode for excursion and thickness, respectively.
Assessments
For patients who had performed a PFT within 1 month of participating in the study, the previous results were used and no retesting was performed. Patients who had no available PFT results within 1 month of participating in this study were reevaluated after enrollment. The Carefusion Vmax 20 (VIASYS Healthcare Inc. Sensormedics; Yorba Linda, CA, USA) was used for PFTs and FEV1, FVC, diffusing capacity of the lungs for CO, and total lung capacity were measured using the body plethysmography test. Regarding spirometry, the patients sat in a small booth and breathed into a mouthpiece. One technical expert from the Department of Respiratory Medicine conducted all the tests to maintain the consistency of the results.
MIP (PONY FX, COSMED Inc.; Rome, Italy) and MEP (PONY FX, COSMED Inc.; Rome, Italy) were measured in the sitting position using a portable mouth pressure meter. Three consecutive MIP and MEP measurements were taken, and the best result was recorded. The PFT was measured in a sitting position. A flanged mouthpiece was applied to the short and rigid tube of the measuring instrument and air leakage was checked around the mouthpiece before testing. The test was performed by an experienced examiner who has conducted the test for more than 8 years. MIP was measured by exhaling as deep as possible and inhaling as hard as possible for at least 1.5 s. MEP was measured by inhaling as deep as possible and exhaling as hard as possible for at least 1.5 s. Both measurements were made three times, and patients recovered to normal breathing patterns with at least a minute of break between measurements. The highest of the three measurements was recorded [ 17 ].
The 6MWT was performed according to the American Thoracic Society standards under the direction of a well-trained respiratory therapist at a 30 m indoor walking course [ 18 ]. Patients were encouraged by the instructor every minute and were allowed to rest or quit the test at any point. We measured the total distance and peripheral saturation with the portable oxygen meter. The patients’ body compositions were estimated indirectly using the BIA from a supine position (InBody S10, InBody, Co. Ltd., Seoul, Korea).
Diaphragm function was assessed using ultrasonography (LOGIQ E9, GE Healthcare; Chicago, IL, USA) obtained from both supine and sitting positions. It is generally accepted that there are positional differences in diaphragm contractility. The effects of gravitational loading on the diaphragm length-tension and body position-mediated changes in intra-abdominal pressure may explain the differences found. Not only that there is also a difference in the excursion between right and left. The excursion of the right diaphragm shows a lower value than that of the left diaphragm because the liver in the abdominal cavity restricts the movement of the right diaphragm. We also measured the diaphragm function in two positions based on this information. The supine position involved lying on the back or with the face upward while the sitting position was semi-seated (45–60 degrees). Both M-mode and B-mode imaging were used to evaluate diaphragmatic excursion and thickness, respectively. The mid-clavicular line and the liver were used as anatomical landmarks on the right side and the spleen on the left side to visualize the diaphragm in the M-mode. B-mode ultrasonography was used to measure the diaphragmatic thickness at the bilateral zone of apposition [ 19 ]. The diaphragm thickness was measured during quiet spontaneous breathing without peak inspiratory or expiratory maneuvers. The diaphragmatic thickness fraction was calculated as the difference between thickness at the end of inspiration and thickness at the end of expiration divided by thickness at the end of expiration x 100. The diaphragmatic excursion was measured as follows. The highest position of the diaphragm movement taken by the M-mode was considered to be the end-expiratory phase, whereas the lowest position was considered as the end-inspiratory phase.
The dyspnea scale used St. George's Respiratory Questionnaire (SGRQ) and the modified Medical Research Council scale (mMRC scale). The SGRQ is a self-administered questionnaire with 76 items [ 20 ]. This can identify the patient’s symptoms and the activities of daily life. mMRC scale is most commonly used in the assessment of dyspnea in chronic respiratory diseases and is a very useful and unrecognized dyspnea scale [ 21 ].
Statistical analysis
The data were analyzed using IBM SPSS (version 27.0; Chicago, IL, USA). The level of significance was set at p < 0.05. Descriptive statistics, including numbers, percentages, means, and standard deviations, were used to summarize each variable (demographics, PFTs, 6MWT, and diaphragmatic ultrasound results). The results were analyzed by independent t-test, cross-analysis, and frequency analysis. The correlation between the variables was analyzed by Pearson’s Correlation Coefficient, which confirmed the linear relationship between two variables using a scatterplot. The cut-off value was calculated using the receiver operating characteristic (ROC) curve analysis. The reference plane was 0.5 or more in the ROC curve, and the p -value < 0.05; hence, this result was adopted. Consequently, the cut-off value was confirmed when sensitivity and specificity were plotted in a line chart, which is the point where the two graphs meet.
Ethics statement
We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed throughout this study. The study procedures were reviewed and approved by our Pusan National University Yangsan Hospital Institutional Review Board [IRB No. 05–2020-217].
FEV1 and diaphragm function
We assessed whether diaphragm function was associated with FEV1 (Fig. 1 ). In the total group analysis, both diaphragmatic excursion and thickness were associated with FEV1. However, the diaphragmatic excursion was more associated with FEV1 than thickness. Diaphragmatic excursion during forced breathing and in the supine position had a greater association with FEV1 than breathing at rest and in the sitting position. Additionally, when comparing the right and left under the same conditions, the right was more significant during forced breathing and in the supine position ( r = 0.370, p = 0.004,). Moreover, diaphragmatic thickness at right end-expiration was associated with FEV1. In summary, right ( r = 0.370, p = 0.004) and left ( r = 0.257, p = 0.048) diaphragmatic excursion during forced breathing in the supine position and diaphragmatic thickness at right end-expiration ( r = 0.310, p = 0.016) were significantly associated with FEV1.
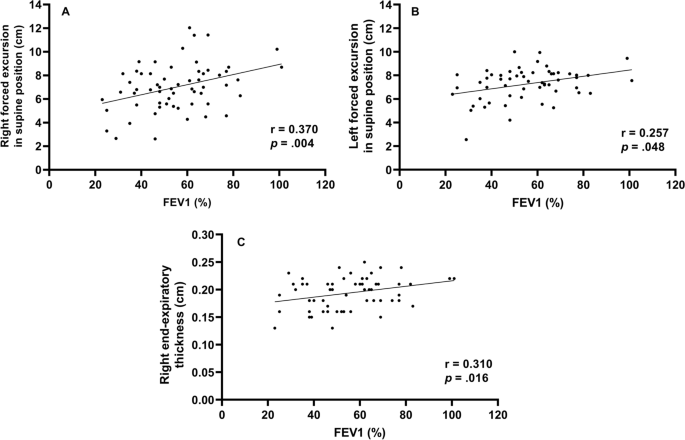
Correlation between forced expiratory volume in 1 s and diaphragm function Right forced excursion, and left forced excursion in the supine position and right end-expiratory thickness were correlated to forced expiratory volume in 1 s
Diaphragmatic function and BMI (body mass index)
To evaluate the function of the diaphragm muscle [ 22 ], the diaphragmatic excursion was measured at rest and during forced expiration (Supplement Table 1 ). In 60 patients, diaphragmatic excursion at rest in the supine position was 3.5 cm ± 1.2 on the right side and 3.5 cm ± 1.2 on the left side. During forced breathing, diaphragmatic excursion in the supine position was 6.9 cm ± 2.0 on the right side and 7.6 cm ± 1.6 on the left side. The total percent body fat was 24.2% ± 6.9. Segmental lean mass analysis was performed by direct segmental multi-frequency BIA. The lean mass was 90.5% ± 9.7 on the right arm, 88.1% ± 9.2 on the left arm, 94.5% ± 5.8 on the trunk, 95.7% ± 131.3 on the right leg, and 9.51% ± 8.8 on the left leg.
Cutoff value-associated characteristics
The ROC curve analysis of the diaphragm function variables was performed to identify the cutoff value for differentiating between FEV1 ≥ 50% and < groups. The cutoff value was ≤ 6.7 cm on the right diaphragmatic excursion at forced breathing with an area under the curve of 0.5 or more and p -value was 0.043. Right diaphragmatic excursion during forced breathing was less than the cut-off value of 6.7 cm for 26 patients and ≥ 6.7 cm for 43 patients (Table 1 ). There were no differences in age, sex, or smoking history between the two groups. The dyspnea scales such as mMRC, SGRQ, and GOLD were not significantly different between both groups. There were no differences in body mass index, percent body fat, or lean mass of the right or left legs between the groups. However, among the pulmonary function indicators, there were significant differences between the two groups. Specifically, FEV1, FVC, and MIP were significantly different (< 6.7 cm group vs. ≥ 6.7 cm group, FEV1: 49.2% ± 16.2 vs. 59.5% ± 17.2, p = 0.021; FVC: 76.2% ± 19.1 vs. 86.0% ± 15.5, p = 0.032; MIP: 67.4 cm H 2 O ± 25.1 vs. 86.5 cm H 2 O ± 28.7, p = 0.010). Concerning the 6MWT, there was a significant difference in SpO2 before 6MWT and the number of interruptions (SpO2 before 6MWT: 94.1% ± 2.7 vs. 95.3% ± 1.6, p = 0.038; number of interruptions: 4 [15.4%] vs. 0 [0%], p = 0.018). The left diaphragmatic excursion during forced breathing was also different between the two groups (6.8 cm ± 1.5 vs. 7.6 cm ± 1.3, p = 0.022) as well as the diaphragmatic thickness during right end-inspiration (0.3 cm ± 0.1 vs. 0.4 cm ± 0.1, p = 0.006). In addition, the ROC ≥ 6.7 cm group left diaphragmatic excursion was also measured with a value greater than that of the ROC < 6.7 cm group.
Subgroup characteristics according to FEV1
To identify the clinical significance of diaphragm function with the relationship between lung function, and COPD severity, the two groups classified as a right diaphragmatic excursion at 6.7 cm of forced breathing were further divided into groups based on FEV1 (< 50% or ≥ 50%) (Table 2 ). There were significant differences in age (65.0 ± 7.8 years vs. 72.7 ± 6.2 years, p = 0.011), the GOLD score ( p < 0.001), FEV1/FVC (40.1% ± 14.7 vs. 55.%4 ± 11.4, p = 0.007), peak expiratory flow rate (183.3 L/min ± 80.4 vs. 275.8 L/min ± 113.8, p = 0.027), SpO2 after the 6MWT (85.9% ± 6.5 vs. 91.5% ± 2.2, p = 0.011), and left diaphragmatic excursion during forced breathing (6.2 cm ± 1.6 vs. 7.4 cm ± 1.0, p = 0.038).
When the group with the right diaphragmatic excursion ≥ 6.7 cm was further divided into subgroups according to FEV1 (< 50% or ≥ 50%) and analyzed, mMRC, GOLD score, FEV1/FVC, MIP, peak expiratory flow rate, 6MWT, SpO2 before and after the 6MWT, and right diaphragmatic thickness at end-expiration subgroups were significantly different between the two groups.
This study contains the following: 1) evidence that FEV1 is significantly correlated with diaphragm movement, 2) cutoff values for diaphragm movement in patients with COPD, and 3) evidence to support the claim that the function of the diaphragm should be considered when interpreting the patient’s condition based on their FEV1.
First, FEV1 was significantly correlated with diaphragm movement. Studies on the relationship between the diaphragm and pulmonary function in patients with COPD are ongoing and have consistently reported that the severity of COPD and diaphragm function are closely related. Some previous studies have evaluated the direct relationship between FEV1 and diaphragm function [ 23 , 24 ].
The results of this study is also consistent with those of previous studies showing that diaphragm movement and FEV1 are related. However, beyond the findings of previous results [ 23 ], in our study, diaphragmatic excursion and thickness were found to be more correlated to FEV1 on the right side than on the left side.
Like the previous study that the thickness of the diaphragm is related to the ventilator weaning mechanical ventilation [ 9 , 10 ], this result has confirmed that the right diaphragm thickness was significantly related not only to the weaning of the ventilator and the prognosis of the patient but also to FEV1.
Second, we provided a cutoff value for a right diaphragmatic forced excursion in patients with COPD. Although there are studies that have presented a reference [ 23 ] value for healthy persons, the significant contribution of this study is the proposed reference value for patients with COPD.
We analyzed the correlation using Pearson’s correlation coefficient and confirmed the factors of diaphragmatic function-related components side (right, left), thickness, and excursion that were most-related to FEV1. Among them, Rt. forced excursion (supine), Lt. forced excursion (supine) and Rt. end-expiratory thickness showed meaningful p -value in association with FEV1. In addition, these three factors were analyzed in the linear relationship with the scattered plot and showed a proportional relationship between FEV1. Finally, when all factors related to the diaphragmatic function were analyzed, the right forced excursion was statistically determined as the most meaningful factor in relation to FEV1. We also obtained the cut-off value of 6.7 cm through the ROC curve.
The range in diaphragmatic excursion values varies considerably depending on the patient’s condition. A previous study has suggested normal values based on sex and the side of the diaphragm using healthy individuals. When breathing deeply, the right diaphragmatic excursion was 7 cm ± 1.1 in men and 5.7 cm ± 1 in women ( p < 0.001) and the left diaphragmatic excursion were 7.5 cm ± 0.9 and 6.4 cm ± 1 in men and women, respectively ( p < 0.01) [ 23 ]. In our study, we also assessed excursion during deep breathing to provide a cut-off value for patients with COPD.
When analyzed by dividing them into two groups based on a cut-off value, the following evaluation factors showed significant differences ( p < 0.05): FEV1, FVC, MIP, left forced excursion, right diaphragmatic thickness during end-inspiration, 6MWT, the SpO2 before and after 6MWT, and interruption of the 6MWT.
These factors can be broadly divided into PFT-related and performance-related factors. As mentioned above, PFT-related factors such as MIP, left diaphragmatic forced excursion and right diaphragmatic thickness during end-inspiration were lower in the < 6.7 cm group. Moreover, the SpO2 level before the 6MWT was lower in the < 6.7 cm group, the overall 6MWT was shorter, and there were many interruptions in the 6MWT. These factors might reflect activity as a performance evaluation factor. Although generalizability is limited given the few patients and the fact that all the participants were outpatients who could walk; these results may reflect an actual patient’s status. However, these findings are intended for patients who can walk, suggesting that the cut-off value of 6.7 cm may be reliable in this population.
Finally, results concerning the degree of pulmonary function and correlations with the diaphragmatic movement were noteworthy. The two groups were analyzed based on the right diaphragmatic forced excursion (6.7 cm) and divided into subgroups based on FEV1 (< 50% vs. ≥ 50%). As a result, in the group that had maintained diaphragm function (≥ 6.7 cm), the MIP, portable peak flow meter, 6MWT, SpO2 before and after the 6MWT, and right diaphragmatic thickness at end-expiration were different between the two FEV1 groups. In summary, the difference between the two FEV1 groups was large when diaphragm function was maintained; when it was not maintained, there were no differences between the two FEV1 groups. Therefore, even in patients who maintained their FEV1 > 50%, when diaphragm function deteriorated, the patient’s 6MWT, SpO2 before and after the 6MWT were less predictable (they either deteriorated or were maintained). The patients whose FEV1 decreased < 50%, if the diaphragm function was maintained, the 6MWT could be better than that in patients with an FEV1 ≥ 50% and a reduced diaphragm function.
In conclusion, when interpreting a patient’s condition based on FEV1, it is important to assess diaphragm function, since the effect of the FEV1 value on the patient depends on how well the diaphragm function has been maintained.
In this study, when the diaphragm function was maintained, there were significant differences in MIP, peak expiratory flow rate, 6MWT, SpO2 before and after the 6MWT, and right diaphragmatic thickness at end-expiration according to FEV1 in patients with COPD. Even if the diaphragm function was not maintained, because there are still differences in the FEV1, it may be beneficial to consider diaphragmatic function measured by right diaphragm excursion as an additional indicator of function beyond the FEV1. Therefore, it can be clinically helpful to check whether the diaphragm is functioning properly when determining a patient’s condition based on FEV1.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
Chronic obstructive pulmonary disease
Pulmonary function test
- 6-minute walk test
Forced expiratory volume in the first second
Maximal inspiratory pressure
International Classification of Diseases 11TH
Forced vital capacity
Global Initiative for Chronic Obstructive Lung Disease
Computed tomography
Maximal expiratory pressure
Bioelectrical impedance analysis
Modified Medical Research Council
Receiver operating characteristic
Body mass index
St. George's Respiratory Questionnaire
Niedermeyer J. Dyspnea in airway and pulmonary diseases. Internist. 2015;56(8):882–9.
Article CAS Google Scholar
Antoniu SA. Descriptors of dyspnea in obstructive lung diseases. Multidisciplinary respiratory medicine. 2010;5(3):216–9.
Article Google Scholar
Lopez AD, Shibuya K, Rao C, Mathers CD, Hansell AL, Held LS, et al. Chronic obstructive pulmonary disease: current burden and future projections. Eur Respir J. 2006;27(2):397–412.
Decramer M. Respiratory muscles in COPD: regulation of trophical status Verhandelingen. Koninklijke Academie voor Geneeskunde van Belgie. 2001;63(6):577–602 discussion −4.
CAS Google Scholar
Corbellini C, Boussuges A, Villafañe JH, Zocchi L. Diaphragmatic mobility loss in subjects with moderate to very severe COPD may improve after in-patient pulmonary rehabilitation. Respir Care. 2018;63(10):1271–80.
Crimi C, Heffler E, Augelletti T, Campisi R, Noto A, Vancheri C, et al. Utility of ultrasound assessment of diaphragmatic function before and after pulmonary rehabilitation in COPD patients. Int J Chronic Obstruct Pulmon Dis. 2018;13:3131–9.
He L, Zhang W, Zhang J, Cao L, Gong L, Ma J, et al. Diaphragmatic motion studied by M-mode ultrasonography in combined pulmonary fibrosis and emphysema. Lung. 2014;192(4):553–61.
Laghi F, Tobin MJ. Disorders of the respiratory muscles. Am J Respir Crit Care Med. 2003;168(1):10–48.
Goligher EC, Fan E, Herridge MS, Murray A, Vorona S, Brace D, et al. Evolution of diaphragm thickness during mechanical ventilation. Impact Inspiratory Effort Am J Respirat Cri Care Med. 2015;192(9):1080–8.
Goligher EC, Dres M, Fan E, Rubenfeld GD, Scales DC, Herridge MS, et al. Mechanical ventilation-induced diaphragm atrophy strongly impacts clinical outcomes. Am J Respir Crit Care Med. 2018;197(2):204–13.
Boussuges A, Rives S, Finance J, Brégeon F. Assessment of diaphragmatic function by ultrasonography: current approach and perspectives. World J Clin Cases. 2020;8(12):2408–24.
Sarwal A, Walker FO, Cartwright MS. Neuromuscular ultrasound for evaluation of the diaphragm. Muscle Nerve. 2013;47(3):319–29.
Criner G. 6-minute walk testing in COPD: is it reproducible? Eur Respir J. 2011;38(2):244–5.
Hernandes NA, Wouters EF, Meijer K, Annegarn J, Pitta F, Spruit MA. Reproducibility of 6-minute walking test in patients with COPD. Eur Respir J. 2011;38(2):261–7.
Chen H, Liang BM, Tang YJ, Xu ZB, Wang K, Yi Q, et al. Relationship between 6-minute walk test and pulmonary function test in stable chronic obstructive pulmonary disease with different severities. Chin Med J. 2012;125(17):3053–8.
Google Scholar
Minami T, Manzoor K, McCool FD. Assessing diaphragm function in Chest Wall and neuromuscular diseases. Clin Chest Med. 2018;39(2):335–44.
ATS/ERS Statement on Respiratory Muscle Testing. Am J Respir Crit Care Med. 2002;166(4):518–624.
ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–7.
Matamis D, Soilemezi E, Tsagourias M, Akoumianaki E, Dimassi S, Boroli F, et al. Sonographic evaluation of the diaphragm in critically ill patients. Technique and clinical applications. Intensive Care Med. 2013;39(5):801–10.
Jones PW, Quirk FH, Baveystock CM. The St George's respiratory questionnaire. Respir Med. 1991;85 Suppl B(25-31):discussion 3-7.
Launois C, Barbe C, Bertin E, Nardi J, Perotin JM, Dury S, et al. The modified Medical Research Council scale for the assessment of dyspnea in daily living in obesity: a pilot study. BMC pulmonary medicine. 2012;12:61.
Dhungana A, Khilnani G, Hadda V, Guleria R. Reproducibility of diaphragm thickness measurements by ultrasonography in patients on mechanical ventilation. World J Critical Care Med. 2017;6(4):185–9.
Boussuges A, Gole Y, Blanc P. Diaphragmatic motion studied by m-mode ultrasonography: methods, reproducibility, and normal values. Chest. 2009;135(2):391–400.
Rocha FR, Brüggemann AK, Francisco DS, Medeiros CS, Rosal D, Paulin E. Diaphragmatic mobility: relationship with lung function, respiratory muscle strength, dyspnea, and physical activity in daily life in patients with COPD. Jornal brasileiro de pneumologia : publicacao oficial da Sociedade Brasileira de Pneumologia e Tisilogia. 2017;43(1):32–7.
Download references
Acknowledgements
Abstract has been published/presented in the Korean tuberculosis and respiratory society, the Korean tuberculosis and respiratory society fall academic presentation | 129 volume 0342 ~ 343, total 2 PAGES, 2021
https://journal.kstudy.com/ISS_Detail.asp?key=3921544&tname=kiss2002&code=YqldZWtoSqVtJTNEOTEnMSUmN/B%20Z%20xLJTNEVHJpZSUmNbNj2bRU4XB/JTNEMA ==
This study was supported by the Research Institute for Convergence of Biomedical Science and Technology (30–2020-003), Pusan National University Yangsan Hospital. The funding body played no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Author information
Taehwa Kim and Sungchul Huh contributed equally to this work.
Authors and Affiliations
Division of Respiratory, Allergy and Critical Care Medicine, Department of Internal Medicine, Pusan National University Yangsan Hospital and Pusan National University School of Medicine, Geumo-ro 20, Beomeo-ri, Yangsan-si, Gyeongsangnam-do, 50612, Republic of Korea
Taehwa Kim, Jae Heun Chung, Yun Seong Kim & Seung Eun Lee
BioMedical Research Institute for Convergence of Biomedical Science and Technology, Pusan National University Yangsan Hospital, Yangsan, South Korea
Department of Rehabilitation Medicine, Rehabilitation Hospital, Pusan National University Yangsan, Yangsan, South Korea
Sungchul Huh & Ra Yu Yun
Pusan National University School of Medicine, Yangsan, South Korea
College of Nursing, Pusan National University, Pusan National University Yangsan Hospital, Yangsan, South Korea
You can also search for this author in PubMed Google Scholar
Contributions
Conceptualization: TK, SEL. Data acquisition and analysis: TK, OP, RYY, SH, JHC, SEL. Data interpretation: TK, RYY, SH, JHC, SEL. Validation: TK, JHC. Writing – original draft: SH, TK. Writing – review: SEL, JHC, YSK. The author(s) read and approved the final manuscript.
Corresponding author
Correspondence to Seung Eun Lee .
Ethics declarations
Ethics approval and consent to participate.
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) [ 17 ]. The study was approved by Pusan National University Yangsan Hospital (PNUYH) Institutional Review Board (IRB No. 05–2020-217) and individual consent for this retrospective analysis was waived.
Consent for publication
Not applicable.
Competing interests
The authors have no conflicts of interest or funding sources to declare.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Kim, T., Huh, S., Chung, J.H. et al. Clinical values of diaphragmatic movement in patients with chronic obstructive pulmonary disease. BMC Pulm Med 23 , 33 (2023). https://doi.org/10.1186/s12890-022-02220-7
Download citation
Received : 25 April 2022
Accepted : 02 November 2022
Published : 27 January 2023
DOI : https://doi.org/10.1186/s12890-022-02220-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Cut-off value
BMC Pulmonary Medicine
ISSN: 1471-2466
- Submission enquiries: [email protected]
- General enquiries: [email protected]
- TheFreeDictionary
- Word / Article
- Starts with
- Free toolbar & extensions
- Word of the Day
- Free content
- diaphragmatic
di·a·phrag·mat·ic
- abdominodiaphragmatic breathing
- accelerated respiration
- asynchronous pacemaker
- bare area of (diaphragmatic surface of) liver
- Bochdalek, Vincent
- Bochdalek's hernia
- Bornholm disease
- breathing pattern, ineffective
- breathing-related sleep disorder
- cardiac impression of diaphragmatic surface of liver
- cerebral hernia
- clavicular respiration
- cogwheel respiration
- costophrenic angle
- crural hernia
- demand pacemaker
- diaphragmatic breathing
- diaphanography
- diaphanometer
- diaphanometry
- diaphanoscope
- diaphanoscopy
- diaphemetric
- diaphen hydrochloride
- diaphoresis
- diaphoretic
- Diaphragm (Birth Control)
- diaphragm breath
- Diaphragm breathing
- diaphragm contraceptive
- diaphragm muscle
- diaphragm pessary
- diaphragm phenomenon
- diaphragma sellae
- diaphragmalgia
- diaphragmatic constriction of esophagus
- diaphragmatic eventration
- diaphragmatic excursion
- diaphragmatic flutter
- diaphragmatic hernia
- diaphragmatic ligament of mesonephros
- diaphragmatic ligament of the mesonephros
- diaphragmatic pacemaker
- diaphragmatic part of parietal pleura
- diaphragmatic peritonitis
- diaphragmatic pleurisy
- diaphragmatic respiration
- diaphragmatic surface (of heart, liver, lung, spleen)
- diaphragmatic surface of heart
- diaphragmatocele
- diaphragmitis
- diaphyseal aclasis
- diaphyseal dysplasia
- diaphyseal-epiphyseal fusion
- diaphysectomy
- diaphysial aclasis
- diaphysial center
- diaphragm of sella turcica
- Diaphragm of the pelvis
- diaphragm pacing
- diaphragm plate
- Diaphragm pump
- diaphragm pumps
- diaphragm sellae
- diaphragm setting
- diaphragm stop
- Diaphragm valve
- Diaphragm-Operated Valve
- diaphragma oris
- diaphragma pelvis
- Diaphragma sella
- Diaphragma sellae
- diaphragma urogenitale
- diaphragmal
- diaphragmatic abscess
- Diaphragmatic breathing
- Diaphragmatic Breathing Technique
- diaphragmatic center
- diaphragmatic crus
- diaphragmatic cupula
- Diaphragmatic Electromyography
- Diaphragmatic excursion
- diaphragmatic flexure
- Facebook Share
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.

StatPearls [Internet].
Diaphragm disorders.
Laxmi Kokatnur ; Rishik Vashisht ; Mohan Rudrappa .
Affiliations
Last Update: July 31, 2023 .
- Continuing Education Activity
The diaphragm is a vital organ for mammals as it is the primary muscle for respiration. Diaphragmatic paralysis is the loss of its muscular power and can arise from either weakness of the muscle itself or damage to its nerve supply. Depending on the severity of the paralysis and whether it is unilateral or bilateral, patients can have varied clinical manifestations. A patient may be asymptomatic, while another may be ventilator dependent. This activity explains how to properly evaluate for diaphragm disorders, and highlights the role of the interprofessional team in caring for patients with this condition.
- Review the causes of diaphragm disorders.
- Describe the evaluation of a patient with diaphragmatic paralysis.
- Summarize the treatment of diaphragm disorders.
- Outline the importance of enhancing care coordination among the interprofessional team to ensure proper evaluation and management of diaphragm disorders.
- Introduction
The diaphragm is a vital organ for mammals as it is the primary muscle for respiration. Diaphragmatic paralysis is the loss of its muscular power and can arise from either weakness of the muscle itself or damage to its nerve supply. Depending on the severity of the paralysis and whether it is unilateral or bilateral, patients can have varied clinical manifestations. A patient may be asymptomatic, while another may be ventilator dependent.
The diaphragm is a dome-shaped musculo-fibrous structure located between the thoracic and abdominal cavities. It constitutes the floor of the thorax and the roof of the abdomen. The word “diaphragm” is derived from the Greek words “dia,” meaning in between, and “phragma,” meaning fence. Although a clear anatomical distinction is not evident, the diaphragm functions as two, separate units (right and left), each with different vascular and nerve supplies. The peripheral portion of the diaphragm is muscular and is composed of three distinct muscle groups. The sternal group originates from the xiphoid process, the costal group originates from the inner surface of the lower six ribs, and the lumbar group originates from two crura and arcuate ligaments which are, in turn, attached to the lumbar vertebra. The central part is made of strong aponeurotic tendinous ligaments without any bony attachment. It is C-shaped and has right lateral, middle, and left lateral leaflets. The anterior sternal attachment of diaphragm is located more cranially compared to posterior lumbar attachment. During inhalation, the muscular part of the diaphragm contracts making it flatter and expands the thoracic cavity outward and downward. This creates negative intrathoracic pressure leading to passive movement of air from atmosphere to the respiratory system along the pressure gradient. When the diaphragm relaxes, the thoracic cavity constricts decreasing the subatmospheric pressure and leading to passive egress of air from the respiratory system during expiration.
Although external intercostal muscles aid in inspiration, the diaphragm is the primary muscle of respiration, and its weakness can impede the respiratory functions. Paralysis on both sides of the hemidiaphragm will cause significant respiratory failure, but paresis of one hemidiaphragm can be asymptomatic due to compensatory function from the other half of diaphragm and recruitment from external intercostal muscles. Voluntary contraction of the diaphragm will also increase the intraabdominal pressure and aid in other vital functions like vomiting, urination, and defecation. It also prevents regurgitation by creating pressure at the lower esophageal sphincter.
The diaphragm has several openings allowing the structures to pass from the thorax to the abdomen. At the level of the eight thoracic vertebrae on the right hemidiaphragm, there is a large opening through which the inferior vena cava enters thorax from the abdomen to join the right atrium. At the level of the ten thoracic vertebrae, there is a posterior midline opening between the two crus of the diaphragm called aortic hiatus through which the descending thoracic aorta enters the abdomen from the thorax, the thoracic duct enters the thorax from the abdomen, and the azygous vein enters the thorax from the abdomen. Between the fibers of the right crus of the diaphragm, there is the esophageal hiatus through which the esophagus locates from the thorax to the abdomen.
The diaphragm functions primarily involuntarily with additional voluntary control when needed. It is innervated by two phrenic nerves originating from cervical nerve roots C3 to C6. Right and left phrenic nerves to innervate the respective hemidiaphragm and control both sensory and motor functions. The right phrenic nerve is located lateral to the caval hiatus, and the left phrenic nerve is located lateral to the pericardium. Each phrenic nerve divides into four trunks, the sternal, anterolateral, posterolateral and crural trunks. The main vascular supply for the diaphragm comes from the bilateral phrenic arteries, which are direct branches of the thoracic aorta.The diaphragm is also supplied by tributaries from the internal mammary arteries, and from pericardiophrenic arteries. Venous drainage occurs through phrenic veins, which drain into the inferior vena cava.
Diaphragmatic palsy or paresis can be a result of either direct diaphragmatic muscle weakness and atrophy or damage to the phrenic nerves. Unilateral weakness of one of the hemidiaphragm is more common than bilateral weakness. Depending on the cause, the weakness can be either temporary or permanent. [1] [2] [3] The cause of diaphragmatic weakness can be divided into the following categories:
This is the most common cause of diaphragmatic weakness. Surgery or trauma can cause injury to the phrenic nerve, leading to diaphragmatic weakness. Cardiac bypass surgery is the most common procedure that causes trauma, with up to 20% risk of diaphragmatic weakness. During the cold cardioplegia for bypass, the phrenic nerve gets phrenic frostbite, leading to temporary loss of diaphragmatic functions. Due to the long course of the left diaphragm in the thorax, left-side diaphragmatic weakness is more common. Any mediastinal procedures, esophageal surgeries or lung transplantation carry a risk of diaphragmatic weakness. Penetrating or gunshot injuries to the thorax can also result in phrenic nerve damage. [4]
Compression
Any space-occupying lesion in the thorax, for example, a mediastinal tumor or lung cancer close to the phrenic nerve, can cause physical compression of the nerve leading to loss of function. Phrenic nerve involvement is seen in 5% of lung cancer. Other causes of compressive damage include an aortic aneurysm, substernal goiter, and cervical spondylosis.
Neuropathic
Phrenic nerve damage cane by caused by many neurological issues like diabetic neuropathy, inclusion body myositis, dermatomyositis, multiple sclerosis, anterior horn cell disease, chronic demyelinating disease, and neuralgic myopathy. [5]
Inflammatory
Many systemic diseases can lead to inflammation of the phrenic nerve or diaphragm leading to diaphragmatic palsy. Viral infections like HIV, West Nile virus and poliomyelitis virus, bacterial infections like Lyme disease, and noninfectious causes like sarcoidosis and amyloidosis have been linked to diaphragmatic weakness.
In nearly 20% cases, no obvious cause if detected after extensive investigations and are referred as idiopathic.
- Epidemiology
Diapahamtic palsy is seen in around 20% cases following cardiac surgery.
- History and Physical
Symptoms of diaphragmatic weakness vary depending on the cause and duration. Most patients with unilateral diaphragmatic weakness are asymptomatic and are detected incidentally. One-third of patients report exertional breathlessness. Patients with coexisting debilitating cardiopulmonary conditions can have dyspnea at rest. Most patients with unilateral diaphragmatic weakness show some limitation of exercise capacity and have lower resting oxygen saturation levels. Most patients with bilateral weakness complain of varying degrees of dyspnea from mild exertional breathlessness to dyspnea at rest. As the diaphragm function becomes further compromised during supine position, patients report orthopnea. Progressive hypoventilation can lead to hypercapnia and right heart failure. The hypoxemia and hypercapnia are worse during sleep.
Evaluation usually includes chest radiographs and more advanced imaging. Other tests include pulmonary function tests and electrophysiologic studies. [6]
- Chest radiographs: As the diaphragm is positioned obliquely, the dome of the right diaphragm is at the level of the five rib anteriorly and the level of the ten rib posteriorly. Normally the right hemidiaphragm is located at a slightly higher level compared to left hemidiaphragm by around one intercostal space. If one side of the diaphragm is weak, then the normal negative intrathoracic pressure will suck the diaphragm cranially into the thoracic cavity. So, the paralyzed diaphragm will be at a higher level. If the right side is paralyzed, the distance between the right and left diaphragm will be more than two intercostal space, and if the left side is paralyzed, both the hemidiaphragms will appear on the same level. Up to 90% of unilateral diaphragmatic palsy can be diagnosed based on chest x-ray alone. In bilateral weakness, both hemidiaphragms will at a higher level and might be missed on a routine chest radiograms. As the diaphragms are curved, the costophrenic and craniovertebral angle will be acuter. An upward located diaphragm can also cause secondary atelectasis of the lower sub-segments of the lung.
- Fluoroscopic test: All patients with suspected diaphragmatic weakness should undergo a fluoroscopic test to diagnose the functional weakness. On tidal breathing in normal individuals, diaphragmatic contraction will lead to the descent of both hemidiaphragms by at least one intercostal space. On deep breathing or sniffing, the descent of hemidiaphragm is more rapid and more distal. In case of a unilateral weakening of the hemidiaphragm, the diaphragmatic excursion will be reduced or absent. Sometimes, it can show a paradoxical movement of the diaphragm where the diaphragm moves into thoracic cavity during inspiration while the other normal working diaphragm moves downward toward the abdominal cavity. The intercostal muscles and the normal hemidiaphragm will generate negative intrathoracic pressure and suck the paralyzed diaphragm to the thorax. In bilateral weakness, the diaphragm either does not move or moves paradoxically, depending on the severity of weakness.
- Ultrasound of thorax: Ultrasound examination for the thoracic disease is gaining popularity. The diaphragm appears as a thick echogenic line in B mode. In M mode, a paralyzed hemidiaphragm will show no cranial movements of paradoxical caudal movements, similar to a sniff test.
- CT chest: CT of the chest should be done in all patients with diaphragm weakness to rule out any thoracic etiology. Unfortunately, diaphragmatic weakness is not recognized easily and cannot be detected in static CT images.
- Pulmonary function tests: As the diaphragm accounts for 80% of the muscular power of respiration, its weakness will lead to measure by pulmonary functions tests. Forced vital capacity will decline by 50% predicted in unilateral diaphragmatic weakness and up to 75% in bilateral weakness. Normally, the lung functions decline less than 15% in supine position due to the restriction of thoracic expansion and positive pressure from abdominal structures. In diaphragmatic weakness, this accentuates and on repeating the spirometry in supine position forced vital capacity will further decrease by 15% to 20% in unilateral weakness, and 20% to 30% in bilateral weakness. Maximum inspiratory and expiratory pressure will also be reduced. Total lung capacity, functional residual capacity, residual volume, and diffusion factor remain unchanged in unilateral weakness but can be reduced in bilateral weakness.
- Electrophysiological tests: In diaphragmatic electromyography, the phrenic nerve is stimulated electrically, and the mechanical response of the diaphragm is detected along with trans-diaphragmatic pressure. In phrenic nerve pathology, there is prolonged latency and reduced trans-diaphragmatic pressure. However, in primary diaphragmatic pathology, the phrenic nerve conduction is normal, but compound action potential is reduced.
- Treatment / Management
There are three main treatment strategies for diaphragmatic palsy if the weakness persists. [7] [8] [9]
Noninvasive positive-pressure ventilation: positive-pressure and bilevel positive-pressure ventilation show success in diaphragmatic weakness. This noninvasive pressure ventilation creates dynamic pressure splint in the airways and prevents paradoxical diaphragmatic movements. It also augments the minute ventilation working in coordination with intercostal muscles. This therapy is started in the night as respiration is compromised in the supine position. In severe cases, intermittent daytime therapy can be used. For more severe cases requiring high-pressure settings and the inability to tolerate oral or nasal masks, a tracheostomy is an option. Advancement in noninvasive technology has enabled noninvasive therapy with tracheostomy interface. If the patient cannot tolerate this setting, then mechanical ventilator can be used. Diaphragmatic paralysis following surgeries is usually temporary and can be treated with this noninvasive, positive-pressure ventilation until the diaphragm or phrenic nerve regains function.
Surgical plication of paralyzed diaphragm: This complicated, invasive procedure, which is done either by open thoracotomy or video-assisted thoracic surgery, has been shown to be effective in relieving symptoms and improving lung function. This prevents the paralyzed diaphragm’s mechanical disadvantage in the thoracic cavity.
Diaphragmatic pacing: This can be used in unilateral phrenic nerve palsy. In this procedure, the phrenic nerve is electrically stimulated to generate mechanical power in the diaphragm. The electric pacer can be placed in the neck or near the diaphragm. The location of generator depends on the cause of the site of phrenic nerve damage.
- Differential Diagnosis
- Alveolar hypoventilation
- Anterior horn cell or neuromuscular junction disease
- Cerebral haemorrhage
- Cervical fracture
- Cervical spine fracture
- Decreased pulmonary compliance
- Guillain-Barre syndrome
- Myasthenia gravis
- Peripheral neuropathies
- Pleural adhesions
- Pearls and Other Issues
The prognosis of diaphragmatic paralysis depends on its etiology. Temporary weakness after cardiac bypass improves completely; whereas, a progressive neuromuscular disease can lead to respiratory failure. WHen only supportive treatment is provided, many patients show stable lung function or improvement in lung functions within 1 to 2 years.
- Enhancing Healthcare Team Outcomes
There are many causes of dysfunction of the diaphragm. Because of the complexity in its management, an interprofessional team that includes an internist, surgeon, intensivist, neurologist, pulmonologist and the nurse practitioner should be involved.
The diaphragm is the primary muscle of respiration, and its weakness can lead to respiratory failure. Various causes can cause diaphragmatic palsy. Depending on severity, the presentation varies from entirely asymptomatic to disabling dyspnea requiring continuous mechanical ventilation. Complete lung function tests, both in sitting and supine positions, should be done in all suspected cases. Historical fluoroscopic examination looking for the movements of the diaphragm is replaced by bedside thoracic ultrasound examination which is equally efficient and without risks of radiation. Treatment depends on the cause. Both surgical and non-surgical methods have shown benefit. Overall prognosis is good except with neuromuscular degeneration conditions. [10] [11]
- Review Questions
- Access free multiple choice questions on this topic.
- Comment on this article.
Chest x ray showing elevated diaphragm compared to chest x ray done two years back Contributed by Laxmi Kokatnur
Chest x-ray of a patient who sustained traumatic root avulsion brachial plexus injury. Note the elevated right hemidiaphragm indicating associated phrenic nerve injury. The patient also sustained fractures to the right midshaft clavicle and right 1st (more...)
Disclosure: Laxmi Kokatnur declares no relevant financial relationships with ineligible companies.
Disclosure: Rishik Vashisht declares no relevant financial relationships with ineligible companies.
Disclosure: Mohan Rudrappa declares no relevant financial relationships with ineligible companies.
This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
- Cite this Page Kokatnur L, Vashisht R, Rudrappa M. Diaphragm Disorders. [Updated 2023 Jul 31]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
In this Page
Bulk download.
- Bulk download StatPearls data from FTP
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Similar articles in PubMed
- Elevated Hemidiaphragm. [StatPearls. 2024] Elevated Hemidiaphragm. Patel PR, Bechmann S. StatPearls. 2024 Jan
- Lower Genitourinary Trauma. [StatPearls. 2024] Lower Genitourinary Trauma. Tullington JE, Blecker N. StatPearls. 2024 Jan
- Anatomy, Abdomen and Pelvis: Diaphragm. [StatPearls. 2024] Anatomy, Abdomen and Pelvis: Diaphragm. Shahid Z, Burns B. StatPearls. 2024 Jan
- Review The surgical anatomy and technique of the thoracoabdominal incision. [Surg Clin North Am. 1993] Review The surgical anatomy and technique of the thoracoabdominal incision. Lumsden AB, Colborn GL, Sreeram S, Skandalakis LJ. Surg Clin North Am. 1993 Aug; 73(4):633-44.
- Review Diaphragmatic weakness and paralysis. [Lung. 1989] Review Diaphragmatic weakness and paralysis. Wilcox PG, Pardy RL. Lung. 1989; 167(6):323-41.
Recent Activity
- Diaphragm Disorders - StatPearls Diaphragm Disorders - StatPearls
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers

IMAGES
VIDEO
COMMENTS
Diaphragmatic excursion. Diaphragmatic excursion is the movement of the thoracic diaphragm during breathing. Normal diaphragmatic excursion should be 3-5 cm, but can be increased in well-conditioned persons to 7-8 cm. This measures the contraction of the diaphragm. It is performed by asking the patient to exhale and hold it.
diaphragmatic excursion: In respiration, the movement of the diaphragm from its level during full exhalation to its level during full inhalation. Normal diaphragmatic excursion is 5 to 7 cm bilaterally in adults. It may be seen during fluoroscopic or ultrasonographic examinations of the chest, or percussed during physical examination of the ...
Percussion is the act of tapping on a surface, thereby setting the underlying structures in motion, creating a sound and palpable vibration. Percussion is used to determine whether underlying structures are fluid-filled, gas-filled, or solid. Percussion: Penetrates 5 - 6 centimeters into the chest cavity. May be impeded by a very thick chest wall.
the diaphragm relaxes during expiration: moves upwards. both hemidiaphragms move together. in healthy patients 1-2.5 cm of excursion is normal in quiet breathing 2. 3.6-9.2 cm of excursion is normal in deep breathing 2. up to 9 cm can be seen in young or athletic individuals in deep inspiration 2. excursion in women is slightly less than men 2
Furthermore, diaphragmatic excursion is an index for respiratory muscle fatigue during the SBT. Some authors had reported a lower accuracy for diaphragmatic excursion compared to most of the available data and suggested that this lower accuracy is due to the heterogeneity of the patients included in the meta-analyses , . Therefore, separate ...
Introduction. The diaphragm is the main muscle of respiration [].Diaphragmatic excursion is 1-2 cm during tidal breathing and 7-11 cm during deep inspiration [].The assessment of diaphragmatic function is important for diagnosis and follow up of various physiologic and pathologic conditions [].Several methods exist for the evaluation of diaphragmatic function.
Decreased diaphragmatic excursion may be detected by percussion of the lower rib cage at end expiration and end inspiration. ... PD, Polkey, MI, Moxham, J, Green, M. Long-term recovery of ...
Patients with emphysema or COPD manifest major changes in the mass, thickness, and area of the diaphragm. Diaphragmatic contractions produce muscle shortening and thickening. Ultrasonography has been recently proposed for use in assessing both diaphragmatic excursions [5-7] and diaphragmatic thickness at different lung volumes .
Background In patients with chronic obstructive pulmonary disease (COPD), the maximum level of diaphragm excursion (DEmax) is correlated with dynamic lung hyperinflation and exercise tolerance. This study aimed to elucidate the utility of DEmax to predict the improvement in exercise tolerance after pulmonary rehabilitation (PR) in patients with COPD. Methods This was a prospective cohort study ...
Normal diaphragm excursion is between 3 and 5 cm. An abnormal sniff test is the paradoxic motion of a hemidiaphragm upward by greater than 2 cm which can indicate diaphragm paralysis ... Long-term results of diaphragmatic plication in adults with unilateral diaphragm paralysis. J Cardiothorac Surg. 2010; 5: 111.
Diaphragmatic excursion is a quantitative measure of expiratory effort as validated by both lung and tracheal volumes in asthma patients, and may be more accurate than qualitative assessment based on tracheal morphology. ... Department of Radiology, Columbia University Medical Center, United States. PMID: 33460955 DOI: 10.1016/j.ejrad.2021. ...
Department of Radiology, Columbia University Medical Center, United States. Search for articles by this author. Published: ... Each study was independently and blindly interpreted by a cardiothoracic radiology fellow in terms of diaphragmatic excursion and lung and tracheal volume calculations. Diaphragmatic excursion (DE) was calculated as the ...
Exams were categorized by ICD-9/10 code documented by the pulmonologist in the computerized medical record, to select patients referred with diagnosis of ... Each study was independently and blindly interpreted by a cardiothoracic radiology fellow in terms of diaphragmatic excursion and lung and tracheal volume calculations. Diaphragmatic ...
Ultrasound assessment of diaphragmatic excursion was done by experienced ultrasonologists. Diaphragmatic excursion for patients was measured on GE make, Voluson S8 series ultrasound machine. The assessment was done in supine position using M-mode and B-mode techniques in quiet and deep breathing scenarios.
nty-six COPD patients and 18 self-reported healthy controls were included in this study. After taking the sociodemographic data, measurement of diaphragm excursion was done using M-mode and B-mode ultrasound. Lung function was assessed by spirometry. Results: In the COPD group, diaphragmatic excursion was found to be reduced, and it correlates with forced expiratory volume in 1 s (FEV1)/forced ...
Background The diaphragm muscle whose dysfunction may be very common in patients undergoing mechanical ventilation (Ferrari G, De Filippi G, Elia F, Panero F, Volpicelli G, Aprà F. Crit Ultrasound J 6:8, 2014). Aim: To evaluate real-time ultrasound in the evaluation of diaphragmatic thickening, thickening fraction, and/or excursion to predict extubation outcomes. We aimed to compare these ...
Impaired diaphragmatic excursion at T3, defined as diaphragmatic excursion < 10 mm under mechanical ventilation, occurred in 15 (83.3%) patients who underwent laparoscopic radical hysterectomy and ...
Background: In patients with chronic obstructive pulmonary disease (COPD), the maximum level of diaphragm excursion (DE max) is correlated with dynamic lung hyperinflation and exercise tolerance.This study aimed to elucidate the utility of DE max to predict the improvement in exercise tolerance after pulmonary rehabilitation (PR) in patients with COPD.
In most previous studies, diaphragm ultrasonography was used to assess DE max, i.e., the measurement of the excursion of the right hemidiaphragm, as used in this study, and diaphragm thickness that assessed the length and thickness of the zone of apposition of the diaphragm against the rib cage [35, 36].
Diaphragmatic function and BMI (body mass index) To evaluate the function of the diaphragm muscle [], the diaphragmatic excursion was measured at rest and during forced expiration (Supplement Table 1).In 60 patients, diaphragmatic excursion at rest in the supine position was 3.5 cm ± 1.2 on the right side and 3.5 cm ± 1.2 on the left side.
This article reports the various methods used to assess diaphragmatic function by ultrasonography. The excursions of the two hemidiaphragms can be measured using two-dimensional or M-mode ultrasonography, during respiratory maneuvers such as quiet breathing, voluntary sniffing and deep inspiration. On the zone of apposition to the rib cage for ...
diaphragmatic. [ di″ah-frag-mat´ik] pertaining to a diaphragm. diaphragmatic hernia protrusion of some of the contents of the abdomen through an opening in the diaphragm and into the chest cavity. The condition may be congenital or acquired. Congenital diaphragmatic hernia in newborns is due to failure of the embryonic diaphragm to fuse.
The diaphragm is a vital organ for mammals as it is the primary muscle for respiration. Diaphragmatic paralysis is the loss of its muscular power and can arise from either weakness of the muscle itself or damage to its nerve supply. Depending on the severity of the paralysis and whether it is unilateral or bilateral, patients can have varied clinical manifestations. A patient may be ...