Learn how UpToDate can help you.
Select the option that best describes you
- Medical Professional
- Resident, Fellow, or Student
- Hospital or Institution
- Group Practice
- Patient or Caregiver
- Find in topic

RELATED TOPICS
INTRODUCTION
There were 2161 cases of malaria reported in 2017 to the United States Centers for Disease Control and Prevention (CDC) [ 3 ]. More than half of the reported cases are due to Plasmodium falciparum , which causes the most severe disease; patients with P. falciparum may progress to life-threatening illness within hours [ 4,5 ]. Since 2011, there has been an average of seven malaria deaths per year in the United States.
Prevention efforts should be aimed at all forms of malaria. In addition to P. falciparum , other Plasmodium species that cause human malaria include P. vivax , P. ovale , P. malariae , and P. knowlesi . While P. falciparum is most likely to result in severe disease, all malaria species can cause severe disease and death [ 6,7 ]. In general, most chemoprophylaxis regimens are designed to prevent primary attacks of malaria. Primaquine and tafenoquine can also prevent relapses of malaria caused by P. vivax and P. ovale . (See 'Drug mechanisms' below and 'Primaquine' below and 'Tafenoquine' below.)
Most travelers who develop malaria do so because they do not adhere to an effective chemoprophylactic drug regimen [ 8-10 ]. In addition, many travelers frequently fail to use personal protection measures for mosquito bite prevention.
Issues related to risk assessment, counseling, mosquito bite prevention, and antimalarial chemoprophylaxis for prevention of malaria will be reviewed here. The clinical manifestations, diagnosis, and treatment of malaria are discussed separately. (See "Malaria: Clinical manifestations and diagnosis in nonpregnant adults and children" and "Laboratory tools for diagnosis of malaria" and "Treatment of severe malaria" and "Treatment of uncomplicated falciparum malaria in nonpregnant adults and children" .)

BRETT A. JOHNSON, MD, AND MONICA G. KALRA, DO
A more recent article on malaria is available.
This is a corrected version of the article that appeared in print.
Am Fam Physician. 2012;85(10):973-977
Patient information : See related handout on prevention of malaria , written by the authors of this article.
Author disclosure: No relevant financial affiliations to disclose.
There are approximately 300 million cases of malaria each year, resulting in 1 million deaths worldwide. Family physicians often encounter patients preparing to travel to malaria-endemic regions. Physicians should have basic knowledge of parasite transmission and malaria prevention. The risk of malaria acquisition is based largely on geographic location and travel season. Most cases occur in sub-Saharan Africa, the Indian subcontinent, and Southeast Asia between the months of May and December. Key elements in prevention include barrier protection and chemoprophylaxis. Travelers to malaria-endemic areas should be advised to use mosquito repellent at all times and bed netting at night. Prophylactic medication should be initiated before travel and continued after return. Travelers should be warned that malaria symptoms can present up to one year after a mosquito bite. Symptoms are vague, and may include fever, chills, arthralgias, and headaches. Travelers experiencing symptoms should seek prompt medical attention.
There are approximately 300 million cases of malaria each year, resulting in 1 million deaths worldwide. 1 Reports from the Centers for Disease Control and Prevention (CDC) indicate that there are between 1,200 and 1,600 cases of malaria annually in the United States. 2 In 2009, there was a 14 percent increase in reported cases of malaria (from 1,298 cases in 2008 to 1,484 cases in 2009). 2 One factor contributing to disease resurgence is global climate change. 3 Between 2011 and 2020, the global mean temperature is expected to rise by 0.4°C. 3 This increase in temperature has been projected to lead to a 30 to 100 percent increase in mosquito abundance worldwide. 3
Most malaria infections in this country occur among persons who have traveled to areas with ongoing malaria transmission. In the United States, cases also can occur through exposure to infected blood products, congenital transmission, or local mosquito-borne transmission. 2
Not only are mosquitoes proliferating with environmental change, but recent findings also suggest that malaria is becoming resistant to treatment. Family physicians can address these issues with a preventive approach that includes traveler education, risk assessment, barrier protection, and chemoprophylaxis.
Sources of Transmission
Five main species of parasites are responsible for transmission of malaria in humans: Plasmodium falciparum , Plasmodium vivax , Plasmodium ovale , Plasmodium knowlesi , and Plasmodium malariae . 4 These protozoa are concentrated in different areas of the world, and each produces a different manifestation of infection. P. falciparum is the most life-threatening form of malaria.
These parasites are transmitted to humans by the bite of an infective female Anopheles mosquito. To produce eggs, the mosquito usually consumes a blood meal, thus needing humans and animals as hosts. The development of the protozoa in the mosquito takes 10 to 21 days, depending on the species of the parasite. After the parasites enter the host's liver, the replication stage begins. Subsequent replication occurs in erythrocytes and may last from one week to one year. Symptoms of malaria appear after the parasites leave the liver and start lysing red blood cells.
Risk Assessment
An individual risk assessment should be conducted for every traveler, taking into account the destination and season of travel. 5 Physicians should provide travelers with resources that discuss risk factors for malaria transmission ( Table 1 ) .
According to the World Health Organization, malaria was endemic in 106 countries in 2010. 6 Most cases occur in sub-Saharan Africa, the Indian subcontinent, and Southeast Asia. A map of worldwide malaria endemicity is available on the CDC Web site at http://cdc.gov/malaria/map/ . Malaria accounts for 5 percent of febrile illnesses in Ethiopia between the months of January and April, and up to 30 percent between the months of May and December. 7
Precipitation is also a contributing factor for vector transmission because riverbeds and stagnant pools of water are breeding grounds for the Anopheles mosquito. Travelers should be advised that the highest risk of malaria is during and after the rainy season. 8
Mosquito Bite Prevention
Mosquito sprays and bed netting are effective in preventing malaria transmission. A trial in the Bolivian Amazon showed that episodes of malaria were reduced by 80 percent among persons using insect repellent and insecticide-treated bed netting. 9
The CDC recommends diethyltoluamide (DEET) and picaridin as repellents for malaria prevention. 10 DEET concentrations between 4 and 30 percent are effective for malaria protection. 11 Higher concentrations are not associated with increased levels of toxicity. The effectiveness of DEET plateaus at a concentration of 30 percent. A formulation of 4 percent offers a complete mean protection time of approximately 90 minutes, whereas a 23 percent formulation offers more than five hours of protection. Adverse effects of DEET include dermatitis, allergic reactions, and rare neurotoxicity. The American Academy of Pediatrics does not recommend DEET for infants younger than two months. 12 The recommendations for DEET use in pregnant and lactating women are similar to those for nonpregnant adults. 11
A 20 percent solution of picaridin is comparable to a 35 percent DEET solution. 13 The highest concentration of picaridin sold in the United States is 15 percent, and the data are insufficient to support adequate protection against Anopheles mosquitoes at this concentration. Picaridin does not cause skin irritation and is safe to use in children and pregnant women.
In 2007, scientists in South America developed a mosquito repellent containing p -menthane-3,8-diol (PMD), a eucalyptus plant extract. 14 The formula is less toxic, cheaper, and more effective against malaria than a 20 percent solution of DEET. 14 In the United States, PMD is available as 65 percent and 10 percent concentrations. 15 The U.S. Environmental Protection Agency recommends these products as repellents against mosquitoes, biting flies, and gnats. 15 Adverse effects include skin and eye irritation. 15
Barriers such as insecticide-treated netting and clothing are as important as repellents in the prevention of malaria. A study in sub-Saharan Africa concluded that bed netting reduces the incidence of malaria by at least 50 percent. 16 Use of clothing treated with permethrin (a synthetic mosquito repellent) is effective in preventing mosquito bites. 17
Chemoprophylaxis
All recommended chemoprophylactic regimens involve taking medication before travel, during travel, and for a period of time after leaving the malaria-endemic region ( Table 2 ) . 18 – 22 Beginning the regimen before travel is necessary to allow the antimalaria agent to enter the bloodstream before exposure to malaria-carrying parasites. 18 Atovaquone/proguanil (Malarone), doxycycline, and mefloquine are the drugs of choice for malaria prevention in most malaria-endemic regions. 18
ATOVAQUONE/PROGUANIL
Atovaquone/proguanil is a good choice for last-minute travelers because it can be started one to two days before travel, as opposed to one to two weeks with some of the other drugs. 18 Common adverse effects include abdominal pain, nausea, vomiting, and elevated alanine transaminase levels. It is contraindicated in patients with a creatinine clearance of less than 30 mL per minute per 1.73 m 2 (0.50 mL per second per m 2 ). 18 Atovaquone/proguanil is a U.S. Food and Drug Administration (FDA) pregnancy category C medication.
DOXYCYCLINE
Doxycycline is taken daily and provides additional protection against many infections, including tick-borne illnesses. 18 Travelers should be aware that photosensitivity may increase in persons with prolonged sun exposure. Other adverse effects include vaginal candidiasis, abdominal pain, and diarrhea. Doxycycline is FDA pregnancy category D, and should be used only if maternal benefits outweigh fetal risks. It is contraindicated in children younger than eight years.
Mefloquine is taken weekly. It is considered safe to use during the second and third trimesters of pregnancy. 18 Resistance to mefloquine is found in areas of China, Myanmar, Laos, Vietnam, and Cambodia. 23 Five percent of patients taking mefloquine will experience neuropsychiatric effects (e.g., insomnia, paranoia, hallucinations, seizures) that lead to discontinuation of the drug. 19 , 20
CHLOROQUINE
Chloroquine (Aralen) was the standard of care for malaria prevention for many years. However, as P. falciparum has become largely resistant to chloroquine, it is now recommended only for travelers going to the Middle East, Central America, Haiti, and the Dominican Republic. 18 Chloroquine can be used in all trimesters of pregnancy and in children of all ages. 18 Adverse effects may include blurry vision, tinnitus, and hearing loss.
Primaquine is used mainly in areas where P. vivax is the primary strain of malaria (e.g., parts of Central and South America). Patients must be tested for glucose-6-phosphate dehydrogenase deficiency before taking primaquine because it may cause hemolysis in affected persons. 21 Other adverse effects include nausea, vomiting, and abdominal pain. 21 Primaquine is an FDA pregnancy category C medication.
Five to 80 percent of patients treated for P. vivax malaria will relapse. 22 As a preemptive measure, patients with P. vivax infection should be treated with a 14-day course of primaquine to prevent further disease. 22 Primaquine therapy should be started on the same day as malaria treatment. 22
Recognition of Illness
Travelers should be warned that adequate chemoprophylaxis does not guarantee full protection against malaria. Symptoms may appear from one week to one year after infection with the parasite. Relapsing illness may occur in patients who have completed a course of treatment. 10 Travelers to malaria-endemic areas should seek medical attention for signs and symptoms of malaria, including fever, chills, headaches, and arthralgias. 10
Presumptive Treatment
Travelers who decline malaria prophylaxis or who will be traveling to remote areas with limited access to health care may be prescribed a three-day supply of presumptive malaria treatment before travel. 23 Travelers should be advised that self-treatment of a possible malaria infection is only a temporary measure, and that prompt medical evaluation is imperative. 23 A three-day course of high-dose oral atovaquone/proguanil or artemether/lumefantrine (Coartem) may be prescribed. 23 Travelers should take the medication if they experience high fevers, chills, or myalgias. 23 Physicians who need assistance with the diagnosis or treatment of malaria should call the CDC Malaria Hotline (855-856-4713).
The Future of Malaria Prevention
A malaria vaccine is being developed for delivery through the World Health Organization's Expanded Programme on Immunization. 24 It is being studied in African infants during the first 13 months of life, and has been reported to reduce transmission of malaria by 65 percent with few adverse effects. 24 Along with barrier protection and chemoprophylaxis, vaccination may eventually play a key role in the eradication of malaria worldwide. 24
Data Sources: We searched PubMed, Essential Evidence Plus, the Cochrane database, and UpToDate using variations of the key term malaria prevention. Search dates: July to September 2010, and July 2011.
Centers for Disease Control and Prevention. Malaria—malaria facts. http://www.cdc.gov/malaria/about/facts.html . Accessed December 12, 2011.
Mali S, Tan KR, Arguin PM Division of Parasitic Diseases and Malaria. Center for Global Health; Centers for Disease Control and Prevention. Malaria surveillance—United States, 2009. MMWR Surveill Summ. 2011;60(3):1-15.
Pascual M, Ahumada JA, Chaves LF, Rodó X, Bouma M. Malaria resurgence in the East African highlands: temperature trends revisited. Proc Natl Acad Sci USA. 2006;103(15):5829-5834.
Freedman DO. Clinical practice. Malaria prevention in short-term travelers. N Engl J Med. 2008;359(6):603-612.
Centers for Disease Control and Prevention. Malaria—disease. http://www.cdc.gov/malaria/about/disease.html . Accessed August 15, 2010.
World Health Organization. World Malaria Report: 2010. http://www.who.int/malaria/world_malaria_report_2010/en/index.html . Accessed December 12, 2011.
Muhe L, Oljira B, Degefu H, Enquesellassie F, Weber MW. Clinical algorithm for malaria during low and high transmission seasons. Arch Dis Child. 1999;81(3):216-220.
Briët OJ, Vounatsou P, Gunawardena DM, Galappaththy GN, Amerasinghe PH. Temporal correlation between malaria and rainfall in Sri Lanka. Malar J. 2008;7:77.
Hill N, Lenglet A, Arnéz AM, Carneiro I. Plant based insect repellent and insecticide treated bed nets to protect against malaria in areas of early evening biting vectors: double blind randomised placebo controlled clinical trial in the Bolivian Amazon. BMJ. 2007;335(7628):1023.
Centers for Disease Control and Prevention. Malaria—malaria and travelers. http://www.cdc.gov/malaria/travelers/index.html . Accessed July 8, 2011.
Fradin MS, Day JF. Comparative efficacy of insect repellents against mosquito bites. N Engl J Med. 2002;347(1):13-18.
American Academy of Pediatrics. Follow safety precautions when using DEET on children. AAP News . 2003;22(5):200-399. http://aapnews.aappublications.org/cgi/content/full/e200399v1 (subscription required). Accessed July 1, 2011.
Frances SP, Waterson DG, Beebe NW, Cooper RD. Field evaluation of repellent formulations containing deet and picaridin against mosquitoes in Northern Territory, Australia. J Med Entomol. 2004;41(3):414-417.
Moore SJ, Darling ST, Sihuincha M, Padilla N, Devine GJ. A low-cost repellent for malaria vectors in the Americas: results of two field trials in Guatemala and Peru. Malar J. 2007;6:101.
U.S. Environmental Protection Agency. Pesticides: regulating pesticides— p -Menthane-3,8-diol (011550) fact sheet. http://www.epa.gov/oppbppd1/biopesticides/ingredients/factsheets/factsheet_011550.htm . Accessed July 1, 2011.
Pennetier C, Corbel V, Boko P, et al. Synergy between repellents and non-pyrethroid insecticides strongly extends the efficacy of treated nets against Anopheles gambiae . Malar J. 2007;6:38.
Kimani EW, Vulule JM, Kuria IW, Mugisha F. Use of insecticide-treated clothes for personal protection against malaria: a community trial. Malar J. 2006;5:63.
Centers for Disease Control and Prevention. Malaria—choosing a drug to prevent malaria. http://www.cdc.gov/malaria/travelers/drugs.html . Accessed August 15, 2010.
Gutman J, Green M, Durand S, et al. Mefloquine pharmacokinetics and mefloquineartesunate effectiveness in Peruvian patients with uncomplicated Plasmodium falciparum malaria. Malar J. 2009;8:58.
Nevin RL, Pietrusiak PP, Caci JB. Prevalence of contraindications to mefloquine use among USA military personnel deployed to Afghanistan. Malar J. 2008;7:30.
Hill DR, Baird JK, Parise ME, Lewis LS, Ryan ET, Magill AJ. Primaquine: report from CDC expert meeting on malaria chemoprophylaxis I. Am J Trop Med Hyg. 2006;75(3):402-415.
Baird JK, Hoffman SL. Primaquine therapy for malaria. Clin Infect Dis. 2004;39(9):1336-1345.
Centers for Disease Control and Prevention. Travelers' health—infectious diseases related to travel: malaria. http://wwwnc.cdc.gov/travel/yellowbook/2012/chapter-3-infectious-diseases-related-to-travel/malaria.htm . Acessed July 8, 2011.
Abdulla S, Oberholzer R, Juma O, et al. Safety and immunogenicity of RTS,S/AS02D malaria vaccine in infants. N Engl J Med. 2008;359(24):2533-2544.
Continue Reading

More in AFP
More in pubmed.
Copyright © 2012 by the American Academy of Family Physicians.
This content is owned by the AAFP. A person viewing it online may make one printout of the material and may use that printout only for his or her personal, non-commercial reference. This material may not otherwise be downloaded, copied, printed, stored, transmitted or reproduced in any medium, whether now known or later invented, except as authorized in writing by the AAFP. See permissions for copyright questions and/or permission requests.
Copyright © 2024 American Academy of Family Physicians. All Rights Reserved.
Learn about the flu shot , COVID-19 vaccine , and our masking policy »
- Doctors, Clinics & Locations, Conditions & Treatments
- Patients & Visitors
- Medical Records
- Support Groups
- Help Paying Your Bill
- COVID-19 Resource Center
- Locations and Parking
- Visitor Policy
- Hospital Check-in
- Video Visits
- International Patients
View the changes to our visitor policy »
View information for Guest Services »
New to MyHealth?
Manage Your Care From Anywhere.
Access your health information from any device with MyHealth. You can message your clinic, view lab results, schedule an appointment, and pay your bill.
ALREADY HAVE AN ACCESS CODE?
Don't have an access code, need more details.
Learn More about MyHealth » Learn More about Video Visits »
MyHealth for Mobile
Get the iPhone MyHealth app » Get the Android MyHealth app »
WELCOME BACK
Malaria prevention, can malaria be prevented.
Malaria can often be prevented by the use of antimalarial drugs and use of protection measures against mosquito bites.
Medications
When planning to travel to an area where malaria occurs, talk with your doctor well in advance of your departure. Drugs to prevent malaria can be prescribed for travelers to malarious areas, but travelers from different countries may receive different recommendations, reflecting differences in treatment protocols as well as availability of medicines in different countries. Travelers visiting only cities or rural areas where there is no risk of malaria may not require preventive drugs, but an exact itinerary is necessary to determine what degree of protection may be needed. According to the Centers for Disease Control and Prevention (CDC), there are several medications recommended for prevention of malaria in travelers. Determining which medication is best depends on several factors, such as your medical history and the amount of time before your scheduled departure. Strict adherence to the recommended doses and schedules of the antimalarial drug selected is necessary for effective protection.
Protection from mosquitoes
Be aware that you are still at risk for malaria even with the use of protection.
To avoid mosquito bites, the CDC recommends the following:
- Apply insect repellent to exposed skin. The recommended repellent contains 20-35% percent N,N-Diethyl-meta-toluamide (DEET).
- Wear long-sleeved clothing and long pants if you are outdoors at night.
- Use a mosquito net over the bed if your bedroom is not air-conditioned or screened. For additional protection, treat the mosquito net with the insecticide permethrin.
- Spray an insecticide or repellent on clothing, as mosquitoes may bite through thin clothing.
- Spray pyrethrin or a similar insecticide in your bedroom before going to bed.
Condition Spotlight
Clinical Trials
Clinical trials are research studies that evaluate a new medical approach, device, drug, or other treatment. As a Stanford Health Care patient, you may have access to the latest, advanced clinical trials.
Open trials refer to studies currently accepting participants. Closed trials are not currently enrolling, but may open in the future.
Clinics for Malaria prevention
- Infectious Disease Clinic 650-736-5200
- Travel Medicine Clinic 650-736-5700
- Child Health
- Heart Health
- Men's Health
- Mental Health
- Sexual Health
- Skin Conditions
- Travel Vaccinations
- Treatment and Medication
- Women's Health
- View all categories
- Bones and Joints
- Digestive Health
- Healthy Living
- Signs and Symptoms
Try our Symptom Checker Got any other symptoms?
- Nervous System
- Heart Disease
- Inflammation
- Painkillers
- Muscle Pain
- View all Medicines and Drugs
- Type 2 Diabetes
- Bacterial Vaginosis
- View all Treatments
- BMI Calculator
- Pregnancy Due Date Calculator
- Screening Tests
- Blood Tests
- Liver Function Tests
- Am I Pregnant?
- Am I Depressed?
- View all Tools
- Latest Features
- Health Videos
- Bronchiolitis
- Molluscum Contagiosum
- Actinic Keratosis
- Abdominal Pain in Children
- Subdural Haematoma
- Obesity in Adults
- View all Pro Articles
- View all Medical Calculators
- Login / Register
- Patient Access
- Health Info
- Travel and Vaccinations
Malaria Prevention
Remove from Saved
It is sensible to find out whether there is a risk of malaria in the place you are visiting. If there is a risk, you can avoid getting malaria by taking steps to avoid mosquito bites, and in some cases by taking antimalarial medication. Malaria can be a life-threatening illness, so it is extremely important to consider prevention before travelling to an at-risk area.
In this article
How to prevent malaria, awareness of the risk of malaria, malaria bite prevention, antimalarial tablets, side-effects of antimalarial tablets.
There is an ABCD for prevention of malaria. This is:
- A wareness of risk of malaria.
- B ite prevention.
- C hemoprophylaxis. This means taking antimalarial medication to prevent the disease.
- D iagnosis should be made promptly and treatment started quickly. Seek medical attention urgently if you become unwell after travelling to a high-risk area.
Malaria is a serious infection, so prevention is crucial.
Before travelling it is important to find out if there is a risk of malaria where you are going. You can find this out on the Fitfortravel website , or from your travel clinic or pharmacist.
The risk varies between countries and can depend on the type of trip. For example, back-packing or travelling to rural areas is generally more risky than staying in urban hotels.
In some countries the risk varies between seasons - malaria is more common in the wet season. In other countries, the risk varies depending on which part of the country you visit. One part of the country may be an area where there is a risk, and in other areas there is no or low risk.
In high mountainous areas there is often no risk, whilst in lower warmer areas there may be higher risk. The mosquito involved thrives in warm, humid conditions, so places with this sort of climate tend to be high-risk areas.
- Risk is particularly high in Africa, much of Asia and parts of South America.
- Western Europe and the United States of America are not areas of risk.
The main type of parasite and the amount of resistance to medication vary in different countries. Although risk varies, all travellers to malaria-risk countries should take precautions to prevent malaria.
The mosquitoes which transmit malaria commonly fly from dusk to dawn and therefore evenings and nights are the most dangerous time for transmission.
Book a pharmacy appointment today
Arrange a consultation with your local pharmacist via Patient Access to discuss your travel plans and malaria prevention.
Insect repellent
You should use an effective insect repellent on clothing and any exposed skin. Diethyltoluamide (DEET) is safe, the most effective insect repellent and can be sprayed on to clothes. It lasts up to three hours for 20%, up to six hours for 30% and up to 12 hours for 50% DEET.
There is no further increase in duration of protection beyond a concentration of 50%. Because 50% DEET lasts longer, you do not need to apply it so often. It is also more effective in this higher concentration.
When both sunscreen and DEET are required, DEET should be applied after the sunscreen has been applied. DEET can be used on babies and children over 2 months of age. In addition, DEET can be used, in a concentration of up to 50%, if you are pregnant. It is also safe to use if you are breastfeeding. If you have sensitive skin you may find DEET irritating. Insecticides containing picaridin are a useful alternative.
Mosquito nets
If you sleep outdoors or in an unscreened room, ideally you should use mosquito nets impregnated with an insecticide (such as pyrethroid). The net should be long enough to fall to the floor all around your bed and be tucked under the mattress.
Check the net regularly for holes. Nets need to be re-impregnated with insecticide every six to twelve months (depending on how frequently the net is washed) to remain effective. Long-lasting nets, in which the pyrethroid is incorporated into the material of the net itself, are also available and can last approximately three to five years.
Covering-up bare skin
You should try to cover up bare areas with long-sleeved, loose-fitting clothing, such as long trousers and socks. If you are outside after sunset you should definitely cover up to reduce the risk of mosquitoes biting.
Clothing may also be sprayed or impregnated with permethrin, which reduces the risk of being bitten through your clothes.
Air-conditioners
Sleeping in an air-conditioned room reduces the likelihood of mosquito bites, due to the room temperature being lowered.
Mesh netting
Doors, windows and other possible mosquito entry routes to sleeping accommodation should be screened with fine mesh netting. You should spray the room before dusk with an insecticide (usually a pyrethroid) to kill any mosquitoes that may have come into the room during the day.
Other prevention methods
If electricity is available, you should use an electrically heated device to vaporise a tablet containing a synthetic pyrethroid in the room during the night. The burning of a mosquito coil is not as effective.
Herbal remedies have not been tested for their ability to prevent or treat malaria and are therefore not recommended. Likewise, there is no scientific proof that homeopathic remedies are effective in either preventing or treating malaria and they are also not recommended.
Antimalarial medication (chemoprophylaxis) helps to prevent malaria. The best medication to take depends on the country you visit. This is because the type of parasite varies between different parts of the world. Also, in some areas the parasite has become resistant to certain medicines.
To find out whether there is a risk of malaria in any country you're visiting, and whether you need to take antimalarial tablets, visit the NHS Fitfortravel site.
Be aware that antimalarials that you buy in the tropics or over the internet may be fake. It is therefore recommended that you obtain your antimalarial treatment from your pharmacist or a travel clinic.
Pharmacists can now provide the full range of antimalarial medications, so there's no need to see your GP or practice nurse for a prescription. Medications to protect against malaria are not funded by the NHS. You will need to buy them, regardless of where you obtain them.
So now you can buy this medication over the counter, what might the benefits be? Well first, you should save some money. — Michael Stewart, Getting malaria tablets from your pharmacist
The type of medication advised will depend upon the area to which you are travelling. It will also depend on:
- Any health problems you have.
- Any medication you are currently taking.
- The length of your stay.
- Any problems you may have had with antimalarial medication in the past.
- Whether you are pregnant or breastfeeding.
- Age (some medicines cannot be used in children).
Names of medications which may be used are:
- Chloroquine (or hydroxychloroquine if you already take this for another condition).
- Proguanil .
- Doxycycline .
- Mefloquine .
- Atovaquone and proguanil combination .
You should seek advice for each new trip abroad. Do not assume that the medication you took for your last trip will be advised for your next trip, even to the same country. There is a changing pattern of resistance to some medicines by the parasites.
How to take antimalarial tablets
You must take the medication exactly as advised. This usually involves starting the medication up to a week before you go on your trip. This allows the level of medicine in your body to become effective. It also gives time to check for any side-effects before travelling.
It is also essential that you continue taking the medication for the correct time advised after returning to the UK. This will vary depending on the individual medicine but is likely to be between one and four weeks.
Because of the way the parasite infects your blood, it can still be spreading in your blood several weeks after being bitten. It is important to take your medicines for the correct amount of weeks after leaving an affected country, in order to prevent this.
Antimalarial medication is usually well tolerated. The most common side-effects are minor and include:
- Feeling sick (nausea).
- Diarrhoea .
However, some people develop more severe side-effects. Therefore, always read the information sheet which comes with a particular medicine for a list of possible side-effects and cautions. To reduce possible side-effects, it is usually best to take the medication after meals.
If you are taking doxycycline then you need to use a high-factor sunscreen. This is because this medication makes the skin more sensitive to the effects of the sun.
A few people taking mefloquine may develop headaches or have problems with sleep (including difficulty sleeping ( insomnia ) or abnormal dreams). Mood may be affected.
Note : medication is only a part of protection against malaria. It is not 100% effective and does not guarantee that you will not get malaria. The advice above on avoiding mosquito bites is just as important, even when you are taking antimalarial medication.
Are you protected against flu?
See if you are eligible for a free NHS flu jab today.
Join our weekly wellness digest
from the best health experts in the business
Further reading and references
Guidelines for malaria prevention in travellers from the UK 2022 ; Public Health England Annual report, April 2023
Malaria: guidance, data and analysis ; Public Health England
NHS Fit For Travel: Travel health information for people travelling abroad from the UK ; Health Protection Scotland
Tickell-Painter M, Maayan N, Saunders R, et al ; Mefloquine for preventing malaria during travel to endemic areas. Cochrane Database Syst Rev. 2017 Oct 3010:CD006491. doi: 10.1002/14651858.CD006491.pub4.
World Malaria Report 2023 ; World Health Organization, November 2023
Malaria ; NICE CKS, July 2023 (UK access only)
Related Information
- Malaria Prophylaxis Pro
- Malaria in Pregnancy Pro
- Malaria (Causes, Symptoms and Treatment) Pro
- Proguanil for malaria prevention (Paludrine)

Festival abroad? Have fun and stay safe

Should you worry about side effects with malaria tablets?

Getting malaria tablets from your pharmacist
Hi All, I dont know if anyone has come across this. I have a worm infestation in my face. They travel around under the skin leaving tracks and bursting holes into my skin. they create glass like... nicolamc
Feeling unwell?
Assess your symptoms online with our free symptom checker.
Disclaimer: This article is for information only and should not be used for the diagnosis or treatment of medical conditions. Egton Medical Information Systems Limited has used all reasonable care in compiling the information but make no warranty as to its accuracy. Consult a doctor or other health care professional for diagnosis and treatment of medical conditions. For details see our conditions .
- Português Br
- Journalist Pass
Stay healthy abroad: Why you should see a travel medicine specialist before your trip
Mayo Clinic Staff
Share this:

As you get ready to travel to another country, you probably have many details to coordinate and plan. One essential task, depending on where those travels take you, may be to make an appointment to see a travel medicine specialist.
A travel medicine specialist assesses travel-related risks and provides information to ensure your health and safety while minimizing the potential for health-related situations during your trip.
Adding a consultation to your travel to-do list
A consultation with a travel medicine specialist includes discussing travel-related illnesses, risk factors for infectious and noninfectious diseases, required immunizations , health regulations and drug-resistant organisms you may encounter.
It's crucial to schedule a pretravel consultation at least two weeks — and preferably 4 to 8 weeks — before your trip to ensure you get complete protection from any needed vaccinations. When requesting a travel medicine consultation, be prepared to provide information about your trip, including:
- All countries being visited
- Any transportation, accommodation or other circumstances that are out of the usual
- Dates and duration of travel
A travel medicine specialist will review your itinerary before your consultation to identify country-by-country health risks, such as exotic infectious agents, the potential for altitude sickness or heat exhaustion, as well as appropriate vaccinations and possible need for malaria-prevention medications.
Your opportunity to learn about staying healthy abroad
A consultation gives you the opportunity to learn about health risks you may face while you're traveling and once you reach your destinations. Based on your itinerary, the travel medicine specialist may:
- Explain the risks of infection from mosquito-borne illnesses and the steps for protecting yourself. This includes reviewing medications to prevent malaria, which is a potentially life-threatening illness.
- Ensure you receive protection against vaccine-preventable illnesses, such as hepatitis A or typhoid fever , and verify that other routine vaccinations are current.
- Evaluate your overall health for travel and discuss with you how to manage preexisting conditions.
- Give tips for preventing jet lag, motion sickness, altitude illness and blood clots .
- Go over how to prevent and treat traveler's diarrhea , the most common travel-related illness.
- Help you reduce the chance of becoming ill during travel.
- Provide a yellow fever vaccination and an International Certificate of Vaccination, also known as a yellow card, if you travel to a country where the vaccine is recommended or required.
- Review food and water precautions. Contaminated food and water can pose disease risk for travelers, many of which are transmitted via by swallowing or coming in contact with impure water, such as fresh or sea water and swimming pools.
Be sure to ask the specialist any questions you may have about your personal health and raise any safety concerns about your travel itinerary.
Get sick on your trip? Check-in with a travel medicine specialist
Once you return home, a travel medicine specialist also can conduct a comprehensive post-travel evaluation of any illnesses you may have picked up while away, including parasitic infections and other tropical diseases that are rare in the U.S.
No matter the reason for travel — vacation, business, studying abroad, visiting friends or relatives or medical tourism — always be prepared and take steps to ensure your health and safety.
Raj Palraj, M.B.B.S., M.D. , specializes in infectious diseases in La Crosse, Wisconsin .
This article first appeared on the Mayo Clinic Health System blog .
- Mayo Clinic Minute: Can aspirin make your breathing worse? Mayo Clinic Minute: Tips to safely watch the total solar eclipse
Related Articles

You are using an outdated browser. Upgrade your browser today or install Google Chrome Frame to better experience this site.
- Section 2 - Interactions Between Travel Vaccines & Drugs
- Section 2 - Travelers’ Diarrhea
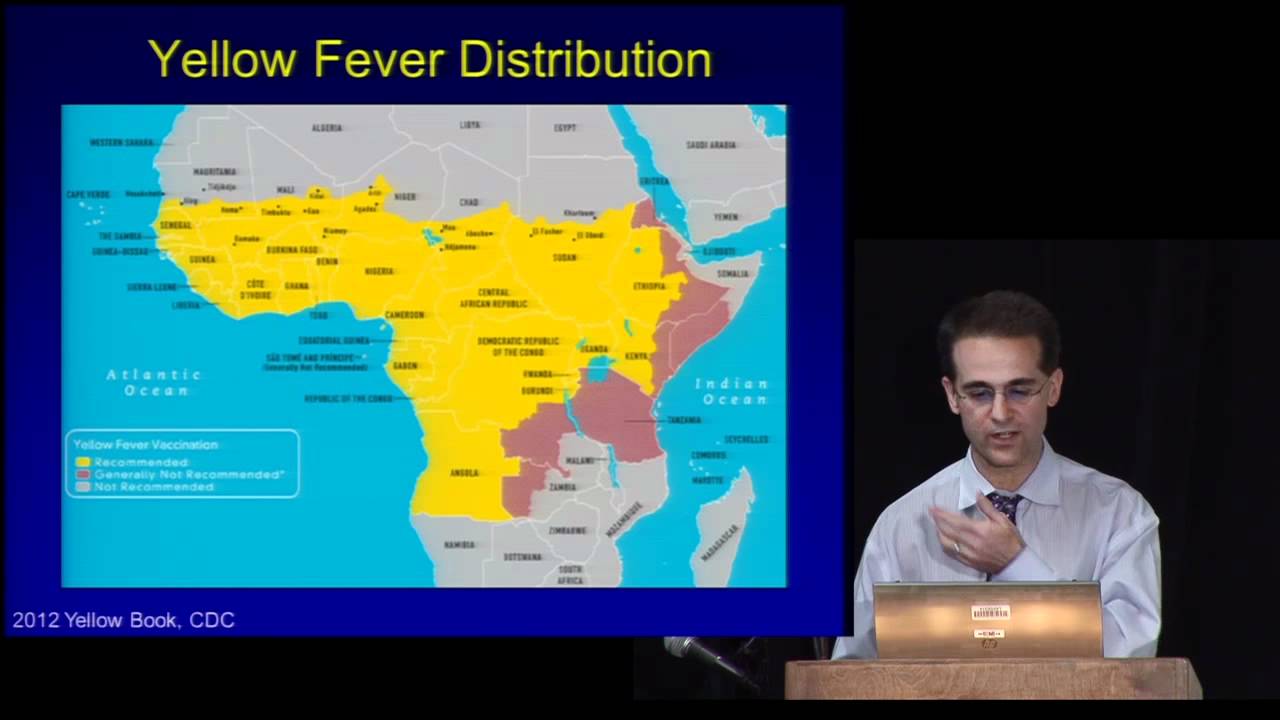
Yellow Fever Vaccine & Malaria Prevention Information, by Country
Cdc yellow book 2024.
Author(s): Mark Gershman, Rhett Stoney (Yellow Fever) Holly Biggs, Kathrine Tan (Malaria)
The following pages present country-specific information on yellow fever (YF) vaccine requirements and recommendations, and malaria transmission information and prevention recommendations. Country-specific maps are included to aid in interpreting the information. The information in this chapter was accurate at the time of publication; however, it is subject to change at any time due to changes in disease transmission or, in the case of YF, changing entry requirements for travelers. Updated information reflecting changes since publication can be found in the online version of this book and on the Centers for Disease Control and Prevention (CDC) Travelers’ Health website. Recommendations for prevention of other travel-associated illnesses can also be found on the CDC Travelers’ Health website .
Yellow Fever Vaccine
Entry requirements.
Entry requirements for proof of YF vaccination under the International Health Regulations (IHR) differ from CDC’s YF vaccination recommendations. Under the IHR, countries are permitted to establish YF vaccine entry requirements to prevent the importation and transmission of YF virus within their boundaries. Certain countries require proof of vaccination from travelers arriving from all countries ( Table 5-25 ); some countries require proof of vaccination only for travelers above a certain age coming from countries with risk for YF virus transmission. The World Health Organization (WHO) defines areas with risk for YF virus transmission as countries or areas where YF virus activity has been reported currently or in the past, and where vectors and animal reservoirs exist.
Unless issued a medical waiver by a yellow fever vaccine provider, travelers must comply with entry requirements for proof of vaccination against YF.
WHO publishes a list of YF vaccine country entry requirements and recommendations for international travelers approximately annually. But because entry requirements are subject to change at any time, health care professionals and travelers should refer to the online version of this book and the CDC Travelers’ Health website for any updates before departure.
CDC Recommendations
CDC’s YF vaccine recommendations are guidance intended to protect travelers from acquiring YF virus infections during international travel. These recommendations are based on a classification system for destination-specific risk for YF virus transmission: endemic, transitional, low potential for exposure, and no risk ( Table 2-08 ). CDC recommends YF vaccination for travel to areas classified as having endemic or transitional risk (Maps 5-10 and 5-11 ). Because of changes in YF virus circulation, however, recommendations can change; therefore, before departure, travelers and clinicians should check CDC’s destination pages for up-to-date YF vaccine information.
Duration of Protection
In 2015, the US Advisory Committee on Immunization Practices published a recommendation that 1 dose of YF vaccine provides long-lasting protection and is adequate for most travelers. The recommendation also identifies specific groups of travelers who should receive additional doses, and others for whom additional doses should be considered (see Sec. 5, Part 2, Ch. 26, Yellow Fever ). In July 2016, WHO officially amended the IHR to stipulate that a completed International Certificate of Vaccination or Prophylaxis is valid for the lifetime of the vaccinee, and YF vaccine booster doses are not necessary. Moreover, countries cannot require proof of revaccination (booster) against YF as a condition of entry, even if the traveler’s last vaccination was >10 years ago.
Ultimately, when deciding whether to vaccinate travelers, clinicians should take into account destination-specific risks for YF virus infection, and individual risk factors (e.g., age, immune status) for serious YF vaccine–associated adverse events, in the context of the entry requirements. See Sec. 5, Part 2, Ch. 26, Yellow Fever , for a full discussion of YF disease and vaccination guidance.
Table 2-08 Yellow fever (YF) vaccine recommendation categories 1
Malaria prevention.
The following recommendations to protect travelers from malaria were developed using the best available data from multiple sources. Countries are not required to submit malaria surveillance data to CDC. On an ongoing basis, CDC actively solicits data from multiple sources, including WHO (main and regional offices); national malaria control programs; international organizations; CDC overseas offices; US military; academic, research, and aid organizations; and the published scientific literature. The reliability and accuracy of those data are also assessed.
If the information is available, trends in malaria incidence and other data are considered in the context of malaria control activities within a given country or other mitigating factors (e.g., natural disasters, wars, the coronavirus disease 2019 pandemic) that can affect the ability to control malaria or accurately count and report it. Factors such as the volume of travel to that country and the number of acquired cases reported in the US surveillance system are also examined. In developing its recommendations, CDC considers areas within countries where malaria transmission occurs, substantial occurrences of antimalarial drug resistance, the proportions of species present, and the available malaria prophylaxis options.
Clinicians should use these recommendations in conjunction with an individual risk assessment and consider not only the destination but also the detailed itinerary, including specific cities, types of accommodations, season, and style of travel, as well as special health conditions (e.g., pregnancy). Several medications are available for malaria prophylaxis. When deciding which drug to use, consider the itinerary and length of trip, travelers’ previous adverse reactions to antimalarials, drug allergies, medical history, and drug costs. For a thorough discussion of malaria and guidance for prophylaxis, see Sec. 5, Part 3, Ch. 16, Malaria .
Afghanistan
Entry requirements : None
CDC recommendations : Not recommended
- All areas <2,500 m (≈8,200 ft) elevation (April–December)
- Chloroquine
- P. vivax (primarily)
- P. falciparum (less commonly)
- Atovaquone-proguanil, doxycycline, mefloquine, tafenoquine 3
Other Vaccines to Consider
See Health Information for Travelers to Afghanistan
Entry requirements : Required for travelers ≥1 year old arriving from countries with risk for YF virus transmission 1
No malaria transmission
See Health Information for Travelers to Albania
Entry requirements : Required for travelers ≥9 months old arriving from countries with risk for YF virus transmission; this includes >12-hour airport transits or layovers in countries with risk for YF virus transmission. 1
See Health Information for Travelers to Algeria
American Samoa (US)
See Health Information for Travelers to American Samoa
See Health Information for Travelers to Andorra
Entry requirements : Required for arriving travelers ≥9 old
CDC recommendations : Recommended for all travelers ≥9 months old
- P. falciparum (primarily)
- P. malariae , P. ovale , and P. vivax (less commonly)
See Health Information for Travelers to Angola
Anguilla (U.K.)
See Health Information for Travelers to Anguilla (U.K.)
See Health Information for Travelers to Antarctica
Antigua and Barbuda
Entry requirements : Required for travelers ≥1 year old arriving from countries with risk for YF virus transmission this includes >12-hour airport transits or layovers in countries with risk for YF virus transmission. 1
See Health Information for Travelers to Antigua and Barbuda
CDC recommendations : Recommended for travelers ≥9 months old going to Corrientes and Misiones Provinces. Generally not recommended for travel to Formosa Province or to designated areas of Chaco, Jujuy, and Salta Provinces. Not recommended for travel limited to provinces and areas not listed above.
Related Maps
Map 2-01 Yellow fever vaccine recommendations for Argentina & neighboring countries
See Health Information for Travelers to Argentina
See Health Information for Travelers to Armenia
Entry requirements : Required for travelers ≥9 months old arriving from countries with risk for YF virus transmission; this includes >12-hour airport transits or layovers in countries with risk for YF virus transmission. 1 Entry will be denied if a valid vaccination certificate cannot be provided.
See Health Information for Travelers to Aruba
Entry requirements : Required for travelers ≥1 year old arriving from countries with risk for YF virus transmission; this includes >12-hour airport transits or layovers in countries with risk for YF virus transmission. 1 Travelers arriving from the Galápagos Islands of Ecuador are exempt from this requirement.
See Health Information for Travelers to Australia
See Health Information for Travelers to Austria
See Health Information for Travelers to Azerbaijan
Azores (Portugal)
See Health Information for Travelers to Azores
Bahamas, The
Entry requirements : Required for travelers ≥1 year old arriving from countries with risk for YF virus transmission; this includes >12-hour airport transits or layovers in countries with risk for YF virus transmission. 1
See Health Information for Travelers to The Bahamas
See Health Information for Travelers to Bahrain
Entry requirements : Required for travelers ≥1 year old arriving from countries with risk for YF virus transmission; this includes airport transits or layovers in countries with risk for YF virus transmission. 1
- Districts of Chittagong Hill Tract (Bandarban, Khagrachari, and Rangamati); and the following districts: Chattogram (Chittagong) and Cox’s Bazar (in Chattogram [Chittagong] Division); Mymensingh, Netrakona, and Sherpur (in Mymensingh Division); Kurigram (in Rangpur Division); Habiganj, Moulvibazar, Sunamganj, and Sylhet (in Sylhet Division)
- No malaria transmission in Dhaka (the capital)
- P. falciparum (90%)
- P. vivax (10%)
- P. malariae (rare)
See Health Information for Travelers to Bangladesh
Entry requirements : Required for travelers ≥1 year old arriving from countries with risk for YF virus transmission. 1 Travelers arriving from Guyana or Trinidad & Tobago are exempt from this requirement, unless an outbreak is occurring.
See Health Information for Travelers to Barbados
See Health Information for Travelers to Belarus
See Health Information for Travelers to Belgium
- Rare transmission
- No malaria transmission in Belize City or on islands frequented by tourists (e.g., Ambergris Caye)
- P. vivax (primarily)
- None (insect bite precautions / mosquito avoidance only) 4
See Health Information for Travelers to Belize
Entry requirements : Required for all arriving travelers ≥9 months old
- P. falciparum (primarily)
- P. malariae , P. ovale, and P. vivax (less commonly)
See Health Information for Travelers to Benin
Bermuda (U.K.)
See Health Information for Travelers to Bermuda (U.K.)
- Rare cases in rural areas <1,700 m (≈5,500 ft) elevation in districts along the southern border shared with India
- P. falciparum (less commonly)
- None (insect bite precautions and mosquito avoidance only) 4
See Health Information for Travelers to Bhutan
Entry requirements : Required for travelers ≥1 year old arriving from countries with risk for YF virus transmission. 1
CDC recommendations : Recommended for travelers ≥9 months old going to areas <2,300 m (≈7,550 ft) elevation, east of the Andes Mountains: the entire departments of Beni, Pando, Santa Cruz, and designated areas in the departments of Chuquisaca, Cochabamba, La Paz, and Tarija. Not recommended for travel limited to areas >2,300 m (≈7,550 ft) elevation and any areas not listed above, including the cities of La Paz (administrative capital) and Sucre (constitutional [legislative and judicial] capital).
- All areas <2,500 m (≈8,200 ft) elevation
- No malaria transmission in La Paz (administrative capital)
- P. vivax (99%)
- P. falciparum (1%)
- Atovaquone-proguanil, doxycycline, mefloquine, primaquine 5 , tafenoquine 3
Map 2-02. Yellow fever vaccine recommendations for Bolivia & neighboring countries
See Health Information for Travelers to Bolivia
See Health Information for Travelers to Bonaire
Bosnia and Herzegovina
See Health Information for Travelers to Bosnia and Herzegovina
Entry requirements : Required for travelers ≥1 year old arriving from countries with risk for YF virus transmission; this includes transits through countries with risk for YF virus transmission. 1
- Districts/ subdistricts of Bobirwa, Boteti, Chobe (including Chobe National Park), Ghanzi, Mahalapye, Ngamiland (Ngami), North East (including its capital, Francistown), Okavango, Serowe/ Palapye, and Tutume
- Rare cases or sporadic foci of transmission in districts/ subdistricts of Kgalagadi North, Kgatleng, Kweneng, and Southern
- No malaria transmission in Gaborone (the capital)
- P. malariae , P. ovale , and P. vivax (less commonly)
- Districts/subdistricts of Bobirwa, Boteti, Chobe (including Chobe National Park), Ghanzi, Mahalapye, Ngamiland (Ngami), North-East (including its capital, Francistown), Okavango, Serowe/Palapye, and Tutume: Atovaquone-proguanil, doxycycline, mefloquine, tafenoquine 3
- Areas with rare cases or sporadic foci of transmission: no chemoprophylaxis recommended (insect bite precautions and mosquito avoidance only) 4
See Health Information for Travelers to Botswana
CDC recommendations : Recommended for travelers ≥9 months old going to the states of Acre, Amapá, Amazonas, Distrito Federal (including the capital city, Brasília), Espírito Santo,* Goiás, Maranhão, Mato Grosso, Mato Grosso do Sul, Minas Gerais, Pará, Paraná,* Piauí, Rio de Janeiro (including the city of Rio de Janeiro and all coastal islands),* Rio Grande do Sul,* Rondônia, Roraima, Santa Catarina,* São Paulo (including the city of São Paulo and all coastal islands),* Tocantins, and designated areas of Bahia*. Vaccination is also recommended for travelers going to Iguaçu Falls. Not recommended for travel limited to any areas not listed above, including the cities of Fortaleza and Recife *In 2017, in response to a large YF outbreak in multiple eastern states, CDC expanded its vaccination recommendations for travelers going to Brazil. The expanded YF vaccination recommendations for these states are preliminary. For updates, refer to the CDC Travelers’ Health website.
- All areas in the states of Acre, Amapá, Amazonas, Rondônia, and Roraima
- Present in the states of Maranhão, Mato Grosso, and Pará, but rare cases in their capital cities (São Luis [capital of Maranhão], Cuiabá [capital of Mato Grosso], Belém [capital of Pará])
- Rural and forested areas in the states of Espírito Santo, Goiás, Minas Gerais, Mato Grosso do Sul, Piauí, Rio de Janeiro, São Paolo, and Tocantins
- No malaria transmission in the cities of Brasília (the capital), Rio de Janeiro, or São Paolo
- No malaria transmission at Iguaçu Falls
- P. vivax (90%)
- P. falciparum (10%)
- Areas with rare cases: No chemoprophylaxis recommended (insect bite precautions and mosquito avoidance only) 4
- Map 2-03 Yellow fever vaccine recommendations for Brazil & neighboring countries
- Map 2-04 Malaria prevention in Brazil
See Health Information for Travelers to Brazil
British Indian Ocean Territory; includes Diego Garcia (U.K.)
See Health Information for Travelers to British Indian Ocean Territory (U.K.)
- No human malaria
- Rare transmission of P. knowlesi 6 in primarily forested or forest-fringe areas
- P. knowlesi 6 (100%)
- None (insect bite precautions and mosquito avoidance only) 4
See Health Information for Travelers to Brunei
See Health Information for Travelers to Bulgaria
Burkina Faso
Entry requirements : Required for all arriving travelers ≥9 months old
CDC recommendations : Recommended for all travelers ≥9 months old.
- P. malariae , P. ovale , and P. vivax (less commonly)
See Health Information for Travelers to Burkina Faso
Burma (Myanmar)
- All areas <1,000 m (≈3,300 ft) elevation, including Bagan
- Rare transmission in areas >1,000 m (≈3,300 ft) elevation
- Chloroquine and mefloquine
- P. vivax (60%)
- P. falciparum (40%)
- P. knowlesi 6 , P. malariae , and P. ovale (rare)
- Areas <1,000 m (≈3,300 ft) elevation in the regions of Bago and Tanintharyi, and in the states of Kachin, Kayah, Kayin, and Shan: Atovaquone-proguanil, doxycycline, tafenoquine 3
- Areas <1,000 m (≈3,300 ft) elevation in all other areas: Atovaquone-proguanil, doxycycline, mefloquine, tafenoquine 3
- Areas >1,000 m (≈3,300 ft) elevation: No chemoprophylaxis recommended (insect bite precautions and mosquito avoidance only) 4
See Health Information for Travelers to Burma (Myanmar)
Entry requirements : Required for all arriving travelers ≥9 months old.
CDC recommendations : Recommended for all travelers ≥9 months old.
See Health Information for Travelers to Burundi
- Present throughout the country
- No (or negligible) malaria transmission in the cities of Phnom Penh (the capital) and Siem Reap
- No (or negligible) malaria transmission at the main temple complex at Angkor Wat
- P. vivax (80%)
- P. falciparum (20%)
- P. knowlesi 6 (rare)
- Atovaquone-proguanil, doxycycline, tafenoquine 3
See Health Information for Travelers to Cambodia
Entry requirements : Required for all arriving travelers ≥1 year old.
See Health Information for Travelers to Cameroon
See Health Information for Travelers to Canada
Canary Islands ( Spain )
See Health Information for Travelers to Canary Islands (Spain)
- No indigenous cases reported since 2018
- Previously, rare cases on Santiago (São Tiago) Island and Boa Vista Island
- Previously, chloroquine
- Previously, P. falciparum (primarily)
See Health Information for Travelers to Cape Verde
Cayman Islands (U.K.)
See Health Information for Travelers to Cayman Islands (U.K.)
Central African Republic
Entry requirements : Required for all arriving travelers ≥9 months old .
See Health Information for Travelers to Central African Republic
Entry requirements : Required for travelers ≥9 months old arriving from countries with risk for YF virus transmission. 1
CDC recommendations : Recommended for travelers ≥9 months old going to areas south of the Sahara Desert. Not recommended for travel limited to areas in the Sahara Desert.
See Health Information for Travelers to Chad
See Health Information for Travelers to Chile
Entry requirements : Required for travelers ≥9 months old arriving from countries with risk for YF virus transmission; this includes >12-hour airport transits or layovers in countries with risk for YF virus transmission. 1 Travelers with itineraries limited to Hong Kong Special Administrative Region (SAR) or Macao SAR are exempt from this requirement.
See Health Information for Travelers to China
Christmas Island (Australia)
See Health Information for Travelers to Christmas Island (Australia)
Cocos (Keeling) Islands (Australia)
See Health Information for Travelers to Cocos (Keeling) Islands (Australia)
Entry requirements : Required for travelers ≥1 year old arriving from Angola, Brazil, Democratic Republic of the Congo, or Uganda; this includes >12-hour airport transits or layovers in any of these countries.
CDC recommendations : Recommended for all travelers ≥9 months old except as follows. Generally not recommended for travel limited to the cities of Barranquilla, Cali, Cartagena, or Medellín. Not recommended for travel limited to areas >2,300 m (≈7,550 ft) elevation, the archipelago department of San Andrés and Providencia, or the city of Bogotá (the capital).
- All areas <1,700 m (≈5,600 ft) elevation
- No malaria transmission in the cities of Bogotá (the capital), Cartagena, or Medellín
- P. falciparum (50%)
- P. vivax (50%)
Map 2-05 Yellow fever vaccine recommendations for Colombia & neighboring countries
See Health Information for Travelers to Colombia
- P. malariae and P. vivax (rare)
See Health Information for Travelers to Comoros
Congo, Republic of the (Congo-Brazzaville)
Entry requirements : Required for all arriving travelers ≥9 months old.
See Health Information for Travelers to Congo, Republic of the
Cook Islands (New Zealand)
See Health Information for Travelers to Cook Islands (New Zealand)
Entry requirements : Required for travelers ≥9 months old arriving from countries with risk for YF virus transmission. 1 Included in this requirement are travelers arriving from Tanzania and Zambia, and designated areas of: Colombia (the entire country, except the cities of Barranquilla, Bogotá, Cali, Cartagena, and Medellín, and the archipelago department, San Andrés and Providencia); Ecuador (the provinces of Morona-Santiago, Napo, Orellana, Pastaza, Sucumbíos, and Zamora-Chinchipe, and excluding the rest of the country); Paraguay (the entire country, except the city of Asunción); Peru (the entire country, except the cities of Cusco and Lima, the regions of Cajamarca, Lambayeque, Piura, and Tumbes, and the highland tourist areas of Machu Picchu and the Inca Trail); Trinidad & Tobago (the entire country, except the urban areas of Port of Spain; travelers with itineraries limited to the island of Tobago, and travelers with airport transits or layovers are also exempt from this requirement). Travelers arriving from Argentina and Panama are exempt from this requirement.
- Present in the provinces of Alajuela and Limón
- Rare to no transmission in other parts of the country
- P. falciparum (86%)
- P. vivax (14%)
- Alajuela and Limón Provinces: Atovaquone-proguanil, chloroquine, doxycycline, mefloquine, tafenoquine 3
- All other areas: None (insect bite precautions and mosquito avoidance only) 4
See Health Information for Travelers to Costa Rica
Côte d'Ivoire (Ivory Coast)
See Health Information for Travelers to Côte d'Ivoire
See Health Information for Travelers to Croatia
See Health Information for Travelers to Cuba
Curaçao, Netherlands
See other recommended vaccines and medicines for travelers to Curaçao
See Health Information for Travelers to Cyprus
See Health Information for Travelers to Czech Republic
Democratic Republic of the Congo (Congo-Kinshasa)
CDC recommendations : Recommended for all travelers ≥9 months old
See Health Information for Travelers to Democratic Republic of the Congo
See Health Information for Travelers to Denmark
- P. falciparum (60–70%)
- P. vivax (30–40%)
- P. ovale (rare)
See Health Information for Travelers to Djibouti
See Health Information for Travelers to Dominica
Dominican Republic
Entry requirements : Required for travelers ≥1 year old arriving from the following states in Brazil: Espírito Santo, Mina Gerais, Rio de Janeiro, São Paulo; this includes >12-hour airport transits or layovers in any of these states
- Primarily in the provinces near the border with Haiti, and the provinces (including resort areas) of La Altagracia, San Cristóbal, San Juan, and Santo Domingo
- In the Distrito Nacional, city of Santo Domingo (the capital), primarily in the La Ciénaga and Los Tres Brazos areas
- Rare transmission in other provinces
- P. falciparum (100%)
- Provinces near the border with Haiti, and the provinces (including resort areas) of La Altagracia, San Cristóbal, San Juan, and Santo Domingo: Atovaquone-proguanil, chloroquine, doxycycline, mefloquine, tafenoquine 3
- All other areas: No chemoprophylaxis recommended (insect bite precautions and mosquito avoidance only) 4
See Health Information for Travelers to Dominican Republic
Easter Island (Chile)
Entry requirements : Easter Island has not stated its YF vaccination certificate requirements
See Health Information for Travelers to Easter Island (Chile) .
Ecuador, including the Galápagos Islands
Entry requirements : Required for travelers ≥1 year old arriving from Brazil, Democratic Republic of the Congo, or Uganda; this includes >12-hour airport transits or layovers in any of these countries .
CDC recommendations : Recommended for travelers ≥9 months old going to areas <2,300 m (≈7,550 ft) elevation, east of the Andes Mountains, in the provinces of Morona-Santiago, Napo, Orellana, Pastaza, Sucumbíos, Tungurahua,* and Zamora-Chinchipe. Generally not recommended for travel limited to areas <2,300 m (≈7,550 ft) elevation, west of the Andes Mountains, in the provinces of Esmeraldas,* Guayas, Los Ríos, Manabí, Santa Elena, Santo Domingo de los Tsáchilas, and designated areas in the provinces of Azuay, Bolívar, Cañar, Carchi, Chimborazo, Cotopaxi, El Oro, Imbabura, Loja, and Pichincha. Not recommended for travel limited to areas >2,300 m (≈7,550 ft) elevation, the cities of Guayaquil or Quito (the capital), or the Galápagos Islands *CDC recommendations differ from those published by WHO .
- Areas <1,500 m (≈5,000 ft) elevation in the provinces of Carchi, Cotopaxi, Esmeraldas, Morona-Santiago, Orellana, Pastaza, and Sucumbíos
- Rare cases <1,500 m (≈5,000 ft) in all other provinces
- No malaria transmission in the cities of Guayaquil or Quito (the capital)
- No malaria transmission on the Galápagos Islands
- P. vivax (85%)
- P. falciparum (15%)
- Transmission areas in the provinces of Carchi, Cotopaxi, Esmeraldas, Morona-Santiago, Orellana, Pastaza, and Sucumbíos: Atovaquone-proguanil, doxycycline, mefloquine, tafenoquine 3
- All other areas with reported malaria transmission: No chemoprophylaxis recommended (insect bite precautions and mosquito avoidance only) 4
Map 2-06 Yellow fever vaccine recommendations for Ecuador & neighboring countries
See Health Information for Travelers to Ecuador .
See Health Information for Travelers to Egypt .
El Salvador
See Health Information for Travelers to El Salvador .
Equatorial Guinea
- P. malariae, P. ovale , and P. vivax (less commonly)
See Health Information for Travelers to Equatorial Guinea .
CDC recommendations : Generally not recommended for travel to the regions of: Anseba, Debub (also known as South or Southern Region), Gash Barka, Ma’ekel (also known as Ma’akel or Central Region), or Semenawi K’eyih Bahri (also known as Northern Red Sea Region). Not recommended for travel to any areas not listed above, including the Dahlak Archipelago.
- All areas <2,200 m (≈7,200 ft) elevation
- No malaria transmission in Asmara (the capital)
- P. falciparum (80–85%)
- P. vivax (15–20%)
- P. malariae and P. ovale (rare)
Map 5-10 Yellow fever vaccine recommendations for Africa
See Health Information for Travelers to Eritrea .
See Health Information for Travelers to Estonia .
Eswatini (Swaziland)
Entry requirements : Required for travelers ≥9 months old arriving from countries with risk for YF virus transmission; this includes airport transits or layovers in countries with risk for YF virus transmission. 1
- Eastern areas bordering Mozambique and South Africa, including the entire region of Lubombo and the eastern half of Hhohho, Manzini, and Shiselweni Regions
- P. malariae , P. ovale , and P. vivax (less commonly)
See Health Information for Travelers to Swaziland .
CDC recommendations : Recommended for all travelers ≥9 months old except as follows. Generally not recommended for travel limited to the regions of Afar or Somali.
- All areas <2,500 m (≈8,200 ft) elevation, except none in Addis Ababa (the capital)
- P. falciparum (80%)
- P. vivax (20%)
- P. malariae and P. ovale (rare)
Map 2-07 Yellow fever vaccine recommendations for Ethiopia & neighboring countries
See Health Information for Travelers to Ethiopia .
Falkland Islands (Islas Malvinas), UK Overseas Territory (also claimed by Argentina)
See Health Information for Travelers to Falkland Islands (Islas Malvinas) .
Faroe Islands (Denmark)
See Health Information for Travelers to Faroe Islands (Denmark) .
See Health Information for Travelers to Fiji .
See Health Information for Travelers to Finland .
See Health Information for Travelers to France .
French Guiana
- Areas associated with gold mining, primarily the communes near the border with Brazil and Suriname, especially Régina and Saint-Georges-de-l’Oyapock; also, the communes of Kourou, Matoury, and Saint-Élie
- No malaria transmission in coastal areas west of Kourou
- No malaria transmission in Cayenne City (the capital)
- P. falciparum (15%)
See Health Information for Travelers to French Guiana (France) .
French Polynesia, including the Society Islands [Bora-Bora, Moorea & Tahiti]; Marquesas Islands [Hiva Oa & Ua Huka]; and Austral Islands (Tubuai & Rurutu), France
See Health Information for Travelers to French Polynesia (France) .
- P. malariae , P. ovale , and P. vivax (less commonly)
See Health Information for Travelers to Gabon .
Gambia, The
See Health Information for Travelers to The Gambia .
See Health Information for Travelers to Georgia .
See Health Information for Travelers to Germany .
- P. malariae, P. ovale, and P. vivax (less commonly)
See Health Information for Travelers to Ghana .
Gibraltar (U.K.)
See Health Information for Travelers to Gibraltar (U.K.) .
- Rare, local transmission in agricultural areas, associated with imported malaria (May–November)
- No malaria transmission in tourist areas
- Not applicable
- P. vivax (100%)
See Health Information for Travelers to Greece .
Greenland (Denmark)
See Health Information for Travelers to Greenland (Denmark) .
See Health Information for Travelers to Grenada .
Guadeloupe (including Marie-Galante, La Désirade & Îles des Saintes)
See Health Information for Travelers to Guadeloupe .
Guam (U.S.)
See Health Information for Travelers to Guam (U.S.) .
- Primarily in the departments of Alta Verapaz, Escuintla, Izabal, Petén, Quiche, and Suchitapéquez
- Few cases reported in other departments
- No malaria transmission in the cities of Antigua or Guatemala City (the capital)
- No malaria transmission at Lake Atitlán
- P. vivax (99%)
- P. falciparum (1%)
- Departments of Alta Verapaz, Escuintla, Izabal, Petén, Quiche, and Suchitapéquez: Atovaquone-proguanil, chloroquine, doxycycline, mefloquine, primaquine 5 , tafenoquine 3
- Other areas with reported malaria transmission: No chemoprophylaxis recommended (insect bite precautions and mosquito avoidance only) 4
See Health Information for Travelers to Guatemala .
Entry requirements : Required for travelers ≥1 year old arriving from countries with risk for YF virus transmission. 1 Required for all arriving travelers from all countries if traveler is ≥9 months of age and arriving at Ahmed Sékou Touré International Airport in Conakry.
See Health Information for Travelers to Guinea .
Guinea-Bissau
See Health Information for Travelers to Guinea-Bissau .
Entry requirements : Required for travelers ≥1 year old arriving from countries with risk for YF virus transmission; this includes >4-hour airport transits or layovers in countries with risk for YF virus transmission. 1
- Rare cases in the cities of Georgetown (the capital) and New Amsterdam
- All areas (except the cities of Georgetown and New Amsterdam): Atovaquone-proguanil, doxycycline, mefloquine, tafenoquine 3
- Cities of Georgetown and Amsterdam: No chemoprophylaxis recommended (insect bite precautions and mosquito avoidance only) 4
See Health Information for Travelers to Guyana .
- All (including Labadee, also known as Port Labadee)
- P. falciparum (99%)
- P. malariae (rare)
- Atovaquone-proguanil, chloroquine, doxycycline, mefloquine, tafenoquine 3
See Health Information for Travelers to Haiti .
Entry requirements : Required for travelers 1-60 years old arriving from countries with risk for YF virus transmission; this includes >12-hour airport transits or layovers in countries with risk for YF virus transmission. 1
- Throughout the country and on the island of Roat á n and other Bay Islands
- No malaria transmission in the cities of San Pedro Sula or Tegucigalpa (the capital)
- P. vivax (93%)
- P. falciparum (7%)
- Atovaquone-proguanil, chloroquine, doxycycline, mefloquine, tafenoquine 3
See Health Information for Travelers to Honduras .
Hong Kong Special Administrative Region, China
See Health Information for Travelers to Hong Kong SAR (China) .
See Health Information for Travelers to Hungary .
See Health Information for Travelers to Iceland .
- Arrive within 6 days of leaving an area with risk for YF virus transmission, or
- Have been in such an area in transit (exception: passengers and members of flight crews who, while in transit through an airport in an area with risk for YF virus transmission, remained in the airport during their entire stay and the health officer agrees to such an exemption), or
- Arrive on a ship that started from or touched at any port in an area with risk for YF virus transmission ≤30 days before its arrival in India, unless such a ship has been disinsected in accordance with the procedure recommended by the World Health Organization (WHO), or
- Arrive on an aircraft that has been in an area with risk for YF virus transmission and has not been disinsected in accordance with the Indian Aircraft Public Health Rules, 1954, or as recommended by WHO.
- Africa: Angola, Benin, Burkina Faso, Burundi, Cameroon, Central African Republic, Chad, Congo, Côte d’Ivoire, Democratic Republic of the Congo, Equatorial Guinea, Ethiopia, Gabon, The Gambia, Ghana, Guinea, Guinea-Bissau, Kenya, Liberia, Mali, Mauritania, Niger, Nigeria, Rwanda, Senegal, Sierra Leone, South Sudan, Sudan, Togo, Uganda
- Americas: Argentina, Bolivia, Brazil, Colombia, Ecuador, French Guiana, Guyana, Panama, Paraguay, Peru, Suriname, Trinidad & Tobago (Trinidad only), Venezuela
- Throughout the country, including the cities of Bombay (Mumbai) and New Delhi (the capital)
- No malaria transmission in areas >2,000 m (≈6,500 ft) elevation in Himachal Pradesh, Jammu and Kashmir, or Sikkim
- P. vivax (50%)
- P. falciparum (>40%)
See Health Information for Travelers to India .
- All areas of eastern Indonesia (the provinces of Maluku, North Maluku, East Nusa Tenggara, Papua, and West Papua), including the town of Labuan Bajo and the Komodo Islands in the Nusa Tenggara region
- Rural areas of Kalimantan (Borneo), West Nusa Tenggara (includes the island of Lombok), Sulawesi, and Sumatra
- Low transmission in rural areas of Java, including Pangandaran, Sukabumi, and Ujung Kulon
- No malaria transmission in the cities of Jakarta (the capital) or Ubud
- No malaria transmission in the resort areas of Bali or Java, the Gili Islands, or the Thousand Islands (Pulau Seribu)
- Chloroquine ( P. falciparum and P. vivax )
- P. falciparum (60%)
- P. vivax (40%)
See Health Information for Travelers to Indonesia .
- No indigenous cases reported since 2017
- Previously, in rural areas of the provinces of Fars and Sistan va Baluchestan, and southern, tropical regions of the provinces of Hormozgan and Kerman (March– November)
- Previously, P. vivax (93%)
- Previously, P. falciparum (7%)
See Health Information for Travelers to Iran .
See Health Information for Travelers to Iraq .
See Health Information for Travelers to Ireland .
See Health Information for Travelers to Israel, including the West Bank and Gaza .
Italy (including Holy See [Vatican City])
See Health Information for Travelers to Italy .
See Health Information for Travelers to Jamaica .
See Health Information for Travelers to Japan .
See Health Information for Travelers to Jordan .
Entry requirements : Required for travelers arriving from countries with risk for YF virus transmission; this includes airport transits or layovers in countries with risk for YF virus transmission. 1
See Health Information for Travelers to Kazakhstan .
CDC recommendations : Recommended for all travelers ≥9 months old except as follows. Generally not recommended for travel limited to: the city of Nairobi (the capital); the counties of the former North Eastern Province (Mandera, Wajir, and Garissa); or the counties (except Taita-Taveta) of the former Coast Province (Kilifi, including the city of Malindi; Kwale; Lamu; Mombasa, including the city of Mombasa; Tana River) .
- All areas (including game parks) <2,500 m (≈8,200 ft) elevation, including the city of Nairobi (the capital)
- Map 2-08 Yellow fever vaccine recommendations for Kenya & neighboring countries
- Map 2-09 Malaria prevention in Kenya
See Health Information for Travelers to Kenya .
Kiribati (formerly Gilbert Islands), includes Tarawa, Tabuaeran (Fanning Island), and Banaba (Ocean Island)
See Health Information for Travelers to Kiribati .
See Health Information for Travelers to Kosovo .
See Health Information for Travelers to Kuwait .
See Health Information for Travelers to Kyrgyzstan .
- All, except in Vientiane (the capital) where there is no transmission
- P. vivax (55%)
- P. falciparum (45%)
- P. knowlesi 6 , P. malariae, and P. ovale (rare)
- Areas bordering Burma (the provinces of Bokeo and Luang Namtha), Cambodia; Thailand (the provinces of Champasak and Salavan); and Vietnam: Atovaquone-proguanil, doxycycline, tafenoquine 3
- All other areas with malaria transmission: Atovaquone-proguanil, doxycycline, mefloquine, tafenoquine 3
See Health Information for Travelers to Laos .
See Health Information for Travelers to Latvia .
See Health Information for Travelers to Lebanon .
See Health Information for Travelers to Lesotho .
See Health Information for Travelers to Liberia .
See Health Information for Travelers to Libya .
Liechtenstein
See Health Information for Travelers to Liechtenstein .
See Health Information for Travelers to Lithuania .
See Health Information for Travelers to Luxembourg .
Macau Special Administrative Region, China
See Health Information for Travelers to Macau SAR (China) .
- All; except in Antananarivo (the capital) where malaria transmission is rare
- P. ovale and P. vivax (less commonly)
- All areas (except the city of Antananarivo): Atovaquone-proguanil, doxycycline, mefloquine, tafenoquine 3
- Antananarivo: No chemoprophylaxis recommended (insect bite precautions and mosquito avoidance only) 4
See Health Information for Travelers to Madagascar .
Madeira Islands (Portugal)
See Health Information for Travelers to Madeira Islands (Portugal) .
See Health Information for Travelers to Malawi .
- No indigenous cases of human malaria since 2017
- Zoonotic transmission of simian malaria occurs in rural, forested areas
- No malaria transmission in other areas, including Kuala Lumpur (the capital), in Penang State, on Penang Island, or in George Town (capital of Penang State)
- P. knowlesi 6 (primarily)
- Previously, P. falciparum , P. malariae , P. ovale , and P. vivax
- In rural, forested areas: atovaquone-proguanil, doxycycline, mefloquine, tafenoquine 3
See Health Information for Travelers to Malaysia .
See Health Information for Travelers to Maldives .
See Health Information for Travelers to Mali .
See Health Information for Travelers to Malta .
Marshall Islands
See Health Information for Travelers to Marshall Islands .
See Health Information for Travelers to Martinique (France) .
- All; except in the regions of Dakhlet Nouadhibou and Tiris Zemmour where there is no transmission
See Health Information for Travelers to Mauritania .
See Health Information for Travelers to Mauritius .
Mayotte (France)
See Health Information for Travelers to Mayotte (France) .
- Chiapas and southern part of Chihuahua state
- Rare in the states of Campeche, Durango, Nayarit, Quintana Roo, Sinaloa, Sonora, and Tabasco
- No malaria transmission along the U.S.–Mexico border
- Chiapas and southern part of Chihuahua state: Atovaquone-proguanil, chloroquine, doxycycline, mefloquine, primaquine 5 , tafenoquine 3
- All other areas with malaria transmission: No chemoprophylaxis recommended (insect bite precautions and mosquito avoidance only) 4
Map 2-10 Malaria prevention in Mexico
See Health Information for Travelers to Mexico .
Micronesia, Federated States of (including Chuuk, Kosrae, Pohnpei & Yap)
See Health Information for Travelers to Micronesia, Federated States of .
See Health Information for Travelers to Moldova .
See Health Information for Travelers to Monaco .
See Health Information for Travelers to Mongolia .
See Health Information for Travelers to Montenegro .
Montserrat, United Kingdom
See Health Information for Travelers to Montserrat (U.K.) .
See Health Information for Travelers to Morocco .
See Health Information for Travelers to Mozambique .
- In the regions of Kavango (East and West), Kunene, Ohangwena, Omaheke, Omusati, Oshana, Oshikoto, Otjozondjupa, and Zambezi
- Rare in other parts of the country
- No malaria transmission in Windhoek (the capital)
- Kavango (East and West), Kunene, Ohangwena, Omaheke, Omusati, Oshana, Oshikoto, Otjozondjupa, and Zambezi: Atovaquone-proguanil, doxycycline, mefloquine, tafenoquine 3
See Health Information for Travelers to Namibia .
See Health Information for Travelers to Nauru .
- Throughout the country in areas <2,000 m (≈6,500 ft) elevation
- No malaria transmission in Kathmandu (the capital) or on typical Himalayan treks
- P. falciparum (<10%)
See Health Information for Travelers to Nepal .
Netherlands
See Health Information for Travelers to The Netherlands .
Netherlands Antilles (Bonaire, Curaçao, Saba, St. Eustasius, and St. Maarten)
Entry requirements : See Bonaire, Curaçao, Saba, St. Eustasius, and St. Maarten for yellow fever information.
- See Bonaire, Curaçao, Saba, St. Eustasius, and St. Maarten for malaria information.
New Caledonia (France)
Entry requirements : Required for travelers ≥1 year old arriving from countries with risk for YF virus transmission; this includes >12-hour airport transits or layovers in countries with risk for YF virus transmission. 1 In the event of an epidemic threat to the territory, a specific vaccination certificate may be required.
See Health Information for Travelers to New Caledonia (France) .
New Zealand
See Health Information for Travelers to New Zealand .
- Región Autónoma Atlántico Norte (RAAN) and Región Autónoma Atlántico Sur (RAAS)
- Rare cases in the departments of Boaco, Chinandega, Estelí, Jinotega, León, Matagalpa, and Nueva Segovia
- No malaria transmission in Managua (the capital)
- P. falciparum (20%)
- Región Autónoma Atlántico Norte (RAAN) and Región Autónoma Atlántico Sur (RAAS): Atovaquone-proguanil, chloroquine, doxycycline, mefloquine, tafenoquine 3
See Health Information for Travelers to Nicaragua .
See Health Information for Travelers to Niger .
CDC recommendations : Recommended for all travelers ≥9 months old.
See Health Information for Travelers to Nigeria .
Niue (New Zealand)
See Health Information for Travelers to Niue (New Zealand) .
Norfolk Island (Australia)
See Health Information for Travelers to Norfolk Island (Australia) .
North Korea
- Southern provinces
- P. vivax (100%)
- Atovaquone-proguanil, chloroquine, doxycycline, mefloquine, primaquine 5 , tafenoquine 3
See Health Information for Travelers to North Korea .
North Macedonia
See Health Information for Travelers to North Macedonia .
Northern Mariana Islands (U.S.), includes Saipan, Tinian, and Rota Island
See Health Information for Travelers to Northern Mariana Islands (U.S.) .
See Health Information for Travelers to Norway .
Entry requirements : Required for travelers ≥9 months old arriving from countries with risk for YF virus transmission, with the addition of Rwanda and Tanzania; this includes >12-hour airport transits or layovers in countries with risk for YF virus transmission. 1
- Rare sporadic transmission after importation only
- Previously, P. falciparum and P. vivax
See Health Information for Travelers to Oman .
- All areas (including all cities) <2,500 m (≈8,200 ft) elevation
See Health Information for Travelers to Pakistan .
See Health Information for Travelers to Palau .
CDC recommendations : Recommended for travelers ≥9 months old going to all mainland areas east of the Canal Zone including Darién Province, the indigenous provinces (comarcas indígena) of Emberá and Kuna Yala (also spelled Guna Yala), and areas of the provinces of Colón and Panamá, east of the Canal Zone. Not recommended for travel limited to the Canal Zone; areas west of the Canal Zone; Panama City (the capital); Balboa district (Pearl Islands) of Panamá Province; or the San Blas Islands of Kuna Yala Province.
- The provinces of Bocas del Toro, Chiriquí, Colón, Darién, Panamá, and Veraguas
- The indigenous provinces (comarcas indígena) of Emberá, Kuna Yala (also spelled Guna Yala) and Ngäbe-Buglé
- No malaria transmission in the province of Panamá Oeste, in the Canal Zone, or in Panama City (the capital)
- Chloroquine (east of the Panama Canal)
- P. vivax (97%)
- P. falciparum (3%)
- Darién, Emberá, Kuna Yala, and eastern Panamá Provinces : Atovaquone-proguanil, doxycycline, mefloquine, primaquine 5 , tafenoquine 3
- Bocas del Toro, Chiriquí, Colón, Veraguas, and Ngäbe-Buglé Provinces : Atovaquone-proguanil, chloroquine, doxycycline, mefloquine, primaquine 5 , tafenoquine 3
- Map 2-11 Yellow fever vaccine recommendations for Panama & neighboring countries
- Map 2-12 Malaria prevention in Panama
See Health Information for Travelers to Panama .
Papua New Guinea
- Chloroquine (both P. falciparum and P. vivax )
- P. falciparum (75%)
- P. vivax (25%)
See Health Information for Travelers to Papua New Guinea .
Entry requirements : Required for travelers ≥1 year old arriving from Bolivia, Brazil, Peru, or Venezuela; this includes this includes >24-hour transits or layovers in those countries 1
CDC recommendations : Recommended for all travelers ≥9 months old except as follows. Generally not recommended for travel limited to the city of Asunción (the capital).
See Health Information for Travelers to Paraguay .
CDC recommendations : Recommended for travelers ≥9 months old going to areas <2,300 m (≈7,550 ft) elevation in the regions of Amazonas, Cusco, Huánuco, Junín, Loreto, Madre de Dios, Pasco, Puno, San Martín, and Ucayali, and designated areas of Ancash (far northeast), Apurímac (far north), Ayacucho (north and northeast), Cajamarca (north and east), Huancavelica (far north), La Libertad (east), and Piura (east). Generally not recommended for travel limited to the following areas west of the Andes: the regions of Lambayeque and Tumbes, and designated areas of Cajamarca (west-central), and Piura (west). Not recommended for travel limited to areas >2,300 m (≈7,550 ft) elevation, areas west of the Andes not listed above, the city of Lima (the capital), and the highland tourist areas (the city of Cusco, the Inca Trail, and Machu Picchu).
- All areas of the country <2,500 m (≈8,200 ft) elevation, including the cities of Iquitos and Puerto Maldonado, and only the remote eastern areas in the regions of La Libertad and Lambayeque
- No malaria transmission in the following areas: Lima Province; the cities of Arequipa, Ica, Moquegua, Nazca, Puno, or Tacna; the highland tourist areas (the city of Cusco, Machu Picchu, Lake Titicaca); along the Pacific Coast
- Map 2-13 Yellow fever vaccine recommendations for Peru & neighboring countries
- Map 2-14 Malaria prevention in Peru
See Health Information for Travelers to Peru .
Philippines
- Palawan and Mindanao Islands
- No malaria transmission in metropolitan Manila (the capital) or other urban areas
- P. falciparum (85%)
- P. vivax (15%)
See Health Information for Travelers to Philippines .
Pitcairn Islands (U.K.)
See Health Information for Travelers to Pitcairn Islands (U.K.) .
See Health Information for Travelers to Poland .
See Health Information for Travelers to Portugal .
Puerto Rico (U.S.)
See Health Information for Travelers to Puerto Rico (U.S.) .
See Health Information for Travelers to Qatar .
Réunion (France)
See Health Information for Travelers to Réunion (France) .
See Health Information for Travelers to Romania .
See Health Information for Travelers to Russia .
CDC recommendations : Generally not recommended for travel to Rwanda.
See Health Information for Travelers to Rwanda .
Saba, Netherlands
See Health Information for Travelers to Saba .
Saint Barthelemy, France
Saint helena, united kingdom.
Entry requirements : Required for travelers ≥1 year old arriving from countries with risk for YF virus transmission. 1 *For YF vaccine entry requirements and recommendations and malaria prevention information for Ascension Island and Tristan da Cunha archipelago, see: UNITED KINGDOM (including CHANNEL ISLANDS, ISLE OF MAN, ASCENSION ISLAND & TRISTAN DA CUNHA ARCHIPELAGO)
See Health Information for Travelers to Saint Helena (U.K.) .
Saint Kitts (Saint Christopher) & Nevis
See Health Information for Travelers to Saint Kitts and Nevis .
Saint Lucia
See Health Information for Travelers to Saint Lucia .
Saint Martin, France
Saint pierre and miquelon (france).
See Health Information for Travelers to Saint Pierre and Miquelon (France) .
Saint Vincent and the Grenadines
See Health Information for Travelers to Saint Vincent and the Grenadines .
Samoa (formerly Western Somoa)
See Health Information for Travelers to Samoa (formerly Western Samoa) .
See Health Information for Travelers to San Marino .
São Tomé and Príncipe
CDC recommendations : Generally not recommended for travel to São Tomé and Príncipe.
See Health Information for Travelers to São Tomé and Príncipe.
Saudi Arabia
- Asir and Jazan (also spelled Jizan) Regions near the Yemen border only
- No malaria transmission in the cities of Jeddah, Mecca, Medina, Riyadh (the capital), or Ta’if
- P. vivax (rare)
See Health Information for Travelers to Saudi Arabia .
See Health Information for Travelers to Senegal .
See Health Information for Travelers to Serbia .
See Health Information for Travelers to Seychelles .
Sierra Leone
Entry requirements : Required for all arriving travelers.
See Health Information for Travelers to Sierra Leone .
See Health Information for Travelers to Singapore .
Sint Eustatius, Netherlands
Entry requirements : Required for travelers ≥6 months old arriving from countries with risk for YF virus transmission. 1
See Health Information for Travelers to Sint Eustatius .
Sint Maarten, Netherlands
See Health Information for Travelers to Sint Maarten .
See Health Information for Travelers to Slovakia .
See Health Information for Travelers to Slovenia .
Solomon Islands
- P. vivax (70%)
- P. falciparum (30%)
- P. ovale (<1%)
See Health Information for Travelers to Solomon Islands .
CDC recommendations : Generally not recommended for travel to the regions of Bakool, Banaadir, Bay, Galguduud, Gedo, Hiiraan (also spelled Hiran), Lower Juba (also known as Jubbada Hoose), Middle Juba (also known as Jubbada Dhexe), Lower Shabelle (also known as Shabeellaha Hoose), or Middle Shabelle (also known as Shabeellaha Dhexe). Not recommended for travel to areas not listed above.
- P. vivax (5–10%)
See Health Information for Travelers to Somalia .
South Africa
- Along the border with Mozambique and Zimbabwe
- KwaZulu-Natal Province: uMkhanyakude District; the districts of King Cetshwayo and Zululand (few cases) Limpopo Province: the districts of Mopani and Vhembe; the districts of Capricorn, Greater Sekhukhune, and Waterberg (few cases)
- Mpumalanga Province: Ehlanzeni District
- Kruger National Park
- KwaZulu-Natal Province (uMkhanyakude District); Limpopo Province (the districts of Mopani and Vhembe); Mpumalanga Province (Ehlanzeni District); and Kruger National Park: Atovaquone-proguanil, doxycycline, mefloquine, tafenoquine 3
- All other areas with malaria transmission (including the districts of King Cetshwayo and Zululand in KwaZulu-Natal Province, and the districts of Capricorn, Greater Sekhukhune, and Waterberg in Limpopo Province): No chemoprophylaxis recommended (insect bite precautions and mosquito avoidance only) 4
Map 2-15 Malaria prevention in South Africa
See Health Information for Travelers to South Africa .
South Georgia & the South Sandwich Islands, UK Overseas Territory (also claimed by Argentina)
Entry requirements : South Georgia & the South Sandwich Islands has not stated its YF vaccination certificate requirements.
See Health Information for Travelers to South Georgia and the South Sandwich Islands (U.K.) .
South Korea
Entry requirements : Required if traveling from a country with risk of YF virus transmission and ≥1 year of age. 1
- Limited to the months of March– December in rural areas in the northern parts of the provinces of Inch’ŏn (also spelled Incheon), Kangwŏn (also spelled Gangwon), and Kyŏnggi (also spelled Gyeonggi), including the demilitarized zone (DMZ)
- Atovaquone-proguanil, chloroquine, doxycycline, mefloquine, primaquine 5 , or tafenoquine 3
See Health Information for Travelers to South Korea .

South Sudan
See Health Information for Travelers to South Sudan .
See Health Information for Travelers to Spain .
See Health Information for Travelers to Sri Lanka .
CDC recommendations : Recommended for travelers ≥9 months old going to areas south of the Sahara Desert. Not recommended for travel limited to areas in the Sahara Desert or the city of Khartoum (the capital).
See Health Information for Travelers to Sudan .
- Primarily in Sipaliwini District, near the border with French Guiana
- Limited transmission in Brokopondo, Marowijne, and Para (near the border with French Guiana)
- No malaria transmission in the districts along the Atlantic Coast or in Paramaribo (the capital)
- Sipaliwini District near the border with French Guiana: Atovaquone-proguanil, doxycycline, mefloquine, tafenoquine 3
- All other areas with malaria transmission: No chemoprophylaxis recommended (insect bite precautions / mosquito avoidance only) 4
See Health Information for Travelers to Suriname .
See Health Information for Travelers to Sweden .
Switzerland
See Health Information for Travelers to Switzerland .
See Health Information for Travelers to Syria .
See Health Information for Travelers to Taiwan .
- No indigenous cases reported since 2014
- Previously, P. vivax (90%)
- Previously, P. falciparum (10%)
See Health Information for Travelers to Tajikistan .
CDC recommendations : Generally not recommended for travel to Tanzania.
- All areas below 1,800 m (≈5,900 ft) elevation
- P. malariae and P. ovale (less commonly)
See Health Information for Travelers to Tanzania .
- Primarily the provinces that border Burma, Cambodia (few cases in Buri Ram Province), and Malaysia (few cases in Satun Province) Also, the provinces of Phitsanulok and Ubon Ratchathani (bordering Laos), and Surat Thani (especially in the rural forest and forest-fringe areas of these provinces)
- Rare to few cases in other parts of Thailand, including the cities of Bangkok (the capital), Chiang Mai, and Chiang Rai, or on the islands of Koh Pha Ngan, Koh Samui, or Phuket
- No malaria transmission on the islands of Krabi Province (Ko Lanta, Koh Phi, Koh Yao Noi, Koh Yao Yai) or in Pattaya City
- P. falciparum (<20%)
- Provinces that border Burma, Cambodia (except Buri Ram Province), and Malaysia (except Satun Province); the provinces of Phitsanulok, Ubon Ratchathani, and Surat Thani: Atovaquone-proguanil, doxycycline, tafenoquine 3
- All other areas with malaria transmission (including the provinces of Buri Ram and Satun): No chemoprophylaxis recommended (insect bite precautions and mosquito avoidance only) 4
Map 2-16 Malaria prevention in Thailand
See Health Information for Travelers to Thailand .
Timor-Leste
- Rare cases; outbreak in Indonesia border area in mid-2020
- Previously, P. falciparum (50%)
- Previously, P. vivax (50%)
- Previously, P. malariae and P. ovale (each <1%)
See Health Information for Travelers to Timor-Leste (East Timor) .
See Health Information for Travelers to Togo .
Tokelau (New Zealand)
See Health Information for Travelers to Tokelau (New Zealand) .
See Health Information for Travelers to Tonga .
Trinidad and Tobago
CDC recommendations : Recommended for travelers ≥9 months old going to densely forested areas on Trinidad. Not recommended for cruise ship passengers, airplane passengers in transit, or travel limited to Tobago.
See Health Information for Travelers to Trinidad and Tobago .
See Health Information for Travelers to Tunisia .
See Health Information for Travelers to Turkey .
Turkmenistan
See Health Information for Travelers to Turkmenistan .
Turks and Caicos Islands (U.K.)
See Health Information for Travelers to Turks and Caicos Islands (U.K.) .
See Health Information for Travelers to Tuvalu .
See Health Information for Travelers to Uganda .
See Health Information for Travelers to Ukraine .
United Arab Emirates
See Health Information for Travelers to United Arab Emirates .
United Kingdom (including Channel Islands, Isle of Man, Ascension Island & Tristan Da Cunha Archipelago)
See Health Information for Travelers to United Kingdom .
United States of America
See Health Information for Travelers to United States .
See Health Information for Travelers to Uruguay .
See Health Information for Travelers to Uzbekistan .
- P. vivax (75%–90%)
- P. falciparum (10-25%)
See Health Information for Travelers to Vanuatu .
Entry requirements : Required for travelers ≥1 year old arriving from Brazil; this includes >12-hour airport transits or layovers in Brazil
CDC recommendations : Recommended for all travelers ≥9 months old except as follows. Generally not recommended for travel limited to the Distrito Capital or the states of Aragua, Carabobo, Miranda, Vargas, or Yaracuy. Not recommended for travel limited to areas >2,300m (≈7,550 ft) elevation in the states of Mérida, Táchira, or Trujillo; the states of Falcón or Lara; Margarita Island; or the cities of Caracas (the capital) or Valencia .
- All areas <1,700 m (≈5,600 ft) elevation and Angel Falls
- P. vivax (75%)
- P. falciparum (25%)
Map 2-17 Yellow fever vaccine recommendations for Venezuela & neighboring countries
See Health Information for Travelers to Venezuela .
- Rural areas only. Rare cases in the Mekong and Red River Deltas
- None in the cities of Da Nang, Hai Phong, Hanoi, Ho Chi Minh City (Saigon), Nha Trang, and Quy Nhon.
- P. falciparum (65%)
- P. vivax (35%)
- Provinces of Bình Dương, Bình Phước, Đắk Lắk, Đắk Nông, Gia Lai, Khánh Hòa, Kon Tum, Lâm Đồng, Ninh Thuận, Tây Ninh: Atovaquone-proguanil, doxycycline, tafenoquine 3
- All other areas with malaria transmission (except Mekong and Red River Deltas): Atovaquone-proguanil, doxycycline, mefloquine, tafenoquine 3
- Mekong and Red River Deltas: No chemoprophylaxis recommended (insect bite precautions / mosquito avoidance only) 4
See Health Information for Travelers to Vietnam .
Virgin Islands, British
See Health Information for Travelers to Virgin Islands, British .
Virgin Islands, U.S.
See Health Information for Travelers to Virgin Islands, U.S. .
Wake Island, U.S.
See Health Information for Travelers to Wake Island .
- All areas <2,000 m (≈6,500 ft) elevation
- No malaria transmission in Sana’a (the capital)
See Health Information for Travelers to Yemen .
Entry requirements : Required for travelers ≥1 year of age arriving from countries with risk for YF virus transmission; this includes >12-hour airport transits or layovers in countries with risk for YF virus transmission. 1
CDC recommendations : Generally not recommended for travel to North-Western Province or Western Province. Not recommended for travel to any areas not listed above.
See Health Information for Travelers to Zambia .
See Health Information for Travelers to Zimbabwe .
1 Current as of November 2022. This is an update of the 2010 map created by the Informal WHO Working Group on the Geographic Risk of Yellow Fever.
2 Refers to Plasmodium falciparum malaria, unless otherwise noted.
3 Tafenoquine can cause potentially life-threatening hemolysis in people with glucose-6-phosphate-dehydrogenase (G6PD) deficiency. Rule out G6PD deficiency with a quantitative laboratory test before prescribing tafenoquine to patients.
4 Mosquito avoidance includes applying topical mosquito repellant, sleeping under an insecticide-treated mosquito net, and wearing protective clothing (e.g., long pants and socks, long-sleeve shirt). For additional details on insect bite precautions, see Sec. 4, Ch. 6, Mosquitoes, Ticks & Other Arthropods.
5 Primaquine can cause potentially life-threatening hemolysis in people with G6PD deficiency. Rule out G6PD deficiency with a quantitative laboratory test before prescribing primaquine to patients.
6 P. knowlesi is a malaria species with a simian (macaque) host. Human cases have been reported from most countries in Southwest Asia and are associated with activities in forest or forest-fringe areas. P. knowlesi has no known resistance to antimalarials.
Yellow Fever Maps
2 In 2017, the Centers for Disease Control and Prevention (CDC) expanded its YF vaccination recommendations for travelers going to Brazil because of a large YF outbreak in multiple states in that country. Please refer to the CDC Travelers’ Health website for more information and updated recommendations.
3 YF vaccination is generally not recommended for travel to areas where the potential for YF virus exposure is low. Vaccination might be considered, however, for a small subset of travelers going to these areas who are at increased risk for exposure to YF virus due to prolonged travel, heavy exposure to mosquitoes, or inability to avoid mosquito bites. Factors to consider when deciding whether to vaccinate a traveler include destination-specific and travel-associated risks for YF virus infection; individual, underlying risk factors for having a serious YF vaccine–associated adverse event; and destination entry requirements.
The following authors contributed to the previous version of this chapter: Mark D. Gershman, Emily S. Jentes, Rhett J. Stoney (Yellow Fever) Kathrine R. Tan, Paul M. Arguin (Malaria)
File Formats Help:
- Adobe PDF file
- Microsoft PowerPoint file
- Microsoft Word file
- Microsoft Excel file
- Audio/Video file
- Apple Quicktime file
- RealPlayer file
- Zip Archive file
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.

Understanding malaria: Causes, symptoms and prevention
It is transmitted to humans through the bite of infected female Anopheles mosquitoes.
Once transmitted to humans, the parasites travel to the liver, where they undergo maturation and multiplication.
The Kenya Medical Supplies Agency (KEMSA) in March embarked on an exercise to distribute mosquito nets in malaria-endemic areas to prevent the spread of the disease.
KEMSA chief executive officer Andrew Mulwa said it is a national government program where they distribute malaria mosquito nets every three years.
There are some 22 counties that have high malaria incidents in the country which are targeted for the mass net distribution every three years.
"This program was last done in 2020/2021. We have several clusters and now we are doing Mombasa then move to Taita Taveta and Kwale as cluster two," he said.
The CEO who was speaking in Mombasa during the flagging off of 932,000 malaria mosquito nets said that the program is being initiated in clusters.
Malaria is a potentially life-threatening infectious disease caused by parasites of the Plasmodium genus.
They then enter the bloodstream, infecting red blood cells and causing the characteristic symptoms of malaria.
These symptoms typically include fever, chills, sweats, headache, muscle aches, and fatigue.
In severe cases, malaria can lead to complications such as severe anemia which can be fatal if not promptly treated.
According to WHO, malaria occurs primarily in tropical and subtropical countries.
The vast majority of malaria cases and deaths are found in the WHO African Region, with nearly all cases caused by the Plasmodium falciparum parasite.
This parasite is also dominant in other malaria hotspots, including the WHO regions of Southeast Asia, Eastern Mediterranean and Western Pacific.
In the WHO region of the Americas, the Plasmodium vivax parasite is predominant.
The threat of malaria is highest in sub-Saharan Africa, and 4 countries in that region accounted for nearly half of all malaria deaths worldwide in 2022: Nigeria (31.1%), the Democratic Republic of the Congo (11.6%), Niger (5.6%) and the United Republic of Tanzania (4.4%).
Preventing malaria primarily involves avoiding mosquito bites and taking preventive medications if you're traveling to areas where malaria is prevalent.
Here are some effective measures to prevent malaria:
Wear protective clothing
Cover your skin as much as possible, especially during peak mosquito activity times (dusk and dawn).
Wear long sleeves, pants, socks, and closed-toe shoes.
Sleep under mosquito nets
Sleep under mosquito nets and make sure the nets are properly tucked under the mattress to prevent mosquitoes from entering.
Stay in air-conditioned or screened accommodations
Choose lodging with air conditioning or screened windows and doors to keep mosquitoes out.
Take antimalarial medications
If you're traveling to an area where malaria is prevalent, consult with a healthcare professional well in advance of your trip to determine if you need to take antimalarial drugs.
These medications can prevent the parasite from establishing itself in your body if you're bitten by an infected mosquito.
Follow medication instructions
If prescribed antimalarial medication, take it exactly as directed. Start before your trip, continue during your stay in the affected area, and continue for a specified time after leaving the area.
Stay informed
Research the malaria risk in the specific area you're traveling to and follow any additional preventive measures recommended by local health authorities.
Reduce mosquito breeding sites
In areas where you live or stay for an extended period, help reduce mosquito populations by eliminating standing water where mosquitoes breed.
Empty or cover containers that can collect water, such as buckets, flower pots, or tires.
By following these preventive measures, you can significantly reduce your risk of contracting malaria.
If you develop symptoms such as fever, chills, headache, muscle aches, nausea, or vomiting after visiting a malaria-endemic area, seek medical attention promptly, as early diagnosis and treatment are crucial for managing malaria.
- Open access
- Published: 18 March 2020
Re-introduction of vivax malaria in a temperate area (Moscow region, Russia): a geographic investigation
- Varvara A. Mironova 1 ,
- Natalia V. Shartova 1 ,
- Andrei E. Beljaev 2 ,
- Mikhail I. Varentsov 1 , 3 , 4 ,
- Fedor I. Korennoy 5 &
- Mikhail Y. Grishchenko 1 , 6
Malaria Journal volume 19 , Article number: 116 ( 2020 ) Cite this article
4269 Accesses
15 Citations
12 Altmetric
Metrics details
Between 1999 and 2008 Russia experienced a flare-up of transmission of vivax malaria following its massive importation with more than 500 autochthonous cases in European Russia, the Moscow region being the most affected. The outbreak waned soon after a decrease in importation in mid-2000s and strengthening the control measures. Compared with other post-eradication epidemics in Europe this one was unprecedented by its extension and duration.
The aim of this study is to identify geographical determinants of transmission. The degree of favourability of climate for vivax malaria was assessed by measuring the sum of effective temperatures and duration of season of effective infectivity using data from 22 weather stations. For geospatial analysis, the locations of each of 405 autochthonous cases detected in Moscow region have been ascertained. A MaxEnt method was used for modelling the territorial differentiation of Moscow region according to the suitability of infection re-emergence based on the statistically valid relationships between the distribution of autochthonous cases and environmental and climatic factors.
In 1999–2004, in the beginning of the outbreak, meteorological conditions were extremely favourable for malaria in 1999, 2001 and 2002, especially within the borders of the city of Moscow and its immediate surroundings. The greatest number of cases occurred at the northwestern periphery of the city and in the adjoining rural areas. A significant role was played by rural construction activities attracting migrant labour, vegetation density and landscape division. A cut-off altitude of 200 m was observed, though the factor of altitude did not play a significant role at lower altitudes. Most likely, the urban heat island additionally amplified malaria re-introduction.
The malariogenic potential in relation to vivax malaria was high in Moscow region, albeit heterogeneous. It is in Moscow that the most favourable conditions exist for vivax malaria re-introduction in the case of a renewed importation. This recent event of large-scale re-introduction of vivax malaria in a temperate area can serve as a case study for further research.
Plasmodium vivax is one of the species of human malaria that is evolutionary well adapted to temperate climatic conditions. Its agent has lower temperature requirements in its extrinsic cycle than other human malaria species. The presence of hypnozoites that are responsible for its long latency allows the parasite to survive seasons when air temperatures prohibit malaria transmission [ 1 ]. Vivax malaria was widespread during the period of its greatest presence in Europe (19th Century), occurring even in the North (in southern England [ 2 , 3 , 4 ], southern Sweden [ 4 ], and being a serious problem in Finland [ 5 ]).
There is very little information on malaria in Russia before the mid-19th century [ 6 ]. Favr [ 7 ] observed that “the incidence of malaria in Russia should be considered at least 5 million cases per year; it takes first place among all the diseases of the Russian people”. After the Bolshevik Revolution of 1917, the incidence of malaria in the USSR, formerly Imperial Russia, showed repeated ups and downs while remaining quite high. The highest level of malaria cases was recorded in 1934 (about 10 million cases) [ 8 ]. At that time, P. vivax largely predominated in temperate areas of European Russia and Siberia, whereas Plasmodium falciparum was widespread in sub-tropical Central Asia and Transcaucasia, which did not belong to Russia proper. The limit of transmission of vivax malaria corresponded roughly to the border of the southern and middle taiga; it was endemic in the southern parts of Arkhangelsk region and occasionally it reached the city of Arkhangelsk itself (64º N) [ 7 ].
A misconception exists among non-specialists, which is shared and fostered by mass media, by which the northern limit of malaria is attributable to the limit of the vector’s distribution. This is not the case since Anopheles mosquito populations are much less demanding of heat and may thrive in areas where the development of P. vivax in mosquitoes is impossible due to the shortness of the warm season.
Malaria was widespread in the Moscow region in the first half of the 20th Century, especially in its central parts and in the east, where large-scale peat extraction was under way at that time. It attracted migrants from neighbouring provinces and created numerous anopheline breeding sites [ 9 ]. Areas of peat production accounted for up to 50% of the total number of malaria cases in the Moscow region [ 10 ]. The cessation of peat mining significantly improved the situation in its eastern part, although malaria transmission persisted in the region until the early 1950s when it was interrupted.
In the course of the global malaria eradication campaign in mid-20th Century, the disease was eliminated on the European continent. The term “Europe” is used in a strictly geographic sense throughout this text i.e. the western part of Eurasia which is limited by the Urals mountains, Ural river and the Great Caucasian chain. This is to be distinguished from the WHO European Region, which includes some areas that do not belong to Europe proper, such as Asian Turkey, Asiatic Russia, the countries of the Caucasus and Central Asia, Israel and Cyprus. However, at the beginning of the 21st Century, several countries in the WHO European Region faced re-introduction and even re-establishment of malaria, most of them belonging to former Soviet republics. Malaria re-emergence was observed in 9 countries by 2000 [ 11 ]. Greece, which had been free from malaria since 1974, experienced a malaria recurrence in 2010–2013 [ 12 ]. In those cases, only vivax malaria restarted transmission, whereas falciparum malaria, which is a species most often imported into Europe from the tropics, was not transmitted by local vectors, probably because of the incompatibility of Afrotropical and Oriental P. falciparum with Palearctic mosquitoes [ 13 ].
Russia has been exposed to large-scale importation of vivax malaria since the dissolution of the USSR in 1991. The greatest challenge was importation from Tajikistan, the country that faced a malaria epidemic in the post-Soviet era. The peak of malaria cases in Tajikistan was officially registered as nearly 30,000 in 1997 [ 14 ], although the true number of cases, according to expert estimates, could have exceeded 100,000 per year [ 15 ].
The dynamics of imported cases is presented in Fig. 1 . By origin, the cases have been split into two groups, viz cases from the “near abroad” (newly independent states of the ex-USSR) and cases from the “far abroad” (all other countries). Imported cases from the “near abroad” were always due to P. vivax, whereas P. falciparum and other species were present in the latter group. The importation reached its peak in 1998 and fell gradually afterwards. This process accelerated in 2005 due to an improvement of the situation in the donor countries [ 14 ]. The curve of autochthonous cases in Russia mirrored that of the cases imported from the “near abroad” with a lag of 3 to 4 years.
The dynamics of imported and autochthonous cases in Russia, 1994–2007
Due to an increase in the influx of refugees and labour migrants from ex-USSR republics and a weakening of epidemiological control, cases of autochthonous malaria began to emerge in various areas of Russia (mainly in the European area). During the period from 1995 to 2008, 525 autochthonous malaria cases were registered in the European part of Russia [ 16 ]. The most difficult situation arose in the Moscow region, which accounted for about half of all recorded autochthonous malaria cases. A sharp deterioration in the situation occurred in 2001 when transmission of malaria resumed not only in rural communities of Moscow region, but also in Moscow city itself, which is exceptional for temperate latitudes. Vivax malaria transmission in the region occurred every year until 2008, although a few sporadic cases were observed later, too.
It should be noted that malaria transmission in temperate and sub-tropical zones of Europe is not rare. After malaria elimination in 1950s, cases of transmission were reported in Corsica [ 17 , 18 , 19 ], Italy [ 20 ], Spain [ 21 ], Bulgaria [ 22 ], and Greece [ 12 , 23 , 24 ]. Cases of transmission were observed in rural communities and small towns almost exclusively. The situation in Russia was significantly worse than these outbreaks, in terms of duration, number of cases, territorial extent and in involvement of large cities.
The post-elimination history of malaria in Europe indicates that Russia, and Moscow region in particular, are especially prone to re-introduction of vivax malaria. The situation in Moscow region once again demonstrated that this disease is able to re-emerge quite easily in those areas where it was widespread in the past. The aim of the study is to present this large-scale malaria re-introduction in Moscow region at the beginning of the 21st Century (1999–2008) to the scientific community and to analyse the geographical determinants of this outbreak. There was no intention to produce a full epidemiological description of the event.
Moscow region includes two administrative units of the Russian Federation that have considerable autonomy: the city of Moscow and Moscow Oblast. The border between them was changed in 2012, but this text refers to the pre-reform status. At the beginning of 2012, the population of Moscow and Moscow Oblast was 11.6 million and 7.2 million, respectively, and the areas were 2561.5 sq km and 44,329 sq km, respectively (data of Russian Federal State Statistics Service) [ 25 ]. The region is in the centre of the East European Plain, at altitudes from 97 to 310 m above sea level. It is characterized by presence of various types of settlements ranging from metropolitan areas of Moscow to small villages. The region is highly developed economically and attracts large numbers of labour migrants, primarily from the countries of Central Asia.
The climate of the region is temperate continental and sufficiently wet (average temperature is –10 ℃ in January and + 19 ℃ in July, average annual precipitation is 713 mm). Over the past decades, Moscow region was subject to significant climatic changes and is characterized by spatial heterogeneity of the thermal regime. A specific feature of urban climate is the presence of an urban heat island (UHI) causing substantial difference in the temperatures of the city and the suburban or rural areas [ 26 ]. On average, the centre of Moscow megacity is 2 ℃ warmer than surrounding rural areas [ 27 ], however, the urban–rural temperature difference could reach up to 14 ℃ [ 28 ]. According to the recent observational and modelling studies, the urban-induced temperature anomaly covers the whole city and its nearest suburbs, beyond its administrative limits [ 29 , 30 ]. Moreover, there has been an intensification of the UHI of Moscow, which is caused by urban growth and development and is especially pronounced in summer [ 27 , 31 ]. Moscow region has a dense and extensive network of rivers and streams, numerous lakes with a total area of more than 130 sq km, as well as many ponds. Many of these water bodies are suitable for Anopheles breeding. In general, natural conditions for existence of both vectors and the pathogen ( P. vivax ) are favourable throughout the region.
Entomological and epidemiological data
Fauna, distribution, abundance, and phenology of vectors of malaria in Moscow region were studied in detail in the 1950–60s. Unfortunately, updated entomological information is scanty, due primarily to lack of interest in malaria in the 1990s. The observations, however, continued uninterrupted, albeit patchy, and they did not demonstrate any major change in vector bionomics or in proxy indicators (such as anophelogenic surfaces). At least four species of Anopheles are present in Moscow region, including three belonging to the complex of Anopheles maculipennis s.l . ( Anopheles maculipennis , Anopheles messeae , Anopheles beklemishevi ) and Anopheles claviger . Of these, An. messeae is believed to play a central role in malaria transmission. The key epidemiological role belongs to females of the first and partly second generation hatched in May–June [ 32 , 33 , 34 , 35 ].
In Central Russia, mosquitoes of Anopheles maculipennis complex breed usually along the banks of moderate-sized water bodies (ponds, lakes) with shallow, warm and clean water. Semi-aquatic vegetation (reeds) is essential. Larvae may also thrive around floating islets of duckweed. They never breed in small containers, like neglected pots or barrels. In general, they rarely breed within the households, unless small dugouts exist for decorative purposes. Due to generally enough water supply for domestic needs, owners do not make dugouts for storing water.
As a rule, every rural settlement has at least one pond or small lake serving for firefighting and recreational purposes, which are perfect breeding places for anophelines. In cities and towns breeding places do exist, mostly in park areas, but their anophelogenic productivity is not high, due to industrial and domestic pollution, periodic removal of aquatic vegetation, streamlining banks and larviciding in some of them.
Due to the lack of updated information on vectors, an entomological factor has not been included in this study. Accordingly, this study is based on official records of Rospotrebnadzor, the organization responsible for surveillance of infectious diseases in Russian Federation. All autochthonous cases of vivax malaria have been parasitologically confirmed and epidemiologically investigated. Autochthonous cases are those cases that occur due to a transmission through local vectors. They are the aggregate of (i) introduced cases that originate immediately from an imported case and, (ii) indigenous cases that originate from any other case due to transmission by mosquitoes within a given area [ 36 ]. Cases of vivax malaria occur either early (10–14 days after infection), or later, mostly within the next transmission season (usually 9–12 months after the inoculation). They are denoted as short and long incubation cases, respectively. However, they are not distinguished in official records. Relapses along with primary cases are being counted as one case, i.e. only once. In total, 405 autochthonous cases of vivax malaria recorded in Moscow and Moscow Oblast from 1999 to 2008 have been analysed. Cases have been mapped using ArcGIS software.
Climate and environmental data
The choice of indicators for analysing the factors that influenced the occurrence and the distribution of cases during the recent re-introduction of malaria in the region relates to the following considerations:
the impetus to malaria transmission was provided by a large-scale importation from countries of the former USSR affected by malaria epidemics amid favourable meteorological conditions in the recipient regions;
spatial heterogeneity of the distribution of cases caused by several environmental factors.
To analyse the degree of climatic favourability for development of sporozoites, observational data from Moscow weather stations was used in the period from the start to the peak of the outbreak (1999–2004). The stations are in the city centre (Balchug), city parks (VDNKh and MSU) and other urban areas (4 stations), as well as 15 stations located in Moscow Oblast.
The integrated database of continuous (8 times a day) meteorological observations was created using the archives of the Russian Institute for Hydrometeorological Information—World Data Centre (RIHMI-WDC), Central Department for Hydrometeorology and Environmental Monitoring, Meteorological Observatory of Lomonosov Moscow State University.
To analyse the territorial heterogeneity of the distribution of cases the following variables of climate and environmental data that were available in continuous spatial resolution were used (Table 1 ). All variables were presented in the form of rasters, cropped according to the shape of the territory (Moscow region), reduced to a total resolution of 1 × 1 km and converted to ASCII format. The selection of these particular climatic variables was guided by long-established consensus that malaria is associated with summer temperatures in the most predictable way [ 43 , 44 ]. Precipitation has a strong influence on malaria, which has long been well-known [ 43 , 45 ], however its linkage to malaria is not linear. All depends on the breeding habits of the local vectors. Altitude and vegetation density have been referred to by the same authors as important factors of malaria.
A popular WorldClim gridded data set with 1-km spatial resolution were used [ 38 ] as a source of climatic information for geospatial analysis. It is emphasized that despite high spatial resolution, the WorldClim data on air temperature is not perfect, especially for urban areas, where significant underestimation of temperature spatial variability was revealed [ 46 ]. WorldClim data reasonably resolves the elevation-induced local climate features, e.g., lower daytime temperatures in the lowlands, however the representation of Moscow UHI was found to be unsatisfactory. The decision to use WorldClim data on daily maximum temperatures is driven by a reason that they are less affected by urban-induced effects in comparison to daily-mean and nocturnal temperatures. WorldClim data allow accounting for regional-scale temperature gradients, small-scale orography-induced effects, but not for urban climate features.
As postulated by Beklemishev [ 47 ], each landscape unit has its own combination of physiographic and ecological features that determines a particular quality of that unit vis-à-vis malaria. Therefore, the landscape grid is tantamount to a malariological stratification grid.
The landscape stratification of the Moscow region is well developed [ 41 ] and provided with appropriate cartographic material. The borders between the landscape units are usually well distinguishable even for an observer in the field.
Building density is used as an indirect indicator of human population and development. The density of roads and railways, as well as the distance to railway stations, are used as indicators of the presence and intensity of mobility of the population. The density of the so-called ‘cottage communities’ and the distance to them are used to identify links with possible sources of infection.
Cottage communities are a new type of human settlements in Russia that proliferated around big cities in the 1990s. Typically, cottage communities consist of a few dozen two-story standardized buildings with a small plot of land attached and are located 20–50 km from the border of Moscow. Each house is usually occupied by one or two families. Their occupants belong to the wealthy segment of Moscow city dwellers who usually work in Moscow and own apartments in the megacity. The developers preferred to use migrant labour for the construction works, mostly from Tajikistan, which experienced large-scale epidemics of malaria at that time.
Water bodies are numerous in Moscow region, especially near rural settlements. However, not all of them are suitable for anopheline breeding. Since there are no simple means to tell anophelogenic reservoirs from innocuous ones using only remote sensing, the proximity to water bodies could not be considered as one of malaria determinants in this geospatial analysis.
Based on the research concept and the need to solve two independent tasks: to analyse the degree of favourability of climatic conditions for extrinsic development of vivax malaria pathogen at the time of the outbreak and to perform the spatial analysis of the distribution of autochthonous cases, two different methods were used.
Climate favourability assessment
To assess the degree of favourability of climatic conditions for vivax malaria, the Moshkovsky’s method has been used [ 48 ], the method, which was being used in Russia for routine monitoring conditions for extrinsic development of malaria parasites since the 1950s. Moshkovsky adapted to malaria the ideas of Bodenheimer [ 49 ], who had developed practical ways to predict timing of development of insects and plants. Information required are average daily temperatures (ADTs) for the period of development of the organism in question (sporozoites in this case). The threshold temperature of development for each particular species is derived from experimental data. The difference between the ADT for each day and the threshold temperature is called an effective temperature. When accumulated effective temperatures measured in degree-days (or the sum of temperatures , as it is usually denoted in Russian or French texts) reach a particular level, this heralds the accomplishment of a particular developmental stage of the organism.
For the purpose of malaria, the method has been recommended by the WHO [ 50 , 51 ]. According to Moshkovsky, extrinsic development of P. vivax requires the accumulated sum of 105 degree-days above the threshold of 14.5 ℃. In this study, the sums of effective temperatures ( P. vivax) have been calculated for each year between the beginning and the peak of the outbreak (1999–2004). As a result, the temporal limits of the following overlapping elements of malaria season have been identified for each year.
the season of effective temperatures: the part of year during which the ADTs are consistently above the threshold temperature;
the season of effective infectivity of mosquitoes: the period during which a full development of malaria parasites in mosquitoes (from gametocytes to mature sporozoites) is possible.
- Geospatial analysis
To analyse the spatial heterogeneity of the distribution of cases caused by various environmental factors, the method for modelling ecological niches with optimization based on the maximum entropy principle [ 52 ] was applied using MaxEnt software. This instrument performs the selection of the probability distribution of a biological species in question over the study area on the basis of: (a) the known locations in which this species was found (presence data), and, (b) a set of spatial variables characterizing the territory in question. The method is widely used for: (i) modelling the potential range of distribution of a species; and, (ii) modelling the potential area of distribution of the disease based on the assumption of its coincidence with the pathogen’s geographical range. Examples of the latter approach are studies by Peterson et al. and Rose and Wall on viral diseases [ 53 , 54 ]; Du et al. on myasis [ 55 ]; Abdrakhmanov et al. [ 56 , 57 ]; Mwakapej et al. [ 58 ] on anthrax. A conceptual review of the application of the MaxEnt method in biogeography is given in [ 59 ]. For malaria, this method was applied in predicting environmentally suitable areas for several Anopheles species in Iran [ 60 ].
All variables were checked for multicollinearity using the Raster Correlation procedure from the additional SDMtoolbox toolkit for ArcGIS [ 61 ]. To determine the possible correlation between all analysed variables and malaria cases, toolkit Band collection statistics analysis in ArcGis software was used.
MaxEnt modelling was carried out using 10 replications, the results of which determined the average values and the confidence interval boundaries of the distribution of the territory suitability for malaria transmission. Assessment of the contribution of each variable in the model reflects a change in its quality when the value of the variable in question changes and when the value of the others is fixed. The weight of the variables for constructing the models was estimated using the jackknife method, based on a comparison of the simulation results with the sequential exclusion of each variable (or when modelling using only one variable).
The predictive ability of the MaxEnt model was estimated by comparing the model’s ability to correctly predict the presence and absence points using the area under the curve indicator (AUC) [ 62 ], which represents the area under the receiver operating characteristic (ROC). Since the MaxEnt model is based on the presence data only, randomly generated points (pseudo-absent points) are used as the absence data. The ROC curve shows the relationship between the fraction of presence data correctly predicted by the model (sensitivity) and the proportion of the incorrectly predicted pseudo-absence data (1-specificity). The AUC value lies between 0.5 and 1.0 where 0.5 denotes a bad classifier and 1.0 denotes an excellent classifier [ 63 ].
In order to obtain the best model complexity, a regularization coefficient is used that modulates the fit of the model and allows reducing over fitting. The higher values of the coefficient provide simpler models resulting in broader areas predicted to be suitable for the species under study [ 64 ]. Eight regularization coefficient values from 0.5 to 4.0 in 0.5 steps were tested. The best value was chosen based on the highest AUC provided.
Spatial distribution of malaria cases
Between 1999 and 2008, 405 cases of vivax malaria transmission were recorded in Moscow region, of which 93 were observed within the borders of the city of Moscow (Fig. 2 ). Reported autochthonous cases of vivax malaria in Moscow region were unevenly distributed (Fig. 3 ). Within the boundaries of the city of Moscow, the bulk of the cases were concentrated in a continuous peripheral area in the western, northwestern and northern parts. It is worth noting that cases of malaria transmission were observed in the same locations during the previous post-eradication outbreaks in 1972 and 1981 [ 65 ]. This time, the greatest number of cases was registered not only in the aforesaid parts of Moscow city but in its immediate surroundings. Further to the east, in the northern part of Moscow city, the largest number of cases were confined to the valley of Yauza River, the main tributary of the Moskva River, and related ponds. In the east of the city, a few cases were confined almost exclusively to park and forest park areas. Finally, in the southern part of Moscow, all cases are mainly related to the Borisov ponds located on the Gorodnya River. In the central, most urbanized, part of Moscow and in the southeast part of the city where large industrial enterprises were located at the time of the outbreak, there were no recorded cases of malaria apart from a few large parks.
Autochthonous cases of vivax malaria in Moscow region, 1999–2008

Spatial heterogeneity of malaria cases distribution in Moscow region, 1999–2008. Purple marks the areas with high compactness of autochthonous cases calculated by ArcGis kernel function
The area of the greatest concentration of malaria cases in the western part of the city of Moscow is an extension of the area of concertation of autochthonous cases outside the city (Krasnogorsk, Odintsovo and Istra districts). In that area, malaria transmission was recorded every year during the outbreak.
To the north of Moscow, the incidence was associated with the nearest satellite cities of Moscow (Mytishchi, Khimki, Dolgoprudny). The number of cases in smaller towns decreased in parallel with increasing distance from Moscow. The only relatively remote site (about 75 km to the north of Moscow) with a significant number (15) of cases was the town of Dmitrov with a few adjacent villages.
There were fewer autochthonous cases in the southern part of the Moscow region and they were also recorded mainly in the areas nearest to Moscow. The smallest number of cases was observed in the eastern part of the region.
Dynamics of climate favourability for malaria transmission
The accumulated temperatures of 105 degree-days make it possible the maturation of one generation of P. vivax sporozoites, in other words, emergence of introduced cases cannot be excluded. When the sum surpasses 210 degree days, this is above the requirements for two consecutive generations of sporozoites, which is needed for emergence of indigenous cases, in addition to the introduced ones, that means the possibility of perpetuation of the transmission in the next year.
The season of 1999 was characterized by a very high sum of effective temperatures unusual for Moscow region. On average, the sum of effective temperatures amounted to 444.8 degree-days in the Oblast. It exceeded 500 degree-days in some areas in the south of the region and surpassed the level of 700 degree-days in the centre of Moscow, due to UHI. After a slight decline in 2000, two hot summers followed, during which the sum of effective temperatures averaged 370 and 257 degree-days over the Oblast, in 2001 and 2002, respectively. For the city centre, this indicator reached 633 degree-days in 2002 (Fig. 4 ). Despite the significant urban–rural temperature differences, everywhere the sum of effective temperatures was significantly higher than 210 degree-days in 1999, 2001 and 2002. There was a significant decline to 200.6 degree-days in rural areas and 355.0 degree-days in the city in 2003, but in 2004 the average sums of temperatures rose again, up to 274.2 and 426.6 degree-days, respectively. In short, meteorological conditions in the city were favourable for a stable malaria transmission, whereas in rural areas, only the year of 2003 was unfavourable.
The sums of effective temperatures accumulated per season in the Moscow region, 1999–2003
Spatial heterogeneity is noteworthy not only in sums of effective temperatures in Moscow megacity versus the surrounding suburbs and rural areas, but also in longer seasons of effective infectivity in the city (Table 2 ). However, during warm summers at the beginning and the peak of the outbreak (1999–2002), the season of effective infectivity was longer than 1 month both in rural and urban areas.
Model of spatial distribution of malaria
Geospatial analysis of the relationship between cases of malaria transmission and each variables separately revealed very weak connection; there is no association with the absolute height of the area (r = − 0.004), it is weakly expressed with the density of cottage construction (r = 0.297) and is moderately expressed with the density of buildings (r = 0.465).
Most autochthonous cases of malaria are linked to four landscapes (No. 29—Moskvoretsko-Klyazminsky, No. 54—Moskvoretsky, No. 56—Aprelevsky-Kuntsevsky and No. 80—Shchelkovsky) (Table 3 , Fig. 5 ). The names of the landscapes used in this work were borrowed from the inventory of landscape units [ 41 ]. The units were named by scientists in the course of the stratification to reflect the names of various prominent physiographic or urban objects.
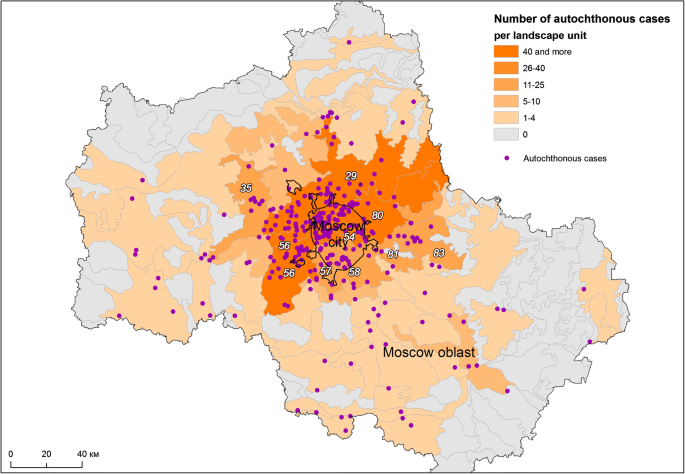
Distribution of malaria cases by landscape units The most affected landscape units are listed in Table 3
A somewhat smaller number of cases belong to five more landscape areas directly adjacent to those three mentioned above. The remaining foci are usually represented by isolated malaria cases and are scattered over other landscape areas, and their number does not exceed 2 or 3 in each of them.
From the physiographical point of view, the landscape units that are good for malaria restoration belong to variants of moraine and water–ice plains with the humidity regime ranging from normal to excessive, with a predominance of elevated terrains at absolute altitudes from 120 to 200 m above sea level. Forest vegetation is largely replaced by arable land and garden plots as well as urban development areas. Among the residual vegetation, coniferous-deciduous forests prevail, as well as floodplains, upland meadows and occasional swamps. A distinctive feature of all landscapes is a strong degree of anthropogenic disturbance [ 66 ].
More meaningful results were obtained when MaxEnt modelling was applied for multivariate analysis. The final MaxEnt model was calibrated with a regularization coefficient of 1.5. It demonstrates the AUC of 0.870 ± 0.015, which indicates a good predictive ability. Figure 6 a–j shows the response curves for each variable demonstrating how the magnitude of the probability predicted by the model changes with the participation of only one given variable in the model.
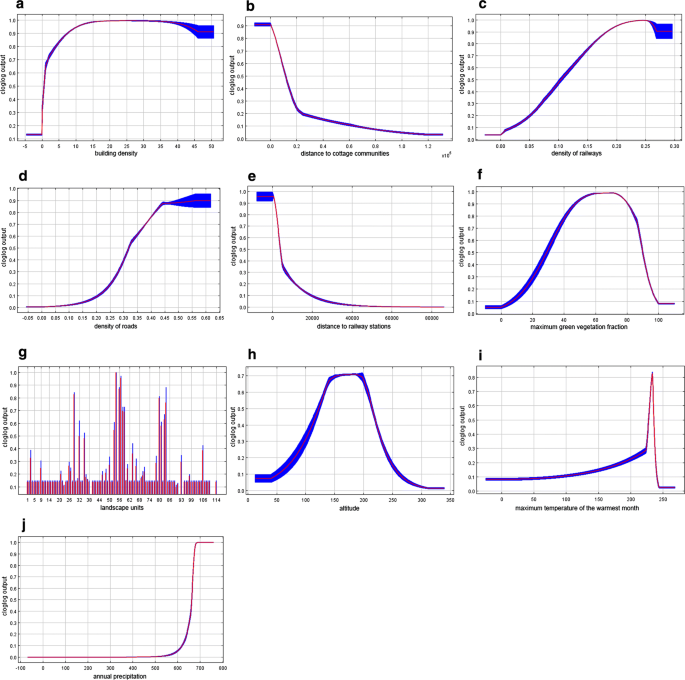
Response curves based on MaxEnt simulation results reflecting the influence of each of the significant spatial factors on the likelihood of appearance of autochthonous cases a building density; b distance to cottage communities; c density of railways; d density of roads; e distance to railway stations; f maximum green vegetation fraction; g landscape units; h altitude; i maximum temperature of the warmest month; j annual precipitation. Colour indicates mean value (red), standard deviation limits (blue)
The most significant factor in this modelling was building density that reflects populated areas, significance of 40.5% (Fig. 6 a). The next most significant factor is the distance to the cottage communities: 25.1% (Fig. 6 b). It demonstrates the expected relationship with the likelihood of outbreaks: it reaches maximum in a radius of about 2 km around the communities, significantly decreasing with increasing distance (Fig. 7 ).
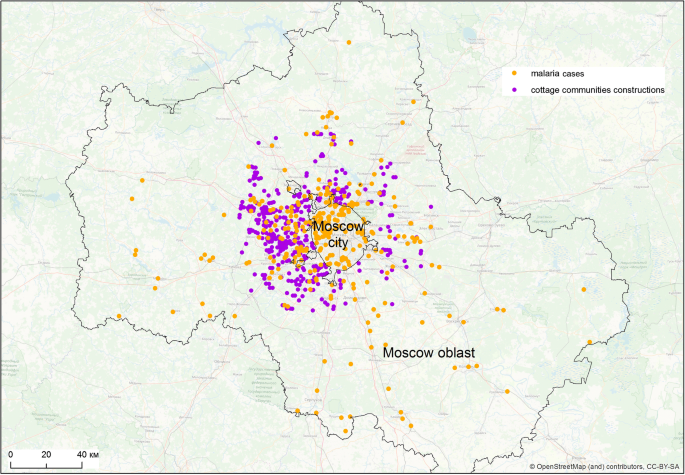
Distribution of malaria cases and cottage communities
The maximum likelihood of malaria cases is also positively associated with natural environmental variables: maximum green vegetation fraction (10.4%) and landscape units (9.1%) (Fig. 6 f, g). For the maximum green vegetation fraction, a high probability response to values ranging from 50 to 80% is observed with a subsequent decrease in the response, which can be interpreted as the absence of malaria cases in places with too thin vegetation as well as too dense one. The highest response is demonstrated by landscapes with the index 57, 58, 56, 80, 29, 54, which generally corresponds to the distribution of the number of malaria cases observed over the landscape. The influence of altitude (1.6%) is of the least importance among environmental variables, with the highest response being in the range of height from 100 to 200 m (Fig. 6 h).
The influence of mobility of the population on malaria cases is rather small (Fig. 6 c–e): 6.9% for density of railways, less than 2% for density of roads and distance to railway stations. Nevertheless, the probability of outbreaks concentrated near railway stations decreases with the distance from them. Notably, malaria cases are not associated with population density. So, autochthonous cases occurred both in the cities of the Moscow region, where the population density is high, and in less densely populated rural areas.
The influence of climatic factors (maximum temperature of the warmest month and annual precipitation) on spatial heterogeneity of cases is less pronounced in the model (significance of 0.6 and 3.1%, respectively). Nevertheless, the dependence of the likelihood of an outbreak on the values of these variables is quite predictably increasing, which demonstrated an insignificant probability in the zone of low temperatures/precipitation and a sharply ascending probability with the increase in temperatures/precipitation. Noteworthy is the absence of the influence of low values of the variable in precipitation, which can be interpreted as the predominant attraction of malaria sites to wet locations (Fig. 6 i, j). The possible impact of the local temperature features on the outbreak development is further discussed below.
Based on the modelling results, a map was created reflecting the territorial differentiation of Moscow region according to the suitability of infection re-emergence (Fig. 8 ). This map shows the statistically valid relationships between the distribution of autochthonous malaria cases and environmental and climatic factors. Malaria re-introduction is most likely to occur in rural area in the immediate vicinity of Moscow, along the main transport routes, and in satellite cities.

Modelling the degree of favourable conditions for the occurrence of malaria cases. Average values from 10 replications (red denotes a high degree of suitability; blue is a low degree). The values represent a probability that a set of explanatory variables within the certain cell is treated by the model as suitable for the emergence of a malaria case
Malaria re-emerging after many years of the absence of its transmission is a subject of great interest to researchers and health authorities. Attempts to assess the environment in terms of predicting malaria re-introduction have been made regularly, especially for Europe where malaria has been eliminated [ 67 ], but has proven able to restore its transmission.
The most common approach to the assessment of possible malaria re-introduction is the analysis of distribution of vectors and changes in their bionomics [ 68 , 69 ]. Climatic and social factors are often added [ 70 , 71 ], but they are first considered from the point of view of their possible effect on vectors. However, in recent post-eradication outbreaks in Europe, Russia included, changes in vector factors have never been the trigger for the resumption of transmission, and re-appearance of autochthonous malaria was due to either intensification of its importation, or meteorological factors favouring the parasite maturation, or a combination thereof [ 72 ].
The approach implemented in this study is distinctive, as there is an attempt to contemplate the integrity of factors that may be involved. The results based on MaxEnt modelling showed that the combined influence of both natural and man-made environmental conditions played a key role in the Moscow outbreak.
Conceptually, the ability of parasitic diseases, including malaria, to thrive in a particular area (a malariogenic potential in case of malaria) may be expressed as a product of two parameters: receptivity and vulnerability [ 73 ]. Receptivity is the ability of ecosystems to incorporate the malaria parasite as their member [ 74 , 75 ]. Vulnerability, or importation risk, is a measure of probability of the pathogen being imported from endemic areas, which is determined by the frequency, numbers and seasonality of gametocyte carrier arrival (less commonly, of infected mosquitoes). It is noteworthy that these terms have been recently reviewed by the newly formed Drafting Committee on Malaria Terminology. It was observed that the term of “vulnerability” may have several conflicting meanings in different medical sciences. Therefore, it has been suggested to replace it, as concerns the malaria terminology, by the “importation risk” [ 75 ]. This term is used throughout the text as a synonym of “vulnerability”.
In this study, the significance of the importation risk is corroborated by the observed high value (25.1%) of the parameter of the model called” distance to cottage communities”. This correlations in space can possibly be explained by the presence of labour migrants. During the studied period, the cottage communities were the sites of the most intensive construction activities in rural areas attracting huge numbers of labour migrants, mostly from Tajikistan [ 16 , 76 ], which was the scene of major post-eradication outbreaks at that time [ 77 ]. Arrival of seasonal workers and illegal migrants in Russia from some countries of the CIS adversely affected the malaria situation. There were no reliable figures on the actual size of illegal immigration in Russia, as estimations differed significantly from official registration data. Some researchers estimated the number of illegal immigrants in Russian Federation in late nineties between 400,000 and 7 million, and in Moscow alone there were no less than one million from the “near abroad” [ 78 ]. Migration control was very weak in late nineties in Russia, which contributed to an increase of imported cases from 218 in 1990 to 1042 in 1998 [ 76 ].
Migrants coming from the areas of intensive transmission were often more knowledgeable about malaria than Moscow medics did. Some of them had had malaria in the recent past and had residual immunity that mitigated malaria in case of new infections. Many had antimalarials in their possession. They knew that in case of positive diagnosis they would be subjected to a long hospitalization, which was not in their interest. Illegal or semi-legal existence of many of them and absence of medical insurance prevented them from seeking medical care. At the same time, local medical facilities were not interested in delivering services to migrants [ 16 ]. As the result, detection of malaria among migrants was very incomplete.
Migrants were aggregated mostly in construction of housing in cities and summer houses for urban dwellers in the countryside. During the construction work in the countryside, usually in summers, they dwelled, as a rule, in semi-constructed structures open to attacks from vectors.
The association of autochthonous malaria and cottage construction seems to corroborate the hypothesis that migrants were the major source of malaria. A similar situation was observed during some of the other post-eradication outbreaks in Europe, where labour migrants and refugees were at the origin of renewed malaria transmission, for example, in Bulgaria [ 22 ] and Greece [ 12 ].
The receptivity of ecosystems is determined by several natural factors. It is generally recognized that the main factor in temperate areas are summer temperatures (while rainfall may be of decisive importance in sub-tropics and tropics) [ 79 ]. An outbreak of vivax malaria in Moscow region has developed amid exceptionally favourable weather conditions for the vivax pathogen’s extrinsic development. It was previously demonstrated that by the start of the 21st Century, conditions for malaria transmission in Moscow region improved compared with 1970–80s [ 51 ]. The most dramatic changes in the sums of effective temperatures and duration of season of effective mosquito infectivity have occurred since the mid-1990s, which is consistent with general climatic warming in Moscow region [ 27 ]. The highly favourable conditions existing during two consecutive seasons (2001 and 2002) might have a crucial significance for the formation of a steady infection reservoir, due to an unusual accumulation of hypnozoite carriers.
The role of the climatic factor in malaria transmission in the model is not so clearly traced. It may be caused firstly by temperature conditions that were uniformly favourable for the development of P. vivax throughout the region (urban and rural areas) during the beginning of the outbreak. Secondly, there is a relatively small variation in the considered variables of maximum temperature of the warmest month according to the gridded WorldClim data. The local climate features, such as UHI, are weakly pronounced by maximum temperatures that are observed at daytime [ 26 ]. More information can give daily mean and minimum temperature that are more sensitive to local climate features. Unfortunately, available datasets do not allow explicit consideration of the urban anomaly of air temperature and temperature-dependent malaria indicators in the MaxEnt model. The network of weather stations in Moscow region is too sparse for such a task, while globally available gridded temperature datasets are unable to adequately represent temperature heterogeneity in urban areas [ 46 ]. Development of detailed and reliable climatic datasets is essential for better understanding of epidemiological threats in urban areas.
More detailed analysis of the sums of effective temperatures and the duration of season of effective infectivity based on temperature data from rural and urban weather stations, shows a spatial heterogeneity that is clearly noted in the distribution of these indicators. This is a clear manifestation of the UHI effect. The influence of UHI is expressed in significantly higher values of indicators in the city centre compared to city parks and the countryside. The year 2003 illustrates that heterogeneity of the territory is manifested mainly during unfavourable weather conditions. That year the sums of effective temperatures, needed for the development of the parasite could be accumulated only in Moscow city (due to the effect of UHI) and in the east of the Oblast. As a result, the number of cases was considerably less in 2003 than in very favourable 2001 and, especially, 2002. In warm years, when the thermal conditions are rather uniform across the region, there is not much difference between the areas under the influence of UHI and the periphery, whereas this difference provokes a sharp decrease of cases at cooler areas during malaria’s lean years.
It can be assumed that in conjunction with human-related environmental factors, the urban–rural temperature difference may explain why areas most affected by malaria were located at the peripheral districts of the city (northwest of Moscow) or near city borders. On the one hand, these are the territories that are most attractive in terms of building density, reflecting areas of population concentration, and more suitable places for anopheline breeding in comparison with the city centre while, on the other hand, the temperatures in these built-up areas are more favourable for the development of the pathogen in comparison with rural areas. In addition, it was there that most large-scale cottage construction unfolded. Despite that, UHI was not represented explicitly in the MaxEnt model, its influence should not be excluded.
The influence of landscape on the distribution of endemic malaria is, in general, well known [ 79 , 80 , 81 ]. The issue of the influence of landscape division on malaria re-introduction in temperate latitudes is not so well studied because there are not so many well-documented cases of such re-emergence. An assessment of environmental suitability for malaria transmission in Greece [ 82 ], which used several climatic and environmental parameters, showed that the highest risk of malaria transmission was confined to particular landscapes of coastal recreational zones.
In Moscow region, some landscapes are especially prone to re-introduction of malaria. The role of the landscape factor is demonstrated by an accumulation of cases in and around the city of Dmitrov, 75 km north of Moscow, an area of a remote protuberance of the most malaria-prone landscape, Moskvoretsko-Klyazminsky (No 29), that lies amid the least affected landscapes. It is noteworthy that the territories inside the city of Moscow and beyond belonging to the same landscape unit have similar malariogenic potential, despite the effects of the urbanization.
Suitability of particular types of landscape for malaria transmission may change over time. For example, the areas in the east of the region which were most affected at the beginning of the 20th Century [ 9 , 10 ], were not affected during the outbreak a century later. In this case, the cessation of peat mining and the subsequent land reclamation in the 1950s played a decisive role. In addition, in the eastern part of the Moscow agglomeration, intensive industrial development took place during the Soviet period which produced considerable industrial pollution creating an environment that was unfavourable for malaria vectors. Similarly, in industrial zones within Moscow city, there were very few cases of malaria transmission during the recent malaria re-emergence.
In Moscow region, malaria re-introduction begins firstly in the landscape units with elevated humidity in well-drained territories, gravitating to the valleys of relatively large rivers. This agrees with a long-standing observation by Favr that malaria is a disease of river valleys in Central Russia [ 7 ]. At the turn of the 19th Century, this assumption was widespread among Muscovites, as evidenced by A.P.Chekhov, the famous writer, who was also a medical practitioner in Melikhovo, 75 km south of Moscow. He notes in a letter to A. S. Suvorin on 1 April, 1897 that “Melikhovo is a healthy place; it is just on the watershed, it stands high, so there is never a fever in it.” [ 83 ].
The main water artery in the area is the Moskva River which crosses the megacity from northwest to southeast. Its waters are relatively clean on their entry in the city but become polluted by industrial and municipal discharges downstream. Because of this gradient of pollution, the rural districts bordering Moscow city from the northwest and west (that were most affected by malaria in 1999–2008) are more suitable for anopheline breeding. At the same time, those areas have greater recreational attractiveness, and were the scene of extensive construction of cottage communities at the turn of the century.
Further to the periphery, in relatively remote areas that do not attract numbers of migrants, malaria transmission is mostly associated with moist river valleys. However, in some of those areas conditions for malaria transmission worsened due to land reclamation/drainage and industrial and possibly domestic pollution.
The altitude of the terrain is a well-known factor of malaria [ 79 , 84 , 85 , 86 ]. Its importance has been demonstrated recently in Greece with respect to the suitability of territories for malaria transmission [ 82 ] and in Iran with respect to the distribution of the most important vectors species [ 60 ]. Despite the fact that the response of probability of the model did not show high significance (it may happen due to more pronounced influence of other variables in the model, such as distance to cottage communities, indirectly reflecting the spread and concentration of labour migrants), the curve of the distribution of cases by altitude (Fig. 6 h) shows that the cases were confined to a range of 100–200 m. In addition, the most affected landscapes (Table 3 ) are linked to the same altitudes. It may be assumed that such model response is due to insufficient number of malaria transmission cases and a small range of absolute heights. In Moscow region, this factor has no particular meaning below 200 m, a cut-off level, above which the re-establishment of malaria is least probable.
Although the receptivity of the Moscow region may be deemed medium [ 73 ], the risk of importation of P. vivax (a species most adapted to the local vectors) is very high, since Moscow is becoming attractive to economic migrants [ 77 ]. As a result, malariogenic potential, which is the product of receptivity and the risk of importation is quite high, irrespective to the latitude.
Whereas the historic post-elimination outbreaks (including those in Russia in the 1970s and 1980s) involved limited territories, this particular outbreak in Central Russia is unique in terms of its great extent and variety of ecosystems involved.
The loss of interest for malaria control was one more reason for the recent malaria re-introduction in Moscow region. After the last autochthonous cases in the 1980s related to the Afghan war [ 87 ] malaria re-introduction ceased to be regarded as a significant threat to public health which led to inadequate staffing, especially of medical entomologists. Despite the high malariogenic potential, the epidemic waned as soon as importation of new cases from ex-Soviet republics came to the stop around 2009 [ 77 ]. Even though the summer of 2010 was extremely hot [ 88 ], no transmission of malaria occurred at that time.
Conclusions
One of the contributing factors for the re-introduction of malaria in Moscow region in 1999–2008 was highly intensive importation, coupled with a decrease in epidemiological awareness against the background of extremely favourable meteorological conditions during 1999, 2001, and 2002. However, both in the past and at the present time, a combination of natural and human-related factors, as well as territorial heterogeneity in relation to malaria transmission affects the development of outbreaks.
In the conditions prevailing in the opening years of the 21st Century, the malariogenic potential in relation to vivax malaria was high in Moscow region, albeit heterogeneous in this regard. During the 1999–2008 outbreak the most affected areas were those with a high concentration of population.
A significant role was also played by rural construction (the attraction of labour migrants from Tajikistan, among whom there were many parasite carriers, including asymptomatic ones), vegetation density, belonging to a particular landscape division, and the altitude below 200 m above sea level. Most likely, the intensive UHI of Moscow megacity was an additional factor which amplified the outbreak in urban and suburban areas. In the future, in case of a renewed massive importation, it is in Moscow that the most favourable conditions are expected to be existing for vivax malaria re-introduction, compared with other regions of Russia.
Availability of data and materials
All documents and publications in Russian are available at the Lomonosov Moscow State University, Moscow, Russian Federation.
Abbreviations
Average daily temperature
American standard code for information interchange
Area under the curve
Commonwealth of independent states
Moscow State University
Russian institute for hydrometeorological information—world data center
Receiver operating characteristic
Urban heat island
Union of the soviet socialist republics
Vystavka Dostizheniy Narodnogo Khozyaystva , exhibition of achievements of national economy
World Health Organization
Beljaev AE, Rybalka VM, Lysenko AJ, Abrashkin-Zhuchkov RG, Alekseeva MI, Arsenyeva LP, et al. Plasmodium vivax : further observations on polymorphism in relation to the duration of exo-erythrocytic development (In Russian). In Malaria Parasites of Mammals; Academy of sciences of the USSR Protozoology series; Leningrad: Nauka; 1986: 11; 40–157. (In Russian) .
Reiter P. From Shakespeare to Defoe: malaria in England in the little ice age. Emerg Infect Dis. 2000;6:1–11. https://doi.org/10.3201/eid0601.000101 .
Article CAS PubMed PubMed Central Google Scholar
Lindsay S, Joyce A. Climate change and the disappearance of malaria from England. Global Change Hum Health. 2000;1:184–7.
Article Google Scholar
Bruce-Chwatt LJ, de Zulueta J. The rise and fall of malaria in Europe. A historico-epidemiological study. London: Oxford University Press; 1980.
Google Scholar
Hulden L, Hulden L. The decline of malaria in Finland–the impact of the vector and social variables. Malar J. 2009;8:94.
Article PubMed PubMed Central Google Scholar
Vasiliev KG, Segal AE. The history of epidemics in Russia. Moscow: Medgiz; 1960 (in Russian) .
Favr VV. Study of malaria in Russia from the public health angle. Kharkov: 1903. (in Russian) .
Sergiev PG, Dukhanina NN, Demina NN, Shipitsina NK, Ozeretskovskaya NN, Lysenko AY. Malaria. A multi-volume guide to microbiology, clinic and epidemiology of infectious diseases. Moscow: Medicine; 1968: 9; 37–115. (in Russian) .
Skibnevsky AI. Materials on the incidence of specific diseases vol. 2: The spread and manifestation of malaria in the population of the Moscow governorate. Moscow: 1903. (in Russian) .
Oganov LI. Malaria in the Moscow region and its seasonal periodicity. PhD Thesis, 1947. Martsinovski Institute for Medical Parasitology and Tropical Medicine, Moscow, USSR. (in Russian) .
WHO. Malaria in the WHO European region. Fact sheet. 2016. http://www.euro.who.int/__data/assets/pdf_file/0009/246168/Fact-sheet-Malaria-Eng.pdf?ua=1 . Accessed 08 Oct 2019.
Danis K, Lenglet A, Tseroni M, Baka A, Tsiodras S, Bonovas S. Malaria in Greece: historical and current reflections on a re-emerging vector borne disease. Travel Med Infect Dis. 2013;11:8–14.
Article PubMed Google Scholar
de Zulueta J, Ramsdale CD, Coluzzi M. Receptivity to malaria in Europe. Bull World Health Organ. 1975;52:109–11.
PubMed PubMed Central Google Scholar
WHO. Regional strategy: from malaria control to elimination in the WHO European Region 2006–2015. Copenhagen: World Health Organization Regional Office for Europe, 2006. http://www.euro.who.int/__data/assets/pdf_file/0011/98750/E88840.pdf . Accessed 8 Oct 2019.
Kondrashin AV, Morozova LF, Stepanova EV, Turbabina NA, Maksimova MS, Morozov EN. On the epidemiology of Plasmodium vivax malaria: past and present with special reference to the former USSR. Malar J. 2018;17:346.
Mironova VA, Beljaev AE. Migration processes and malaria in Russia. In: Yushchenko GV, editor. Current issues in the epidemiology of infectious diseases: Shaposhnikov AA. Moscow: RMAPO; 2011. p. 680–90 (in Russian) .
Ambroise-Thomas P, Quilici M, Ranque P. Réapparition du paludisme en Corse. Bull Soc Pathol Exot. 1972;65:533–42.
CAS Google Scholar
Armengaud A, Legros F, D’Ortenzio E, Quatresous I, Barre H, Houze S. A case of autochthonous Plasmodium vivax malaria, Corsica, August 2006. Travel Med Infect Dis. 2008;6:36–40.
Article CAS PubMed Google Scholar
Toty C, Barré H, Le Goff G, Larget-Thiéry I, Rahola N, Couret D, et al. Malaria risk in Corsica, former hot spot of malaria in France. Malar J. 2010;9:231.
Baldari M, Tamburro A, Sabatinelli G, Romi R, Severini C, Cuccagna G, et al. Malaria in Maremma, Italy. Lancet. 1998;351:1246–7.
Santa-Olalla Peralta P, Vazquez-Torres MC, Latorre-Fandos E, Mairal-Claver P, Cortina-Solano P, Puy-Azon A. First autochthonous malaria case due to Plasmodium vivax since eradication, Spain, October 2010. Euro Surveill. 2010;15:19684.
CAS PubMed Google Scholar
Kurdova R, Vutchev D, Petrov P. Malaria situation in Bulgaria and surveillance measures (1991–2000). Global Nest Intern J. 2001;3:153–62.
Kampen H, Maltezos E, Pagonaki M, Hunfeld K-P, Maier W, Seitz H. Individual cases of autochthonous malaria in Evros Province, northern Greece: serological aspects. Parasitol Res. 2002;88:261–6.
Olaso A, Ramos JM, López-Ballero MF, Olaso I. Malaria in Europe: follow-up of autochthonous malaria in Greece and new risks. Enferm Infecc Microbiol Clin. 2017;35:543–4.
http://www.gks.ru/wps/wcm/connect/rosstat_main/rosstat/ru/statistics/population/demography/ Accessed 11 Sep 2019.
Oke TR, Mills G, Christen A, Voogt JA. Urban climates. Cambridge: Cambridge University Press; 2017.
Book Google Scholar
Kislov AV. Climate of Moscow in the context of global warming. Moscow: Publishing House of Moscow University; 2017 (in Russian) .
Lokoshchenko MA. Urban “heat island” in Moscow. Urban Clim. 2014;10:550–62.
Varentsov M, Wouters H, Platonov V, Konstantinov P. Megacity-induced mesoclimatic effects in the lower atmosphere: a modeling study for multiple summers over Moscow, Russia. Atmosphere. 2018;9:50.
Varentsov MI, Grishchenko MY, Wouters H. Simultaneous assessment of the summer urban heat island in Moscow megacity based on in situ observations, thermal satellite images and mesoscale modeling. Geography, Environment, Sustainability. 2019; https://ges.rgo.ru/jour/article/view/762 . Accessed 26 Dec 2019. https://doi.org/10.24057/2071-9388-2019-10 .
Kislov AV, Varentsov MI, Gorlach IA, Alekseeva LI. The heat island” of the Moscow agglomeration and the urban increase of global warming. Bull Mosc Univ Geogr. 2017;4:12–9 (in Russian) .
Gornostaeva RM, Danilov AV. Mosquitoes of Moscow and the Moscow region. Moscow: KMK Scientific Press; 1999 (in Russian) .
Artemiev MM, Baranova AM, Darchenkova NN, Dremova VP, Ganushkina LA, Markovich NY, et al. The malarial mosquitoes of Russia the genus Anopheles. Med Parazitol (Mosk). 2000;2:40–5 (in Russian) .
Gordeev MI, Ejov MN, Zvantsov AB, Perevozkin VP. Malaria mosquitoes in Moscow and in the Moscow Region: cytogenetic analysis. Med Parazitol (Mosk). 2005;1:30–4 (in Russian) .
Moskaev AV, Gordeev MI, Kuzmin OV. Chromosomic composition of populations of the mosquito Anopheles messeae in the center and periphery of its geographical range. Vestnik MGOU. Nat Sci. 2015;1:29–35 (in Russian) .
WHO. Practical guidelines on malaria elimination in countries of WHO European region. Geneva: WHO-EURO; 2010 (in Russian) .
WorldClim–Global climate data. http://worldclim.org/www.worldclim.org .
Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. Very high resolution interpolated climate surfaces for global land areas. Int J Climatol. 2005;25:1965–78.
The USGS land cover institute https://archive.usgs.gov/archive/sites/landcover.usgs.gov/green_veg.html ). Accessed 26 Aug 2019.
Broxton P, Zeng X, Scheftic W, Troch P. A MODIS-based global 1-km maximum green vegetation fraction dataset. J Appl Meteorol Climatol. 2014;53:1996–2004.
Mamai II, editor. Landscapes of the Moscow region and their current state. Smolensk: Publishing House of Smolensk State University; 1997 (in Russian) .
Makhrova AG. Organized cottage communities: a new type of settlement (on the example of the Moscow region). Reg Stud. 2008;2:13–20 (in Russian) .
Macdonald G. The epidemiology and control of malaria. London: Oxford University Press; 1957.
Lysenko AJ, Semashko IN. Geography of malaria: a medical-geographical study of an ancient disease. Itogi nauki: meditsinskaya geografija. Moscow: VINITI, 1968;5-146. English translation. (in Russian) . https://www.who.int/malaria/publications/atoz/lysenko.pdf?ua=1 . Accessed 20 Nov. 2019.
Russell P, West L, Manwell R, Macdonald G. Practical malariology. 2nd ed. London: Oxford University Press; 1963.
Morgan B, Guénard B. New 30 m resolution Hong Kong climate, vegetation, and topography rasters indicate greater spatial variation than global grids within an urban mosaic. Earth Syst Sci Data. 2019;11:1083–98.
Beklemishev VN. The problem of typization of malaria foci and some types of malariogenic landscapes. Med Parazitol (Mosk). 1947;16:231–42 (in Russian) .
Moshkovsky SD. The dependence upon temperature of the speed of development of malaria plasmodia in the mosquito. Med Parazitol (Mosk). 1946;15:19–32 (in Russian) .
Bodenheimer FS. On predicting the development cycles of insects. I. Ceratitis capitata Wied. Bull Soc R Entomol Egypt. 1925;1924:147–59.
WHO. Guidelines on prevention of the reintroduction of Malaria. Cairo: WHO Regional Office for the Eastern Mediterranean; 2007.
Mironova VA, Shartova NV, Beljaev AE, Varentsov MI, Grishchenko MY. Effects of climate change and heterogeneity of local climates on the development of malaria parasite ( Plasmodium vivax ) in Moscow megacity region. Int J Environ Res Public Health. 2019;16:694.
Article PubMed Central Google Scholar
Phillips S, Anderson R, Schapire R. Maximum entropy modeling of species geographic distributions. Ecological Model. 2006;190:231–59.
Peterson AT, Bauer JT, Mills JN. Ecologic and geographic distribution of filovirus disease. Emerg Infect Dis. 2004;10:40–7.
Rose H, Wall R. Modelling the impact of climate change on spatial patterns of disease risk: sheep blowfly strike by Lucilia sericata in Great Britain. Int J Parasitol. 2011;41(7):739–46.
Du Z, Wang Z, Liu Y, Wang H, Xue F, Liu Y. Ecological niche modeling for predicting the potential risk areas of severe fever with thrombocytopenia syndrome. Int J Infect Dis. 2014;26:1–8.
Abdrakhmanov SK, Mukhanbetkaliyev YY, Korennoy FI, Sultanov AA, Kadyrov AS, Kushubaev DB, et al. Maximum entropy modeling risk of anthrax in the Republic of Kazakhstan. Prev Vet Med. 2017. https://doi.org/10.1016/j.prevetmed.2017.06.003 .
Abdrakhmanov SK, Sultanov AA, Beisembayev KK, Korennoy FI, Kushubaev DB, Kadyrov AS. Zoning the territory of the Republic of Kazakhstan as to the risk of rabies among various categories of animals. Geospat Health. 2016;11:174–81.
Mwakapeje ER, Ndimuligo SA, Mosomtai G, Ayebare S, Nyakarahuka L, Nonga HE, et al. Ecological niche modeling as a tool for prediction of the potential geographic distribution of Bacillus anthracis spores in Tanzania. Int J Infect Dis. 2019;79:142–51.
Escobar LE, Craft ME. Advances and limitations of disease biogeography using ecological niche modeling. Front Microbiol. 2016;7:1174.
Hanafi-Bojd AA, Sedaghat MM, Vatandoost H, Azari-Hamidian S, Pakdad K. Predicting environmentally suitable areas for Anopheles superpictus Grassi (s.l.), Anopheles maculipennis Meigen (s.l.) and Anopheles sacharovi Favre (Diptera: Culicidae) in Iran. Parasit Vectors. 2018;11:382.
Brown JL, Bennett JR, French CM. SDMtoolbox 2.0: the next generation Python-based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. PeerJ. 2017;5:e4095.
Pearson RG. Species’ distribution modeling for conservation educators and practitioners. In: lessons in conservation, 2010; 3:54–89. https://www.amnh.org/research/center-for-biodiversity-conservation/resources-and-publications/lessons-in-conservation/lessons-in-conservation-volume-iii . Accessed 20 Dec 2019.
Araújo MB, Pearson RG, Thuiller W, Erhard M. Validation of species-climate impact models under climate change. Glob Change Biol. 2005;11:1504–13.
Merow C, Smith M, Silander J. A practical guide to MaxEnt for modeling species’ distributions: what it does, and why inputs and settings matter. Ecography. 2013;36:1058–69.
Mironova VA. Geographical determinants of malaria re-introduction in different ecosystems: evaluation and prognosis. Ph.D. thesis. Moscow: Lomonosov Moscow State University; 2006. (in Russian) .
Moscow Region: History, Culture, Economics. Moscow, IPC “Dizain. Informatsiya. Kartografiya”. 2004. (in Russian) .
WHO. World malaria report 2018. Geneva: World Health Organization; 2018. https://www.who.int/malaria/publications/world-malaria-report-2018/en/ . Accessed 26 Nov 2019.
Kavran M, Zgomba M, Weitzel T, Petric D, Manz C, Becker N. Distribution of Anopheles daciae and other Anopheles maculipennis complex species in Serbia. Parasitol Res. 2018;117:3277–87.
Tagliapietra V, Arnoldi D, Di Luca M, Toma L, Rizzoli A. Investigation on potential malaria vectors ( Anopheles spp.) in the province of Trento, Italy. Malar J. 2019;18:151.
Linard C, Ponçon N, Fontenille D, Lambin EF. A multi-agent simulation to assess the risk of malaria re-emergence in southern France. Ecol Model. 2009;220:160–74.
Pergantas P, Tsatsaris A, Malesios C, Kriparakou G, Demiris N, Tselentis Y. A spatial predictive model for malaria resurgence in central Greece integrating entomological, environmental and social data. PLoS ONE. 2017;12:e0178836.
Article PubMed PubMed Central CAS Google Scholar
Cohen JM, Smith DL, Cotter C, Ward A, Yamey G, Sabot OJ, et al. Malaria resurgence: a systematic review and assessment of its causes. Malar J. 2012;11:122.
WHO. Regional office for Europe. Receptivity to malaria and other parasitic diseases: report on a WHO working group, Izmir, 11–15 September 1978. Copenhagen, WHO Regional Office for Europe, “1979”. https://apps.who.int/iris/handle/10665/204466/ Accessed 20 Oct 2019.
Lysenko AY, Kondrashin AV, Ejov MN. Malariology. Copenhagen: WHO/MAL/03.1089; 2003 (in Russian) .
WHO. WHO malaria terminology. 2016 (updated March 2018). https://www.who.int/malaria/publications/atoz/malaria-terminology/en/ Accessed 12 Nov 2019.
Syskova TG. The impact of migration on the incidence of parasitic diseases and the development of preventive measures. Synopsis of a Ph.D. thesis, 2005. Martsinovski Institute for Medical Parasitology and Tropical Medicine, Moscow.
Ejov MN, Sergiev VP, Baranova AM, Kurdova-Mintcheva R, Emiroglu N, Gasimov E. Malaria in the WHO European region. On the road to elimination, 2000–2015. Copenhagen: WHO EURO; 2017 (in Russian) .
Krassinets E. Illegal migration and employment in Russia. Informal network on foreign labour in Central and Eastern Europe, ILO/Luxemburg co-operation: project rer/97/mo2/lux. https://www.ilo.org/wcmsp5/groups/public/—ed_protect/—protrav/—migrant/documents/publication/wcms_201973.pdf . Accessed 12 Feb 2020.
Beljaev AE. Determinants of malaria in the middle East and North Africa. In: Casman EA, Dowlatabadi H, editors. The contextual determinants of malaria. Washington, DC: Resources for the Future Press; 2002. p. 137–66.
Kitron U. Landscape ecology and epidemiology of vector-borne diseases: tools for spatial analysis. J Med Entomol. 1998;35:435–45.
Schapira A, Boutsika K. Malaria ecotypes and stratification. Adv Parasitol. 2012;78:97–167.
Sudre B, Rossi M, Van Bortel W, Danis K, Baka A, Vakalis N, et al. Mapping environmental suitability for malaria transmission, Greece. Emerg Infect Dis. 2013;19:784–6.
Chekhov AP. Collected Works. Moscow. Goslitizdat. 1957;11:155.
The relation of malaria to altitude. Lancet, 1924; 203: 37–8.
Bakradze TL. Special features of the epidemiology of malaria in the process of its elimination in the Georgian SSR. Tbilisi: Metzniereba; 1974.
Hay SI, Noor AM, Simba M, Busolo M, Guyatt HL, Ochola SA, et al. Clinical epidemiology of malaria in the highlands of western Kenya. Emerg Infect Dis. 2002;8:543–8.
Sergiev VP, Baranova AM, Orlov VS, Mihajlov LG, Kouznetsov RL, Neujmin NI, et al. Importation of malaria into the USSR from Afghanistan, 1981–1989. Bull World Health Organ. 1993;71:385–8.
CAS PubMed PubMed Central Google Scholar
Grumm RH. The central European and Russian heat event of July–August 2010. Bull Am Meteorol Soc. 2011;92:1285–96.
Download references
Acknowledgements
Not applicable.
This research was funded by the Russian Science Foundation (Grant 17-77-20070 “Assessment and Forecast of the Bioclimatic Comfort of Russian Cities under Climate Change in the 21st Century”)
Author information
Authors and affiliations.
Faculty of Geography, Lomonosov Moscow State University, Moscow, 119991, Russia
Varvara A. Mironova, Natalia V. Shartova, Mikhail I. Varentsov & Mikhail Y. Grishchenko
WHO Global Malaria Programme, Geneva, Switzerland
Andrei E. Beljaev
A.M, Obukhov Institute of Atmospheric Physics, 3 Pyzhyovskiy Pereulok, Moscow, 119017, Russia
Mikhail I. Varentsov
Research Computing Center, Lomonosov Moscow State University, Moscow, 119991, Russia
FGBI Federal Center for Animal Health (FGBI ARRIAH), Vladimir, 600901, Russia
Fedor I. Korennoy
Faculty of Geography and Geoinformatics, Higher School of Economics, Moscow, 101000, Russia
Mikhail Y. Grishchenko
You can also search for this author in PubMed Google Scholar
Contributions
VAM, NVS and AEB conceptualized the paper and had overall responsibility for the study. VAM and AEB wrote the first draft of the manuscript. MIV provided meteorological data, carried out meteorological modeling and wrote the appropriate section of the paper. FIK implemented MaxEnt model, developed the risk map and prepared graphs. MYG and NVS carried out geospatial analysis and prepared maps. All authors took part in the preparation of the final draft of the paper. NVS, AEB and VAM edited the final draft of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Natalia V. Shartova .
Ethics declarations
Ethics approval and consent to participate, consent for publication.
All authors have given their consent for this publication.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Mironova, V.A., Shartova, N.V., Beljaev, A.E. et al. Re-introduction of vivax malaria in a temperate area (Moscow region, Russia): a geographic investigation. Malar J 19 , 116 (2020). https://doi.org/10.1186/s12936-020-03187-8
Download citation
Received : 29 December 2019
Accepted : 09 March 2020
Published : 18 March 2020
DOI : https://doi.org/10.1186/s12936-020-03187-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Vivax malaria
- Autochthonous cases
- Re-introduction
- Environmental determinants
- Climate favourability
Malaria Journal
ISSN: 1475-2875
- Submission enquiries: [email protected]
Loading metrics
Open Access
Viewpoints are opinion pieces grounded in evidence on topics of broad interest to the journal's readership.
See all article types »
Amidst spreading infectious diseases and climate change, US FDA should renew its focus on neglected tropical diseases
* E-mail: [email protected]
Affiliation US Federal Government, Rockville, Maryland, United States of America
- Mitchell Berger

Published: March 21, 2024
- https://doi.org/10.1371/journal.pntd.0012005
- Reader Comments
Citation: Berger M (2024) Amidst spreading infectious diseases and climate change, US FDA should renew its focus on neglected tropical diseases. PLoS Negl Trop Dis 18(3): e0012005. https://doi.org/10.1371/journal.pntd.0012005
Editor: Elizabeth J. Carlton, University of Colorado - Anschutz Medical Campus, UNITED STATES
This is an open access article, free of all copyright, and may be freely reproduced, distributed, transmitted, modified, built upon, or otherwise used by anyone for any lawful purpose. The work is made available under the Creative Commons CC0 public domain dedication.
Funding: The author received no specific funding for this work.
Competing interests: The authors have declared that no competing interests exist.
In 2015, the United States Food and Drug Administration (US FDA) and other Department of Health and Human Services agencies helped respond to a public health emergency concerning Zika Virus during which 5,600 cases of the virus were reported in the United States mainland and 37,000 cases in Puerto Rico and other US Territories [ 1 ]. In June 2023, the US Centers for Disease Control and Prevention alerted health providers to locally acquired malaria infections in Florida and Texas [ 2 ]. In October 2023, California (Pasadena) reported a case of dengue infection in a resident who apparently did not travel outside the United States [ 3 ]. In November 2023, an additional case report of cutaneous leishmaniasis in Texas seemed to confirm earlier suggestions that this disease had become endemic to the US [ 4 ]. These recent cases highlight one reason for US FDA to renew its past focus on developing policies and encouraging new diagnostics and treatments for neglected tropical diseases (NTDs).
Though primarily focused on the United States, US FDA considers itself to have an important global role. The agency, for example, supports a global program and international offices (see e.g., https://www.fda.gov/international-programs ). Yet, even though, according to the World Health Organization’s 2023 Global Report on Neglected Tropical Diseases, nearly 1.65 billion people worldwide needed treatment for NTDs, and even amid such case reports as above, US FDA’s response to NTDs has for many years been fairly limited in scope and prominence [ 5 ].
In a well-written recent review in this journal, Mukherjee discusses US FDA programs relevant to NTDs and discusses existing or potential efforts such as additional incentive programs, enhancing the tropical disease Priority Review Voucher program, and use of authorities such as those covering orphan products that could spur additional NTD product development [ 6 ]. Yet, thus far as Mukherjee points out, most attention has focused on tuberculosis and malaria that are considered NTDs under US FDA’s definition but not those of the World Health Organization [ 5 , 6 ]. As explained by Mukherjee and other sources, Section 524 of the US Federal Food, Drug and Cosmetic Act and current US FDA guidance documents define neglected tropical diseases. The US FDA’s definition of NTDs includes malaria, tuberculosis, dengue/dengue haemorrhagic fever, leishmaniasis, and soil-transmitted helminthiasis (e.g., hookworm) as well as “[a]ny other infectious disease for which there is no significant market in developed nations and that disproportionately affects poor and marginalized populations” as designated by the Secretary of the US Department of Health and Human Services [ 6 , 7 ]. Thus, while Zika virus, tuberculosis, and malaria are considered NTDs by US FDA, they are not included on WHO’s list. Conversely, snakebite envenoming is considered an NTD by WHO but not US FDA.
Mukherjee points to several barriers to NTD product such as limited incentives and a difficult and lengthy process for bringing products to market [ 6 ]. However, another possible reason why NTD product development within the US has for been limited is lack of significant attention to NTDs by Congress and US FDA senior leadership. US FDA’s last major Report to Congress on NTDs, for example, dates to 2011 [ 8 ]. Its most pertinent guidance on the topic has not been updated since 2014 [ 9 ]. The last major US FDA hearing or workshop on NTDs was in 2010 [ 10 ]. While it is true that other US FDA programs may have overlap with and implications for NTD efforts, lacking in US FDA’s current or recent programs and policy development is an express focus on NTDs as has been the case in the past.
There are several reasons why US FDA’s taking a strong interest in NTDs or, seen from another perspective, resuming and updating its past activities would have benefits globally, for the American people, for drug, vaccine, medical device, and other manufacturers and even for US FDA and US FDA staff.
For one thing, climate change, development, and globalization are increasing the potential range of infectious diseases [ 11 ]. One recent review, for example, concluded that nearly 60% of 375 pathogens infecting humans may be exacerbated by climate change [ 11 ]. Focus on NTDs also would be consistent with US FDA’s efforts to enhance public health preparedness and response, including development of medical countermeasures [ 12 ].
US FDA could take several steps to bolster NTD research and product development. For example, US FDA could hold a new hearing or workshop on NTDs, updating its 2010 efforts. US FDA could update its guidances related to NTDs, including especially its 2014 Neglected Tropical Diseases of the Developing World: Developing Drugs for Treatment or Prevention. US FDA also could hold a new workshop or public hearing on this topic or issue a request for information to seek public input about ways it could enhance its NTD-related policies and programs.
US FDA also could form a working group comprised of US federal agencies that includes not only the National Institutes of Health but also, among others, representatives from the US Departments of Agriculture (animal health), Defense (research and efforts to protect military and dependents) and State (global diplomacy and health), US Patent and Trademark Office, US President’s Malaria Initiative, the Advanced Research Projects Agency for Health ( ARPA-H ) (Innovation), the US Agency for International Development (global programs), the Federal Emergency Management Agency (disaster response and planning), the US State Department’s US President’s Emergency Plan for AIDS Relief (PEPFAR) program, the Department of Health and Human Services’ Administration for Strategic Preparedness and Response and its Biomedical Advanced Research and Development Authority (emergency response and new product development), and the Centers for Disease Control and Prevention that includes its One Health program.
Ideally, a workgroup or collaborative, perhaps under the auspices of the Reagan-Udall Foundation for the [US] FDA ( https://reaganudall.org/ ) or National Academies of Sciences, Engineering, and Medicine ( https://www.nationalacademies.org/about ) also could include representatives from the philanthropic and private sectors as well as medical and public health associations and state, local, tribal, and territorial public health agencies.
Even within US FDA, cross-agency partnerships and collaboration as NTDs span at least 4 US FDA Centers including those for Biologics Evaluation and Research, Devices and Radiological Health, Veterinary Medicine (i.e., Zoonoses), and Drug Evaluation and Research. CDER’s Medical Product Council could be one venue for holding such discussions as workgroups can include representatives from other Centers and Offices [ 13 ]. US FDA also can harmonize its NTD efforts with those of other national and international authorities such as the European Medicines Agency (EMA) and World Health Organization.
Recognizing that many existing drug, device, and biologic products could potentially be repurposed or repositioned for neglected tropical disease treatment, US FDA could build on and enhance its focus on drug, biologic, and medical device repurposing such as its December 2019 workshop with the National Institutes of Health and Reagan-Udall Foundation on repurposing off-patent drugs [ 14 ]. As some NTD treatments have developed over time without an ideal evidence base, US FDA, WHO, and others, including philanthropic and private sector partners, could review, where warranted, the evidence base for these treatments [ 15 ]. Through these and other steps, US FDA could significantly enhance its work on NTDs, providing critical leadership, coordination, and expertise at a time when such efforts are increasingly and greatly needed.
The author is a former consumer safety officer/senior policy analyst at U.S. FDA (CBER) and now works for another U.S. federal agency. The opinions expressed above are solely those of the author in his private capacity and should not be imputed to any public or private entity.
- 1. Government Accountability Office. Zika Supplemental Funding: Status of HHS Agencies’ Obligations, Disbursements, and the Activities Funded. Government Accountability. Office. 2018 May 14. PubMed PMID: [cited 2023 Nov 5]. Available from: https://www.gao.gov/products/gao-18-389 .
- View Article
- Google Scholar
- 5. Global Report on Neglected Tropical Diseases 2023. World Health Organization. [cited 2023 Nov 5]. Available from: https://www.who.int/teams/control-of-neglected-tropical-diseases/global-report-on-neglected-tropical-diseases-2023 .
- PubMed/NCBI
- 12. Food and Drug Administration. Medical Countermeasures Initiative. [cited 2023 Nov 5]. Available from: https://www.fda.gov/emergency-preparedness-and-response/counterterrorism-and-emerging-threats/medical-countermeasures-initiative-mcmi .
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Int J Environ Res Public Health

Effects of Climate Change and Heterogeneity of Local Climates on the Development of Malaria Parasite ( Plasmodium vivax ) in Moscow Megacity Region
Varvara mironova.
1 Faculty of Geography, Lomonosov Moscow State University, Moscow 119991, Russia; [email protected] (V.M.); moc.liamg@19ravm (M.V.); [email protected] (M.G.)
Natalia Shartova
Andrei beljaev.
2 WHO Consultant on malaria, Former WHO Advisor on malaria, WHO EMRO, Cairo 11371, Egypt; [email protected]
Mikhail Varentsov
3 A.M. Obukhov Institute of Atmospheric Physics, 3 Pyzhyovskiy Pereulok, Moscow 119017, Russia
4 Research Computing Center, Lomonosov Moscow State University, Moscow 119991, Russia
Mikhail Grishchenko
The article presents the results of a spatio-temporal analysis of the changes of the favorability of climatic conditions for the transmission of vivax malaria in the Moscow megacity and its surroundings during the period from 1977 to 2016. Using the historical temperature records at urban and rural weather stations, we calculated the key indicators of climate favorability for malaria transmission, viz . the sum of effective temperatures, the duration of the season of effective infectiveness, and a new integral index of climate favorability. We demonstrated a dramatic increase of all three indicators, which accelerated after 1984, and a high spatial heterogeneity among them. Due to the urban heat island effect, the degree of climatic favorability is especially high in the densely urbanized areas of Moscow megacity compared with the suburban and rural areas. Climatic conditions for vivax malaria in Moscow are better now than before. The season of effective infectiveness continues in the central part of the city for 25 days longer, and the integral index of climate favorability is 85% higher in comparison to mean values over the rural surroundings. The study contains an alert regarding the risk of malaria resurgence in the Moscow region in the case of the sufficient importation of cases from abroad.
1. Introduction
Human malaria is a group of four infections caused by protozoan parasites of the genus Plasmodium and transmitted by anopheline mosquitoes. On a global scale, practically all cases of malaria are caused by either P. falciparum or P. vivax . The former is the predominant of the two ( ca 90%). The share of infections caused by human parasites P. malariae and P. ovale is negligible. Humans occasionally become infected with Plasmodium species that normally infect animals, such as P. knowlesi . As of yet, there are no reports of human–mosquito–human transmission of such zoonotic species of malaria [ 1 ]. Particular parasite species have their specific strategies for survival [ 2 ], as well as their own features of pathology, epidemiology, public health significance, and amenability to control.
Malaria reintroduction in the territories that had been freed from it is one of the critical issues of malaria control in the 21st century [ 3 ]. In the pre-elimination era, malaria was endemic in most of the European countries, including a considerable part of Russia. In Europe, all of the species of malaria ever present (that is P. malariae, P. falciparum , and P. vivax ) had been eliminated in the mid-20th century, and vivax malaria was the last one to disappear. Since then, short-living episodes of the autochthonous transmission of P. vivax have been documented in a number of European countries, and, among them, Russia was the most affected.
Between 1979 and 1990, episodes of local transmission of P. vivax in Russia occurred mostly due to importation from Afghanistan by the returning Soviet soldiers [ 4 ]. Importation of malaria became a problem again after the dissolution of the Union of the Soviet Socialist republics (USSR), which triggered epidemics of vivax malaria in a number of former member states, including Tajikistan and Azerbaijan in particular. During the post-Soviet epoch, the local transmission of malaria in Russia followed ups and downs of malaria in Asiatic ex-members of USSR, with a lag of about two years [ 5 ]. Since the dissolution of the USSR in 1991, there were no autochthonous cases of malaria in Russia until 1996. An upsurge of malaria in post-Soviet states provoked secondary transmission in Russia, which happened due to the importation of malaria by migrants and often coincided with hot summer weather. From 1996 to 2009, there was a wave of transmission with more than 700 autochthonous cases. After that, only five episodic autochthonous cases occurred within Russia ( Figure 1 ).

Malaria cases in Russia. Source: Russian Federal Service for Surveillance on Consumer Rights Protection and Human Wellbeing (Rospotrebnadzor). * CIS—Commonwealth of Independent States, consisting of a part of former Soviet Republics.
The reintroduction of vivax malaria remains possible in temperate regions; this is why the question of determinants and drivers of malaria re-emergence does not lose its relevance and requires new approaches.
Malaria is often referred to as a climate-dependent disease, which is primarily because a certain sum of temperatures is needed for the complete maturation of sporozoites in mosquitoes. Plasmodium vivax requires lower temperatures for its development in the vector than other human malaria species. At temperatures between 16 °C and 18 °C, P. vivax would develop, albeit slowly, whereas the development of P. falciparum is possible only at 18 °C and above. P. falciparum used to be widespread in Europe, including the southernmost areas of Russia. In warm years, it could spread in Russia even up to 61° N [ 6 ]. However, the Palaearctic variety of P. falciparum had been probably eliminated throughout Europe by the end of the 1950s. The importation of P. falciparum from elsewhere is arguably impossible, because the mosquitoes of the Palaearctic region are insusceptible to P. falciparum varieties from the Afrotropical and Oriental regions [ 7 , 8 , 9 ]. Therefore, we discuss here the climatic conditions favoring the possible reintroduction of P. vivax only, and leave the question of P. falciparum beyond the scope of this work.
Malaria vectors are much more resistant to low temperatures than malaria parasites. Temperature affects the bionomics of Anopheles mosquitoes, such as the speed of development of the aquatic stages (which depends on the temperature of the place of breeding), the speed of blood digestion (which depends on the temperature of the resting place), and their survival in general [ 10 ]. However, the minimum temperature requirements of malaria parasites during the extrinsic part of the cycle are significantly higher than those of the mosquitoes. For example, aquatic stages of the principal malaria vector in the Palaearctic region, A. maculipennis s.l , require no less than +10 °C, and adults actively feed at this temperature, whereas stages of P. vivax in mosquitoes die out at temperatures below 16 °C. This was the explanation of the phenomenon of “anophelism without malaria” that puzzled European malariologists in the 1920s and 1930s [ 11 , 12 ].
Hence, in temperate regions, by the time of the year when the temperatures become suitable for the development of the parasite in the mosquito, active vectors are already present in ample quantities. Therefore, we focus on the temperature requirements of parasites, not the vectors, as only the former present the limiting factor.
Malaria transmission occurs both in rural and urban areas, although the rural environment is, as a rule (but not always), more favorable for malaria [ 13 ]. The reasons for this are not the same in the tropics and in temperate/subtropical areas. Generally, conditions for urban malaria are less favorable due to the paucity of suitable breeding places and widespread pollution, to which anophelines are much more sensitive than other mosquitoes. On the other hand, the urban areas are usually significantly warmer (by up to 2 to 3 °C) than their rural surroundings due to specific land-cover modifications and anthropogenic factors. Such a temperature anomaly, which is known as an urban heat island effect [ 14 , 15 ], may favor the transmission of malaria. The magnitude of the urban heat islands is comparable with the magnitude of the observed climate changes. The latter allows suggesting the significant effect of the urban temperature anomaly on the climate favorability for malaria transmission.
In temperate and subtropical climates, malaria transmission in large cities is not an exception. In recent decades, it has been repeatedly documented in New York [ 16 ], Houston [ 17 ], etc. As for Russia, there was an outbreak in Nizhny Novgorod in 1998 [ 18 ], Perm between 1998 and 2003 [ 19 ], Moscow between 1972 and 1973 and in 1981, and the largest outbreak was between 1999 and 2009 [ 20 ].
The Moscow region has faced significant climate changes over the past decades. Regional climate warming is now most pronounced since the 1970s, and is especially pronounced in the summer ( Figure 2 a,b). Between 1977 and 2016, the average rate of growth of the mean summer temperature (June–August) was 0.6 °C per decade for rural areas [ 21 ]. The Moscow megacity forms an intensive urban heat island [ 22 ], which manifests itself as a mesoscale temperature anomaly that covers the whole city and even its neighboring regions [ 21 , 23 ]. The urban–rural temperature contrasts could reach up to 13 °C in favorable weather conditions, while the annual mean temperature difference between the city center and rural surroundings is about 2 °C [ 21 , 24 ]. Urban growth in recent decades has resulted in the intensification of the urban heat island ( Figure 2 b) and caused additional amplification of the climate warming rates for urban areas [ 21 , 25 ].

Dynamics of the average summer air temperature at the Mozhaisk station between 1936 and 2016. ( a ); the dynamics of air temperature at Mozhaisk and Balchug stations and the average background temperature (averaged over the Naro-Fominsk, New Jerusalem, Klin, Dmitrov, Aleksandrov, Pavlovsky Posad, Kolomna, Serpukhov, Maloyaroslavets stations), and the heat island’s intensity, which is defined as the temperature difference between the station Balchug and average background values between 1977 and 2016. ( b ). Black dashed lines show linear trends for between 1977 and 2016. UHI is Urban Heat Island.
Hence, the Moscow megacity is a good example of a densely populated urban area, which is affected by pronounced climate changes and by a variability of local climates, and is threatened by vivax malaria outbreaks. This study is based on actual weather observations over the past 40 years. It evaluates the significance of the regional recent climate changes and the heterogeneity of local climates in the Moscow region in terms of climatic favorability for the transmission of vivax malaria.
2. Materials and Methods
2.1. study area.
Administratively, the Moscow region consists of two units, which are both plenipotentiary members of the Russian Federation viz. the Moscow city and the Moscow oblast ’.
The border between them was changed in 2012, but in the text, we refer to the pre-reform status. By the beginning of 2012, the population of the city and the oblast ’ was 11.6 million and 7.2 million inhabitants, and the areas were 2561.5 sq km and 44.329 sq km, respectively (data of Russian Federal State Statistics service) [ 26 ] ( Figure 3 ). The territories of the neighboring regions are also considered.

Moscow region geographical position.
2.2. Meteorological Data
To analyze the change in the degree of favorable climatic conditions for the extrinsic development of the malaria parasite, we used long-term observational data from 16 weather stations, which are operated under the standards of World Meteorological Organization (WMO). Three of them are located in Moscow city, including Balchug station (WMO ID 27605), which is located in a densely built area just in the city center, VDHKh station (WMO ID 27612), which is located in a park area nine km to the north from the city center, and the meteorological observatory of Lomonosov Moscow State University (MSU), which is located in another park area 7.5 km to the southwest from the city center. These three stations represent the climate of the urban environment affected by the urban heat island effect. The annual mean temperature anomaly (deviation from the mean rural value) is 2 °C for Balchug station and about 1 °C for the park stations MSU and VDHKh [ 24 ]. According to the recent detailed studies of Moscow climate features [ 21 , 23 ], the temperature observations at Balchug station are representative of the whole central part of the city, and of some especially densely built areas beyond. Most of the other built areas within the city are typically cooler than the city center, but warmer than the urban parks, and, moreover, rural areas. For rural areas, we use data for 13 weather stations that are located in Moscow oblast ’ or in the neighboring parts of Vladimir oblast ’ and Kaluga oblast ’: Volokolamsk (WMO ID 27502) Klin (27417), Dmitrov (27419), Pavlovsky Posad (27523), Cherusti (27538), New Jerusalem (27511), Mozhaisk (27509), Naro-Fominsk (27611), Serpukhov (27618), Kolomna (27625), Maloyaroslavets (27606), Aleksandrov (27428), and Petushki (27526). Listed stations are located in rural areas or at the edges of the small towns, so they represent almost natural environments in different parts of the Moscow region.
The further analysis is based on the time period from 1977 to 2016. It was selected according to the availability of observation data. This time period also allows examining a rather monotonous climate-warming pattern (see Figure 2 ), which simplifies the analysis of trends.
A database of temperature observations on three-hourly intervals (at zero hours, three hours, six hours, 12 h, 15 h, 18 h, and 21 hours UTC) for the period between 1977 and 2016 was created on the basis of archives of RIHMI-WDC (Russian Institute for Hydrometeorological Information—World Data Center), Central Department for Hydrometeorology and Environmental Monitoring, website “Weather Schedule”, and the meteorological observatory of Moscow State University.
Average daily temperatures (ADT) were calculated based on observations on three-hourly intervals. The missing data ratio did not exceed 5% for all the weather stations considered, and we applied a gap-filling algorithm to restore missing ADT values. This algorithm is based on the method of [ 27 ]. Missing values for a given station were filled using the multiple linear regressions between existing ADT values for this station and a number of the nearest stations. Such an approach allows restoring missing values while taking into account the typical local climate features.
In order to quantify the baseline rural conditions, we consider the mean rural ADT, averaging nine rural stations (Klin, Dmitrov, Alexandrov, Pavlovsky Posad, Kolomna, Serpukhov, Maloyaroslavets, Naro-Fominsk, and New Jerusalem).
2.3. Quantitative Indicators of Climatic Favorability for Malaria Transmission
As mentioned above, the spread of malaria in temperate areas is limited by the temperature requirements of Plasmodia at the extrinsic part of their life cycle. For the development of the sporozoites of P. vivax to occur, the ADT should be no lower than +16 °C. If this condition is met, the ADTs excesses over the threshold of 14.5 °C are summed up, and when the sum reaches 105 °C, this indicates that the sporogony is over [ 28 ]. If ADTs fall below 16 °C, this particular day is excluded. In this case, sporogony is interrupted, but it can resume if the break is shorter than a week. It is important to note that when the ADT is close to the lower threshold, the maturation of sporozoites goes so slowly that the time required surpasses the life span of mosquitoes, and the possibility of transmission becomes negligible. At higher ADTs, the development of sporozoites continue to accelerate up to about 30 °C. Temperatures above 30 °C are deleterious for both parasites and mosquitoes.
The structure of each malaria season over the 40-year period was evaluated according to [ 29 ]. This method is recommended by the WHO [ 30 ], and is optimal for temperate areas where malaria transmission is possible for only one or a few months per year. At the same time, this method is of little use for other areas such as tropical climates, where ADTs always or almost always permit the development of sporozoites, and where the dynamics of malaria are often defined by rainfall. It calls for other methods of forecasting, such as for example, the Gradient Model Risk Index, which is based on a detailed analysis of the environmental conditions for the vector (the dynamics of vector populations, vector’s ability to transmit the infection), and, occasionally, taking into account human cases [ 31 , 32 , 33 , 34 , 35 ].
The following elements of the malaria season have been identified and calculated ( Figure 4 ):
- Season of manifestations: the period of the year during which most malaria manifestations start. It begins before the activity of mosquitoes, since a significant proportion of vivax malaria cases occurs nine to 12 months after the inoculation due to the long latency period [ 36 ];
- Season of effective temperatures (SET): the period of the year during which ADTs consistently keep above the parasite development threshold (+16 °C for P. vivax );
- Season of effective infectiveness of mosquitoes (SEI): the period during which a mosquito infected on a person can generate mature sporozoites. It starts at the same moment as SET, but stops earlier; this is because mosquitoes that contracted parasites nearer to the end of the SEI do not have enough time at a favorable temperature to generate fully developed parasites (the infection of mosquitoes is not effective in this case).
- Malaria transmission season: the period during which mosquitoes with mature sporozoites can infect humans. It starts later than the SEI by the lag equal to the duration of the period for development of a single generation of sporozoites, which may take three to four weeks at the beginning of the warm period.

Interrelation of the elements of malaria season (e.g., in Central Russia), adopted from [ 30 ].
The timing of events of the malaria season gives a useful basis for comparisons and forecast for malaria control in those situations in which the transmission is deemed possible. However, this approach does not differentiate between situations in which the above calculations indicate that the transmission seems impossible. In fact, when the calculations predict the impossibility of the transmission, this may be accounted for by different circumstances. There would be occasions when the probability of the maturation of sporozoites is definitively zero (say, if ADT does not reach 16° all over the period). On the other hand, there would be occasions when the sums of temperatures come very near to the required values. On such occasions, due to the spatial variation of microclimatic conditions, in which mosquitoes dwell during their incessant journeys between the feeding and breeding places (which may be only coarsely appreciated by the temperatures at the meteorological stations), vectors may occasionally find themselves in warmer conditions than those predicted by the records. As a result, the maturation of sporozoites may occasionally take place, despite the prediction.
In order to take into account those marginal situations when the probability of the transmission is slightly above zero, we propose the following integral index of favorability of thermal conditions, which is based on the assumptions that in order to complete one infection turnover (from the ingestion of gametocytes by a mosquito to the mosquito becoming infective), no less than a sum of effective temperatures (ET) of 105 °C is required. When it happens, this may generate a small number of secondary cases, but for the transmission to become established, another round of maturation of sporozoites would be required. In other words, no less than 210 °C would be needed (that is notwithstanding that at least additional 10 days would be needed for an infected person to generate gametocytes, but since this span is temperature-independent, it cannot be absorbed into the model).
K f be the index of favorability of thermal conditions;
S obs be the observed sum of ET;
S min be the required sum of ET for a particular species of malaria: 210 °C in case of P. vivax
The interpretation of particular values of the index is shown in Table 1 .
The extent of favorability of meteorological conditions for autochthonous transmission of malaria.
Finally, temporal trends and their significance were analyzed for individual malaria seasons. For each station, the slope coefficient of the linear trend k, the coefficient of determination R 2 , and the confidence level p at which the trend would be statistically significant by the Student’s criterion have been calculated.
Over the past 40 years (1977–2016), all three indicators of climatic suitability in relation to P. vivax ( viz ., the sums of effective temperatures, the duration of the season of effective infectivity, and the index of favorability (K f )) continued to rise.
3.1. Sums of Effective Temperatures (ET)
The dynamics of the sums of ETs accumulated is presented in Figure 5 .

Sums of effective temperatures accumulated in Moscow city and suburban areas during the years 1977 to 2016 (dashed lines show linear trends).
The following salient points should be noted:
- Sums of ETs for the city center remain well above the average rural values every year, whereas the values for the park zones stay in between the two.
- For the city center, temperatures allowed the full development of at least one generation of sporozoites every year. For individual rural stations, there were years when the temperatures remained below the threshold of 105 °C, i.e., the temperatures were insufficient to ensure the transmission of P. vivax . The average rural values moved below the threshold only twice, in 1978 and in 1984 (before 1977 not shown on the graph); there were regular cases of years when temperatures remained prohibitive to the development of the parasite.
- There was a growing trend of sums of ETs, especially after 1997. For average rural values, the coefficient of the linear trend k equals 65 °C/10 years, which is statistically significant ( R 2 = 0.31, p < 0.01). This trend is further emphasized by the sums of ETs reaching below the threshold only once after 1997, in the year 2015, and in one place, i.e., in Volokolamsk (which is known as a “cold” station, where this indicator is always lower than in the other points).
- The growth rate of the sums of ET for the urban stations exceeded the growth rate in the rural stations: the trend slope coefficient and its significance for these two stations was greater than for the rural ones, revealing the intensification of the urban heat island.
The geographical distribution of the phenomenon is shown in Figure 6 . Here, the average values of the sums of ET are shown by shadings, and the tempo of their increase during the 40-year period is shown by the size of the circles (a similar approach is applied further for the other two indicators in figures 8 and 10). A marked spatial heterogeneity may be seen, with the values being consistently higher in Moscow city and in the eastern parts of the region. For Moscow city, this may be explained by the effect of the urban heat island; for the east of Moscow region, by the rise in the continentality of the climate: west to east, the winters grow colder, but summers are hotter. At the same time, the rate of change of the indicator is more significant in the central and western parts of the region.

Spatial distribution of changes in the sum of effective temperatures in the Moscow region in 1977–2016 (figures for each station indicate the average amount of effective temperature, or ET, for the period).
3.2. Duration of the Season of Effective Infectivity (SEI)
Over the period of 40 years, the duration of the SEI displays a similar tendency of rise ( Figure 7 ). This is true for all the points under consideration, but there is a marked variation between them. During the same given year, the difference may be up to 37 days. The SEI is longer in Moscow city than at the periphery, and longer in the eastern and southern parts of the Moscow oblast ’ than elsewhere. At the same time, the rate of increase in the duration of the season is more significant in its western and southwestern parts.

Change in the duration of season of effective infectivity (SEI) at urban and suburban stations, in comparison with the average background conditions in the Moscow region in 1977–2016 (dashed lines show linear trends, thin gray lines indicate the rest of the analyzed stations).
Balchug station in the city center has consistently demonstrated the longest SEI duration compared with all other stations (67.1 days on the average for the whole period of 40 years). However, in contrast to the sum of ET, the tendencies to increase the duration of the season in the city center are less pronounced than those in the rural areas ( k = 8.2 days/10 years, which is less than in any of the stations considered; R 2 = 0.27) (for rural areas k = 14.1 days/10 years; R 2 = 0.40) ( Figure 8 ). It is probable that a further increase in the duration of the SEI becomes impossible, since the SEI occupies most of the warm period of the year anyway, due to the influence of the urban heat island. In other words, the longer the SEI, the less is the sensitivity of malaria to further climate warming. This pattern, though with some exceptions, is also shown by rural stations.

Spatial distribution of changes in the duration of the SEI in the Moscow region (the numbers for each station indicate the average duration of the SEI for the period).
There is a tendency for the entire Moscow region for a shift of the date of the beginning of the SEI to an earlier date, but the significance of these trends is small. In the rural areas, on the average, this date advanced by k = −6 days/10 years ( R 2 = 0.16, p = 0.013). In other words, the average date of the beginning of the SET was the first week of June at the beginning of the 40-year observation period, and the last week of May at the end. Within the limits of Moscow city, the SEI always starts earlier than in the rural areas; the southern parts of Moscow oblast’ are also marked by earlier dates. However, the spatial variability is not so great, with the maximum differences within the whole of the area not exceeding nine days.
The date of the end of the SEI for the rural average was the first half of July at the beginning of the 40-year period, and the end of July/the beginning of August at its end. The trends of the SEI ending later over the years were more pronounced than the trends of SEI commencing earlier. For the rural average, k = 7 days/10 years, R 2 = 0.26, p < 0.01.
The spatial variability of the dates of the end of the SEI largely repeats the patterns of the change in its beginning and its total duration: the season ends later in the southern parts of the region and in Moscow city. In other words, warmer autumns are the main contributor to the increase in the duration of the malaria season.
3.3. Index of Favorability (K f )
The change in the degree of favorability of the thermal regime is better demonstrated by spatio-temporal analysis of the integrated index K f . The graph of K f . ( Figure 9 ) clearly confirms the conclusions made earlier for the two other indicators.

Changes in the K f (index of favorability) in the Moscow region between 1977 and 2016 (dashed lines show linear trends, and thin gray lines indicate the rest of the analyzed stations).
First, the climate of the Moscow city (especially of its center) is much more favorable for malaria than that of the periphery. Second, there is a significant ( p < 0.01) trend of increase of K f , but its speed is very variable ( Figure 10 ).

Spatial distribution of changes in the K f (index of favorability).
When analyzing data by the 10-year periods, there is a noticeable progressive increase in the degree of favorability of the climate ( Figure 11 ). Whereas the change was insignificant in the period from 1977 to 1996, it is more noticeable during the period from 1997 to 2016. At the same time, for the western and northern (less continental) parts of the region, the greatest changes related to between 1997 and 2006, especially in the Klin, Mozhaisk, and New Jerusalem stations, while for the rest of the territory, the shift was more pronounced during the period from 2007 to 2016. Finally, due to the intensification of the heat island, the growth trend of the index was most pronounced in the city center, especially during the last decade.

Changes in the degree of favorability of thermal regime for the development of the vivax malaria parasite in the Moscow region over the 10-year periods between 1977 and 2016.
These changes are also reflected in a considerable growth trend of the K f index for all the stations in the Moscow region, as well as its variation within the region ( Figure 11 ).
4. Discussion
A great number of scenarios depicting the changes inflicted by global warming have been published during the past three decades. By and large, they predict that the global warming ipso facto would result in a deterioration of the malaria situation.
However, these works did not always address the following:
The two main species of human malaria are rather distant ecologically: that is why we consider only one species, P. vivax ;
The limiting factors of malaria are not the same in the tropics and in the temperate areas (rainfall versus temperature, respectively): that is why we examine a particular case of environment of Central Russia;
In temperate areas, malaria is limited by the requirements of the parasite, and not those of the vector: that is why we do not consider the vector.
There has been a considerable number of studies on the influence of climatic change on the future malaria situation. They are based on various prognostic scenarios that appear more or less realistic. For instance, Lindsay et al. [ 37 ] provided risk maps indicating the territory that was climatically suitable for vivax malaria distribution, which nowadays includes central and southern England. This territory will probably increase in extent in the future, reaching southwestern Scotland by 2030. According to this scenario, in some places in southern England, the duration of malaria transmission season may exceed three to four months.
Caminade et al. [ 34 ] forecasted an overall global net increase in climate suitability for malaria transmission and an increase in the population at risk from the 2050s to the 2080s. They believed that, according to some scenarios, malaria epidemic belt may shift northward over central–northern Europe, Russia, northern Asia, and northern America. It is specially highlighted in their work that the results of such prognoses are quite uncertain due to differences in the initial malaria impact models that were selected for the prognosis, especially over the periphery of the malaria distribution range. Unfortunately, in this paper the authors regarded malaria as a single entity, ignoring the differences in the temperature requirements of particular parasite species, which may have invalidated their prognosis.
On the contrary, some forecasters doubted a climate-driven increase of the area of distribution of malaria. For example, Semenov et al. [ 38 ] suggested that climate change is unlikely to lead to considerable changes in the territory suitable for malaria transmission in the Russian Federation.
There was an assumption supported by many researchers that while climatic factors may favor autochthonous transmission by increasing vector densities and accelerating the parasite development, the re-emergence of malaria in Europe (e.g. temperate regions) would hardly occur due to other counteractive factors, primarily the socioeconomic changes in land-use and agriculture practices, and effective functioning of the public health system [ 37 , 39 , 40 , 41 ].
We feel that the climatic prerequisites should be considered separately from the other prerequisites e.g. those related to socioeconomic factors and public health. The availability of climatic prerequisite is a conditio sine qua non , but their presence does not automatically mean that the area is at malaria risk. That is why we limit ourselves by analyzing climatic prerequisites only.
The real danger of occasional outbreaks following the importation of malaria was demonstrated in a recent episode of vivax malaria transmission in Greece [ 42 ]. Climate change may act as a trigger factor for malaria reintroduction in countries with insufficient malaria surveillance, uncontrolled migrations, and a lack of malaria awareness among health professionals and travelers. Van Lieshout et al. [ 43 ] considered Russia among the “countries where malaria remains at a low level despite poor control efforts” with previously “effective control program [having] declined in recent years”. In the authors’ view, such countries may be migrating toward a high malaria status, and it is unlikely that they will have “the structural or economic capacity to cope with any increases in malaria that climate change will bring”. In this regard, it can be concluded that the authors consider climate change to be an important cause of the deterioration of malaria status. However, we feel that malaria control in Russia, in fact, has been quite reasonable in the past, and has not changed much in strategy and scope since the period of massive importation of malaria from Afghanistan in the 1980s. This “low level” was an achievement by itself in view of an unprecedented influx of infected individuals from Tajikistan and Azerbaijan experiencing severe epidemics at that time, which was aggravated by climatic change.
In the case of Russia, the reintroduction of malaria manifested itself in the most aggressive form in the Moscow region [ 44 ]. Historically, thermal conditions for the transmission of tertian malaria have been, in general, moderately favorable in Moscow region, although there were years when the transmission was impossible due to cold summers. The infection was endemic in this area, including the megapolis (which means that its transmission was observed practically annually) right until its elimination in the mid-1950s [ 45 , 46 , 47 , 48 ].
Thermal conditions started to change in the region since the beginning of the 1980s. In 1981, thermal conditions for malaria were better than ever. Since then, a rising trend has been observed ( Figure 8 ). This is demonstrated by the SEI becoming longer. This prolongation is due, to some extent, to an earlier beginning of SEI, but is mostly due to its delayed ending. The index of favorability K f that we proposed is also on the rise, and seems to be a reliable indicator of the quality of weather in relation to malaria.
The trends of the three indicators is generally consistent with the general warming of the Moscow region, which has accelerated since the early 1990s, with an increase in summer temperature and an intensification of the summer urban heat island [ 21 ].
These indicators, as well as the speed of their change, demonstrated a spatial heterogeneity. The climatic conditions of Moscow city and, especially, of its central part are much more favorable for the development of the malaria parasite than the conditions in rural areas. This is probably due to the influence of the urban heat island. However, better thermal conditions might be counterbalanced by the deterioration of the conditions for mosquito proliferation, due to the urbanization and pollution.
For rural areas, conditions are more favorable in relation to malaria in the south than in the north of the oblast ’, which is due to the general warming of the climate in southern direction. At the same time, the conditions are more favorable in the east than in the west, due to a more continental climate in the east. As a result of the interaction of these factors, the thermal conditions for malaria are more favorable in the city of Moscow and least favorable in the western and northern parts of the Moscow oblast ’.
Besides the increase in the favorability of climatic conditions for malaria parasites, warming may also affect the vector populations. The phenology of urban mosquitoes demonstrates a shift to earlier terms comparing rural and suburban areas, and this was associated with an urban heat island effect, which raised urban air and water temperatures [ 49 ]. However, the increase of climatic favorability for malaria vectors per se doesn’t lead to any deterioration of the malaria situation. This is because malaria transmission is limited by the thermal requirements of the parasite, which are narrower than those of the vector. This is often ignored by some researchers who have equated the climate-driven vectors’ range transformations and the changes in potential malaria range [ 50 , 51 ].
It is generally believed that the causes of decline of malaria in Europe during the first half of the 20th century were related mostly to socioeconomic improvements, whereas the changes in climate and land use played a less important role [ 52 ]. However, it seems that the climatic changes may play a more significant role in malaria resurgence nowadays.
It is true that the causes for the resurgence of malaria transmission in the Moscow region after about two decades of its total absence obviously point to the widespread epidemics in Central Asia and in Azerbaijan, on the background of intensive labor migration. However, this process was likely to have been modulated by climatic change.
As it was mentioned above, the transmission of vivax malaria in Moscow city and oblast ’ lasted from 1999 to 2008. During this period, there were four years with unusually hot summers, viz . 1999, 2002, 2007, and 2010. During these warm summers, important epidemiological events took place. In 1999, malaria transmission restarted, and in 2002, it reached its peak. At the same time, nothing remarkable happened during the hot summers of 2007 and 2010. This is probably due to a radical improvement of the situation in Central Asia and Azerbaijan, and the cessation of the importation of malaria in Russia [ 48 ].
Climate change may act as a trigger factor for malaria reintroduction in countries with insufficient malaria surveillance, uncontrolled population movement, and lack of malaria awareness among health professionals and travelers. Despite the predominant perception that urbanization would rather decline the possibilities of malaria transmission in big cities mostly due to socioeconomic improvements [ 52 , 53 , 54 ], the lessons being learned from the recent re-emergence of vivax malaria in Moscow region, where the degree of antimalaria preparedness was traditionally high), demonstrate that malaria transmission can restart due to some triggering factors, such as climate change.
5. Conclusions
In this research, we have demonstrated the patterns of spatial variability and long-term dynamics of the climatic favorability to malaria in Moscow megacity and its surroundings. Due to the climate warming, the climate of the region has become significantly more conducive to a new resurgence of malaria. This mandates the continuation of antimalaria preventive activities, despite the interruption of malaria transmission that had been achieved. The most favorable climatic conditions were found within Moscow megacity, especially in the city center, which is warmer than the rural surroundings due to the urban heat island effect. In the central part of the city, the season of effective infectiveness is longer by 25 days, and the integral index of climate favorability is higher by 85% in comparison to mean values over the rural surroundings. Moreover, the observed intensification of the urban heat island additionally amplifies the rates of summer climate warming and the growth of favorability for malaria transmission. Such favorable conditions seem to be mostly counterbalanced by the hostility of the highly urbanized environment toward the anopheline vector. The presented results show the importance of taking the local climatic features of urban environment into consideration in malaria studies, as well as monitoring antimalaria preventive activities.
Acknowledgments
The automated calculation algorithm was developed by V.N. Krainov, an engineer at the Department of Biogeography, Faculty of Geography, Lomonosov Moscow State University.
Author Contributions
Conceptualization, V.M., N.S. & A.B.; Methodology, N.S., M.V. & A.B.; Software, M.V. & M.G.; Validation, N.S., M.V. & M.G.; Formal Analysis, N.S., M.V. & M.G.; Investigation, V.M., N.S. & A.B.; Resources, M.V. & M.G.; Data Curation, M.V. & M.G.; Writing—Original Draft Preparation, V.M. & N.S.; Writing—A.B.; Visualization, M.V. & M.G.; Supervision, V.M., N.S. & A.B.; Project Administration, N.S.; Funding Acquisition, N.S.
This research was funded by the Russian Science Foundation (Project No. 17-77-20070 “Assessment and Forecast of the Bioclimatic Comfort of Russian Cities under Climate Change in the 21st Century”).
Conflicts of Interest
The authors declare no conflict of interest.
We’re in Myanmar right now and it’s SO epic… click here to follow along on Instagram.
- Meet the Team
- Work with Us
- Czech Republic
- Netherlands
- Switzerland
- Scandinavia
- Philippines
- South Korea
- New Zealand
- South Africa
- Budget Travel
- Work & Travel
- The Broke Backpacker Manifesto
- Travel Resources
- How to Travel on $10/day
Home » Europe » Moscow
EPIC MOSCOW Itinerary! (2024)
Moscow is the heart of Mother Russia. Just the mention of this city conjures images of colorful bulbous pointed domes, crisp temperatures, and a uniquely original spirit!
Moscow has an incredibly turbulent history, a seemingly resilient culture, and a unique enchantment that pulls countless tourists to the city each year! Although the warmer months make exploring Moscow’s attractions more favorable, there’s just something about a fresh snowfall that only enhances the appearance of the city’s iconic sites!
If you’re a first-time visitor to Moscow, or simply wanting to see as much of the city as possible, this Moscow itinerary will help you do just that!

Unlock Our GREATEST Travel Secrets!
Sign up for our newsletter and get the best travel tips delivered right to your inbox.
Best Time To Visit Moscow
Where to stay in moscow, moscow itinerary, day 1 itinerary in moscow, day 2 itinerary in moscow, day 3 and beyond, staying safe in moscow, day trips from moscow, faq on moscow itinerary.
Here is a quick look at the seasons so you can decide when to visit Moscow!
The summer months (June-August) are a great time to travel to Moscow to take advantage of the enjoyable mild temperatures. This is considered peak travel season. Bear in mind that hotel prices rise along with the temperatures!

If you’re planning a trip to Moscow during fall (September-November) try to plan for early fall. This way the temperatures will still be pleasant and winter won’t be threatening.
Russian winters (December-February) are not for the faint of heart as Napoleon learned to his peril. Some days the sun will be out for less than an hour, and snow is guaranteed. Although winters are exceptionally cold, this is when you’ll get a true glimpse of the Moscow experience!
The best time to visit Moscow is during spring (March-May). The temperatures will begin to creep up and the sun begins to shine for significant portions of the day. Hotel rates will also have yet to skyrocket into peak ranges!

With a Moscow City Pass , you can experience the best of Moscow at the CHEAPEST prices. Discounts, attractions, tickets, and even public transport are all standards in any good city pass – be sure invest now and save them $$$ when you arrive!
Moscow is a large city with many accommodation options to choose from. Staying in a location that fits with your travel plans will only enhance your Moscow itinerary. Here is a brief introduction to a few great areas of the city we recommend checking out!
The best place to stay in Moscow to be close to all the action is Kitay-Gorod. This charming neighborhood will put you within walking distance to Moscow’s famous Red Square, thus cutting down on travel time. This will allow you to see more of the city in a shorter amount of time!

It’s surrounded by restaurants, cafes, bars, and shops. If you’re a first-time visitor to Moscow, or just planning a quick weekend in Moscow, then this area is perfect for you!
Another great area to consider is the Zamoskvorechye district. This area of the city offers a blend of new and old Moscow. It has an artsy vibe and there are plenty of fun sites you can explore outside of the main touristy areas of Moscow.
Of course, as in all areas of Moscow, it’s close to public transportation that will quickly connect you with the rest of the city and make your Moscow itinerary super accessible!
Best Airbnb in Moscow – Exclusive Apartment in Old Moscow

Modern and cozy, this apartment is in the heart of Old Moscow. Bordering the Basmanny and Kitay-Gorod districts, this two-bedroom flat is walking distance to the Kremlin and Red Square. Safe, quiet, and comfortable, this is the best Airbnb in Moscow, no question!
Best Budget Hotel in Moscow – Izmailovo Alfa Hotel

The Izmailovo Alfa Hotel is a very highly rated accommodation that provides all the components necessary for a comfortable trip to Moscow. There is an on-site restaurant, bar, fitness center, and an airport shuttle service. The rooms are modern and spacious and are equipped with a TV, heating/air conditioning, minibar, and more!
Best Luxury Hotel in Moscow – Crowne Plaza Moscow World Trade Centre

If you’re touring Moscow in luxury, the Crowne Plaza Moscow World Trade Centre is the hotel for you! Elegantly furnished rooms are equipped with a minibar, flat-screen TV, in-room safes, as well as tea and coffee making facilities! Bathrooms come with bathrobes, slippers, and free toiletries. There is also an onsite restaurant, bar, and fitness center.
Best Hostel in Moscow – Godzillas Hostel

Godzillas Hostel is located in the center of Moscow, just a short walk from all the major tourist attractions and the metro station. Guests will enjoy all the usual hostel perks such as self-catering facilities, 24-hour reception, Free Wi-Fi, and security lockers. This is one of the best hostels in Moscow and its wonderful social atmosphere and will make your vacation in Moscow extra special!
Godzillas Hostel is one of our favourites in Moscow but they’re not taking guests right now. We’re not sure if they’re closed for good but we hope they’ll come back soon.
An important aspect of planning any trip is figuring out the transportation situation. You’re probably wondering how you’re going to get to all of your Moscow points of interest right? Luckily, this sprawling city has an excellent network of public transportation that will make traveling a breeze!
The underground metro system is the quickest and most efficient way to travel around Moscow. Most visitors rely exclusively on this super-efficient transportation system, which allows you to get to pretty much anywhere in the city! It’s also a great option if you’re planning a Moscow itinerary during the colder months, as you’ll be sheltered from the snow and freezing temperatures!

If you prefer above-ground transportation, buses, trams, and trolleybuses, run throughout the city and provide a rather comfortable alternative to the metro.
Moscow’s metro, buses, trams, and trolleybuses are all accessible with a ‘Troika’ card. This card can be topped up with any sum of money at a metro cash desk. The ticket is simple, convenient, and even refundable upon return to a cashier!
No matter which method you choose, you’ll never find yourself without an easy means of getting from point A to point B!
Red Square | Moscow Kremlin | Lenin’s Mausoleum | St. Basil’s Cathedral | GUM Department Store
Spend the first day of your itinerary taking your own self guided Moscow walking tour around the historic Red Square! This is Moscow’s compact city center and every stop on this list is within easy walking distance to the next! Get ready to see all of the top Moscow landmarks!
Day 1 / Stop 1 – The Red Square
- Why it’s awesome: The Red Square is the most recognizable area in Moscow, it has mesmerizing architecture and centuries worth of history attached to its name.
- Cost: Free to walk around, individual attractions in the square have separate fees.
- Food nearby: Check out Bar BQ Cafe for friendly service and good food in a great location! The atmosphere is upbeat and they’re open 24/7!
The Red Square is Moscow’s historic fortress and the center of the Russian government. The origins of the square date back to the late 15th century, when Ivan the Great decided to expand the Kremlin to reflect Moscow’s growing power and prestige!
During the 20th century, the square became famous as the site for demonstrations designed to showcase Soviet strength. Visiting the Red Square today, you’ll find it teeming with tourists, who come to witness its magical architecture up close!

The square is the picture postcard of Russian tourism, so make sure to bring your camera when you visit! No matter the season, or the time of day, it’s delightfully photogenic!
It’s also home to some of Russia’s most distinguishing and important landmarks, which we’ve made sure to include further down in this itinerary. It’s an important center of Russia’s cultural life and one of the top places to visit in Moscow!
In 1990, UNESCO designated Russia’s Red Square as a World Heritage site. Visiting this historic site is a true bucket-list event and essential addition to your itinerary for Moscow!
Day 1 / Stop 2 – The Moscow Kremlin
- Why it’s awesome: The Moscow Kremlin complex includes several palaces and cathedrals and is surrounded by the Kremlin wall. It also houses the principal museum of Russia (the Kremlin Armory).
- Cost: USD $15.00
- Food nearby: Bosco Cafe is a charming place to grat a casual bite to eat. They have excellent coffee and wonderful views of the Red Square and the Moscow Kremlin!
The iconic Moscow Kremlin , also known as the Kremlin museum complex, sits on Borovitsky Hill, rising above the Moscow River. It is a fortified complex in the center of the city, overlooking several iconic buildings in the Red Square!
It’s the best known of the Russian Kremlins – citadels or fortress’ protecting and dominating a city. During the early decades of the Soviet era, the Kremlin was a private enclave where the state’s governing elite lived and worked.
The Kremlin is outlined by an irregularly shaped triangular wall that encloses an area of 68 acres! The existing walls and towers were built from 1485 to 1495. Inside the Kremlin museum complex, there are five palaces, four cathedrals, and the enclosing Kremlin Wall with Kremlin towers.
The Armoury Chamber is a part of the Grand Kremlin Palace’s complex and is one of the oldest museums of Moscow, established in 1851. It showcases Russian history and displays many cherished relics. Definitely make sure to check out this museum while you’re here!

The churches inside the Moscow Kremlin are the Cathedral of the Dormition, Church of the Archangel, Church of the Annunciation, and the bell tower of Ivan Veliki (a church tower).
The five-domed Cathedral of the Dormition is considered the most famous. It was built from 1475–1479 by an Italian architect and has served as a wedding and coronation place for great princes, tsars, and emperors of Russia. Church services are given in the Kremlin’s numerous cathedrals on a regular basis.
The Grand Kremlin Palace was the former Tsar’s Moscow residence and today it serves as the official workplace of the President of the Russian Federation (Vladimir Putin seems to have bagged that title for life) .
Insider Tip: The Kremlin is closed every Thursday! Make sure to plan this stop on your Moscow itinerary for any other day of the week!
Day 1 / Stop 3 – Lenin’s Mausoleum
- Why it’s awesome: The mausoleum displays the preserved body of Soviet leader Vladimir Lenin .
- Cost: Free!
- Food nearby: Khinkal’naya is a charming Georgian restaurant with vaulted ceilings and exposed brick. It’s a popular place with locals and right next to the Red Square!
Lenin’s Mausoleum, also known as Lenin’s Tomb, is the modernist mausoleum for the revolutionary leader Vladimir Lenin. It’s located within the Red Square and serves as the resting place for the Soviet leader! His preserved body has been on public display since shortly after his death in 1924.
It’s located just a few steps away from the Kremlin Wall and is one of the most controversial yet popular Moscow attractions!
Admission is free for everyone, you’ll only need to pay if you need to check a bag. Before visitors are allowed to enter the mausoleum, they have to go through a metal detector first. No metal objects, liquids, or large bags are allowed in the mausoleum!

Expect a line to enter the building, and while you’re inside the building, you’ll be constantly moving in line with other visitors. This means you won’t be able to spend as long as you’d like viewing the mausoleum, but you’ll still be able to get a good look. Pictures and filming while inside the building are strictly prohibited, and security guards will stop you if they see you breaking this rule.
The mausoleum is only open on Tuesday, Wednesday, Thursday, and Saturday – unless it’s a public holiday or a day scheduled for maintenance. The hours it’s open for each day are limited, make sure to check online before you visit to make sure you can fit this into your Moscow itinerary for that day!
Insider Tip: The Lenin’s Museum is there for people to pay their respect; remember to keep silent and move along quickly, it’s not intended for people to congregate around. Also, men are not allowed to wear hats and everyone must take their hands out of their pockets when inside the building.
Day 1 / Stop 4 – St. Basil’s Cathedral
- Why it’s awesome: A dazzling designed cathedral that showcases Russia’s unique architecture. This cathedral is one of the most recognizable symbols of the country!
- Cost: USD $8.00
- Food nearby: Moskovskiy Chaynyy Klub is a cozy cafe serving food items and pipping hot tea; it’s the perfect place to go if you’re visiting Moscow during the winter months!
Located in the Red Square, the ornate 16th-century St. Basil’s Cathedral is probably the building you picture when you think of Moscow’s unique architecture. Its colorful onion-shaped domes tower over the Moscow skyline!
The cathedral was built from 1555-1561 by order of Tsar Ivan the Terrible. It was designed with an iconic onion dome facade and enchanting colors that captivate all who see it. Fun fact: If you’re wondering why Russian churches have onion domes, they are popularly believed to symbolize burning candles!
This iconic cathedral has become a symbol of Russia due to its distinguishing architecture and prominent position inside the Red Square. It’s one of the most beautiful, wonderful, and mesmerizing historical cathedrals in the world!

The interior of the church surprises most people when they visit. In contrast to the large exterior, the inside is not so much one large area, but rather a collection of smaller areas, with many corridors and small rooms. There are 9 small chapels and one mausoleum grouped around a central tower.
Visiting the inside is like walking through a maze, there are even small signs all around the cathedral tracing where to walk, and pointing you in the right direction! The walls are meticulously decorated and painted with intricate floral designs and religious themes.
The church rarely holds service and is instead a museum open for the public to visit.
Insider Tip: During the summer months the line to go inside the cathedral can get quite long! Make sure to arrive early or reserve your tickets online to guarantee quick access into the cathedral!
Day 1 / Stop 5 – GUM Department Store
- Why it’s awesome: This is Russia’s most famous shopping mall! It’s designed with elegant and opulent architecture and provides a real sense of nostalgia!
- Cost: Free to enter
- Food nearby: Stolovaya 57 is a cafeteria-style restaurant with a variety of inexpensive Russian cuisine menu items including soups, salads, meat dishes, and desserts. It’s also located inside the GUM department store, making it very easily accessible when you’re shopping!
The enormous GUM Department Store is located within the historic Red Square. It has a whimsical enchantment to it that sets it apart from your typical department store.
A massive domed glass ceiling lines the top of the building and fills the interior with natural sunlight. There are live plants and flowers placed throughout the mall that give the shopping complex a lively and cheerful feel! A playful fountain sits in the center, further adding to the malls inviting a sense of wonder and amusement!
The GUM department store opened on December 2, 1893. Today, it includes local and luxury stores, including Fendi, Louis Vuitton, Prada, and many more! There are numerous cafes, restaurants, and even a movie theater inside!

For a special treat, head into Gastronom 1. This 1950s-style shop sells gourmet food items, like wine, freshly-baked pastries, cheese, Russian chocolate, and of course, vodka! Also, be on the lookout for a bicycle pedaling ice cream truck with an employing selling ice cream!
The ambiance is simply amazing, a trip to this idyllic shopping mall is an absolute must on any Moscow itinerary!
Insider Tip: Make sure to carry some small change on you in case you need to use the restroom, you’ll need to pay 50 rubles – or about USD $0.80 to use the bathroom in GUM.

Wanna know how to pack like a pro? Well for a start you need the right gear….
These are packing cubes for the globetrotters and compression sacks for the real adventurers – these babies are a traveller’s best kept secret. They organise yo’ packing and minimise volume too so you can pack MORE.
Or, y’know… you can stick to just chucking it all in your backpack…
Novodevichy Convent | Gorky Park | State Tretyakov Gallery | All-Russian Exhibition Center | Bolshoi Theater
On your 2 day itinerary in Moscow, you’ll have a chance to use the city’s excellent public transportation service! You’ll explore a few more of Moscow’s historic highlight as well as some modern attractions. These sites are a little more spread out, but still very easily accessible thanks to the metro!
Day 2 / Stop 1 – Novodevichy Convent
- Why it’s awesome: The Novodevichy Convent is rich in imperial Russian history and contains some of Russia’s best examples of classical architecture!
- Cost: USD $5.00
- Food nearby: Culinary Shop Karavaevs Brothers is a cozy and simple place to have a quick bite, they also have vegetarian options!
The Novodevichy Convent is the best-known and most popular cloister of Moscow. The convent complex is contained within high walls, and there are many attractions this site is known for!
The six-pillared five-domed Smolensk Cathedral is the main attraction. It was built to resemble the Kremlin’s Assumption Cathedral and its facade boasts beautiful snowy white walls and a pristine golden onion dome as its centerpiece. It’s the oldest structure in the convent, built from 1524 -1525, and is situated in the center of the complex between the two entrance gates.
There are other churches inside the convent as well, all dating back from many centuries past. The convent is filled with an abundance of 16th and 17th-century religious artworks, including numerous large and extravagant frescos!

Just outside the convent’s grounds lies the Novodevichy Cemetery. Here, you can visit the graves of famous Russians, including esteemed authors, composers, and politicians. Probably the most intriguing gravestone belongs to Russian politician Nikita Khruschev!
The Novodevichy Convent is located near the Moscow River and offers a peaceful retreat from the busy city. In 2004, it was proclaimed a UNESCO World Heritage Site. The convent remains remarkably well-preserved and is an outstanding example of Moscow Baroque architecture!
Insider Tip: To enter the cathedrals inside the complex, women are advised to cover their heads and shoulders, while men should wear long pants.
Day 2 / Stop 2 – Gorky Central Park of Culture and Leisure
- Why it’s awesome: A large amusement area in the heart of the city offering many attractions!
- Cost: Free!
- Food nearby: Check out Mepkato, located inside Gorky Central Park for a casual meal in a cozy setting. There are indoor and outdoor seating options and the restaurant is child-friendly!
Gorky Central Park of Culture and Leisure is a large green space in the heart of Moscow. The park opened in 1928, and it stretches along the scenic embankment of the Moskva River. It covers an area of 300-acres and offers a lovely contrast from the compact city center.
You’ll find all sorts of wonderful attractions, from boat rides to bike rentals to tennis courts and ping-pong tables, and much more! there are an open-air cinema and festive events and concerts scheduled in the summer months. A wide selection of free fitness classes is also offered on a regular basis, including jogging, roller skating, and dancing!
Although many of the options you’ll find here are more suited for outdoor leisure during the summer, you’ll also a selection of winter attractions, including one of Europe’s largest ice rinks for ice-skating!

If you’re trying to decide what to do in Moscow with kids, the park also offers several venues designed specifically for kids. Check out the year-round Green School which offers hands-on classes in gardening and art! You can also feed the squirrels and birds at the Golitsinsky Ponds!
The park is very well maintained and kept clean and the entrance is free of charge, although most individual attractions cost money. There is also Wi-Fi available throughout the park.
With so many attractions, you could easily spend all day here! If you’re only planning a 2 day itinerary in Moscow, make sure to plan your time accordingly and map out all the areas you want to see beforehand!
Day 2 / Stop 3 – The State Tretyakov Gallery
- Why it’s awesome: The gallery’s collection consists entirely of Russian art made by Russian artists!
- Food nearby : Brothers Tretyakovs is located right across the street from the gallery. It’s a wonderfully atmospheric restaurant serving top quality food and drinks!
The State Tretyakov Gallery was founded in 1856 by influential merchant and collector Pavel Tretyakov. The gallery is a national treasury of Russian fine art and one of the most important museums in Russia!
It houses the world’s best collection of Russian art and contains more than 130, 000 paintings, sculptures, and graphics! These works have been created throughout the centuries by generations of Russia’s most talented artists!

The exhibits range from mysterious 12th-century images to politically charged canvases. The collection is rich and revealing and offers great insight into the history and attitudes of this long-suffering yet inspired people!
All pictures are also labeled in English. If you plan to take your time and see everything inside the museum it will take a good 3-4 hours, so make sure to plan your Moscow trip itinerary accordingly! This gallery is a must-see stop for art lovers, or anyone wanting to explore the local culture and history of Russia in a creative and insightful manner!
Insider Tip: When planning your 2 days in Moscow itinerary, keep in mind that most museums in Moscow are closed on Mondays, this includes The State Tretyakov Gallery!
Day 2 / Stop 4 – All-Russian Exhibition Center
- Why it’s awesome: This large exhibition center showcases the achievements of the Soviet Union in several different spheres.
- Food nearby: Varenichnaya No. 1 serves authentic and homestyle Russian cuisine in an intimate and casual setting.
The All-Russian Exhibition Center is a massive park that presents the glory of the Soviet era! It pays homage to the achievements of Soviet Russia with its many different sites found on the property.
The center was officially opened in 1939 to exhibit the achievements of the Soviet Union. It’s a huge complex of buildings and the largest exhibition center in Moscow. There are several exhibition halls dedicated to different achievements and every year there are more than one hundred and fifty specialized exhibitions!

The Peoples Friendship Fountain was constructed in 1954 and is a highlight of the park. The stunning gold fountain features 16 gilded statues of girls, each representing the former Soviet Union republics.
The Stone Flower Fountain was also built in 1954 and is worth checking out. The centerpiece of this large fountain is a flower carved from stones from the Ural Mountains! Along the side of the fountain are various bronze sculptures.
You will find many people zipping around on rollerblades and bicycles across the large area that the venue covers. It’s also home to amusement rides and carousels, making it the perfect place to stop with kids on your Moscow itinerary! Make sure to wear comfortable shoes and allow a few hours to explore all the areas that interest you!
Day 2 / Stop 5 – Bolshoi Theater
- Why it’s awesome: The Bolshoi Theater is a historic venue that hosts world-class ballet and opera performances!
- Cost: Prices vary largely between USD $2.00 – USD $228.00 based on seat location.
- Food nearby: Head to the Russian restaurant, Bolshoi for high-quality food and drinks and excellent service!
The Bolshoi Theater is among the oldest and most renowned ballet and opera companies in the world! It also boasts the world’s biggest ballet company, with more than 200 dancers!
The theater has been rebuilt and renovated several times during its long history. In 2011 it finished its most recent renovation after an extensive six-year restoration that started in 2005. The renovation included an improvement in acoustics and the restoration of the original Imperial decor.
The Bolshoi Theater has put on many of the world’s most famous ballet acts! Tchaikovsky’s ballet Swan Lake premiered at the theater in 1877 and other notable performances of the Bolshoi repertoire include Tchaikovsky’s The Sleeping Beauty and The Nutcracker!

Today, when you visit the theater, you can expect a magical performance from skilled singers, dancers, and musicians with the highest level of technique!
If you don’t have time to see a show, the theater also provides guided tours on select days of the week. Tours are given in both Russian and English and will provide visitors with a more intimate look at the different areas of the theater!
The stage of this iconic Russian theater has seen many outstanding performances. If you’re a fan of the performing arts, the Bolshoi Theater is one of the greatest and oldest ballet and opera companies in the world, making it a must-see attraction on your Moscow itinerary!

Godzillas Hostel
Godzillas Hostel is located in the center of Moscow, just a short walk from all the major tourist attractions and the metro station.
- Towels Included
Cosmonautics Museum | Alexander Garden | Ostankino Tower | Izmaylovo District | Soviet Arcade Museum
Now that we’ve covered what to do in Moscow in 2 days, if you’re able to spend more time in the city you’re going to need more attractions to fill your time. Here are a few more really cool things to do in Moscow we recommend!
Memorial Museum of Cosmonautics
- Hear the timeline of the ‘space race’ from the Russian perspective
- This museum is fun for both adults and children!
- Admission is USD $4.00
The Memorial Museum of Cosmonautics is a museum dedicated to space exploration! The museum explores the history of flight, astronomy, space exploration, space technology, and space in the arts. It houses a large assortment of Soviet and Russian space-related exhibits, and the museum’s collection holds approximately 85,000 different items!

The museum does an excellent job of telling the full story of the exciting space race between the USSR and the US! It highlights the brightest moments in Russian history and humanity and is very interesting and fun for all ages!
If you’re a fan of space or just curious about gaining insight into Russia’s fascinating history of space exploration, make sure to add this to your 3 day itinerary in Moscow!
The Alexander Garden
- A tranquil place to relax near the Red Square
- Green lawns dotted with sculptures and lovely water features
- The park is open every day and has no entrance fee
The Alexander Garden was one of the first urban public parks in Moscow! The garden premiered in 1821 and was built to celebrate Russia’s victory over Napoleon’s forces in 1812!
The park is beautiful and well maintained with paths to walk on and benches to rest on. The park contains three separate gardens: the upper garden, middle garden, and lower garden.

Located in the upper garden, towards the main entrance to the park is the Tomb of the Unknown Soldier with its eternal flame. This monument was created in 1967 and contains the body of a soldier who fell during the Great Patriotic War!
The park stretches along all the length of the western Kremlin wall for about half a mile. Due to its central location in the city, it’ll be easily accessible when you’re out exploring The Red Square.
It provides a bit of relief from the city’s high-energy city streets. Bring a picnic lunch, go for a walk, or just sit and people watch, this is one of the best Moscow sites to wind-down and relax!
Ostankino Television Tower
- Television and radio tower in Moscow
- Currently the tallest free-standing structure in Europe
- Make sure you bring your passport when you visit, you can’t go up without it!
For spectacular views of the city, make sure to add the Ostankino Television Tower to your itinerary for Moscow! This impressive free-standing structure provides stunning views of the city in every direction. The glass floor at the top also provides great alternative views of the city!

It takes just 58 seconds for visitors to reach the Tower’s observation deck by super fast elevator. The tower is open every day for long hours and is a great site in Moscow to check out! There is even a restaurant at the top where you can enjoy rotating views of the city while you dine on traditional Russian cuisine or European cuisine!
The tower is somewhat of an architectural surprise in a city that is not known for skyscrapers! To see the city from a new perspective, make sure to add this stop to your Moscow itinerary!
Izmaylovo District
- The most popular attractions in this district are the kremlin and the flea market
- Outside of the city center and easy to reach via metro
- Most popular during the summer and on weekends
Travel outside the city center and discover a unique area of the city! The Izmaylovo District is a popular destination for locals and tourists alike, and one of the coolest places to see in Moscow! The two main attractions we recommend checking out are the Kremlin and the flea market.
The Izmailovo Kremlin was established as a cultural center and molded after traditional Russian architecture. This colorful complex is home to several single-subject museums, including a Russian folk art museum and a vodka museum!

Next to the Kremlin is the Izmailovo open-air market, which dates back to the 17th century! The market is connected to the Izmailovo Kremlin by a wooden bridge. Pick up all your Russian souvenirs here, including traditional handicrafts, paintings, books, retro toys, and Soviet memorabilia!
You will find many hand-made and hand-painted options available at higher prices, as well as mass-produced souvenir options at lower prices!
Museum of Soviet Arcade Games
- Closed on Mondays
- Filled with old arcade games that visitors get to try out!
- The museum also includes a small cafe and burger shop
For something a little different, check out the Museum of Soviet Arcade Games! The museum features roughly 60 machines from the Soviet era, including video games, pinball machines, and collaborative hockey foosball! The machines inside the museum were produced in the USSR in the mid-1970s.

The best part is, most of the games are still playable! Purchase tickets and try the games out for yourself! The museum also has a neat little screening room that plays old Soviet cartoons and an area with Soviet magazines! This unique attraction is a fun addition to a 3 day itinerary in Moscow, and an attraction that all ages will enjoy!
Whether you’re spending one day in Moscow, or more, safety is an important thing to keep in mind when traveling to a big city! Overall, Moscow is a very safe place to visit. However, it is always recommended that tourists take certain precautions when traveling to a new destination!
The police in Moscow is extremely effective at making the city a safe place to visit and do their best to patrol all of the top Moscow, Russia tourist attractions. However, tourists can still be a target for pickpockets and scammers.
Moscow has a huge flow of tourists, therefore there is a risk for pickpocketing. Simple precautions will help eliminate your chances of being robbed. Stay vigilant, keep your items close to you at all times, and don’t flash your valuables!
If you’re planning a solo Moscow itinerary, you should have no need to worry, as the city is also considered safe for solo travelers, even women. Stay in the populated areas, try and not travel alone late at night, and never accept rides from strangers or taxis without a meter and correct signage.
The threat of natural disasters in Moscow is low, with the exception of severe winters when the temperature can dip below freezing! Bring a good, warm jacket if you visit in Winter.
However, please note that Russian views on homsexuality are far less accepting than those in Western Europe. Likewise, Non-Caucasian travellers may sadly encounter racism in Russia .
Don’t Forget Your Travel Insurance for Moscow
ALWAYS sort out your backpacker insurance before your trip. There’s plenty to choose from in that department, but a good place to start is Safety Wing .
They offer month-to-month payments, no lock-in contracts, and require absolutely no itineraries: that’s the exact kind of insurance long-term travellers and digital nomads need.

SafetyWing is cheap, easy, and admin-free: just sign up lickety-split so you can get back to it!
Click the button below to learn more about SafetyWing’s setup or read our insider review for the full tasty scoop.
Now that we’ve covered all the top things to see in Moscow, we thought we’d include some exciting day trips to other areas of the country!
Sergiev Posad (Golden Ring)

On this 7-hour guided tour, you’ll visit several scenic and historic areas of Russia. Start your day with hotel pick-up as you’re transferred by a comfortable car or minivan to Sergiev Posad. Admire the charming Russian countryside on your drive and enjoy a quick stop to visit the Russian village, Rudonezh!
You’ll see the majestic Saint Spring and the Church of Sergiev Radonezh. You’ll also visit the UNESCO World Heritage Site, Trinity Lavra of St. Sergius, one of the most famous Orthodox sites in Russia!
Lastly, you’ll swing by the local Matreshka market and enjoy a break in a nice Russian restaurant before returning to Moscow!
Day Trip to Vladimir and Suzdal

On this 13-hour trip, you’ll discover old Russia, with its picturesque landscapes and white-stoned beautiful churches! You’ll visit the main towns of the famous Golden Ring of Russia – the name for several cities and smaller towns north-east of Moscow.
Your first stop will be in the town of Vladimir, the ancient capital of all Russian principalities. The city dates back to the 11th century and is one of the oldest and the most important towns along the Ring! Next, you’ll visit Suzdal, a calm ancient Russian town north of Vladimir with only 13,000 inhabitants!
The old-style architecture and buildings of Suzdal are kept wonderfully intact. If you’re spending three days in Moscow, or more, this is a great option for exploring the charming areas outside the city!
Zvenigorod Day Trip and Russian Countryside

On this 9-hour private tour, you’ll explore the ancient town of Zvenigorod, one of the oldest towns in the Moscow region! As you leave Moscow you’ll enjoy the stunning scenery along the Moscow River, and make a few stops at old churches along the way to Zvenigorod.
Upon arrival, you’ll explore the medieval center, including the 14th-century Savvino-Storozhevsky Monastery. Next, you’ll take a break for lunch (own expense) where you’ll have the chance to try out the Russian cuisine! Next, you’ll visit the Museum of Russian Dessert and sip on tea at a Russian tea ceremony.
The final stop of the day is at the Ershovo Estate, a gorgeous place to walk around and enjoy nature!
Day Trip to St Petersburg by Train visiting Hermitage & Faberge

On this full-day tour, you’ll enjoy a a full round trip to St Petersburg where you’ll spend an exciting day exploring another popular Russian city! You’ll be picked up from your hotel in Moscow and be transferred to the train station where you’ll ride the high-speed train ‘Sapsan’ to St Petersburg.
Upon arrival, you’ll start the day by touring the Hermitage Museum and the Winter Palace. Next, you’ll visit the Faberge Museum, where you’ll explore the impressive collection of rare Faberge Eggs! In the afternoon, enjoy a sightseeing boat ride and a traditional 3-course Russian lunch.
If you’re spending 3 days in Moscow, or more, this is an excellent trip to take!
Trip to Kolomna – Authentic Cultural Experience from Moscow

On this 10-hour tour, you’ll escape the city and travel to the historic town of Kolomna! First, you’ll visit the 14th-century Kolomna Kremlin, home to the Assumption Cathedral and an abundance of museums!
Next, enjoy lunch at a local cafe (own expense) before embarking on a tour of the Marshmallow Museum – of course, a marshmallow tasting is provided! Your final stop is the Museum of Forging Settlements, where displays include armor and accessories for fishing and hunting.
Discover this beautiful Russian fairytale city on a private trip, where all of the planning is taken care of for you!

Stash your cash safely with this money belt. It will keep your valuables safely concealed, no matter where you go.
It looks exactly like a normal belt except for a SECRET interior pocket perfectly designed to hide a wad of cash, a passport photocopy or anything else you may wish to hide. Never get caught with your pants down again! (Unless you want to…)
Find out what people want to know when planning their Moscow itinerary.
How many days you need in Moscow?
We recommend that you spend at least two or three days in Moscow to take it all in.
What’s the best month to visit Moscow?
The best time to visit Moscow is over the spring, from March to May as temperatures are mild, crowds are thin and prices are reasonable.
What are some unusual things to do in Moscow?
I mean, queuing up to see an almost 100 year old corpse is pretty unsual! Check out Lenin’s Mausoleum if you fancy it!
What are some fun things to do in Moscow?
The Memorial Museum of Cosmonautics is a fun place to explore the famous space race from the perspective of the ‘other side’!
We hope you enjoyed our Moscow itinerary! We’ve made sure to cover all the Moscow must-sees as well as some unique attractions in the city! Our addition of insider tips, favorite food stops, and day trips from Moscow is an added bonus and will guarantee you make the most out of your exciting Russian vacation!
Immerse yourself in the modern and traditional Russian lifestyle! Get lost in museums, witness awe-inspiring architecture, and indulge in Russian cuisine! Spend the day strolling through all of the charming sites of Moscow, admiring the beautiful scenery and discovering the city’s fairytale-like enchantment!

And for transparency’s sake, please know that some of the links in our content are affiliate links . That means that if you book your accommodation, buy your gear, or sort your insurance through our link, we earn a small commission (at no extra cost to you). That said, we only link to the gear we trust and never recommend services we don’t believe are up to scratch. Again, thank you!

Alya and Campbell

Share or save this post

Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Notify me of followup comments via e-mail.
- Brief Report
- Open access
- Published: 10 April 2024
A nopheles sacharovi in Italy: first record of the historical malaria vector after over 50 years
- Donato Antonio Raele 1 ,
- Francesco Severini 2 ,
- Luciano Toma 2 ,
- Michela Menegon 2 ,
- Daniela Boccolini 2 ,
- Giovanni Tortorella 3 ,
- Marco Di Luca 2 na1 &
- Maria Assunta Cafiero 1 na1
Parasites & Vectors volume 17 , Article number: 182 ( 2024 ) Cite this article
Metrics details
Anopheles sacharovi , a member of the Anopheles maculipennis complex, was a historical malaria vector in Italy, no longer found since the last report at the end of 1960s. In September 2022, within the Surveillance Project for the residual anophelism, a single specimen of An. maculipennis sensu lato collected in Lecce municipality (Apulia region) was molecularly identified as An. sacharovi . This record led to implement a targeted entomological survey in September 2023.
Investigation was conducted in the areas around the first discovery, focusing on animal farms, riding stables and potential breeding sites. Adult and immature mosquitoes were collected, using active search or traps, in several natural and rural sites. Mosquitoes belonging to An. maculipennis complex were identified morphologically and molecularly by a home-made routine quantitative polymerase chain reaction (qPCR) assay, developed specifically for the rapid identification of An. labranchiae , and, when necessary, by amplification and sequencing of the ITS-2 molecular marker.
Out of the 11 sites investigated, 6 were positive for Anopheles presence. All 20 An. maculipennis s.l. (7 adults, 10 larvae and 3 pupae) collected in the areas were identified as An. sacharovi by ITS-2 sequencing.
Conclusions
The discovery of An. sacharovi , considered to have disappeared from Italy for over 50 years, has a strong health relevance and impact, highlighting an increase in the receptivity of the southern areas. As imported malaria cases in European countries are reported every year, the risk of Plasmodium introduction by gametocyte carriers among travellers from endemic countries should be taken into greater consideration. Our findings allow rethinking and building new models for the prediction and expansion of introduced malaria. Furthermore, to prevent the risk of reintroduction of the disease, the need to strengthen the surveillance of residual anophelism throughout the South should be considered.
Graphical Abstract
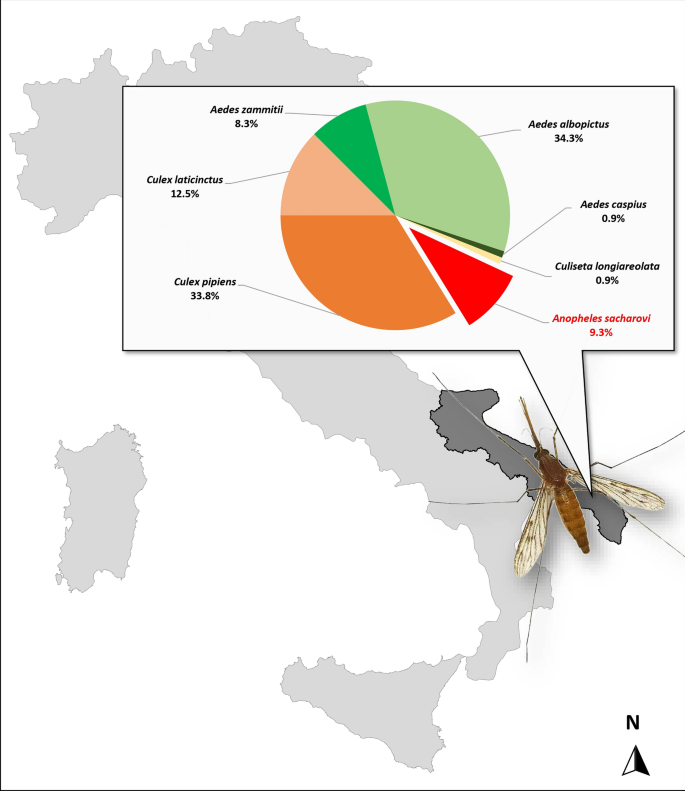
Malaria was endemic in Italy until the middle of the last century. Human cases, mainly due to Plasmodium falciparum and P. vivax , were widespread especially along the coastal plains, and before the decisive interventions of reclamation and the launch of the National Anti-malarial Campaign, the disease affected hundreds of people every year [ 1 ]. Although Italy was officially declared malaria free by the World Health Organization (WHO) in 1970, since then a few sporadic cases of non-imported malaria have been recorded. Among the most recent cases, four non-travel related malaria cases occurred in Ginosa (Taranto, Apulia region) in October 2017. Integrated entomological surveys were performed, and the presence of the potential malaria vector, Anopheles labranchiae , was recorded in the involved areas [ 2 ]. In Italy, there are currently six anopheline sibling species belonging to the Anopheles maculipennis Meigen complex that cannot be distinguished morphologically, including An. atroparvus Van Thiel, 1927, An. labranchiae Falleroni, 1926, An. maculipennis sensu stricto Meigen, 1818, An. melanoon Hackett, 1934, An. messeae Falleroni, 1926, and An. daciae species inquirenda Linton, Nicolescu & Harbach, 2004 [ 3 , 4 ]. A seventh sibling taxon, Anopheles sacharovi Favre, 1903, once widely distributed throughout the country, progressively disappeared probably because of the progressive modification of its larval habitats [ 5 , 6 , 7 , 8 , 9 , 10 ]. Among the An. maculipennis complex, An. labranchiae and An. sacharovi were historically considered the two most competent malaria vectors in Italy, always associates with endemic malaria and involved in P. falciparum and P. vivax transmission [ 11 , 12 , 13 ]. These two species were common and sometimes found in sympatry in Central and South Italy, including the Apulia region [ 10 , 11 , 12 , 13 , 14 ]. Anopheles labranchiae is still abundant and widespread in the region, especially in the Gargano, as recently documented [ 12 , 15 ]. Contrarily, despite two doubtful findings reported in Northern and Central Italy [ 16 , 17 ], An. sacharovi has not been found since the last report in the late 1960s. This mosquito thrived in several rural areas, mostly along the north and southwestern coastal plains and in northern Sardinia [ 5 ]. Although exophagic activity has been described for this species, it commonly exhibited endophagic and endophilic behaviour, resting in human dwellings, flooded basements and animal shelters where it could bite both day and night [ 18 , 19 ]. This paper describes the investigation that led to the rediscovery of An. sacharovi in Italy, specifically in the Apulia region.
In 2022, the Italian Ministry of Health funded a research project (RC IZSPB 06/2022) to monitor the presence and the extent of An. maculipennis complex mosquitoes in Apulia and Basilicata territories. The Centers for Disease Control light trap (CDC-LT) network was extended to the area of Lecce Province in southeastern Italy. During the year, only one Anopheles specimen was collected in early September, in a horse-riding stable, and it was morphologically identified as An. maculipennis s.l. according to Severini et al. [ 20 ]. The mosquito was subsequently analysed by a home-made qPCR, an assay developed for routine rapid screening of An. labranchiae identification, the most widespread species of the complex in the region. A primer set was designed to amplify a 95-bp fragment of nuclear ribosomal internal transcribed spacer 2 (ITS-2) of An. labranchiae . The reaction was carried out in a 20-µl reaction using the SsoAdvanced Universal ProbeMix (BIORAD) with primers L1: 5′-GGTCATCGTGAGGCGTTATC-3′ and R1: 5′-GCTAGGAGCCGGTCTTGTAT-3′ at concentrations of 0.5 µM each and the probe P1: FAM-5′-AAGCACTCGCTGCTGCGCGT-BHQ1′ at a concentration of 0.2 µM under the following conditions: 95 ℃ for 3 min, 30 cycles at 95 ℃ for 10 s and 60 ℃ for 20 s. The negative qPCR sample was tested by conventional PCR. ITS-2 amplicon was sequenced at Eurofins Genomics (Ebersberg, Germany) and analysed by National Center for Biotechnology Information’s (NCBI) Basic Local Alignment Search (BLAST) for the identification of mosquito species [ 21 ]. The resulting sequence was deposited in GenBank (accession no. OQ748043). In September 2023, the entomological investigation was extended from the positive horse-riding stable (site 6) to neighbouring coastal areas of Lecce Province, including ten other different sites. In Fig. 1 , the study area with the geographical distribution of the visited sites, for both adult and larval collections, is shown. The collection sites were selected in areas characterized by the presence of several marshes, brackish water basins and natural lakes and farms with animals (cattle, horses, sheep and poultry). The adult collections were carried out in animal shelters and human dwellings, using manual or battery-powered aspirators (modified Katcha® Bug Buster Spider Vacuum Blue) or CDC light traps. In potential breeding sites (i.e. irrigation and drainage canals, streams, marshes, large ponds and other permanent water collections), larvae and pupae were collected using a 500-ml standard dipper. All mosquito specimens were morphologically identified [ 20 ] and, when necessary, screened by molecular tools for species detection. Rapid DNA extraction for a single specimen was performed using a microwave method [ 22 ]. Culex and Aedes species and all specimens belonging to the An. maculipennis complex caught in 2023 were directly tested by conventional PCR, according to Marinucci et al. [ 21 ]. ITS-2 amplicons were sequenced at Eurofins Genomics (Ebersberg, Germany) and analysed using NCBI’s Basic Local Alignment Search (BLAST) for the identification of mosquito species.
Mosquito collection sites in the 2-year period, 2022–2023. Black dot: adult collection sites; black square: larval breeding sites
The single An. maculipennis s.l. collected in September 2022, at site 6 (Lecce Province), was molecularly analysed and identified as An. sacharovi (GenBank accession no. OQ748043). During the subsequent 2023 entomological survey, a total of 216 mosquitoes (125 immatures and 91 adults) were captured and identified morphologically, when possible. For Culex pipiens, Cx. laticinctus , Aedes mariae complex and An. maculipennis complex, targeted molecular analyses allowed the exact identification of the different taxa. Seven An. maculipennis s.l. females were collected on four farms (sites 4, 5, 8 and 9) and in a human dwelling (site 11); only one larval breeding site was positive for Anopheles presence, with 10 larvae and 3 pupae detected (site 2). All An. maculipennis s.l. were molecularly analysed and identified as An. sacharovi . ITS2 sequences obtained shared 100% nucleotide sequence identity with the sequence from a single specimen collected in 2022 (GenBank accession no. OQ748043). Table 1 summarizes the result obtained in this study, showing all sites visited in 2023, the number of specimens collected and the mosquito species detected. Overall, seven species were identified: An. sacharovi (9.3%), Cx. pipiens (33.8%), Cx. laticinctus (12.5%), Aedes zammitii (8.3%), Ae. albopictus (34.3%), Ae. caspius (0.9%), Culiseta longiareolata (0.9%).
Even after the anti-malarial campaign following the Second World War, An. labranchiae and An. sacharovi were recorded in Apulia region, often found in sympatry [ 23 ]. However due to the diking and draining of marshes and retrodunal ponds, the use of insecticides, urbanization and pollution, many larval habitats, typical of An. sacharovi , have been progressively reduced, and the species was reported in the region until the late 1960s [ 14 ]. This heavy anthropic impact has contributed to the rarefaction of An. sacharovi in its distribution, perhaps confining small and limited mosquito populations in restricted natural sites. More recently, a reduction in anthropic pressure, the expansion of natural areas and the creation of new ones, together with other favourable climatic and environmental factors, could have contributed to the slow reconquest of territories and reappearance of the species in Apulia region. The record of Anopheles algeriensis Theobald, 1903, a potential secondary malaria vector, recently reported in Apulia, confirms how these changes can positively allow the Anopheles species to spread and/or recolonize some areas [ 14 , 24 ]. The recolonization of areas with characteristics favourable to the larval development of An. algeriensis could be a positive signal of environmental requalification, which could also be beneficial for the spread of An. sacharovi . As supported by the literature, An. sacharovi can colonise different types of water collections, such as swamps, marshes, river margins, streams, pools and ditches, and can develop in both freshwater and brackish-salt water collections [ 18 ].
Although research projects have often been short and discontinuous, in recent years the collaborative activities conducted by the Experimental Zooprophylactic Institute of Puglia and Basilicata and the Istituto Superiore di Sanità have been able to clearly document the presence of potential malaria vectors, such as An. labranchiae , Anopheles superpictus Grassi, 1899 and An. algeriensis in the Apulia and Basilicata regions [ 14 , 24 ]. Therefore, after decades of entomological investigations, this notable rediscovery in the coastal areas of southeastern Italy allows us to reintegrate An. sacharovi in the Italian Culicidae fauna. Adults of An. sacharovi were found indoors, either resting in animal shelters or landing on a human during dusk. This highlights not only an endophagic and endophilic behaviour but also a certain degree of anthropophilia of the species. Furthermore, a typical natural breeding site was identified, completing the entomological investigation. Beyond the exceptionality of such findings, it is necessary to underline the importance of strengthening and maintaining constant surveillance of residual anophelism, especially in vulnerable areas where the occurrence of sporadic malaria transmission could be possible. Further investigations will need to assess the distribution and seasonal abundance of An. sacharovi along the southeastern coasts of Italy. However, our findings, confirmed by two point-in-time investigations in 2 consecutive years, represent a valid basis for rethinking and building new models for the prediction and expansion of introduced malaria and for reconsidering the receptivity of the studied areas to malaria to prevent the risk of reintroduction of the disease. Although vector densities do not currently appear to be epidemiologically relevant enough to pose a health threat, the conditions for a renewal of transmission in several Mediterranean countries still exist, as reported in Greece [ 25 ], and the occurrence of foci of introduced malaria (in particular by P. vivax ) in some regions of our country should not to be underestimated [ 13 ].
Data availability
The datasets generated and analysed during the current study are available in the NCBI (GenBank) repository by accession no. OQ748043. https://www.ncbi.nlm.nih.gov/nuccore/OQ748043.1
Majori G. Short history of malaria and its eradication in Italy with short notes on the fight against the infection in the Mediterranean basin. Mediterr J Hematol Infect Dis. 2012;4:e2012016. https://doi.org/10.4084/MJHID.2012.016 .
Article PubMed PubMed Central Google Scholar
Boccolini D, Menegon M, Di Luca M, Toma L, Severini F, Marucci G, et al. Non-imported malaria in Italy: paradigmatic approaches and public health implications following an unusual cluster of cases in 2017. BMC Public Health. 2020;20:857. https://doi.org/10.1186/s12889-020-08748-9 .
Toma L, Severini F, Di Luca M, Menegon M. Insecta Diptera Culicidae. In: Bologna MA, Zapparoli M, Oliverio M, Minelli A, Bonato L, Cianferoni F, Stoch F, editors. Checklist of the Italian Fauna. Version 1.0. 2021. https://www.lifewatchitaly.eu/en/initiatives/checklist-fauna-italia-en/checklist/ .
Calzolari M, Desiato R, Albieri A, Bellavia V, Bertola M, Bonilauri P, et al. Mosquitoes of the Maculipennis complex in Northern Italy. Sci Rep. 2021;11:6421. https://doi.org/10.1101/2020.11.02.365262 .
Article CAS PubMed PubMed Central Google Scholar
Romi R, Pierdominici G, Severini C, Tamburro A, Cocchi M, Menichetti D, et al. Status of malaria vectors in Italy. J Med Entomol. 1997;34:263–71. https://doi.org/10.1093/jmedent/34.3.263 .
Article CAS PubMed Google Scholar
Zamburlini R, Cagnus E. Residual mosquitoes in the northern Adriatic seacoast 50 years after the disappearance of malaria. Parassitologia. 1998;40:431–7.
CAS PubMed Google Scholar
Romi R, Sabatinelli G, Majori G. Could malaria reappear in Italy? Emerg Infect Dis. 2001;7:915–9. https://doi.org/10.3201/eid0706.010601 .
Romi R, Sabatinelli G, Majori G. Malaria epidemiological situation in Italy and evaluation of malaria incidence in Italian travelers. J Travel Med. 2001;8:6–11. https://doi.org/10.2310/7060.2001.5140 .
Bietolini S, Zamburlini R, Candura F, Musella V, Rinaldi L, Cringoli G, et al. Is Anopheles sacharovi disappeared from Italy? Retrospective analysis and new field data. Parassitologia. 2004;46:81.
Google Scholar
Bietolini S, Candura F, Coluzzi M. Spatial and long term temporal distribution of the Anopheles maculipennis complex species in Italy. Parassitologia. 2006;48:581–608.
Hackett LW, Missiroli A. The varieties of Anopheles maculipennis and their relation to the distribution of malaria in. Europe Riv Malariol. 1935;14:45–109.
Raffaele G. Note sull’eradicazione della malaria in Italia. Riv Malariol. 1964;43:1–27.
Jetten TH, Takken W. Anophelism without malaria in Europe: a review of the ecology and distribution of the genus Anopheles in Europe. Wageningen Agricultural University Papers. 1994. p. 69.
Rivosecchi L, Stella E. Artropodi ematofagi delle aree naturali da proteggere. Nota II. La zona del Massiccio Garganico. In Proceedings of the Atti del IV Simposio Nazionale sulla Conservazione della Natura, Bari, Italy, 23–28 April 1974; Volume I, Cacucci Editore. pp. 149–170.
Raele DA, Severini F, Boccolini D, Menegon M, Toma L, Vasco I, et al. Entomological surveillance in former malaria-endemic areas of southern Italy. Pathogens. 2021;10:1521. https://doi.org/10.3390/pathogens10111521 .
Talbalaghi A, Shaikevich E. Molecular approach for identification of mosquito species (Diptera: Culicidae) in Province of Alessandria, Piedmont, Italy. Eur J Entomol. 2011;108:35–40.
Article CAS Google Scholar
Möhlmann TWR, Wennergren U, Tälle M, Favia G, Damiani C, Bracchetti L, et al. Community analysis of the abundance and diversity of mosquito species (Diptera: Culicidae) in three European countries at different latitudes. Parasit Vectors. 2017;10:510. https://doi.org/10.1186/s13071-017-2481-1 .
Bertola M, Mazzucato M, Pombi M, Montarsi F. Updated occurrence and bionomics of potential malaria vectors in Europe: a systematic review (2000–2021). Parasit Vectors. 2022;15:88. https://doi.org/10.1186/s13071-022-05204-y .
Boreham PF, Garrett-Jones C. Prevalence of mixed blood meals and double feeding in a malaria vector ( Anopheles sacharovi Favre). Bull World Health Organ. 1973;48:605–14.
CAS PubMed PubMed Central Google Scholar
Severini F, Toma L, Di Luca M. Zanzare in Italia: raccolta, identificazione e conservazione delle specie più comuni. Roma: Istituto Superiore di Sanità; 2022. (Rapporti ISTISAN 22/3).
Marinucci M, Romi R, Mancini P, Di Luca M, Severini C. Phylogenetic relationships of seven palearctic members of the maculipennis complex inferred from ITS2 sequence analysis. Insect Mol Biol. 1999;8:469–80. https://doi.org/10.1046/j.1365-2583.1999.00140.x .
Dashti AA, Jadaon MM, Abdulsamad AM, Dashti HM. Heat treatment of bacteria: a simple method of DNA extraction for molecular techniques. Kuwait Med J. 2009;41:117–22.
Romi R, Severini C, Pierdominici G, Marchi A, Erbi G, Mantega V, et al. Anofelismo residuo in Italia: distribuzione in quattro regioni meridionali. Ann Ist Super Sanità. 1994;30:237–42.
Menegon M, Tomazatos A, Severini F, Raele DA, Lilja T, Werner D, et al. Molecular characterization of Anopheles algeriensis theobald, 1903 (Diptera: Culicidae) populations from Europe. Pathogens. 2022;30:990. https://doi.org/10.3390/pathogens11090990 .
Tseroni M, Georgitsou M, Baka A, Pinaka O, Pervanidou D, Tsironi M, et al. The importance of an active case detection (ACD) programme for malaria among migrants from malaria endemic countries: the Greek experience in a receptive and vulnerable area. Int J Environ Res Public Health. 2020;17:4080.
Download references
Acknowledgements
We are grateful to Inspectors of ASL of Lecce for their help with field surveys and farm contacts and all the breeders, who have always cordially made their stables accessible for the inspections. We also acknowledge the assistance and cooperation provided by the personnel of the Carabinieri Biodiversity Protection Unit of San Cataldo, on duty at the San Cataldo State Nature Reserve, Lecce. Finally, special thanks to Roberto Romi, whose teachings guided and inspired us.
The surveillance study was supported by funding obtained through IZS PB 06/2022 RC by Ministry of Health, Italy, and partially by EU funding within the NextGeneration EU-MUR PNRR Extended Partnership Initiative on Emerging Infectious Diseases (project no. PE00000007, INF-ACT).
Author information
Marco Di Luca and Maria Assunta Cafiero have contributed equally to this article and share last authorship.
Authors and Affiliations
Istituto Zooprofilattico Sperimentale della Puglia e Della Basilicata, Via Manfredonia, 20, 71121, Foggia, Italia
Donato Antonio Raele & Maria Assunta Cafiero
Dipartimento di Malattie Infettive, Reparto di Malattie Trasmesse da Vettori, Istituto Superiore di Sanità, Viale Regina Elena, 299, 00161, Rome, Italia
Francesco Severini, Luciano Toma, Michela Menegon, Daniela Boccolini & Marco Di Luca
Azienda Sanitaria Nazionale (ASL), Servizio Veterinario Sanità Animale, Viale Don Minzoni N. 8, 73100, Lecce, Italia
Giovanni Tortorella
You can also search for this author in PubMed Google Scholar
Contributions
Marco Di Luca (MD) and Maria Assunta Cafiero (MAC) contributed equally to this article and share last authorship. Conceptualization, DAR, MD and MAC; methodology, DAR, FS, LT, GT, MM, DB and MAC; formal analysis, DAR, FS, LT, DB, MM and MD; writing—original draft preparation, DAR.; writing—review and editing, MDL, FS, LT, DAR, DB, MM and MAC; project administration, MAC and DAR; funding acquisition, DAR, MAC and MDL. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Donato Antonio Raele .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Additional information, publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Raele, D.A., Severini, F., Toma, L. et al. A nopheles sacharovi in Italy: first record of the historical malaria vector after over 50 years. Parasites Vectors 17 , 182 (2024). https://doi.org/10.1186/s13071-024-06252-2
Download citation
Received : 15 February 2024
Accepted : 19 March 2024
Published : 10 April 2024
DOI : https://doi.org/10.1186/s13071-024-06252-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Anopheles sacharovi
- Anopheles maculipennis complex
- Residual anophelism
Parasites & Vectors
ISSN: 1756-3305
- Submission enquiries: [email protected]
Insect Repellents Help Prevent Malaria and Other Diseases Spread by Mosquitoes

Every year, millions of U.S. residents travel to countries where malaria and other diseases spread by mosquitoes (mosquito-borne) are found. These diseases can cause serious illness and death in some cases. Preventing mosquito-borne infections is very important to staying well when you travel.
Why do I need to use an insect repellent when I travel to an area with malaria?
An insect repellent will help protect you from mosquitoes that spread malaria and other diseases, such as dengue, chikungunya, and Yellow fever. You can use an insect repellent on your skin and clothes to keep away (repel) insects.
Your doctor may also prescribe you a medication to prevent malaria (antimalarial drug).
Although antimalarial drugs are very effective, they are not 100% effective in preventing malaria. This is why you need to also use an insect repellent and take other steps to keep mosquitoes from biting you (mosquito avoidance).
In some areas where only a few cases of malaria occur, CDC recommends mosquito avoidance as the only way to prevent malaria.
Are insect repellents safe?
Most insect repellents that can be put on the skin must be registered by the U.S. Environmental Protection Agency (EPA) before they can be sold in stores.
EPA registration means the insect repellent has been tested and approved for human safety and is effective when used according to directions on the label. Before you buy an insect repellent, be sure to look on the label for EPA approval.
Which insect repellents will give me long-lasting protection against mosquitoes?
For hours of long-lasting protection, look for insect repellents with the following active ingredients:
- Oil of lemon eucalyptus (OLE)*
- Picaridin (KBR 3023)
Some brand names of repellents include:
- DEET products: Off!, Cutter, Sawyer, Ultrathon.
- IR3535 products: Skin So Soft Bug Guard Plus Expedition, SkinSmart.
- OLE products: Repel, Off! Botanicals.
- Picaridin products (Autan, Bayrepel and icaridinoutside U.S.): Cutter Advanced, Skin So Soft Bug Guard Plus.
*”Pure” oil of lemon eucalyptus (essential oil) is not recommended. It has not been tested by EPA for safety and effectiveness
See a doctor or other healthcare provider 4-6 weeks before your trip to get any prescriptions, shots, and information you may need to stay healthy while you travel. Even if your trip is sooner, see a doctor to be safe. For more information visit CDC Travelers’ Health website at www.cdc.gov/travel
How do I use an insect repellent?
- Always follow directions on the label.
- Spray or rub the insect repellent onto your skin that is NOT covered by your clothes. Do not use under clothing.
- Use just enough insect repellent to cover your skin not covered by clothing. Heavy use of insect repellent or pouring it all over your body is not necessary.
- Never use insect repellents over cuts, wounds, or irritated skin.
- Do not spray insect repellents directly on your face—spray it onto your hands first and then pat the insect repellent onto your face.
- Do not spray or put insect repellents on your eyes or mouth, and put only a little around your ears.
- Use separate sunscreen and insect repellent products. Put the sunscreen on first, then spray on the insect repellent.
- After returning indoors, wash the insect repellent off your skin with soap and water or take a bath. This is especially important when you use insect repellents daily.
- Wash any clothes you treated with insect repellent before wearing them again.
Can I use an insect repellent if I am pregnantor breastfeeding
Yes. Pregnant and breastfeeding women can use EPA-approved insect repellents. Always follow directions on the label.
Can I put insect repellent on my baby?
- Do not put insect repellent on babies younger than 2 months of age.
- Protect small babies under the age of 2 months by placing a fitted mosquito net around their infant seat or carrier.
How do I put insect repellent on a child?
- Follow directions on the label.
- See directions for how to use an insect repellent on this page.
- Use no more than 30% DEET on a child.
- Do not use insect repellent with lemon eucalyptus on children under the age of 3 years.
What other things can I do to prevent mosquito bites?
- Wear a long-sleeved shirt, long pants, and a hat when you are outdoors.
- Spray insect repellent on your clothes for extra protection or buy a product with permethrin to treat your clothes and bed net to repel insects.
- Sleep in a well-screened or air-conditioned room, or sleep under a permethrin-treated bed net. This is because malaria mosquitoes mostly bite at night (dusk until dawn).
- Stay indoors during the times biting mosquitoes are most active.
Always spray insect repellents in open areas and wash your hands after using
What should I do if I develop a rash or reaction to the insect repellent?
Stop using the insect repellent and wash the area with soap and water. If you see a doctor about the reaction, take the repellent container with you. You can also call the Poison Help Hotline at 1-800-222-1222 .
FOR MORE INFORMATION:
Visit the EPA website at www2.epa.gov/insect-repellents
Check out the CDC malaria website at www.cdc.gov/malaria or call toll-free the CDC Malaria Hotline at 1-855-856-4713
To receive email updates about this page, enter your email address:
New! Locally Acquired Cases of Malaria in Florida, Texas, Maryland, and Arkansas
New! Update to Guidance for use of Artemether-Lumefantrine (Coartem®) in Pregnancy for Uncomplicated Malaria New! Discontinuation of CDC’s Distribution of Intravenous Artesunate as Commercial Drug Guidance for Malaria Diagnosis in Patients Suspected of Ebola Infection in the United States -->
See all Malaria Notices
- New! Malaria is a Serious Disease
- New! La malaria (paludismo) es una enfermedad grave
- How to Report a Case of Malaria
- CDC Yellow Book
- Red Pages: Malaria-endemic areas by country
- Drugs for Prevention
- Choosing a Drug to Prevent Malaria
- Drugs for Treatment in the U.S.
- Frequently Asked Questions (FAQs)
- Blood Banks
Click here for contact information
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.
Planned outage: Files stored in our eDOCS platform may be temporarily unavailable today from noon to 1 p.m.
Skip to Content

511 Travel Info
News releases
April 10, 2024
Latest news releases
Hwy 10 lane closures, delays begin east of St. Cloud April 15
Cable median barrier to prevent crossover crashes ST. CLOUD, Minn. – Motorists will encounter periodic lane closures on eastbound Highway 10 from St. Cloud to Clear Lake as crews install high-tension cable median barrier, reports the Minnesota Department of Transportation.
On Monday, April 15, motorists should begin to watch for roadwork signs, lane closures, lane shifts and reduced speeds. Expect multiple crews and equipment working at different locations within the nine-mile work zone. Work will occur on good weather days during daylight hours until mid-May. Both traffic lanes will be open on Sundays.
High-tension cable median barriers are made of three or four steel cables strung on posts. When a vehicle hits the barrier, the posts break and cables flex, absorbing much of a crash’s kinetic energy. This redirects the vehicle along the median, preventing a severe or fatal cross-median crash. When complete, the $2.8 million safety improvement project is intended to prevent crossover collisions with on-coming motorists and save lives. To learn more about the benefits of cable median barriers, visit MnDOT’s traffic engineering web page .
- International
June 24, 2023 - Wagner head says group standing down
By Helen Regan , Andrew Raine , Sophie Tanno, Hafsa Khalil, Tori B. Powell , Adrienne Vogt and Kaanita Iyer , CNN
Anti-terrorist regime introduced in Moscow and Voronezh regions
From CNN's Uliana Pavlova
The Russian National Anti-Terrorism Committee announced the introduction of a counter-terrorist operation regime in Moscow, the Moscow region and Voronezh region.
"In order to prevent possible terrorist acts on the territory of the city of Moscow, the Moscow and Voronezh regions, a counter-terrorist operation regime has been introduced," the statement said.
According to Russian state media, this is the first time that the counter-terrorist regime has been announced in these regions.
The measures were announced as the head of the Wagner private military group, Yevgeny Prigozhin, was accused of mounting an armed revolt against the Russian state.
The counter-terrorist regime includes, but is not limited to:
- document checks
- strengthened protection of public order
- monitoring telephone conversations
- restricting communications
- restricting the movement of vehicles and pedestrians on the streets.
Key lines from Putin's address
Russian President Vladimir Putin warned that those on the “path of treason” or armed rebellion will be “punished” after the head of the Wagner paramilitary group said his troops had taken control of military facilities in two Russian cities, plunging the country into crisis.
Here are some of the key lines from Putin's address:
- Putin vowed a harsh response and punishment of those involved in armed rebellion. "Any actions that fracture our unity" are "a stab in the back of our country and our people,” he said.
- Putin said the armed forces "have been given the necessary orders" and "decisive action will also be taken to stabilize the situation in Rostov."
- He said, "additional anti terrorism, security measures have been imposed in Moscow, Moscow region and a number of other regions."
- Putin appealed to Wagner forces "pushed into the provocation of a military rebellion," saying at this time, "we require unity, consolidation, and responsibility."
- Putin described events in Rostov as an insurrection. He said the situation in Rostov "remains difficult during the armed uprising" and "the work of civil and military administration is basically blocked."
- Putin said the country had been "betrayed by those who are trying to organize a mutiny, pushing the country toward anarchy and fratricide." He said "excessive ambition and vested interests have led to treason."
- Putin said it was a "blow to Russia," adding, "Internal turmoil is a mortal threat to our statehood, to us as a nation."
Putin says Wagner's "betrayal" is a "stab in the back of our country and our people"
From CNN's Josh Pennington
Russian President Vladimir Putin said Wagner's "betrayal" and "any actions that fracture our unity," are "a stab in the back of our country and our people."
"What we are facing is precisely betrayal. Excessive ambition and vested interests have led to treason. Betrayal of one's country, one's people, and the cause for which the soldiers and commanders of the Wagner group had fought and died, side by side with our other units," Putin said.
Putin called Wagner actions "internal treachery," saying that "all kinds of political adventurers and foreign forces, who divided the country and tore it apart, profited from their own interests. We will not let this happen again. We will protect both our people and our statehood from any threats, including internal treachery."
Putin says situation in Rostov remains difficult because of "armed uprising"
From CNN's Uliana Pavlova

Russian President Vladimir Putin said in a televised address Saturday, "the situation in Rostov-on-Don remains difficult during the armed uprising."
"In Rostov, the work of civil and military administration is basically blocked," Putin said.
Russian President Putin says those on a path of treason or preparing armed rebellion will be punished

Russian President Vladimir Putin appealed to Wagner forces in an address Saturday.
"I appeal to those pushed into the provocation of a military rebellion," he said.
Putin added that at this time, "we require unity, consolidation, and responsibility."
The Russian President said, "any internal turmoil is a deadly threat to our statehood for us as a nation; it is a blow to Russia for our people and our actions to protect our homeland. Such a threat will face a severe response."
Wagner chief claims to have seized control of key military facilities in 2 Russian cities. Here's the latest

The simmering conflict between Moscow’s military leadership and Yevgeny Prigozhin , the bombastic chief of private mercenary group Wagner, has dramatically escalated into an open insurrection that plunges Russia into renewed uncertainty.
Moscow's mayor said the capital is reinforcing security as Prigozhin claimed to have seized control of key military facilities in the Russian cities of Rostov and Voronezh Saturday.
Here's the latest:
- Wagner claims control in Rostov: Prigozhin said in a video he is in Rostov-on-Don, in southern Russia close to the Ukraine border, and that his forces have control of military facilities and the airfield there. He pledged to blockade Rostov and move on to Moscow if Russia's Defense Minister and top general did not meet with him in the city, where Russia’s Southern Military District is headquartered. He said his men are not stopping the officers from carrying out their duties. It comes after Prigozhin said his fighters were entering the Rostov region on Friday and that Russian Guards and military police have joined the Wagner group. CNN cannot independently verify his claims. Videos circulating on social media and geolocated to Rostov city show military vehicles on the streets and helicopters over the city Saturday morning. It is currently unclear whose command the vehicles are under the control of.
- Claims of control in Voronezh: The Wagner group later said it had taken control of Russian military facilities in the city of Voronezh, in southwestern Russia, saying "the army switches to the side of the people." Earlier, the governor of Voronezh oblast said that "a convoy of military equipment is moving along the M-4 Don Federal Highway." The M-4 is a highway connecting Voronezh and Rostov-on-Don. Voronezh is directly north of the Rostov region.
- Alleged helicopter attack: Prigozhin also claimed a helicopter fired at a civilian column and was downed by his forces, but did not give any further details. He accused the Russian military's chief of staff of ordering an aerial attack "in the middle of civilian cars." Later, he said that his units were hit by a helicopter on a highway. CNN cannot independently verify these claims.
- Prigozhin accuses Russia of killing his forces: The Wagner chief accused Russia's military leadership of killing a "huge amount" of his mercenary forces in a strike on a camp and vowed to retaliate. "Many dozens, tens of thousands of lives, of Russian soldiers will be punished," Prigozhin said. "I ask that nobody put up any resistance." In a later Telegram post, Prigozhin said that his criticism of the military leadership was a “march of justice” and not a coup. Russia’s Ministry of Defense denied Prigozhin’s claims, calling it an “informational provocation."
- Russia accuses Prigozhin of "armed rebellion": The Federal Security Service (FSB), Russia’s domestic intelligence service, responded by urging Wagner fighters to detain their leader and on Friday it opened a criminal case against the Prigozhin, accusing the mercenary force's chief of calling for " armed rebellion ," the state news agency TASS reported. Russia's Ministry of Defense appealed to Wagner forces to "safely return to their points of permanent deployment," saying they were "tricked into Prigozhin's criminal adventure."
- Russia steps up security: Moscow's mayor said "anti-terrorist measures" are being carried out in the city. A local journalist said the streets appear calm in Moscow, but that there is heightened security at government agencies. In the Russian city of Rostov , military vehicles could be seen driving the streets. Posts were organized on Saturday in the area of the headquarters of the Southern Military District in Rostov where military personnel and law enforcement officers are keeping order, a TASS correspondent reported.
Russian Ministry of Defense appeals to Wagner forces to return to "permanent deployment"
From CNN's Uliana Pavlova and Lizzy Yee
Russia's Ministry of Defense appealed to Wagner forces to "safely return to their points of permanent deployment" on Saturday, after the private mercenary group's chief Yevgeny Prigozhin claimed to have taken control of military facilities in two Russian cities.
"Many of your comrades from several squads have already realized their mistake by asking for help in ensuring the ability to safely return to their points of permanent deployment," the statement said.
"Such assistance from our side has already been provided to all the fighters and commanders who applied," it continued.
The Ministry of Defense said it would "guarantee everyone's safety."
Wagner claims it has taken control of military facilities in Voronezh
From CNN's Isaac Yee and Yulia Kesaieva
The Wagner paramilitary group claimed Saturday it had taken control of Russian military facilities in the Russian city of Voronezh.
"Military facilities in Voronezh are taken under the control of the Wagner PMC. The army switches to the side of the people," read a short statement from Wagner's Telegram channel.
Earlier on Saturday, Wager chief Yevgeny Prigozhin said he was at the military headquarters in Rostov and that the local airfield was under his force's control.
Russian security forces have cordoned off Wagner Center in St. Petersburg
From CNN's Josh Pennington and Lizzy Yee
Russian security forces have cordoned off the building of the Wagner Center in St. Petersburg, according to Russian state media outlet RIA Novosti.
Videos circulating on Telegram channels of the Wagner group show security personnel at the headquarters in St. Petersburg and a cordon around the building.
Please enable JavaScript for a better experience.

IMAGES
COMMENTS
Begin 1-2 days before travel, daily during travel, and for 7 days after leaving. Good for last-minute travelers because the drug is started 1-2 days before traveling to an area where malaria transmission occurs. Some people prefer to take a daily medicine. Good choice for shorter trips because you only have to take the medicine for 7 days after ...
Malaria prevention consists of a combination of mosquito avoidance measures and chemoprophylaxis. Prevention measures must address all malaria species in the travel area and apply to both short-term and long-term travelers. ... Recommendations for drugs to prevent malaria by country of travel can be found in Sec. 2, Ch. 5, Yellow Fever Vaccine ...
INTRODUCTION. Malaria is an important cause of fever and serious illness in returned travelers [].Among nearly 7000 returned travelers with fever seen at a GeoSentinel clinic between 1997 and 2006, for example, malaria was the most common specific etiologic diagnosis, found in 21 percent of cases [].The relative risk of malaria is higher among returned travelers from sub-Saharan Africa than ...
Chapter on malaria in the WHO "International travel and health" ... Vector control is the main approach to prevent malaria and reduce transmission. Two forms of vector control are effective for people living in malaria-endemic countries: insecticide-treated nets, which prevent bites while people sleep and which kill mosquitoes as they try ...
The prevention of malaria in travelers continues to be challenging. A multitude of factors determine the risk of malaria acquisition among travelers. The knowledge of such factors and the available preventive measures are of vital importance in being able to provide evidence-based recommendations to travelers. This knowledge should not be ...
Drugs recommended for prevention of malaria in travelers Mefloquine, Doxycycline, Chloroquine. Strict adherence to the recommended doses and sched- ules of the antimalarial drug selected is necessary for effective protection. . Take tablets on the same day each week or, in the case of tablets to be taken daily, at the same time each day.
Key elements in prevention include barrier protection and chemoprophylaxis. Travelers to malaria-endemic areas should be advised to use mosquito repellent at all times and bed netting at night ...
When planning to travel to an area where malaria occurs, talk with your doctor well in advance of your departure. Drugs to prevent malaria can be prescribed for travelers to malarious areas, but travelers from different countries may receive different recommendations, reflecting differences in treatment protocols as well as availability of ...
C hemoprophylaxis. This means taking antimalarial medication to prevent the disease. D iagnosis should be made promptly and treatment started quickly. Seek medical attention urgently if you become unwell after travelling to a high-risk area. Malaria is a serious infection, so prevention is crucial. Dr Sarah Jarvis MBE, FRCGP.
The disease is rare in the U.S., with about 2,000 cases per year. If you're traveling to an area where malaria is common, talk to your healthcare provider about ways you can prevent being infected. People who are infected and travel to the U.S. can spread the disease if a mosquito bites them and then bites someone else.
For more information regarding the malaria surveillance system, or assistance in completing the form, please call the Malaria Branch at 770-488-7788 or toll-free at 855-856-4713. The CDC provides ...
Dates and duration of travel; A travel medicine specialist will review your itinerary before your consultation to identify country-by-country health risks, such as exotic infectious agents, the potential for altitude sickness or heat exhaustion, as well as appropriate vaccinations and possible need for malaria-prevention medications.
CDC Yellow Book 2024. Preparing International Travelers. Author (s): Mark Gershman, Rhett Stoney (Yellow Fever) Holly Biggs, Kathrine Tan (Malaria) The following pages present country-specific information on yellow fever (YF) vaccine requirements and recommendations, and malaria transmission information and prevention recommendations.
The threat of malaria is highest in sub-Saharan Africa, and 4 countries in that region accounted for nearly half of all malaria deaths worldwide in 2022: Nigeria (31.1%), the Democratic Republic ...
Between 1999 and 2008 Russia experienced a flare-up of transmission of vivax malaria following its massive importation with more than 500 autochthonous cases in European Russia, the Moscow region being the most affected. The outbreak waned soon after a decrease in importation in mid-2000s and strengthening the control measures. Compared with other post-eradication epidemics in Europe this one ...
In June 2023, the US Centers for Disease Control and Prevention alerted health providers to locally acquired malaria infections in Florida and Texas . In October 2023, California (Pasadena) reported a case of dengue infection in a resident who apparently did not travel outside the United States .
Health-care providers needing assistance with diagnosis or management of suspected cases of malaria should call the CDC Malaria Hotline: 770-488-7788 or 855-856-4713 toll-free (M-F, 9am-5pm, Eastern Time). and request to speak with a CDC Malaria Branch clinician. • Take an antimalarial drug. • Prevent mosquito bites.
1. Introduction. Human malaria is a group of four infections caused by protozoan parasites of the genus Plasmodium and transmitted by anopheline mosquitoes. On a global scale, practically all cases of malaria are caused by either P. falciparum or P. vivax.The former is the predominant of the two (ca 90%).The share of infections caused by human parasites P. malariae and P. ovale is negligible.
EPIC MOSCOW Itinerary! (2024) Moscow is the heart of Mother Russia. Just the mention of this city conjures images of colorful bulbous pointed domes, crisp temperatures, and a uniquely original spirit! Moscow has an incredibly turbulent history, a seemingly resilient culture, and a unique enchantment that pulls countless tourists to the city ...
Anopheles sacharovi, a member of the Anopheles maculipennis complex, was a historical malaria vector in Italy, no longer found since the last report at the end of 1960s. In September 2022, within the Surveillance Project for the residual anophelism, a single specimen of An. maculipennis sensu lato collected in Lecce municipality (Apulia region) was molecularly identified as An. sacharovi.
Every year, millions of U.S. residents travel to countries where malaria and other diseases spread by mosquitoes (mosquito-borne) are found. ... Your doctor may also prescribe you a medication to prevent malaria (antimalarial drug). Although antimalarial drugs are very effective, they are not 100% effective in preventing malaria. ...
Latest news releases. Hwy 10 lane closures, delays begin east of St. Cloud April 15 . Cable median barrier to prevent crossover crashes ST. CLOUD, Minn. - Motorists will encounter periodic lane closures on eastbound Highway 10 from St. Cloud to Clear Lake as crews install high-tension cable median barrier, reports the Minnesota Department of Transportation.
The Russian National Anti-Terrorism Committee announced the introduction of a counter-terrorist operation regime in Moscow, the Moscow region and Voronezh region.