Ancient eel migration mystery unravelled
- Published 15 October 2022

Scientists have unravelled a mystery surrounding one of nature's most incredible journeys.
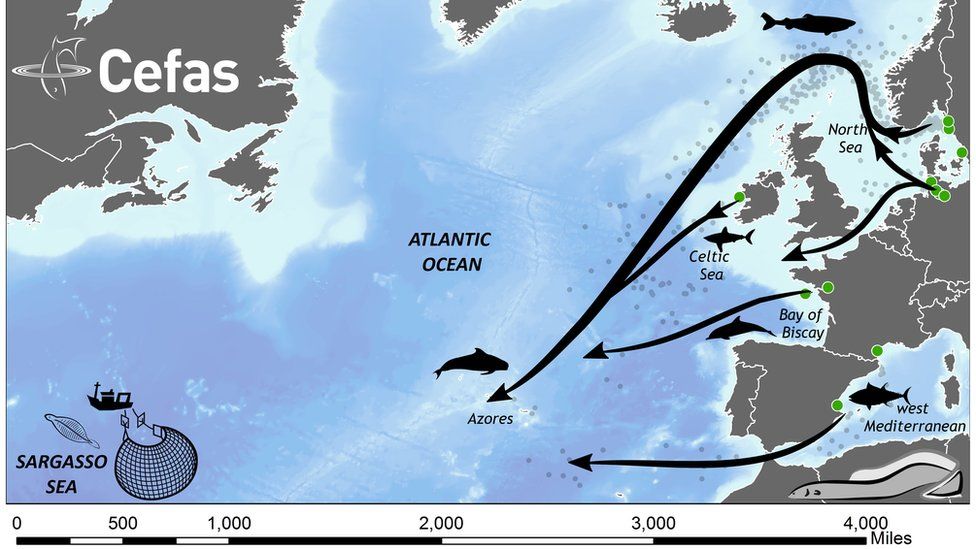
Every year, eels leave European rivers to travel in an epic migration to the Sargasso Sea in the North Atlantic to breed for a single time, then die.
Although this final destination has long been suspected, until now there has been no direct evidence.
By fitting eels with satellite tags, researchers have tracked the creatures on the final leg of the route.
And they say the information will help in the conservation of the critically endangered species.
"This is the first time we've been able to track eels to the Sargasso Sea and we are delighted we have the first direct evidence of adult European eels reaching their spawning area," said Ros Wright of the Environment Agency, who led the research.
"Their journey will reveal information about eel migration that has never been known before."
The European eel faces many hazards throughout its life cycle, including changes to ocean currents caused by climate change, pollution, poaching and obstacles in waterways such as dams and weirs.
Eel specialist at the Environment Agency, Dan Hayter, has been monitoring eels in the River Blackwater in Essex for 20 years and has seen a drastic decline over that time.
"We do catch eels here every single year," he explained. "Compared with the historic numbers, they're very low now, and there's been a 95% decline since the 1980s."

Conservation measures
Eels arrive around the European coast as tiny, fragile and transparent glass eels, having drifted across the Atlantic for two or three years from the Sargasso Sea.
They adapt to freshwater and mature in rivers - growing up to 1m long - until they are ready to swim all the way back to reproduce once and die.

Until now, it has been very difficult to study their migration across the ocean; previous studies have tracked adult eels all the way to the Azores, but from there the trail went cold.
The researchers have now tagged adult eels in the Azores, showing they can swim all the way to the Sargasso Sea.
"We knew they could get as far as the Azores, but that final leg was just undiscovered country," said Ros Wright.
"We thought if we could tag eels in the Azores then we might fill that gap - and we have managed this - we can confirm we have filled in that final leg of the journey to the Sargasso Sea."
Unravelling the routes taken and locating where eels spawn is critical for understanding the reasons behind their decline and to inform conservation measures.
Mysterious life cycle
The life cycle of the eel has long puzzled scientists. Even the Greek philosopher Aristotle pondered the question of where eels came from, deciding that they sprang up spontaneously from the mud.
Almost 100 years ago, it was assumed that their destination was the Sargasso Sea, in the western Atlantic near the Bahamas, but until now final proof had been lacking.
The research is published in the journal, Scientific Reports.
Follow Helen on Twitter @hbriggs.
Related Topics
- Environment
100-year-old mystery solved: Adult eel observed for the first time in the Sargasso Sea
After more than a century of speculation, researchers have finally proved that American eels really do migrate to the Sargasso Sea to reproduce. A team supervised by Professor Julian Dodson of Université Laval and Martin Castonguay of Fisheries and Oceans Canada reports having established the migratory route of this species by tracking 28 eels fitted with satellite transmitters. One of these fish reached the northern boundary of the Sargasso Sea, the presumed reproduction site for the species, after a 2,400 km journey. Details are published in the latest edition of Nature Communications .
The discovery puts an end to more than a hundred years of conjecture regarding the migratory route and location of the only American eel reproduction site. "Eel larvae have been observed in the Sargasso Sea since 1904, suggesting that the species reproduced in this area, but no adult eels had ever been observed in this part of the Atlantic Ocean," explained Professor Dodson of the Faculty of Science and Engineering at Université Laval.
The many expeditions aimed at catching eels in their mysterious gathering site have all failed, but the recent development of sophisticated satellite transmitters opened up new opportunities for researchers. Julian Dodson and his team affixed these transmitters to 22 eels captured in Nova Scotia and 16 from the St. Lawrence Estuary. In the ensuing weeks, 28 of these transmitters resurfaced in different areas of the Atlantic and transmitted the data they had recorded.
Analysis of the data revealed that all the eels adopted similar migratory paths and patterns. Near the coastline they appear to use the salinity level and temperature to find the high seas. A single eel provided data for the ocean segment of the migration. Its transmitter showed that it turned due south upon reaching the edge of the continental shelf, and headed straight to the Sargasso Sea. In 45 days, this eel captured in the province of Quebec covered 2400 km. "This points to the existence of a navigation mechanism probably based on magnetic field detection," asserted Professor Dodson.
Julian Dodson remains cautious about drawing premature conclusions from some thirty eels, only one of which travelled the full migratory route. "Our data nonetheless shows that the eels don't follow the coastline the whole way, they can cover the route in just weeks, and they do go to the Sargasso Sea. We knew that millions of American eels migrated to reproduce, but no one had yet observed adults in the open ocean or the Sargasso Sea. For a scientist this was a fascinating mystery."
The article published in Nature Communications is coauthored by Mélanie Béguer-Pon, José Benchetrit, and Julian Dodson of Université Laval; Martin Castonguay of Fisheries and Oceans Canada; and Shiliang Shan of Dalhousie University.
- Marine Biology
- Oceanography
- Environmental Awareness
- Global Warming
- Deep sea fish
Story Source:
Materials provided by Université Laval . Note: Content may be edited for style and length.
Journal Reference :
- Mélanie Béguer-Pon, Martin Castonguay, Shiliang Shan, José Benchetrit, Julian J. Dodson. Direct observations of American eels migrating across the continental shelf to the Sargasso Sea . Nature Communications , 2015; 6: 8705 DOI: 10.1038/ncomms9705
Cite This Page :
Explore More
- New Circuit Boards Can Be Repeatedly Recycled
- Collisions of Neutron Stars and Black Holes
- Advance in Heart Regenerative Therapy
- Bioluminescence in Animals 540 Million Years Ago
- Profound Link Between Diet and Brain Health
- Loneliness Runs Deep Among Parents
- Food in Sight? The Liver Is Ready!
- Acid Reflux Drugs and Risk of Migraine
- Do Cells Have a Hidden Communication System?
- Mice Given Mouse-Rat Brains Can Smell Again
Trending Topics
Strange & offbeat.
The Utterly Engrossing Search for the Origin of Eels
To save the endangered animals, researchers have been working for decades to figure out where they reproduce
Christina Couch, Hakai
:focal(800x602:801x603)/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer_public/43/b6/43b66271-b2cb-4fb9-adfb-0125e117e05a/gettyimages-549040441_web.jpg)
Every three years, Reinhold Hanel boards a research ship and voyages to the only sea in the world that’s located in the middle of an ocean. The Sargasso, bounded by currents instead of land, is an egg-shaped expanse that takes up about two-thirds of the North Atlantic, looping around Bermuda and stretching east more than 1,000 kilometers. Dubbed the “golden floating rainforest” thanks to the thick tangles of ocher-colored seaweed that blanket the water’s surface, the Sargasso is a slowly swirling sanctuary for over 270 marine species. And each year, the eels arrive.
The European eel and the American eel—both considered endangered by the International Union for Conservation of Nature—make this extraordinary migration. The Sargasso is the only place on Earth where they breed. The slithery creatures, some as long as 1.5 meters, arrive from Europe, North America, including parts of the Caribbean, and North Africa, including the Mediterranean Sea. Hanel, a fish biologist and director of the Thünen Institute of Fisheries Ecology in Bremerhaven, Germany, makes his own month-long migration here alongside a rotating cast of researchers, some of whom hope to solve mysteries that have long flummoxed marine biologists, anatomists, philosophers, and conservationists: What happens when these eels spawn in the wild? And what can be done to help the species recover from the impacts of habitat loss, pollution, overfishing, and hydropower? Scientists say that the answers could improve conservation. But, thus far, eels have kept most of their secrets to themselves.
The idea that eels have sex at all is a fairly modern notion. Ancient Egyptians associated eels with the sun god Atum and believed they sprang to life when the sun warmed the Nile. In the fourth century BCE, Aristotle proclaimed that eels spontaneously generated within “the entrails of the earth” and that they didn’t have genitals.
The no-genital theory held for generations. Roman naturalist Pliny the Elder asserted that eels rubbed against rocks and their dead skin “scrapings come to life.” Others credited eel provenance to everything from horses’ tails to dew drops on riverbanks. In medieval Europe, this presumed asexuality had real economic consequences and helped make the European eel a culturally important species, according to John Wyatt Greenlee, a medieval cartographic historian who wrote part of his dissertation on the subject. Frequent Christian holidays at the time required followers to adhere to church-sanctioned diets for much of the year. These prohibited adherents from eating “unclean” animals or meat that came from carnal acts, which could incite, as Thomas Aquinas put it, “an incentive to lust.” Fish were the exception, Greenlee says, and eels, given their abundance and “the fact that they just sort of appear and that nobody can find their reproductive organs at all,” appealed to anyone trying to avoid a sexy meal.
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer_public/d5/97/d597d0df-b210-408e-8a71-266d69ddf58c/gettyimages-1231886334_web.jpg)
Eels could be practically anything to anyone: dinner or dessert; a cure for hangovers, drunkenness, or ear infections; material for wedding bands or magical jackets. They were even used as informal currency. Since yearly rent and taxes in medieval Europe were often due during Lent—the roughly 40-day period preceding Easter—and monasteries owned land people lived on, tenants sometimes paid with dried eels. Entire villages could pay 60,000 eels or more at once.
Eventually, spontaneous generation theories died. But eel genitals landed in the spotlight again after an Italian surgeon found ovaries in an eel from Comacchio, Italy, and the findings were published in the 18th century. The legitimacy of the so-called Comacchio eel remained in question for decades until an anatomist published a description of ovaries from a different Comacchio eel, launching a race to find testicles. Even the granddaddy of psychosexual development theory got involved: near the beginning of his career, in 1876, Sigmund Freud dissected at least 400 eels in search of gonads. It would be about another two decades before someone discovered a mature male eel near Sicily.
It’s no surprise that it took so long to find eel sex organs. There are more than 800 species, about 15 of which are freshwater varieties, and their bodies change so dramatically with age that scientists long thought the larvae were a different species than adult eels. Eels transform from eggs to transparent willow-leaflike larvae, to wormy see-through babies called glass eels, and onward until full size. Like most eel species, American and European eels don’t fully develop gonads until their last life stage, usually between 7 and 25 years in. Around that time, they leave inland fresh and brackish waters, where people can easily observe them, and migrate up to about 6,000 kilometers—roughly the distance from Canada’s easternmost tip to its westernmost—to the Sargasso.
By now, researchers have seen eels mate in lab settings, but they don’t know how this act plays out in the wild. The mechanisms that guide migration also remain somewhat enigmatic, as do the exact social, physical, and chemical conditions under which eels reproduce. Mature eels die after spawning, and larvae move to freshwater habitat, but when that happens and how each species finds its home continent are also unknown.
“We think that the European eel reproduces in the Sargasso Sea because this is the place where we have found the smaller larvae, but we have never found a European eel egg or the eels spawning,” says Estibaliz Díaz, a biologist at AZTI marine research center in Spain, who studies European eel population dynamics and management. “It’s still a theory that has not been proven.” The same applies to the American eel, and yet more questions remain about how many eels survive migration, what makes the Sargasso so singular, and how factors like climate change might affect it.
Both species have dropped in number, but researchers debate which threat is the biggest. Habitat loss is huge—humans have drained wetlands, polluted waters with urban and agricultural runoff, and built hydropower turbines that kill eels and dams that block the animals from migrating in or out of inland waters. Fishing further reduces eel numbers. Commercial fisheries for adult eels exist, but most eels consumed globally come from the aquaculture industry, which pulls young glass eels from the wild and raises them in farms. American and European eels are among the top three most commercially valuable species alongside the Japanese eel, which is also endangered. While it’s legal to fish for all three, regulations on when, where, and how many eels can be sold vary between countries. The European Union requires member nations to close their marine fisheries for three consecutive months around the winter migration season each year—countries themselves determine exact dates—and prohibits trade outside of member countries, but these management efforts are undermined by black-market traders who illegally export more than 90 tonnes of European eels to Asia every year.
The International Union for Conservation of Nature (IUCN) lists European eels as critically endangered—populations have plummeted more than 90 percent compared with historical levels, and it’s “rather unclear,” as one report notes, whether the decline continues today. By counting glass eels in estuaries and inland waters, researchers found that eel numbers dropped precipitously between the 1980s and 2011, but plateaued afterward without clear cause. American eels are thought to be faring better—they’re considered endangered only by IUCN standards, not by other conservation and research groups—though their numbers have also decreased since the 1970s.
Captive breeding might one day reduce the aquaculture industry’s dependence on wild catches, but isn’t yet viable. Scientists must induce eel gonad development with synthetic hormones. It’s also hard to keep larvae alive. Many researchers believe that, in their natural habitat, larvae eat marine snow—a mélange of decaying organic matter suspended in the water that is impractical to reproduce at commercial scales. Illuminating what happens in the Sargasso could help guide better conservation measures. That’s why Reinhold Hanel heads to sea.
After three years of COVID-19-related delay, in 2023, Hanel will send a research vessel on a 14-day trip from Germany to Bermuda. He’ll fly there and meet up with 11 other eel researchers, then he’ll spend about a month slowly traversing the southern Sargasso, recording ocean conditions, trawling for eel larvae with mesh plankton nets, and sampling for environmental DNA—genetic material shed from skin, mucus, and poop—to track eels by what they leave behind.
Hanel has led voyages like these since 2011. His main goal is to document the abundance of larvae and young eels and, secondarily, to identify possible locations for spawning. By sampling estuaries and inland waters, researchers can identify trends over time to figure out if glass eels in continental waters are increasing or not, but without comparing those trends with similar ones in the Sargasso, it’s impossible to judge whether either American or European eels are bouncing back. Meanwhile, protective regulations aren’t enough, Hanel contends. In 2007, the European Union mandated that member countries develop European eel recovery plans, but several prominent fishery and marine science organizations have criticized the particulars .
In tandem with other measures aimed at reducing eel mortality, provisions like closing fisheries make sense, Hanel says—last year, an international consortium of researchers, of which Hanel is a member, recommended closing fisheries until glass eel stocks recover. But other requirements aren’t rooted in research, including one to ensure 40 percent of adult eels survive to migrate from inland waters to the sea each year. “Scientists cannot say if 40 percent is sufficient to recover the stock,” Hanel says.
That’s why Hanel’s work is so important, says Martin Castonguay, a marine biologist and scientist emeritus at Fisheries and Oceans Canada, who has collaborated with Hanel. Financial obstacles often prevent eel scientists from conducting research outside of inland waters. Research vessels can cost anywhere from CAN $30,000 to $50,000 per day, or just under $1-million for a month-long trip, Castonguay says, requiring scientists to have hefty grants or government support to venture all the way to the Sargasso.
Despite the barriers, scientists keep trying to find answers to how to help eels recover. They have planted hydroacoustic devices in hopes of tracking migrating eels by sound, pored over satellite photos, and injected eels with hormones to induce gonad development before releasing them into the Sargasso to try to study how deep beneath the surface they spawn. Back at home in the lab, they’ve developed algorithms to scan for and spot eels in sonar images of inland waters and built hyperbaric swimming tubes to observe how eels respond to changes in pressure and current strength. They’ve even tried to follow them with satellite transmitters.
In the mid-2010s, Castonguay and four other researchers sewed buoyant trackers to 38 American eels and released them off the coast of Nova Scotia. Every 15 minutes, the trackers recorded the depth at which the eels were swimming, the water temperature, and light levels. The sensors were designed to detach several months later and transmit the data along with the eels’ final location. Unfortunately, they detached before the eels reached any specific spawning locations, though one eel got as close as 100 to 150 kilometers from the spawning region. Still, “it was the first time that an [adult American] eel was documented in the Sargasso,” says Castonguay. Previously, only larvae had been found there. “We were extremely excited.”
If more governments and research institutions were willing to spend the resources, Castonguay adds, these eels wouldn’t be so mysterious. Research on a similar species in Japan offers a case study for how that could work.
On the other side of the globe from the Sargasso, the Japanese eel makes a 3,000-kilometer annual migration from Japan and surrounding countries to the West Mariana Ridge in the western Pacific Ocean. With support from the Japanese government and other scientific institutions, researchers there have identified a spawning location, collected fertilized eggs, and tracked tagged eels swimming to their spawning area—all feats never attained in the Sargasso. They’ve found that Japanese eels spawn over a period of a few days before the new moon, at depths of 150 to 200 meters, and that spawning is triggered in part by temperature shifts that happen as eels move from deep to shallower water. Some eels, they learned, might spawn more than once during a spawning season.
Public outreach efforts have also been important, says University of Tokyo eel biologist Michael Miller. The researcher who led most of the eel work, Katsumi Tsukamoto—a University of Tokyo scientist emeritus known as Unagi Sensei, or Dr. Eel—has worked hard to raise the eels’ public profile. His findings have helped build the case that eels are “something other than just a meal,” Miller says. “It’s something [that’s] part of the Japanese culture and it’s worth conserving,” which has helped boost efforts to protect them.
Hanel is trying to do the same for the eels of the Sargasso and for other species. He speaks to the press and the public as often as he can. He believes, as many others do, that successfully conserving these creatures hinges on whether there’s a unified international effort to do so. But so long as data snapshots come only every few years, answers to questions about spawning and species well-being will stay hidden somewhere in the watery depths, just like the eels themselves.
This article is from Hakai Magazine, an online publication about science and society in coastal ecosystems. Read more stories like this at hakaimagazine.com .
Related stories from Hakai Magazine:
- Want to Find Whales? Look for the Birds
- The Eternal Life of Beached Whales
Get the latest Science stories in your inbox.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 27 October 2015
Direct observations of American eels migrating across the continental shelf to the Sargasso Sea
- Mélanie Béguer-Pon 1 , 2 ,
- Martin Castonguay 3 ,
- Shiliang Shan 2 ,
- José Benchetrit 1 &
- Julian J. Dodson 1
Nature Communications volume 6 , Article number: 8705 ( 2015 ) Cite this article
20k Accesses
62 Citations
313 Altmetric
Metrics details
- Animal migration
- Ichthyology
Since inferring spawning areas from larval distributions in the Sargasso Sea a century ago, the oceanic migration of adult American eels has remained a mystery. No adult eel has ever been observed migrating in the open ocean or in the spawning area. Here, we track movements of maturing eels equipped with pop-up satellite archival tags from the Scotian Shelf (Canada) into the open ocean, with one individual migrating 2,400 km to the northern limit of the spawning site in the Sargasso Sea. The reconstructed routes suggest a migration in two phases: one over the continental shelf and along its edge in shallow waters; the second in deeper waters straight south towards the spawning area. This study is the first direct evidence of adult Anguilla migrating to the Sargasso Sea and represents an important step forward in the understanding of routes and migratory cues.
Similar content being viewed by others

Climate change-driven cooling can kill marine megafauna at their distributional limits

Biogeographic response of marine plankton to Cenozoic environmental changes

The role of biota in the Southern Ocean carbon cycle
Introduction.
At the onset of maturation, American eels ( Anguilla rostrata ) undertake a long-distance migration (up to 4,000 km) from continental waters to their single spawning area in the Sargasso Sea as inferred from the collection of leptocephalus larvae during several marine expeditions that began in 1904 (refs 1 , 2 ). After over a century of research that has failed to catch a single adult in the open ocean, most information concerning their spawning migration remains a complete mystery. Neither the exact location of the spawning site nor the migration routes and the environmental conditions along these routes are known. The American eel is listed as endangered on the International Union for Conservation of Nature (IUCN) red list 3 and assessed as threatened in Canada 4 . Considering the species’ precarious status, filling the knowledge gap with respect to its marine migration is important for both research and management objectives. More specifically, this implies refining information on the extent of the spawning area, describing the environmental conditions experienced during the migration and revealing the migration routes and orientation cues eels use. Acoustic tracking of American eels in the St. Lawrence River and Estuary revealed that maturing (silver) eels must use selective tidal stream transport to successfully leave the Estuary 5 . In 2011, attempts to track silver American eels in marine waters using pop-up satellite archival tags (PSATs) began in the Gulf of St. Lawrence (Canada) 6 . However, a high rate of predation of tagged eels by homeothermic fishes prevented successful tracking of the eels beyond the Gulf. This same technology was used to track European eels ( A. anguilla ) at sea, and that study provided unique behavioural insights into the early phase of their marine migration 7 , 8 .
In an attempt to follow American eels en route to their spawning site and thus document the full-marine migration, a total of 38 silver eels were tracked with PSATs. Two types of PSATs were used, they are: 27 X-tags from Microwave Telemetry ( http://www.microwavetelemetry.com ) and 11 SeaTag-GEOs from Desert Star Company ( http://desertstar.com/ ). The tagged eels were released on the Scotian Shelf, off Nova Scotia (NS), Canada, in the fall of 2012, 2013 and 2014. While eels tagged in 2012 and 2013 were from NS rivers, those released in 2014 were captured in the St. Lawrence Estuary (SLE) and transported by truck to NS (migration shortcut of 1,400 km). The latter approach was used given the considerably larger size and body mass of eels from the SLE relative to NS-native eels (2.9 kg on average versus 1.4 kg), thereby reducing the potential negative impact of carrying the PSAT. To document the horizontal and vertical behaviours of the migrating eels, we analysed data from 28 PSATs that successfully transmitted their data. Daily trajectories were reconstructed by matching the environmental data recorded by the tags (water temperature and either depth or geomagnetic field total intensity) with outputs of the operational global ocean circulation models that have advanced data assimilation components. As satellite tags were unable to collect measurable light intensity because of the propensity of eels to avoid the euphotic zone during daytime, reliable sunset and sunrise estimates were not available, preventing us from inferring the longitude as traditionally performed by other large-scale tracking studies of marine species 9 . Nevertheless, we were able to infer ranges of longitude in certain cases 10 , using sunrise and sunset times estimated from marked diel vertical migration (DVM) for some days and some individuals.
Eight eels were successfully tracked to the open ocean off the continental shelf, including one tracked for 2,400 km to the northern limit of the spawning site in the Sargasso Sea. Our results represent the first direct evidence of adult Anguilla migrating to the Sargasso Sea. The similarity of trajectories and behaviour of migrating eels indicate a degree of consistency in the orientation/navigation mechanism employed throughout the migration. The migratory routes seem to be largely independent of current fields. Although migrating mainly in shallow water over the continental shelf with no marked vertical migratory pattern, eels exhibit DVM down to 700 m in the open ocean.
Pop-off location and tracking success
Of the 38 PSATs attached to eels and released on the Scotian Shelf over 3 years, 28 successfully transmitted their data after popping off at an estimated distance of 5–1,570 km from their release site ( Fig. 1 and Supplementary Table 1 ). The rate of non-reporting was particularly high in 2012 when 70% of the X-tags (7/10) never transmitted to satellites compared with 17% in 2013 (1 SeaTag-GEO) and 6% in 2014 (1 X-tag; Supplementary Table 1 ). The 18 reporting X-tags transmitted on average 97% (range: 56–100%) of their archival data, whereas the average transmitting rate for the 8 SeaTag-GEOs was 48% (range: 7–100%). The PSATs transmitted their data 24.4 days on average after their deployment (range: 0.7–59.7 days), that is, long before their scheduled transmission date (which was 3–5 months after deployment). Reasons for these premature releases were unclear except for two predation events clearly identified (see below). A total of 431 days of eel tracks were obtained, with the longest track reaching 57.2 days and the shortest only 0.5 day ( Supplementary Table 2 ). Six eels were tracked for more than a month (31.8–57.2 days), eight eels were tracked between 12.7 and 28.7 days and the remaining ten eels were tracked for less than 8 days.
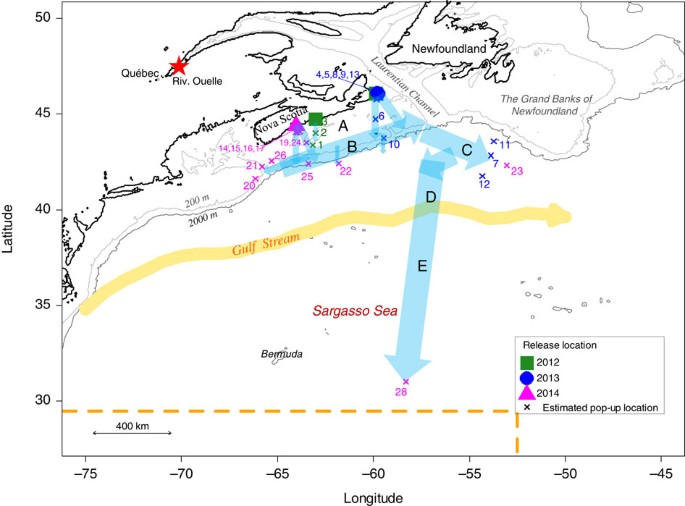
A is the Scotian Shelf, B represents the edge of the Scotian Shelf, C represents the exit of the Laurentian Channel (open ocean), D represents the area after C, which includes the Gulf Stream and E the Sargasso Sea. The orange arrow represents the north wall of the Gulf Stream in November. The red star represents the capture location of the eels used in the 2014 experiments. Eels of other years were captured nearby their release location. The dashed orange line represents the northern and eastern limits of the American eel spawning site inferred from the collection of leptocephalus larvae 11 . Each number represents a satellite tag.
The pop-up locations were estimated to be beyond the Scotian Shelf for five of the eels, at a great-circle distance (that is, the shortest distance between two points on the surface of a sphere) of 555–1,570 km from the release site (2 X-tags and 3 SeaTag-GEOs, Fig. 1 and Supplementary Table 2 ). The uncertainty around these estimated locations were on average 120±94 km in latitude (mean±s.d.; range: 9–288) and 77±37 km in longitude (range: 20–148; Supplementary Table 3 ). The estimated pop-up locations were generally relatively close to the first transmitting location estimated from Argos. For instance, the PSAT that went the furthest began transmitting its data at 32.37°N, 58.26°W and the reconstructed pop-up location was estimated to be 30.33–31.67°N, 58.08–58.50°W ( Figs 1 and 2 , and Supplementary Tables 1 and 2 ), which is very close to the northern limit of the spawning area 2 . This provides the first direct evidence of American eel migrating from coastal waters to the Sargasso Sea spawning site. The pop-up location of six PSATs was estimated to be beyond (or very close to) the edge of the Scotian Shelf, at a distance of between 230 and 354 km from their coastal release sites, whereas the remaining PSATs (17) remained relatively close to their respective release sites (a few km to 111 km).
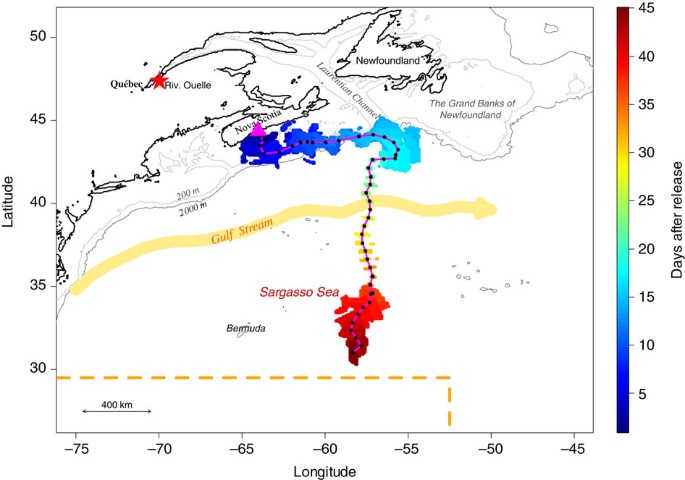
The red star represents the capture location of this eel in the SLE while it was performing its downstream migration. The magenta triangle represents the release location after tagging. The black dots are the mean reconstructed daily locations and the magenta line is the corresponding mean trajectory. A colour gradient is used to show the temporal dimension of the reconstructed trajectory (each day is represented by a colour). The range of potential daily locations is lowest for days 19–33.
Reconstructed paths and behaviour
The migratory paths were reconstructed for 16 eels equipped with X-tags and 4 eels equipped with SeaTag-GEOs ( Supplementary Tables 2 and 3 ). The uncertainty of reconstructed locations varied considerably among tags and days, averaging 120±91 km in latitude and 207±170 km in longitude for X-tags and 206±127 km of latitude and 320±201 km in longitude for SeaTags-GEOs ( Supplementary Table 3 ). In some cases, the uncertainty of the daily reconstructed locations was as little as 9 km of latitude and 7 km of longitude (for example, eel #28 (in the open ocean) equipped with an X-tag), allowing the reconstruction of relatively precise trajectories. However, uncertainty was generally quite high along the shelf break front of the Scotian Shelf (area B, Fig. 1 ), reaching several hundred kilometres in longitude. We were thus unable to evaluate potential bidirectional movements (southwestward or northeastward) at the shelf edge for many eels.
The reconstructed paths indicate a migration distance between ca 1,300 and 1,700 km for four eels and of ca 2,400 km for the eel that reached the Sargasso Sea ( Supplementary Table 2 and Fig. 2 ). The inferred trajectories and behaviour of eels within five different areas are summarized in Table 1 and Fig. 1 (also see Supplementary Table 2 ). Despite differences in release years, locations and eel origins, similar paths and vertical behaviours were observed. Following their release over the Scotian Shelf (area A), all eels immediately headed south and slightly to the east towards the edge of the continental shelf ( Fig. 1 ). During this first stage of the migration, lasting between 3 and 13 days (7.2 days on average, N =11), eels equipped with X-tags generally exhibited DVM, occupying shallow waters at night (<50 m) and bottom waters during the day (maximum of 240 m in the Emerald Basin on the Scotian Shelf, see bathymetry in Supplementary Fig. 1 ). Eels experienced a positive gradient of temperature while travelling from the coast to the edge of the Scotian Shelf:+ca 8 °C in ca 200 km from the southernmost release location ( Supplementary Figs 2–4 ). Once over the edge of the Scotian Shelf (area B), they remained in shallow waters, performing between 5 and 23 dives per day without any obvious diel pattern, and lasting from 4 to 26 days for individuals tracked beyond that area. Although it was not possible to assess the trajectories in that area because of the relatively high uncertainty of daily locations for five eels, seven eels were shown to have headed northeastward, swimming against the southwestward shelf-break current. Five of these eels then migrated to the southeast, reaching the exit of the Laurentian Channel (the so-called Laurentian Fan) and west of the Grand Banks off Newfoundland (area C), 17–57 days after their release (average of 36.4 days). Of those five eels, the smallest three which were equipped with SeaTag-Geo exhibited mean migration speeds relative to the ground between 10 and 17 km per day, whereas the two largest eels, equipped with X-tags, showed much higher speeds of 38 and 50 km per day. Eels again experienced a positive gradient in salinity (ca +3.2 in about 170 km) while travelling in this area ( Supplementary Figs 2–4 ). The two eels equipped with X-tags (which record depth) exhibiting clearly discernable DVM between the surface and ca 400–500 m in depth once the inferred salinity was greater than 35 ( Fig. 3 and Supplementary Figs 3–5 ).
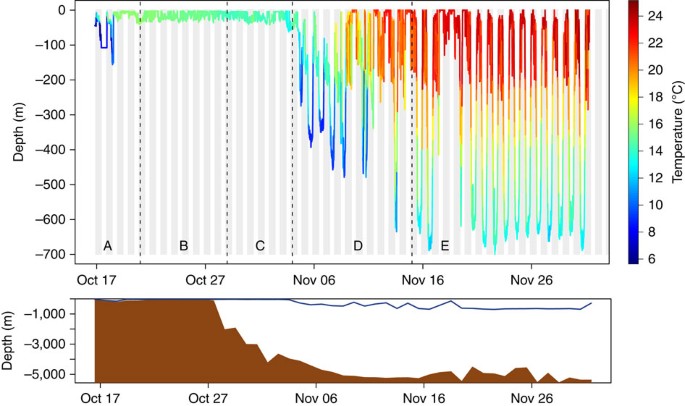
The temperature experienced along the way is superimposed to the depth profile. The bottom panel shows the observed daily maximum eel depth (dark blue line) and the corresponding bottom depth at the daily reconstructed location (brown-shaded curve). A is the Scotian Shelf, B represents the edge of the Scotian Shelf, C represents the exit of the Laurentian Channel (open ocean), D represents the area after C, which includes the Gulf Stream and E represents the Sargasso Sea.
The tracking continued for one eel (eel #28), which suddenly modified its trajectory and headed south while performing vertical migrations that were not clearly diel (area D; Fig. 2 ). It crossed the Gulf Stream 29 days after its release ( Supplementary Fig. 3 ) and reached 45 days after release a latitude of 30.33–31.67°N, that is, 92–242 km from the 29.5°N northern limit of the spawning site 11 , at a mean travel speed of 49 km per day. It performed marked DVM, with an average depth of 141±14 m at night and 618±16 m during the day (maximum of 699 m), during its relatively direct southerly trajectory into the Sargasso Sea (area E, Fig. 3 ). Another eel (eel #10) reached the Gulf Stream 12 days after its release but failed to cross it, and instead was tracked to the edge of the Scotian Shelf several days later, indicating a reverse movement ( Supplementary Fig. 6 ).
Eels experienced a very wide temperature range during their migration, from 2.5 °C over the Scotian Shelf to 25.1 °C in the Sargasso Sea ( Supplementary Table 2 ).
Predation events
Premature releases for two X-tags were clearly due to predation by homeothermic fishes. These predation events were identified from the sudden increase in ambient temperature recorded by the tags ( Supplementary Fig. 7 a,b). The tags were also not able to record any light data during the predation periods. One eel (#18) was eaten while on the Scotian Shelf only 1.5 days after its release and the tag remained inside the predator’s stomach for 6.7 days as identified by the high temperature (between 20.5 and 23.6 °C) during that period. The second eel (#27) was subject to predation after 34 days of activity and the tag remained in the predator’s stomach for 3.3 days with observed temperatures varying between 19.2 and 27.1 °C. The depth recorded by the tag just before this predation event showed that it occurred beyond the Scotian Shelf ( Supplementary Fig. 7 a,b ). The statistical method previously used and detailed in our previous tracking study conducted in the Gulf of St. Lawrence 6 was applied to identify the two predators. The predator of eel #18 was identified as a porbeagle shark ( Lamna nasus ), whereas the predator of eel #27 was most likely a Bluefin tuna ( Thunnus thynnus ). Potential predation events by ectothermic fish could also have occurred for seven of the eels equipped with X-tags, as suggested by their vertical migratory behaviours shortly after their release and over the Scotian Shelf. Indeed, these eels generally started to exhibit DVM immediately after their release for several days (1.5–7 days) before suddenly descending to the bottom ( Supplementary Fig. 7c ). They then remained for 1.5–14 days at the bottom (constant depth, zero light) before suddenly reaching the surface (the release mechanism was then triggered following 7 days at the surface).
This large-scale study has successfully reconstructed daily locations of migrating eels at sea and is the first to have tracked one individual from the coast to the northern limit of the spawning area in the Sargasso Sea. This study therefore represents an important step forward in the understanding of the oceanic migration of anguillid eels and the possible orientation mechanisms used by the species. The similarity of trajectories and behaviour of migrating eels in this study indicate a degree of consistency in the orientation/navigation mechanisms employed throughout the migration. Two distinct migratory phases are identified: one in shallow waters and the other in deep waters off the shelf. The first phase of the marine migration, from the coast to oceanic/deep waters (salinity <35), may simply rely on gradients and fronts associated with salinity and temperature. Both physical factors increase from the coast to open waters and could thus have guided eels towards the edge of the Scotian Shelf away from the coast immediately after their release. The eels then moved mainly eastward along the edge of the Scotian Shelf where they experienced relatively constant temperature and salinity. If the eels were seeking thermohaline gradients, it could explain their departure from the Scotian Shelf at the exit of the Laurentian Channel where the waters exiting the Gulf of St. Lawrence are colder and less saline. Migration along the edge of the continental shelf was also observed for European eels tracked in the North Sea 12 . In that study, all tagged eels but one headed north while on the Norwegian shelf. Assuming that eels were searching for thermohaline gradients, two opposite migration headings (north or south) were possible, such as in our present study over the Scotian Shelf (southwest or northeast). Although the reasons why most American eels headed northeast over the Scotian Shelf edge and European eels headed north over the Norwegian Trench are unknown, this does indicate that orientation cues other than those provided by thermohaline gradients are involved for that portion of the migration. Nevertheless, in both studies some eels were observed to have headed in the opposite direction, reinforcing the hypothesis that thermohaline fronts provide some orientation cues for migrating eels.
During the second phase of the marine migration (salinity >35, bottom >2,000 m), the eel tracked to the Sargasso Sea exhibited a relatively sudden change in direction, heading south to the northern limit of the spawning site in a quasi-straight line from the area adjacent to the exit of the Laurentian Channel. The orientation/navigation cues are unknown but there appears to be clear thermohaline variation for the first part of this second phase from the continental shelf to the southern edge of the Gulf Stream ( Fig. 3 ); so thermohaline cues could be used for orientation up to that point. However, the speed and directionality of the last portion of the track in the Sargasso Sea for a fish that has never before experienced such a migratory trajectory in an ocean with only weak horizontal gradients suggests the involvement of an inherited bi-dimensional map similar to that proposed for Pacific salmon 13 . Indeed, the latter study showed that salmon use a combination of geomagnetic intensity and inclination angle to assess their geographic location. The sensitivity of eels to the geomagnetic field has been known for a long time 14 , 15 and recent experiments by Durif et al . 16 supported the conclusion that eels have a magnetic compass that they can use for orientation. The existence of a neural substrate for a vertebrate magnetic sense was also recently demonstrated 17 . It thus seems likely that eels do possess a magnetic map and true navigation abilities.
In the marine environment, several fish species undertake long-distance migrations and must possess equally impressive navigation abilities 18 . The Atlantic bluefin tuna ( Thunnus thynnus ), for instance, is one of the best documented cases in the Atlantic ocean, with transatlantic seasonal migrations between the Gulf of Mexico and the eastern Atlantic or Mediterannean sea (several thousands of kilometres) and strong interannual fidelity to spawning sites 19 . Another known example involves mature female porbeagle sharks ( Lamna nasus ), which migrate ∼ 2,400 km to a subtropical pupping ground in the Sargasso Sea 20 . Both fish species are known eel predators 6 , 21 , 22 .
The eel tracked into the Sargasso Sea was an eel translocated from the SLE to the Scotian Shelf. Its behaviour during the first phase of the marine migration was similar to the behaviour of other translocated and non-translocated eels (from previous years) suggesting that translocation had no discernable effects on the large-scale behavioural patterns documented here. In contrast, a recent study attempted to track translocated European eels en route to the Sargasso Sea by releasing them in the open ocean several thousand kilometres from their native continental waters 8 . The 19 translocated eels in that study exhibited a broad range of directions and no consistent migratory patterns were observed. However, eels used in the European experiment were artificially matured and kept in captivity for weeks or months before their release, probably interfering with the normal developmental process of migration and maturation. It is also possible that silver eels need cues from continental shelf waters to orientate across open oceanic waters. Translocated American eels used in our study were not artificially matured and were released in continental shelf waters within a few days of capture, thus minimizing the aforementioned problems. Given the relative success of the approach reported in the present study, additional experiments using similar protocols should be pursued to confirm the migratory patterns documented here.
Silver eel migratory routes seem to be largely independent of current fields. Instead of taking advantage of oceanic currents, they may travel against them as was observed for European eels tracked in the North Sea 12 . American eels leaving the Scotian Shelf must cross the Gulf Stream, a strong northeastward current. One of our tracked eels managed to do this and its track was little affected by this crossing even though a slight drift was observed along its path towards the spawning area. However, this eel was among the largest individuals that can be found in North America (2.8 kg, 113 cm) and it is likely that smaller eels tagged with PSAT would have trouble crossing the Gulf Stream as we observed for one eel released in 2013 (1.3 kg, 93 cm).
Our results indicate that DVM does not occur along the whole migratory path. Over the continental shelf and at its edge, most eels did not exhibit DVM and remained in shallow waters, performing multiple dives day and night. In that area, the horizontal salinity gradient is greater near the surface than in deeper waters (100–200 m). Remaining in shallow waters and periodically diving to greater depths may represent a mechanism by which eels sample the salinity gradient to obtain directional information leading offshore. Well-defined patterns of DVM appeared once the salinity was greater than 35 and remained constant to the northern limit of the spawning area. DVM behaviour has been observed among many anguillids in the early stages of their marine migration 7 , 23 , 24 and some authors have speculated that such behaviour is a trade-off between predator avoidance and the necessity to maintain sufficiently high metabolism for migration. The American eel tracked to the Sargasso Sea showed a clear bimodal distribution in ambient temperatures such as seen in European eels directly released in the Sargasso Sea 8 . Such vertical behaviour observed in both American and European eels is consistent with the hypothesized trade-off between predator avoidance and the metabolic requirements of migration.
An important rate of non-reporting by tags and premature releases were two important issues encountered in this study, as in similar studies of eels tagged with PSAT. In our study, the overall loss rate of tags was 26.3%, which is approximately the same as other PSAT studies in eels (11.1–32% (refs 7 , 8 , 23 , 24 , 25 , 26 )) and close to the average from a review conducted on the performance of PSAT on other fish species (21% (ref. 27 )). The reasons for non-reports are unknown but could be due to tag malfunctioning, destruction by predation or inability of the tag to transmit their data to satellites. In our study, the loss rate was particularly high during the 2012 experiment during which eels were released directly from the shore, in shallow waters, unlike in other years. The vertical behaviour of eels immediately after their release in deeper waters suggests that eels released on the shoreline may have taken refuge under rocks and in the substrate where they dislodged their tags. The non-reporting tags may have remained stuck in the substrate or under rocks, therefore preventing data transmission, or they may have washed up on the shore in a position preventing satellite transmission.
Releasing eels 5–10 km offshore over deeper waters appeared to be a more successful method. Nevertheless, premature release occurred for all tags. Such a high rate of premature release was also reported in similar tagging studies in which 50–92.8% of the PSATs started transmitting their data before the scheduled pop-up date. In our study, predation by warm-gutted fish was clearly responsible for the premature release of two tags. Premature release of the other tags could also have been caused by predation by ectothermic fish. For several tags that successfully transmitted their data, a sudden descent to the seabed was observed a few hours or days after their release. These tags remained for several consecutive days on the bottom (100–200 m) before suddenly rising to the surface definitively. These sudden changes in vertical behaviour as well as the absence of light data and the absence of temperature increases may be due to predation by ectothermic fish. Such potential predation events not accompanied by an increase in temperature were also reported in other studies that tracked eels using PSATs 8 , 12 . Finally, for the eels tracked the longest, their tags suddenly reached the surface terminating a continuous period of regular behaviour (DVM), such as in ref. 8 , suggesting predation of the eel without the tag or more likely a failure of the attachment.
Although we have tracked eels with the best telemetry technology available, a certain degree of uncertainty concerning the observations reported here is associated with two limitations of the technology; the potential impact of the tags on the behaviour of fish of relatively small body mass and limited data retrieval from the satellite tags (see ‘Methods’ for a more extensive discussion of potential drawbacks). Several laboratory studies have shown that PSATs increase drag and can significantly impair the swimming performance of relatively small eels 28 , 29 , 30 . This may have contributed to slower speeds of smaller eels and the more westerly pop-up locations on the Scotian Shelf. Although DVM has been reported for eels tagged with much smaller, internally-placed, acoustic tags, it remains unknown if the maximum and minimal depths at which eels swim could be affected by the external PSAT. Another drawback of using PSATs is associated with the limitations of retrieving recorded data and its impact on the reconstruction of migratory paths. For the X-tags, we used the depth data available at 15-min intervals to infer the longitude 10 . Because of the data sampling rate and the individual and daily variability of DVM 10 , the uncertainty in longitude estimates was around 1.2° ( ∼ 400 km). The latitudes were inferred using the temperature recorded at specific depths by X-tags. The uncertainty of the reconstructed latitudes thus comes from the accuracy of the recorded data and from the resolution and accuracy of the operational ocean circulation models used to compare with the archival data (7–9 km). This defines the minimal uncertainty of the reconstructed path and prevents assessment of finer horizontal movements. For the SeaTag-GEOs, temperature data were limited to 3–4 values a day with no depth records, forcing us to consider all depth layers in the search for matching temperature values, thus increasing the uncertainty of locations. Given these limitations, it is essential to conduct additional studies to assess the repeatability of results reported here and to work closely with manufacturers to push for further miniaturization of the tags and improvements in data retrieval capabilities.
In conclusion, despite the limitations imposed by currently available technologies, this study represents a significant step in understanding the migrations of this most enigmatic of species and illustrates the feasibility of revealing in even greater detail the migration routes and orientation cues eels use to complete their life cycle.
Pop-up archival satellite tags (PSATs)
A total of 38 silver eels were equipped with two different kinds of PSATs ( Supplementary Table 1 ): 27 tags were X-tags from Microwave Telemetry ( http://www.microwavetelemetry.com ) and 11 were SeaTag-GEOs from Desert Star Company ( http://desertstar.com/ ). Each X-tag measures 120 mm in length, has a maximum diameter of 32 mm and weighs 45 g in air. On board sensors collect and archive data on depth, water temperature and light every 2 min. X-tags were programmed to record 12-bit resolution measurements of light, temperature (range −4 °C to+40 °C, 0.23 °C accuracy) and pressure (range 0–1,296 m, 0.3–5 m resolution) and to store the records in the 64 Mb FLASH memory. At the end of each day (Universal Time Coordinates), the archived data for the previous 24 h is processed within the tag to build up a subset of the data (15-min intervals for temperature and depth, minimum and maximum light level and sunrise and sunset estimates) for transmission to the Argos low earth orbiting satellite system ( http://www.argos-system.org/ ) after tag release. In case of premature death of the host or detachment of the tag from its host, the X-tags were programmed to initiate the pop-up procedure and transmit data after 7 consecutive days of constant depth readings (±3 m) with a 15-day delay following deployment (that is, the tag ignores constant pressure for the first 15 days). The SeaTag-GEOs are 132 mm in length, 13 mm in diameter for the main section and a weight 29 g in air. Their internal memory allowed to record temperature (−40 to +85 °C, 0.2 °C accuracy) and geomagnetic field values (3 axes) either three or four times a day during 3–4.5 months. Light sensors are also on board so day length and noon estimates are also transmitted. The SeaTag-GEOs were programmed to transmit both raw data and daily average for 2 months after the programmed dates. Unlike the X-tags, the SeaTag-GEOs have a solar battery and transmit their data continuously, that is, as soon as they are at the surface, satellites can pick up the data.
Capture and eel tagging
All eels used in the experiments were wild silver eels caught while performing their downstream migration from fresh or brackish waters. They were all caught by commercial fishermen who used fyke nets and were kept for several days in appropriate basins before retrieval. To minimize the negative effects of drag caused by the external tags, eels were selected for tagging on the basis of their large size and body mass. In 2012 and 2013, the selected eels originated from NS and were caught and released at the same location. In 2012, several locations in NS were visited in order to find the largest eels. In 2014, based on the previous year’s experiments and results, it was decided to use the largest eels that can be found in the entire species range: eels from the St. Lawrence system 31 . Indeed, the latter were on average 109 cm in total length (maximum of 120 cm) and 2.9 kg in body mass (maximum of 3.7 kg), whereas the largest eels that we found in NS reached a maximum of 93 cm in total length (85 cm on average) and 2.0 kg in body mass (1.4 kg on average; Supplementary Table 1 ). The eels from the St. Lawrence system were caught in the brackish estuary (Rivière Ouelle, 47.44°N, 70.03°W, Fig. 1 ) and transported by truck to the tagging and release location in Blandford, NS ( Supplementary Table 1 and Fig. 1 ) at ca 860 km of driving from the capture location. It represents an aquatic shortcut of around 1,400 km for translocated eels, which consequently did not have to cross the lower estuary and the Gulf of St. Lawrence to reach the open ocean, thereby avoiding high predation 6 .
The tagging procedure (surgery and tag attachment method) was the same as previously used and detailed in Béguer-Pon et al . 6 but for the last 2 years two attachment points instead of four were used. Furthermore, based on a recent study about the tag effect 30 , it was decided for the last year of experiment to attach the tag closer to the head of the eels (0.125 body length from the tip of the snout) instead of at their centre of mass (0.35 body length), in order to reduce the potential negative impact due to the drag of the tag.
For all years’ experiments, tagged eels were released at the same time along with 12 non-tagged eels since swimming in schools can provide fish with a number of behavioural and ecological advantages, such as reduced predation risk or energy saving 32 . It is not really known whether or not silver eels swim in schools during their oceanic migration but there are reports indicating eels tend to aggregate in large groups during their seaward migration 33 . It was also observed that silver eels migrating down the St. Lawrence River show a synchrony in the time of their passage in the brackish estuary 5 , 34 , suggesting that eels could travel together during the marine phase of the migration. In 2012, eels were released in very shallow waters, directly from the beaches or docks at the tagging locations. In 2013 and 2014, eels were transported and released 5–10 km offshore, where the water depth is 30–50 m.
This study was carried out in strict accordance with the recommendations of the Canadian Council on Animal Care. The protocol was approved by the Animal Care Committee, Laval University (Permit Number 2011101-01) and Maurice-Lamontagne Institute, Fisheries and Oceans Canada (Permit Number 12-6C). All surgery was performed under acetyleugenol (220 p.p.m.) and all efforts were made to minimize suffering.
Reconstructing the daily locations
Geolocation of ‘pop-up’/detachment events. All tags popped up earlier than the programmed dates. Except for two tags that were ingested by homeothermic fish, the reason for premature release could not be identified and could be various: failure in the attachment system, predation by ectothermic fishes or death of the eels. The release mechanism of X-tags was triggered by constant pressure during 7 consecutive days; they were actually drifting at the surface for 7 days before the first transmitting location was calculated by Argos system. Therefore, the first transmitting locations were not the locations where the tags detached and reached the surface. We thus inferred the location of their detachment using the temperature and light data collected at the surface. Sunset and sunrise estimates were used to calculate the longitude (with a 0.5° uncertainty), whereas the latitude was inferred from the surface water temperature (±1 °C). The package ‘oce’ in R 35 , 36 was used to calculate the longitude from sunrise and sunset. For all X-tags, sunset and sunrise estimates from the day of their detachment or following it could not be used as they were clearly erroneous. We used the maximum drifting distance observed during 5 days after the beginning of the transmission to increase the longitudinal search limits, as well as the directions of currents observed while the tags were drifting. As previously mentioned, data transmitted by the SeaTag-GEOs can be received by satellites as soon as the tags are at the surface, leading to only a few hours of drift before detection in most cases. Their first transmitting Argos location may thus reflect the location where the tag reached the surface. However, for some SeaTag-GEOs it was noticed that reliable sunset and sunrise were provided 2–5 days before the first locations were calculated by the Argos system, indicating these tags were probably drifting at the surface during that period (no depth sensor on these tags) but the data were not transmitted right away (for unknown reasons). We thus used the same method as for the X-tags to infer the geolocation of the pop-up events.
Temperatures recorded by the tags at the surface were matched with strongly assimilated physical models: HYCOM for data of 2012 and 2013 experiments and the operational Mercator global ocean 1/12° analysis and forecast system for 2014 experiments as HYCOM had missing data during our 2014 tracking period.
HYCOM has 1/12° equatorial resolution and latitudinal resolution of 1/12° cos(lat) or ∼ 7 km for each variable at mid-latitudes. It has 40 coordinate surfaces in the vertical. The data assimilation is performed using the Navy Coupled Ocean Data Assimilation 37 system with a model forecast as the first guess. Navy Coupled Ocean Data Assimilation assimilates available satellite altimeter observations (along the track obtained via the NAVOCEANO Altimeter Data Fusion Center), satellite and in situ sea surface temperatures as well as available in situ vertical temperature and salinity profiles from XBTs, ARGO floats and moored buoys.
The operational Mercator global ocean 1/12° analysis and forecast system uses the NEMO 3.1 (Nucleus for European Models of the Ocean) modelling system, coupled to the thermodynamic-dynamic sea ice model LIM2 (Louvain sea Ice Model 2). The ocean model has a horizontal resolution of 9 km at the equator, 7 km at Cape Hatteras (mid-latitudes) and 2 km towards the Ross and Weddell seas. The ocean model has 50 levels in the vertical with 1 m resolution at the surface decreasing to 450 m at the bottom, and 22 levels within the upper 100 m. The 3-hourly atmospheric fields forcing the ocean model are taken from the European Centre for Medium-Range Weather Forecasts Integrated Forecast System. This modelling system assimilates jointly satellite sea level anomaly (Jason2, Cryosat, Saral-Altika) and sea surface temperature (Reynolds AVHRR-AMSR 1/4°), and in situ profiles of temperature and salinity. A detailed description of the modelling system and the quality of its product can be found at http://www.myocean.eu/web/69-myocean-interactive-catalogue.php?option=com_csw&view=details&product_id=GLOBAL_ANALYSIS_FORECAST_PHYS_001_002 . Salinity is reported using the Practical Salinity Scale.
We checked that the modelled sea surface temperature was compatible with the temperature observed by the tags during their free drifting stage. It should be noted that the modelled sea surface temperature represents the daily mean temperature of the top 1 m water column. However, the temperature sensor on the tag measures the water temperature at a depth of ∼ 5 cm while drifting at the surface.
For each daily reconstructed location, we calculated the distance between the minimum and maximum estimates of latitude and longitude. These distances provided a measure of the uncertainty around the daily reconstructed locations. The uncertainty around the estimated pop-up locations varied among tags and was on average 120±94 km in latitude (mean±s.d.; range: 9–288) and 77±37 km in longitude (range: 20–148; Supplementary Table 3 ).
Geolocation of daily tracks. Traditionally, light data are used to infer the longitude 9 but as eels avoid the euphotic zone during daytime and the light sensors on the tags are not sensitive enough to record reliable sunset and sunrise estimates 10 , we developed another method similar to the one used in Westerberg et al . 12 . According to the data recorded by the two kinds of tags, we used the bathymetry, DVM behaviour and temperatures at specific depths to infer the geolocation of eels equipped with X-tags and the temperature (point records, not daily averages) and the geomagnetic field total intensity for eels equipped with SeaTag-GEOs.
In some cases, the depths recorded by X-tags were assumed to be the bottom, as a constant value for several hours and days was observed. This was the case for several tags for only a few days following their release. The daily geolocation in these cases were inferred by matching the observed water depth and associated temperature to the 30 arc-second GEBCO bathymetry and the results from the operational ocean circulation model assuming the maximum distance eels could have travelled in one day in any direction to be 60 km.
When clear DVM patterns were observed, we estimated the sunrise and sunset times from the vertical profiles using the statistical R package ‘breakpoints’, which implements the cross-entropy method 38 . This method is based on a stochastic optimization technique to estimate both the number and their corresponding locations of break-points in biological sequences of continuous and discrete measurements. Estimating sunrise and sunset from vertical profiles of eels was used in Westerberg et al . 12 to calculate the longitude. Furthermore, Chow et al . 10 tracked several Japanese eels ( A. japonica ) using ultrasonic transmitters and determined that eels started descending 55 min before sunrise (±10 min) and started ascending at sunset (±2 min). Considering the individual and daily variability described in Chow et al . 10 and considering our sampling rate (15 min versus 2 min in the study that used acoustic tags) we decided to apply a 15-min uncertainty around the sunrise and sunset estimates. This leads to an average of±1.2° uncertainty in longitude (that is, around 400 km). The possible daily locations of eels equipped with X-tags were then further constrained by searching the modelled temperature field from the operational ocean circulation model within the range of mean±s.d. of observed temperature at the maximum depth that was reached by the eel each day and the temperature in the depth layer 0–200 m.
For eels equipped with SeaTag-GEOs, as there was no depth record, we matched the temperatures recorded by the tags with temperatures from the HYCOM model for all depth layers between the surface and 800 m. The geomagnetic field total intensity data (Gnt) recorded by the SeaTags-GEOs were matched with the modelled values from the International Geomagnetic Reference Field–IRGF- ( www.ngdc.noaa.gov/IAGA/vmod/irgf.html ). The real-time tracking data (while the tags were drifting) were used to calibrate the geomagnetic field values. The discrepancy between the Gnt recorded by the tags at the surface and the modelled data from IGRF was on average of 800 nT (range: 92–4,000 nT). For each tag, the calculated standard deviation of the discrepancy between the tag and the model was used as a measure of uncertainty around the Gnt value recorded by the tag for constraining the geolocation. Some Gnt values were obviously erroneous and thus not taken into account for the constraint. Although the constraint using temperature generally led to latitudinal error estimates, the Gnt constraint led to oblique strips because of the natural gradient of this environmental data.
Following the constraints from the environmental data, the inferred locations were finally constrained using both backward and forward in time tracking procedures with a maximum daily travel speed we assume the eels could have gone in any direction (60 km per day). No hypothesis about preferred directions were made.
Methodological limitations of the PSAT technology
The placing of PSAT on a relatively small marine species such as eel may adversely affect behaviour and produce distorted patterns of movement and erroneous interpretations of migratory behaviour, as observed with other species 39 . Several laboratory studies have shown that PSATs increase drag and can significantly impair the swimming performance of relatively small eels 28 , 29 , 30 . The eels tagged in 2013 were about half the body mass of the eels tagged in 2014. The ground migratory speeds between the edge of Scotian Shelf and the open waters at the exit of the Laurentian Channel was 2.2–5 times slower for the smallest eels compared with the largest eels, potentially reflecting increased drag from the PSAT affecting the smallest eels. The pop-up locations on the western part of the Scotian Shelf could also reflect the difficulty of eels to swim against the main westward current. The potential impact of carrying a PSAT on vertical migratory behaviour is unknown. As the DVM was also exhibited by eels tagged with internal acoustic tags 10 , the PSAT is not responsible for this behaviour. However, it remains unknown if the maximum and minimal depths at which eels swim could be affected by the external tag.
Another limitation of using PSATs comes from the data recorded by the tags and our ability to reconstruct the migratory paths. The X-tags record depth, temperature and light every 2 min but these data cannot be assessed until the tags are physically retrieved, which is just about impossible in our study area because of its vastness. A subset of data is transmitted to satellites: depth and temperature at 15-min intervals, minimal and maximum daily light levels and sunset and sunrise estimates. We used the depth data to infer the longitude, as sunset and sunrise estimates from light sensors were not available. Because of the data sampling rate and the individual and daily variability of DVM observed in another study 10 , the uncertainty in longitude estimate was around 1.2° (around 400 km). This uncertainty could be reduced with a higher sampling rate and better correlations between migration depth and light intensity in our study area. The latitudes were inferred using the temperature recorded at specific depths by X-tags. The uncertainty of the reconstructed latitudes thus comes from the accuracy of the recorded data and from the resolution and accuracy of the operational ocean circulation models used to compare with the archival data. In this study, the ocean models have horizontal resolutions of around 7–9 km, defining thus the minimal uncertainty of the reconstructed path and preventing assessment of finer horizontal movements. For the SeaTag-GEOs, temperature data were limited to 3–4 values a day with no depth records, forcing us to consider all depth layers in the search for matching values, thus increasing the uncertainty of locations. These tags record geomagnetic field total intensity values that allow us to constraint location in oblique strips. We noticed various issues with the geomagnetic data: we had to calibrate the values using real-time tracking data (while the tags were drifting) and high discrepancies between recorded data and the modelled data from the International Geomagnetic Reference Field (up to 4,000 nT) were noted. Furthermore, some of recorded geomagnetic values were obviously erroneous (for instance leading to inferred locations at several thousands of kilometres) and had to be discarded from the analysis. The overall uncertainty of reconstructed trajectories was higher in the case of SeaTag-GEOs compared with X-tags. X-tags have a higher sampling rate, do record depth, have more reliable data and had a higher transmitting rate (97% versus 48%).
Additional information
How to cite this article: Béguer-Pon, M. et al . Direct observations of American eels migrating across the continental shelf to the Sargasso Sea. Nat. Commun. 6:8705 doi: 10.1038/ncomms9705 (2015).
Schmidt, J. The breeding places of the eel. Philos. Trans. R. Soc. Lond. B Biol. Sci. 211 , 179–208 (1923).
Article ADS Google Scholar
Miller, M. J. et al. A century of research on the larval distributions of the Atlantic eels: a re-examination of the data. Biol. Rev. doi:10.1111/brv.12144 (2015).
IUCN. in The IUCN Red List of Threatened Species. Version 2014.3. Available at: http://www.iucnredlist.org . Downloaded on 21 November 2014 (2014).
COSEWIC. COSEWIC assessment and status report on the American Eel Anguilla rostrata in Canada. xii+109 (Committee on the Status of Endangered Wildlife in Canada, Ottawa, 2012).
Béguer-Pon, M. et al. Large scale migration patterns of silver American eels from the St. Lawrence River to the Gulf using acoustic telemetry. Can. J. Fish. Aquat. Sci. 71 , 1–14 (2014).
Article Google Scholar
Béguer-Pon, M. et al. Shark predation on migrating adult American eels ( Anguilla rostrata ) in the Gulf of St. Lawrence. PLoS ONE 7 , e46830 (2012).
Aarestrup, K. et al. Oceanic spawning migration of the European eel ( Anguilla anguilla ). Science 325 , 1660 (2009).
Article CAS ADS Google Scholar
Wysujack, K. et al. The migration behaviour of European silver eels ( Anguilla anguilla ) released in open ocean conditions. Mar. Freshw. Res. 66 , 145–157 (2015).
Nielsen, J. L. et al. Tagging and Tracking of Marine Animals with Electronic Devices Springer (2009).
Chow, S. et al. Light-sensitive vertical migration of the Japanese eel Anguilla japonica revealed by real-time tracking and its utilization for geolocation. PLoS ONE 10 , e0121801 (2015).
McCleave J . in Eel Biology eds Aida K., Tsukamoto K., Yamauchi K Ch. 10 , 141–155Springer (2003).
Westerberg, H., Sjöberg, N. B., Lagenfelt, I., Aarestrup, K. & Righton, D. Behaviour of stocked and naturally recruited European eels during migration. Mar. Ecol. Prog. Ser. 496 , 145–157 (2014).
Putman, N. F. et al. An inherited magnetic map guides ocean navigation in juvenile Pacific salmon. Curr. Biol. 24 , 446–450 (2014).
Article CAS Google Scholar
Tesch, F. W. Influence of geomagnetism and salinity on the directional choice of eels. Helgol. Wiss. Meeresunters 26 , 382–395 (1974).
Souza, J. J., Poluhowich, J. J. & Guerra, R. J. Orientation responses of American eels, Anguilla rostrata , to varying magnetic fields. Comp. Biochem. Phys. A Physiol. 90 , 57–61 (1988).
Durif, C. M. F. et al. Magnetic compass orientation in the European Eel. PLoS ONE 8 , e59212 (2013).
Wu, L.-Q. & Dickman, J. D. Neural correlates of a magnetic sense. Science 336 , 1054–1057 (2012).
Luschi, P. Long-Distance Animal migrations in the oceanic environment: orientation and navigation correlates. ISRN Zool. 2013 , 23 (2013).
Block, B. A. et al. Electronic tagging and population structure of Atlantic bluefin tuna. Nature 434 , 1121–1127 (2005).
Campana, S. E., Joyce, W. & Fowler, M. Subtropical pupping ground for a cold-water shark. Can. J. Fish Aquat. Sci. 67 , 769–773 (2010).
Battaglia, P. et al. Feeding habits of the Atlantic bluefin tuna, Thunnus thynnus (L. 1758), in the central Mediterranean Sea (Strait of Messina). Helgol. Mar. Res. 67 , 97–107 (2013).
Joyce, W. N. et al. Analysis of stomach contents of the porbeagle shark ( Lamna nasus Bonnaterre) in the northwest Atlantic. ICES J. Mar. Sci. 59 , 1263–1269 (2002).
Manabe, R. et al. First observations of the oceanic migration of Japanese eel from pop-up archival transmitting tags. Mar. Ecol. Prog. Ser. 437 , 229–240 (2011).
Schabetsberger, R. et al. Oceanic migration behaviour of tropical Pacific eels from Vanuatu. Mar. Ecol. Prog. Ser. 475 , 177–190 (2013).
Schabetsberger, R. et al. Genetic and migratory evidence for sympatric spawning of tropical Pacific eels from Vanuatu. Mar. Ecol. Prog. Ser. 521 , 171–187 (2015).
Jellyman, D. & Tsukamoto, K. Swimming depths of offshore migrating longfin eels Anguilla dieffenbachii . Mar. Ecol. Prog. Ser. 286 , 261–267 (2005).
Musyl, M. K. et al. Performance of pop-up satellite archival tags. Mar. Ecol. Prog. Ser. 433 , 1–28 (2011).
Methling, C., Tudorache, C., Skov, P. V. & Steffensen, J. F. Pop up satellite tags impair swimming performance and energetics of the European Eel ( Anguilla anguilla ). PLoS ONE 6 , e20797 (2011).
Burgerhout, E. et al. Dramatic effect of pop-up satellite tags on eel swimming. Naturwissenschaften. 98 , 631–634 (2011).
Tudorache, C., Burgerhout, E., Brittijn, S. & van den Thillart, G. The effect of drag and attachment site of external tags on swimming eels: experimental quantification and evaluation tool. PLoS ONE 9 , doi:e11228010.1371/journal.pone.0112280 1–10 (2014).
Jessop, B. M. Geographic effects on American eel ( Anguilla rostrata ) life history characteristics and strategies. Can. J. Fish Aquat. Sci. 67 , 326–346 (2010).
Burgerhout, E. et al. Schooling reduces energy consumption in swimming male European eels, Anguilla anguilla L. J. Exp. Mar. Biol. Ecol. 448 , 66–71 (2013).
Tesch, F. W. The eel. 3rd edn Blackwell Publishing (2003).
Verreault, G., Pettigrew, P., Tardif, R. & Pouliot, G. in Biology, Management, and Protection of Catadromous Eels Vol. 33 , ed. Dixon D. A. 225–234American Fisheries Society Symposium (2003).
Google Scholar
Kelley, D. oce. : Analysis of Oceanographic data. R package version 0.9-14 (2014).
R Core Team. R: A language and environment for statistical computing. Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL http://www.R-project.org/ . (2014).
Cummings, J. A. Operational multivariate ocean data assimilation. Quart. J. R. Met. Soc Part C, 131 , 3583–3604 (2005).
Priyadarshana, W. J. R. M. & Sofronov, G. Package ‘breakpoint’ for R software Package ‘breakpoint’ for R software v.1.1 (2015).
Wilson, R. P. Animal behaviour: the price tag. Nature 469 , 164–165 (2011).
Download references
Acknowledgements
Funding for this project was provided to J.J.D. and M.C. by the Ocean Tracking Network (OTN) through a network project grant (NETGP #375118-08) from the Canadian Natural Sciences and Engineering Research Council (NSERC) with additional support from the Canadian Foundation for Innovation (CFI, Project #13011). Publication charges were partially covered by contributions from the Department of Biology, Laval University, Québec, and the Canadian Department of Fisheries and Oceans, Quebec. S.S. acknowledges support from the Killam Predoctoral Fellowship. We thank the fishermen who provided us with precious eels: Georges-Henri Lizotte, Howard Hawkins, Dale Larade, Gordon MacKay and Clayton McNeil. We acknowledge all people who participated in the field experiments: Lucia Abellan, Serge Higgins, Kyoko Ohashi and Darrin Baker. We also acknowledge Jinyu Sheng, Kyoko Ohashi, Anna Katavouta, Yuan Wang, Mathieu Dever, Dan Kelley, Keith Thompson and Guy Verreault for their advice and help with the physical oceanography aspect of the study.
Author information
Authors and affiliations.
Département de Biologie, Université Laval, Pavillon Vachon, 1045 Avenue de la Médecine, Université Laval, Québec, Québec, G1V OA6, Canada
Mélanie Béguer-Pon, José Benchetrit & Julian J. Dodson
Department of Oceanography, Dalhousie University, 1355 Oxford, Street, PO Box 15000, Halifax, B3H 4R2, Nova Scotia, Canada
Mélanie Béguer-Pon & Shiliang Shan
Institut Maurice-Lamontagne, Pêches et Océans Canada, 850 Route de la Mer, C.P. 1000, Mont-Joli, G5H 3Z4, Québec, Canada
Martin Castonguay
You can also search for this author in PubMed Google Scholar
Contributions
M.C. and J.J.D. are the principal investigators of the project. All co-authors participated in the field experiments (tagging). M.B.P. and S.S. analyzed the data. The paper was initially prepared by M.B.P. and modified by M.C., J.J.D., J.B. and S.S.
Corresponding author
Correspondence to Mélanie Béguer-Pon .
Ethics declarations
Competing interests.
The authors declare no competing financial interests.
Supplementary information
Supplementary information.
Supplementary Figures 1-7 and Supplementary Tables 1-3 (PDF 1622 kb)
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
Reprints and permissions
About this article
Cite this article.
Béguer-Pon, M., Castonguay, M., Shan, S. et al. Direct observations of American eels migrating across the continental shelf to the Sargasso Sea. Nat Commun 6 , 8705 (2015). https://doi.org/10.1038/ncomms9705
Download citation
Received : 09 April 2015
Accepted : 22 September 2015
Published : 27 October 2015
DOI : https://doi.org/10.1038/ncomms9705
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Regional differences in oceanic migratory behavior of japanese silver eel in waters with different vertical temperature gradients.
- Takaaki K. Abe
- Ishmerai Galang
- Hiroshi Hakoyama
Animal Biotelemetry (2023)
The drivers of anguillid eel movement in lentic water bodies: a systematic map
- Michael J. Williamson
- David M. P. Jacoby
- Adam T. Piper
Reviews in Fish Biology and Fisheries (2023)
Coastal water temperature difference, a potential predictor of glass eel recruitment in Anguilla japonica during non-meander periods of the Kuroshio
- Yoichi Miyake
- Marie-Agnès Tellier
- Shingo Kimura
Journal of Oceanography (2023)
The effect of externally attached archival data loggers on the short-term dispersal behaviour and migration speed of European eel (Anguilla anguilla L.)
- Pieterjan Verhelst
- Kim Aarestrup
- Martin Lykke Kristensen
Animal Biotelemetry (2022)
First descriptions of the seasonal habitat use and residency of scalloped hammerhead (Sphyrna lewini) and Galapagos sharks (Carcharhinus galapagensis) at a coastal seamount off Japan
- Yuuki Y. Watanabe
- Austin J. Gallagher
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Advertisement
How we finally tracked European eels all the way to the Sargasso Sea
Where European eels start and end their lives was long a mystery, but an audacious expedition has finally revealed the last details of their incredible migration
By Graham Lawton
16 January 2023

Mathieu Foulquie/Biosphoto/Minden Pictures
RIGHT now, millions of sinuous, silvery fish are swimming determinedly across the Atlantic Ocean. They are snake-like, more than a metre long and have huge, bulbous eyes. They left their homes in Europe in late autumn and have been navigating westwards ever since, often swimming against the currents that once carried them the other way. They travel alone at a languid pace, never stopping to rest. By night they are near the surface; by day in the depths. Their journey will take more than a year. Many won’t make it. But those that do have a reward awaiting them: sex and death in the Sargasso Sea.
This is the ultimate goal, and fate, of the European eel ( Anguilla anguilla ), a remarkable and enigmatic species that has nourished the human imagination, and belly, for millennia. Their life cycle is fascinating and their final journey, the details of which have only recently been discovered, is jaw-dropping. “This is a species which is notoriously difficult to understand ,” says eel expert Jack Wootton of the Forth Rivers Trust in Edinburgh, UK. What we do know for certain is that the European eel is critically endangered and needs help to recover, or woe betide it and the ecosystems it nourishes.
This species starts life far from Europe in the Sargasso Sea, a region in the western Atlantic that is defined by the four ocean currents that form its boundaries. From December to May adult eels spawn there, and their larvae – known as leptocephali – start a long journey to Europe and North Africa. They are largely carried by the prevailing currents, which drag on…
Sign up to our weekly newsletter
Receive a weekly dose of discovery in your inbox! We'll also keep you up to date with New Scientist events and special offers.
To continue reading, subscribe today with our introductory offers
No commitment, cancel anytime*
Offer ends 2nd of July 2024.
*Cancel anytime within 14 days of payment to receive a refund on unserved issues.
Inclusive of applicable taxes (VAT)
Existing subscribers
More from New Scientist
Explore the latest news, articles and features
How the US is preparing for a potential bird flu pandemic
Bowhead whales still harmed from whaling that ended a century ago.
Subscriber-only
5 extraordinary ideas about the mind and what it means to be conscious
Rare mutation that causes short stature may shed light on ageing, popular articles.
Trending New Scientist articles
Cookies on GOV.UK
We use some essential cookies to make this website work.
We’d like to set additional cookies to understand how you use GOV.UK, remember your settings and improve government services.
We also use cookies set by other sites to help us deliver content from their services.
You have accepted additional cookies. You can change your cookie settings at any time.
You have rejected additional cookies. You can change your cookie settings at any time.
- Environment
- Wildlife, animals, biodiversity and ecosystems
- Wildlife and habitat conservation
Ancient mystery of European eel migration unravelled to help combat decline of critically endangered species
New research enables a better understanding of the lifecycle of critically endangered European eels and how to combat their decline.

Adult European eel being released
- Environment Agency’s world-first research will help combat dramatic decline of the critically endangered European eel
- Adult European Eels tracked to the Sargasso Sea for the first time, unravelling a mystery that has perplexed scientists for centuries
- Journey of the European Eel to its spawning area considered one of the most impressive feats of animal migration observed in nature
A team of researchers led by the Environment Agency have taken a major step forward in solving one of nature’s most enduring mysteries – where do European Eels spawn and how do they get there?
Having suffered a 95% decline in numbers returning to Europe’s rivers since the 1980s, the European Eel is now a critically endangered species.
But ground-breaking research published this week enables us to better understand the lifecycle of this globally important but little-known species and ensure effective protection measures are put in place to combat their decline.
Project lead and Environment Agency researcher Ros Wright said:
The European Eel is critically endangered so it is important that we solve the mystery surrounding their complete life-cycle to support efforts to protect the spawning area of this important species. This is the first time we’ve been able to track eels to the Sargasso Sea and we are delighted we have the first direct evidence of adult European eels reaching their spawning area. Their journey will reveal information about eel migration that has never been known before.
The journey of European eels to their breeding place in the Sargasso Sea is up to 10,000km and considered one of the most impressive feats of animal migration observed in nature. It’s a mystery that has perplexed scientists for centuries, with the first recorded evidence of scientists looking into this phenomenon dating back to the 4th century BC.
Working alongside the Zoological Society of London, Defra, Cefas, Natural England, the University of Azores and the Denmark University of Technology, the Environment Agency has produced the first ever direct evidence of European eels navigating the last 2,500km leg of their journey. Up until this point, no eggs or eels had been found to confirm this spawning ground.
Back in December 2018 and 2019, researchers fitted 26 large female European eels with satellite tags and released them from the Azores into the Atlantic Ocean. The Azores islands are close to the furthest known point on the eel migration route that was tracked by previous projects.
Programmed to detach and transmit their data after 6-12 months, data were received from 23 satellite tags at various stages of the journey, with six tagged eels reaching the Sargasso Sea. Data transmitted from these tags reveal the eels migrated consistently towards the Sargasso Sea and, remarkably, that this journey to their breeding grounds takes over a year.
Once eels spawn in the Sargasso Sea, their larvae return to the UK and other European waters via a different route, carried on ocean currents on the North Atlantic Drift. They then migrate into rivers as glass eels.
Unravelling the navigation mechanisms, routes taken and locating where eels spawn is critical for understanding the reasons behind their decline and putting in place targeted conservation measures to protect this globally important species.
Chair of the IUCN Anguillid Eel Specialist Group, Matthew Gollock from the Zoological Society of London said:
Populations of the European eel are at a historic low and the more we understand their life-history, the better we are able to develop conservation measures to address the critical status of the species.

Professor José Manuel N. Azevedo from the University of the Azores said:
This discovery emphasizes the role of the Azores in the life cycle of eels. It will help scientist and conservationists to push for measures to restore eel habitats across the archipelago.
The Environment Agency and partners will now conduct a deeper analysis of the satellite tag data to uncover further clues on how eels navigate to their spawning area. This is part of an ongoing project and field teams have returned to the Azores to satellite tag eels with extended life tags to reveal more data on silver eel migration and spawning sites in the Sargasso Sea.
The Environment Agency continue to carry out research on all life stages of the European eel to inform conservation measures. This complements work around England to protect eels, for example improving eel pass design to enhance upstream eel passage into our rivers.
The full research paper is available online.
Share this page
The following links open in a new tab
- Share on Facebook (opens in new tab)
- Share on Twitter (opens in new tab)
Related content
Is this page useful.
- Yes this page is useful
- No this page is not useful
Help us improve GOV.UK
Don’t include personal or financial information like your National Insurance number or credit card details.
To help us improve GOV.UK, we’d like to know more about your visit today. We’ll send you a link to a feedback form. It will take only 2 minutes to fill in. Don’t worry we won’t send you spam or share your email address with anyone.
Advertisement
- Previous Article
- Next Article
Introduction
Materials and methods, acknowledgements, eel migration to the sargasso: remarkably high swimming efficiency and low energy costs.
- Split-screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- Open the PDF for in another window
- Get Permissions
- Cite Icon Cite
- Search Site
Vincent van Ginneken , Erik Antonissen , Ulrike K. Müller , Ronald Booms , Ep Eding , Johan Verreth , Guido van den Thillart; Eel migration to the Sargasso: remarkably high swimming efficiency and low energy costs. J Exp Biol 1 April 2005; 208 (7): 1329–1335. doi: https://doi.org/10.1242/jeb.01524
Download citation file:
- Ris (Zotero)
- Reference Manager
One of the mysteries of the animal kingdom is the long-distance migration(5000–6000 km) of the European eel Anguilla anguilla L. from the coasts of Europe to its spawning grounds in the Sargasso Sea. The only evidence for the location of the spawning site of the European eel in the Sargasso Sea is the discovery by Johannes Schmidt at the beginning of the previous century of the smallest eel larvae (leptocephali) near the Sargasso Sea. For years it has been questioned whether the fasting eels have sufficient energy reserves to cover this enormous distance. We have tested Schmidt's theory by placing eels in swim tunnels in the laboratory and allowing them to make a simulated migration of 5500 km. We find that eels swim 4–6 times more efficiently than non-eel-like fish. Our findings are an important advance in this field because they remove a central objection to Schmidt's theory by showing that their energy reserves are, in principle, sufficient for the migration. Conclusive proof of the Sargasso Sea theory is likely to come from satellite tracking technology.
At the beginning of the previous century Johannes Schmidt found the smallest eel larvae (leptocephali) of the European eel Anguilla anguilla L. near the Sargasso Sea( Schmidt, 1923 ) and the bigger leptocephali nearer to the European coast. This is the only evidence to date that places the spawning grounds in the Sargasso Sea (neither eggs nor mature adults have ever been found in this area). For Schmidt's theory to be substantiated, the following three conditions must be met. (1) Adult European eels must be able cover a distance of 6000 km in a fasting state, implying that they have sufficient energy reserves to cover this enormous distance( Tucker, 1959 ). (2) Mature European eels and fertilized eggs must be found in the Sargasso Sea. (3) Eel larvae must be shown to migrate towards the European coasts. Concerning condition (3), the most recent observations on larval migration patterns were published by McCleave et al.( 1987 ). Several questions concerning the large variation in age( Antunes and Tesch, 1997 ) and genetic make up ( Wirth and Bernatchez,2001 ) of glass eels collected at different places and times(suggesting the existence of more than one spawning site) have not yet been resolved, however. To test condition (2), Tesch's group( Post and Tesch, 1982 ) have tried, so far without success, to catch adult eels in the Sargasso. Until now there have only been two reports of silver eels A. anguilla caught incidentally in the open Atlantic ( Ernst,1977 ; Bast and Klinkhardt,1988 ). Concerning condition (1), Tucker( 1959 ) expressed severe doubts as to whether the European eel would be able to swim across the ocean and suggested that all European eels are the offspring of the American eel. Tucker's ` new solution to the Atlantic eel problem ' provoked a long debate ( D'Ancona and Tucker,1959 ; Deelder and Tucker,1960 ) and was finally rejected when a distinction was made between the two Atlantic eel species based on allozymes( Williams and Koehn, 1984 ),enzymes ( Comparini and Rodino,1980 ), mitochondrial DNA (Avise et al., 1986 , 1990 ; Tagliavini et al., 1995 ) and genomic DNA (Nieddu et al., 1988). Hence, if we assume a long-distance spawning migration to the Sargasso Sea, energy reserves may easily be critical( Svedä ng and Wickström,1997 ). An estimation of the energy required to cover the distance was presented in a recent paper. Based on the oxygen consumption rates during a 10-day swim trial, the equivalent fat consumption extrapolated to 6000 km was 120 g kg –1 or 40% of the initial fat reserves( van Ginneken and van den Thillart,2000 ). Female silver eels of 0.8 m body length ( BL )swimming at 0.5 BL s –1 would cover a distance of 6000 km in about 180 days. However, direct proof that European eels are able to swim the long distances between Europe and the Sargasso Sea is still missing, and the question remains as to whether eels can continue swimming for up to 180 days at the same low energetic cost. The objective of this study was to determine the energy costs of swimming over a complete 5500 km swim trial in the laboratory.
We conducted two sets of experiments, one over the entire distance of 5500 km, which lasted 173 days, and another set over the duration of 1 week. The second set served as confirmation of the results obtained during the first set by comparing our measurements on eel with the performance of another well-studied fish species, trout. In experiment 1, we performed respirometry in combination with bomb-calorimetry on the eel carcasses. In experiment 2,using trout and eel, we performed respirometry only in the swim tunnels.
For experiment 1, 3 year old hatchery eels Anguilla anguilla L.( N =30, 860±81.9 g, 73.1±3.8 cm BL ) were used. In experiment 2, we used eels A. anguilla ( N =5,155.0±18.3 g, 43.2±3.2 cm) and trout Oncorhynchus mykiss Walbaum ( N =5, 161.5±21.5 g, 24.6±1.0 cm) of the same body mass. The eels used in both experiments 1 and 2 were in the non-migratory yellow stage. Eels were obtained from Royal B.V., Helmond, The Netherlands, and trout were obtained from the Dutch Organization of Fisheries,O.V.B. Geertruidenberg, The Netherlands.
Flow tank experiments
In experiment 1, the oxygen consumption rates of eels ( N =9)swimming at 0.5 BL s –1 were measured over a period of 173 days in 2 m Blazka-type flow tanks with a volume of 127.1±0.9 l. In addition, to establish the routine metabolic rate (RMR), we measured the oxygen consumption, over the same period of 173 days, of six eels resting in Blazka-type flow tanks with continuous water refreshment. The cross section of the flow tanks is circular with an inner diameter of 190 mm. Outside the boundary layer the flow speed is constant over the cross section of the inner tube. The flow tanks were calibrated using a Laser Doppler method. Thus the eels swam at a constant known speed with negligible wall effects( van den Thillart et al.,2004 ). Swimming experiments were performed over 173 days under a 12 h:12 h day:night light cycle at a temperature of 19.0±0.3°C at 0.5 BL s –1 . The illumination in the climatized room was switched to 670 nm light (bandwidth 20 nm) during experiments. Based on pigment changes during silvering it is assumed that this far-red light is invisible for eels ( Pankhurst and Lythgoe,1983 ). The time lapse between the start of migration and the first leptocephali in the Sargasso Sea is about 6 months. Thus to cover a distance of 5000–6000 km in 6 months requires a mean swimming speed of 0.4 m s –1 ( Ellerby et al.,2001 ).
For experiment 2, five eels and five trout were placed in the same 127 l flow tanks at a constant temperature of 18±0.3°C under the same illumination protocol. Eels swam at 0.5 BL s –1 (21.5±1.6 cm s –1 ), whereas the trout swam at a slightly higher speed of 0.7 BL s –1 (17.2±0.7 cm s –1 ). We ensured that both species swam at their maximum range or optimal swimming speed, which is the relevant speed for migration, to facilitate the comparison between costs of transport of eel and trout. The optimal swimming speed of trout is available in the literature( Webb, 1971 ), whereas the value for eel is based on a study of eel muscle efficiency( Ellerby et al., 2001 ; for a more detailed explanation, see Discussion).
Swimming behavior was recorded at regular intervals during the entire period using an infrared video camera (frame rate 25 Hz) during the dark period to document the position of the animal in the flow tank as well as to detect possible long-term changes in swimming behavior.
Before sampling, the animals were quickly anaesthetized with 300 p.p.m. MS222 (3-aminobenzoic-acid-ethyl-ester methane sulfonate salt; Sigma, St Louis, MO, USA). All experiments were approved by the local committee on animal experimentation.
Oxygen consumption
The oxygen level in the tunnel was measured continuously using an oxygen electrode (Mettler Toledo, Tiel, The Netherlands). The oxygen consumption rate was calculated from the oxygen decline after automatic closure of the water-inlet by a magnetic valve. The oxygen levels changed between 85 and 75%air saturation. The valve was normally open allowing a refreshment rate of 5–7 l min –1 and automatically operated between 14:00 h and 17:00 h to measure oxygen consumption. The oxygen value was not allowed to fall below 75% in order to prevent the animals from becoming hypoxic( van den Thillart and van Waarde,1985 ).
From the decrease in O 2 concentration, the rate of oxygen consumption V̇ O 2 (mg O 2 h –1 kg –1 ) was calculated from the formula: V̇ O 2 =127δ[O]δ t –1 ,where δ[O 2 ]δ t –1 is the decrease in oxygen content per hour. Oxygen consumption data were corrected for the decline in mass of the animals.
Carcass analyses
To quantify the energy cost of transportation by a second method,independently from our respiratory measurements, we analyzed the changes in body composition. Carcass analyses were performed according to ISO-standards(International Organization for Standardization, Animal feeding stuffs; ISO 5983 and ISO/DIS 6492, Geneva, Switzerland). After weighing, fish samples were cut into pieces of about 3 cm and nearly submerged in water in a glass beaker. The samples were autoclaved at 2 atm (2.013×10 5 Pa) at 120°C for 4 h. They were then homogenized and subsequently sampled in triplicate for dry matter, protein and fat analyses. Dry matter content was measured by freeze drying of the sub-samples to constant mass. Protein was measured according to procedures described in ISO 5983 (1979). For fat determination, freeze dried sub-samples were extracted as described in ISO/DIS 6492 (1996).
Calculations and statistics
In order to calculate the cost of transportation (COT: mean gross energy costs of transportation; Schmidt-Nielsen,1972 ), total energy consumption was first calculated from oxygen consumption by multiplying the mean measured rate of oxygen consumption( Table 1 , in ml O 2 kg –1 fish h –1 ) with the number of swimming hours (4152 h = 173 days) and the applied energy conversion factor for respirometry of 18.89 kJ ml –1 O 2 ( Elliot and Davison, 1975 ). This gives a total energy consumption of 2316.58 kJ kg –1 fish over 5533.2 km or a COT of 0.42 kJ kg –1 km –1 . Alternatively the energy used during the 5533 km run was calculated by bomb-calorimetry. Bomb-calorimetry could be only used once on eels at the start of the experiment, so a control group of 15 animals was also measured for comparison with the swim- and the rest-groups( Table 2 ).
Energy consumption parameters of female yellow eels after 6 months of rest and after 6 months of swimming at 0.5 BL s –1
The eels swam 5533±354 km over a period of 173 days. For details of the methods used, see text.
COT, mean gross Energy Costs of Transportation( Schmidt-Nielsen, 1972 ).
Body constitution as % of dry mass of female yellow eels at the start and after 6 months swimming or resting
The calculations for the bomb-calorimetry were as follows:
Initial wet body mass (g) × initial dry matter fraction (%) = initial dry matter content (g dry mass fish –1 ).
End wet body mass (g) × end dry matter fraction (%) = end dry matter content (g dry mass fish –1 ).
Initial energy content (kJ g –1 dry mass) × initial dry matter content (g dry mass fish –1 ) = total energy content begin (kJ fish –1 ).
End energy content (kJ g –1 dry mass) × end dry matter content (g dry mass fish –1 ) = total energy content end(kJ fish –1 ).
Total energy content begin (kJ fish –1 ) – total energy content end (kJ fish –1 ) = total energy difference (kJ fish –1 ).
Total energy difference (kJ fish –1 ) / geometric body mass(g) × 1000 = total energy usage (kJ kg –1 ), with:
The `swimming' eels in experiment 1 used 2.1 times more oxygen than the`resting' eels (29.55 vs 14.26 ml O 2 kg –1 h –1 ; Table 1 ). The regression lines show that the oxygen consumption of the swimming eels increased by 2.56% over nearly 6 months, that of the resting group increased by 2.66% ( Fig. 1 ). There was a significant difference in mass loss: 13.1% in the resting group and 19.7% in the swimmers. Analysis of body constituents of the eels at the start and end of the experiment revealed that the ratio of all three substrates (lipid,carbohydrate, and protein) remained constant despite significant mass losses( Table 2 ). This means that body composition did not change during the six months and that fat, protein and carbohydrate were metabolized in the same proportion. A similar result was also found in reef fish migrating over much smaller distances( Stobutzki, 1997 ).

Oxygen consumption of fasting yellow eels from a hatchery (860±81.9 g, 73.1±3.8 cm BL ) during a 6 month period of rest (circles)or 6 months of continuously swimming at 0.5 BL s –1 (diamonds) at 19°C. Regression lines: Rest-group: y =0.0326 x +25.294; Swim-group: y =0.0394 x +54.86.
We obtained two independent estimates for cost of transport. From the first method, oxygen uptake (using the oxycaloric value of the three substrates; Elliot and Davison, 1975 ), we obtained a value of 2317 kJ kg –1 fish for the energy cost of 6 months swimming covering a distance of 5500 km. This corresponds to a COT value of 0.42 kJ kg –1 km –1 . The swimming group lost more total body mass than the resting group (180.3 g compared with 103.9 g; Table 1 ). Hence, based on the second method, which uses mass loss, body composition and energy conversion factors ( Brafield and Llewellyn,1982 ), we calculated that energy used for swimming was 3450 kJ kg –1 fish, corresponding to a COT value of 0.62 kJ kg –1 km –1 . The two COT estimates obtained independently ( Table 1 ) are of the same order of magnitude.
In experiment 2, where eels and trout swam in identical experimental set-ups, we measured an oxygen consumption of 43.9±8.42 mg O 2 kg –1 h –1 and 130.4±9.49 mg O 2 kg –1 h –1 , respectively. During 7 days the eels and trout covered a mean distance of 132.5±12.1 km and 102.8±2.3 km, respectively. From these data we calculated COT values for eel and trout of 0.68 and 2.73 kJ kg –1 km –1 , respectively. Our video recordings of the fish swimming in the flow tank show that the fish were swimming in the free-stream and did not benefit from wall effects: the fish swam mostly in the centre of the flow tank and therefore are more than 2 (eel) up to 3 (trout) tail heights removed from the wall (results not shown).
Efficiency, commonly defined as the rate of useful energy expenditure divided by the total rate of energy consumption, is a crucial parameter for animals migrating over long distances. During long-distance migration, animals are likely to maximize the distance covered per given fuel unit, which corresponds to maximizing efficiency. Amongst the various eel species, the European eel needs to migrate the farthest to reach its spawning grounds:European eel A. anguilla , 5500 km( Schmidt, 1923 ), American eel A. rostrata , 4000 km ( Tucker,1959 ; McCleave et al.,1987 ), Australian eel A. australis , 5000 km( Jellyman, 1987 ) and Japanese eel A. japonica , 4000 km( Tsukamoto, 1992 ). So European eels need to be very efficient swimmers.
There are various levels of energy conversion in a swimming animal. The overall metabolic efficiency (how much heat is generated at a given swimming speed) comprises the efficiencies of various processes, e.g. propeller efficiency (how much momentum is gained by the animal and wasted in the wake)and the muscle efficiency (how many ATP molecules are used per myosin-head cycle). The concept of efficiency used here corresponds to overall metabolic efficiency and encompasses propeller and muscle efficiency, for example. During locomotion, propeller and muscle efficiency are likely to contribute significantly to overall metabolic efficiency, so it is interesting to compare the hydrodynamic and muscle performance of eel to those of other undulatory swimmers.
To reduce costs of transport and increase overall metabolic efficiency, all or some of the processes that determine the costs of transport can be optimized. Efficiency can be improved most effectively by improving those processes in which efficiency increases nonlinearly and progressively with a given performance parameter. Performance parameters that only weakly or linearly affect efficiency are less likely to bring about a drastic increase in efficiency.
In order to explain the remarkable difference in cost of transport between eel and trout, it is important to identify the processes that cause it. To this end, we have studied the literature on propeller efficiency and muscle efficiency in undulatory swimmers.
Hydrodynamic performance and propeller efficiency
A fish can alter its propeller efficiency by changing its structural design and its motion pattern. Both carangiform and anguilliform swimmers undulate their body, the former with a narrower amplitude envelope than the latter. How the shape of the body undulations affect locomotory efficiency has been estimated using analytical approximations. Lighthill's elongated body theory(EBT) concludes that efficient swimmers should undulate only the most posterior section of their body – in the ideal case only their trailing edge – to maximise propeller efficiency( Lighthill, 1971 ; Tytell and Lauder, 2004 ). Daniel's predictions ( Daniel,1991 ) differ in part: the propeller efficiency of undulatory swimming decreases linearly as the rearward speed of the body wave increases relative to the swimming speed, and it is independent of the frequency and the amplitude of the body wave. Given that the swimming kinematics of trout and eel mainly differ in the amplitude envelope of their body wave, but have a similar range of body wave speeds (for a review, see Videler, 1993 ), it is unlikely that kinematic differences between trout and eel can explain the difference in their overall metabolic efficiency.
The combined effect of propeller shape and motion on performance can be studied by visualising the flow generated by anguilliform and carangiform swimmers. The ratio of forward to total momentum of the entire wake provides the mean propeller efficiency over a complete tail beat. This approach,whether using experimental or computational flow fields, requires the quantification of the three-dimensional flow in the complete wake, which so far has not been done. The currently available two-dimensional slices through the wake suggest that eels generate considerable lateral momentum, which do not contribute to the forward motion and therefore reduce efficiency( Müller et al., 2001 ; Tytell and Lauder, 2004 ). Tytell estimated a hydrodynamic efficiency of 0.5 to possibly up to 0.87( Tytell and Lauder, 2004 ). Equivalent estimates for carangiform fish are reported in the range 0.74–0.97 ( Drucker and Lauder,2001 ; Müller et al.,2001 ; Nauen and Lauder, 2002a , b ). These values suggest that trout has a higher propeller efficiency than eel,which does not explain the higher overall metabolic efficiency of eels.
Efficiency is also inversely related to thrust( Lighthill, 1971 ; Daniel, 1991 ). However, a 25%difference in swimming speed is insufficient to explain a fourfold difference in efficiency. So, the currently existing evidence on the hydrodynamics of undulatory swimming contradicts rather than explains the high swimming efficiency of eels.
Muscle performance and efficiency
The efficiency with which a muscle converts chemical energy into mechanical work is important in prolonged aerobic locomotion, such as migration. Cruising is characterized by cyclic contractions at a well-defined frequency. Swimming speed depends linearly on tail-beat frequency, and tail-beat frequency corresponds to contraction frequency. The mechanical efficiency of muscle contractions depends on contraction speed in a non-linear fashion. This relationship can be predicted from Hill's model of muscle contractions( McMahon, 1984 ) and has also been documented in fish swimming muscles( Curtin and Woledge, 1993 ). There is a narrow range of contraction frequencies over which efficiency remains high. At contraction frequencies above and below this range,efficiency drops off progressively( McMahon, 1984 ; Curtin and Woledge, 1993 ). McMahon's calculations ( McMahon,1984 ) show that maximum efficiency occurs at a contraction speed at 13% of the maximum contraction speed of the muscle, which is a slightly lower speed than the speed at maximum power. To swim at maximum muscle efficiency, the fish should maintain a tail-beat frequency that allows the muscle to contract at this optimal speed.
If we take the contraction frequency that maximizes power as a first approximation of the contraction speed that maximizes efficiency, we can compare eel aerobic swimming muscles to those of trout. Eel muscles deliver peak power at much lower contraction frequencies (0.5–0.8 Hz in silver eel, measured at 14°C; Ellerby et al.,2001 ) than the muscles of trout (2–3 Hz, measured at 11°C; Hammond et al.,1998 ). The swimming speeds that correspond to these contraction frequencies are 0.5 l s –1 for eel and 0.4–1.0 l s –1 for trout ( Webb,1971 ). These values confirm that in our experiments both eel and trout were swimming at close to their optimal swimming speed, and hence the much higher COT of trout is probably not due to the trout having been forced to swim under considerably suboptimal conditions for its swimming muscles.
Muscle fibre type recruitment and swimming speed
At the low speeds used in this study, the eels will recruit only the posterior red muscle to swim continuously. As demonstrated in the work of Gillis ( 1998 ), muscle fiber type recruitment was clearly dependent upon swimming speed. A pattern of`posterior-to-anterior' recruitment within a fiber type was observed as eels increased their swimming speed (figs 2, 3A,F in Gillis, 1998 ). For example,eels typically used mainly posteriorly located red muscle (at 0.75 and 0.6 BL ) to power slow-speed swimming, but would then additionally recruit more anteriorly located red muscle (at 0.45 and 0.3 BL ) to swim at the higher speeds (figs 2, 3A in Gillis,1998 ). These unusual muscle activation pattern and kinematics may explain the low COT in eels compared with trout, in which most of the red muscle on each side of the body is stimulated during a tail-beat cycle –assuming that the European and American eels are similar in this regard.
In contradiction to this theory/hypothesis of Gillis( 1998 ) to explain the low swimming efficiency of eel by recruitment patterns of muscle, Wardle et al.( 1995 ) showed that the muscle activity pattern (% time active during one tail-beat cycle) does not differ substantially between different undulatory swimmers. Wardle's values for eel(based on Williams et al.,1989 ) agree with those mentioned by Gillis( 1998 ). Compared with trout and other fish, recruitment in eel is certainly not less by a factor of 2–4. Hence it is not likely that more posterior muscle recruitment in eel can explain the many-fold difference in efficiency between eel and trout.
Metabolism and non-locomotory influences on costs of transport
Overall metabolic efficiency is also influenced by the efficiency of the respiration and energy-conversion processes themselves. The whole-organism locomotory performance is determined by its metabolic machinery, bringing us to the whole-body oxygen consumption (routine metabolic rate, RMR) of the animal. In this study, we found a RMR for eel of 29.55±4.2 ml O 2 kg –1 h –1 , which corresponds to 42.21±6.0 mg O 2 kg –1 h –1 . This value is similar to values reported in literature: 35 mg kg –1 h –1 (for the similar sized animals at 18°C; Degani et al., 1989 ; McKenzie et al., 2000 ) for eel and also the RMR measurements of other fish species( Winberg, 1956 ). Hence, we may conclude that, based on metabolic rate comparisons with other fish species,the mitochondrial capacity remains the same. However, in the wild, eel do not migrate at the surface but in the deep sea: a migrating eel has been photographed in the Bahamas at a depth of 2000 m( Robins et al., 1979 ). There,they experience considerably larger pressures, which might further increase metabolic efficiency at the mitochondrial level by increasing the efficiency of their oxidative phosphorylation ( Theron et al., 2000 ). In a laboratory study in which eels were exposed for 21 days to 10.1 MPa hydrostatic pressure, Theron et al.( 2000 ) demonstrated that the ADP/O ratios, calculated from mitochondrial respiration measurements, were significantly increased.
Eels actually performing the migration will not only experience higher pressures, but also lower temperatures, which will also affect their efficiency. Furthermore, eels might adapt their migratory route to take advantage of favorable sea currents, which would further reduce the energy requirements. However, with the migratory routes unknown, nothing can be said about the possible energy savings from pressure, temperature and sea-current effects.
Metabolic costs and efficiency compared
Our respiratory measurements and the carcass analyses suggest that eels have a much higher metabolic efficiency than trout. In eel, the COT values obtained from oxygen consumption data and carcass analyses are 0.42 and 0.62 kJ kg –1 km –1 , respectively, whereas trout has much higher COT values (respirometry only) of 2.73 kJ kg –1 km –1 , respectively. The COT in trout matches the value measured by Webb( 1971 ), and is similar to other salmonids ( Brett, 1973 )and many adult fish species (for a review, see Videler, 1993 ). This means that eel swim 4–6 times more efficiently than many other fish species,even across swimming styles.
In conclusion, we demonstrate in this study that fasting European eels are able to swim 5500 km, a distance corresponding to their supposed spawning area in the Sargasso Sea, with a remarkably high swimming efficiency and at low energy costs. At this moment, this high efficiency can be explained neither by propeller nor muscle efficiency. How the extremely low optimal contraction frequency of eel aerobic muscle affects muscle efficiency has not yet been studied. So far, the only evidence that eels have a remarkably high swimming efficiency comes from metabolic energy costs. The source of the eel's remarkably high efficiency remains at present unknown, providing ample stimulation for biomechanists and physiologists alike to investigate eel migratory swimming performance.
We thank Prof. Dr K. Dickson (California State University, Fullerton, USA),Prof. Dr R. Blickhan (Friedrich Schiller Universitä t, Jena, Germany), Dr E. D. Tytell (Harvard University, USA), Dr I. L. Y. Spierts (Wageningen University, The Netherlands) and Prof. M. Richardson (Leiden University, The Netherlands) for helpful suggestions and improving the manuscript. This work was supported by the Foundation for Technical Research (LBI.4199), which is subsidized by the Netherlands Organization for Scientific Research (N.W.O.)and the European Commission, project (EELREP, Q5RS-2001-01836).
Email alerts
2023 jeb outstanding paper prize shortlist and winner.

The JEB Editors are delighted to announce the shortlisted authors for the 2023 JEB Outstanding Paper Prize . Read the winning paper - Tiny spies: mosquito antennae are sensitive sensors for eavesdropping on frog calls - by Hoover Pantoja-Sanchez and Brian Leavell from Ximena Bernal's lab at Purdue University, USA.
JEB Science Communication Workshop for ECRs

If you’re an early-career researcher interested in science communication and are attending the SEB Annual Conference in Prague this summer, come a day early and join the JEB Editors at a sci comm workshop to learn the key writing skills needed to promote your research to a broad audience beyond your peers (1 July at 14.30-17.30). Places are limited to 24 attendees, and applicants should apply through the SEB registration page by 30 April 2024.
Bridging the gap between controlled conditions and natural habitats in understanding behaviour

Novel technologies enable behavioural experiments with non-model species, in naturalistic habitats and with underexplored behaviours. In their Commentary , Scholz and colleagues discuss how to obtain a deeper understanding of the natural ecology and lifestyle of study animals.
Beluga metabolic measures could help save species

To help save animals from extinction, it’s important to understand what each species needs to survive. This led Jason John et al. to measure the metabolic rates of captive belugas to develop a ‘fish calculator’ showing that the whales need to eat ~23 salmon per day.
ECR Workshop on Positive Peer Review

Are you an ECR looking for tips on how to write concise, astute and useful manuscript reviews? If so, join the JEB Editors at a 2-hour JEB-sponsored Workshop on Positive Peer Review at the Canadian Society of Zoologists annual meeting in Moncton on 9 May 2024 at 13.00-15.00. There are 25 spaces for ECRs and selection is first come, first serve. To sign up, check the ECR Workshop box when you register for the CSZ meeting.
Social media
Other journals from The Company of Biologists
- Development
- Journal of Cell Science
- Disease Models & Mechanisms
- Biology Open
- Editors and Board
- Aims and scope
- Submit a manuscript
- Manuscript preparation
- Journal policies
- Rights and permissions
- Sign up for alerts
Affiliations
- Journal of Experimental Biology
- Journal Meetings
- Library hub
- Company news

- Privacy policy
- Terms & conditions
- Copyright policy
- © 2024 The Company of Biologists. All rights reserved.
- Registered Charity 277992 | Registered in England and Wales | Company Limited by Guarantee No 514735 Registered office: Bidder Building, Station Road, Histon, Cambridge CB24 9LF, UK
This Feature Is Available To Subscribers Only
Sign In or Create an Account
Scientists Tracked an American Eel's 1500-Mile Migration to the Sargasso Sea
By kirstin fawcett | nov 1, 2015.
The waters of the Northeast were once chock-full of American eels, which lived in freshwater rivers and estuaries before swimming hundreds of miles each year to spawn in the Sargasso Sea. However, thanks to development and hydroelectric dam construction, the eels have lost 80 percent of their habitat, Newsweek reports. Now, the once-plentiful fish is dwindling in population —making it all the more important for conservationists to ensure they make it safely to their only known breeding ground.
Between 1904 to 1922, researchers found eel larvae in the Sargasso Sea, an algae-rich patch of ocean in the North Atlantic that serves as home to a vast array of diverse marine species. For more than a century, scientists have assumed that the sea was the eels' main reproductive hub. However, they had never witnessed the fish actually breeding there, nor did they have scientific data to support that they actually travel so far away to spawn. So Canadian researchers attached satellite transmitters to 38 American eels, and tracked their journey to see where they ended up.
While not all of the eels made it to their final destination, academics were able to follow one fish for 1500 miles—all the way from the Scotian Shelf off Nova Scotia to the Sargasso Sea. The experiment provided researchers with concrete proof that the annual migration exists, the study’s authors explain in the journal Nature Communications .
Not only might this knowledge help protect American eels—which are listed as endangered on the International Union for Conservation of Nature (IUCN) Red List and assessed as "threatened" in Canada—it also sheds new light on the life cycle of an otherwise elusive species.
“We knew that millions of American eels migrated to reproduce, but no one had yet observed adults in the open ocean or the Sargasso Sea. For a scientist this was a fascinating mystery,” one of the study’s authors, Professor Julian Dodson of Université Laval in Québec, said in a press statement .
[h/t Newsweek ]
When you choose to publish with PLOS, your research makes an impact. Make your work accessible to all, without restrictions, and accelerate scientific discovery with options like preprints and published peer review that make your work more Open.
- PLOS Biology
- PLOS Climate
- PLOS Complex Systems
- PLOS Computational Biology
- PLOS Digital Health
- PLOS Genetics
- PLOS Global Public Health
- PLOS Medicine
- PLOS Mental Health
- PLOS Neglected Tropical Diseases
- PLOS Pathogens
- PLOS Sustainability and Transformation
- PLOS Collections
- About This Blog
- About PLOS ONE
- Official PLOS Blog
- EveryONE Blog
- Speaking of Medicine
- PLOS Biologue
- Absolutely Maybe
- DNA Science
- PLOS ECR Community
- All Models Are Wrong
- About PLOS Blogs
How eels navigate to the Sargasso Sea

To test the ability of eels to orient using a magnetic field, the authors used a carefully controlled laboratory setting, in which they eliminated other potential orientation cues, including odors, vibrations, light, and sounds. They also created an artificial magnetic field, the same strength as the Earth’s magnetic field, which could be oriented in different directions. Turning the artificial magnetic field between test runs helped rule out the possibility of the eels using other orientation cues.
The authors found that the eels consistently oriented in a particular direction with respect to magnetic north and that the eel’s particular orientation varied with water temperature. Below 12 o C, which is the temperature range associated with eel migration, the eels oriented in the direction they had been transported from the holding tank to the testing tank. This also corresponded with the direction of increasing water temperature. Above 12 o C, the eels oriented at right angles to the direction they had been transported, which the authors speculate might reflect foraging behavior during times of the year the eels are not migrating.
Thus, eels seem capable of using the Earth’s magnetic field as a navigational guide. They also seem to integrate this information with other cues, such as water temperature, to determine their direction of movement.
While these results provide some insight into how eels navigate to their spawning ground, other mysteries about eel migration remain, including where the spawning ground in the Sargasso Sea is precisely located. For further reading about eel migration, see another paper published in PLOS ONE in October , in which Béguer-Pon et al. tagged adult American eels ( Anguilla rostrata ) to map their migration to the Sargasso Sea but instead learned something about porbeagle shark predation .
Image credit: Steffen Zienert (http://nas.er.usgs.gov/queries/factsheet.aspx?SpeciesID=308)
Citations: Durif CMF, Browman HI, Phillips JB, Skiftesvik AB, Vøllestad LA, et al. (2013) Magnetic Compass Orientation in the European Eel. PLoS ONE 8(3): e59212. doi:10.1371/journal.pone.0059212
Béguer-Pon M, Benchetrit J, Castonguay M, Aarestrup K, Campana SE, et al. (2012) Shark Predation on Migrating Adult American Eels (Anguilla rostrata) in the Gulf of St. Lawrence. PLoS ONE 7(10): e46830. doi:10.1371/journal.pone.0046830
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name and email for the next time I comment.

Juvenile American eels wriggle in a glass dish. New research shows the fish can migrate more than a thousand miles into open sea to spawn.
- WEIRD & WILD
Epic Eel Migration Mapped for the First Time
Scientists use satellite tags to track American eels to an open ocean spawning area—a first for science.
Scientists know that American eels spend most of their adult lives inland or close to the shore, because for thousands of years, that’s where people have caught them. And we know the animals spawn in the open ocean, because that’s where we find their tiny, transparent larvae. But despite decades of searching, no adult American eel ( Anguilla rostrata ) has ever been spotted migrating across the hundreds of miles of ocean between the animals’ adult haunts and their ancestral spawning areas.
That is, until now.
A team of Canadian scientists used satellite tags to track an adult female eel from the coast of Nova Scotia to the northern limits of the Sargasso Sea in the middle of the North Atlantic—a journey of more than 1,500 miles (2,400 kilometers). The team published their results Tuesday in the journal Nature Communications .
“It’s pretty exciting,” says Julian Dodson , a biologist at Laval University in Quebec City and coauthor of the new study. “We’re beginning to catch a glimpse of what’s going on for the first time.”
American eel numbers have plummeted in the last few decades, earning the animals an endangered classification from the International Union for the Conservation of Nature. All the more reason, says Dodson, for us to learn about eel reproduction while we can.
Slippery as an Eel
Adult eels, also called silver eels, spend most of their lives in freshwater habitats such as the Great Lakes and in brackish estuaries, the transition zones between rivers and open sea. The animals are nocturnal, haunting the roots and rocks of riverbeds from the Gulf of Mexico to the St. Lawrence River in Canada. They can live to be twenty years or more before returning to the open ocean to spawn. But it is their final act—the animals die soon after.
At least, that’s what scientists think happens. But American eels have proven exceptionally difficult to keep tabs on. The satellite tags researchers use to track eels’ movements can come off prematurely as the fish slither against mud and rocks. They can also malfunction and pop off before an animal reaches its breeding grounds.
And though American eels can grow to nearly four feet (1.2 meters) long, they are a tasty snack for many open ocean predators. Previous studies that have used temperature and depth measurements from the eels’ satellite tags show that they sometimes wind up in the bellies of the sharks that frequent the Gulf of St. Lawrence, Dodson says. Those findings led Dodson’s team to start releasing their tagged eels in different locations, further from the sharks’ hunting grounds. “We didn’t want to keep feeding our rather expensive satellite tags to porbeagle sharks,” says Dodson.
Another Piece of the Puzzle
Finally, after years of trying, the researchers recently captured data from a female eel—eel No. 28—that made it from Nova Scotia to the northern edge of the Sargasso Sea.
According to her tag, No. 28 made the journey by swimming 30 miles (49 kilometers) a day. Depth measurements showed that—probably to avoid predators—she dove to deeper waters while the sun was up, sometimes reaching depths of more than 2,000 feet (699 meters). Such deep dives may help explain why no one has so far sighted the eels en route to their spawning grounds.
After 45 days at sea, No. 28’s tag popped off and started beaming all of its GPS coordinates, depth, water temperature and salinity, and other data up to a satellite. Dodson says this could be an example of premature detachment, since the tags were designed to stay attached for up to five months. Or perhaps it indicates that No. 28 was attacked by a predator.
Even though No. 28’s tag detached before she could reach the heart of the breeding grounds, American eel expert Steven Shepard praised the researchers for succeeding where similar studies had been stymied by premature tag loss or high mortality.
“This study advances the state of knowledge of silver eel migration at sea,” says Shepard, a biologist at the U.S. Fish and Wildlife Service and co-author of the agency’s recent status review for the species.
As for No. 28, Dodson remains optimistic.
“Whether she survived and made it to the migration or something else happened, we don’t know,” says Dodson, “but I’d like to think she made it all the way.”
Follow Jason Bittel on Twitter and Facebook .
FREE BONUS ISSUE
Related topics.
- ANIMAL MIGRATION
- ANIMAL BEHAVIOR
- SCIENCE AND TECHNOLOGY
You May Also Like

Follow the monarch on its dangerous 3,000-mile journey across the continent

Meet Simon and Jekyll, two great white sharks on a 4,000-mile journey together. Are they … friends?

In Japan, an endangered eel was hiding in plain sight

Cougar travels 1,000 miles in one of longest recorded treks

What lurks beneath the surface of these forest pools? More than you can imagine.
- Environment
- Perpetual Planet
History & Culture
- History & Culture
- History Magazine
- Mind, Body, Wonder
- Paid Content
- Terms of Use
- Privacy Policy
- Your US State Privacy Rights
- Children's Online Privacy Policy
- Interest-Based Ads
- About Nielsen Measurement
- Do Not Sell or Share My Personal Information
- Nat Geo Home
- Attend a Live Event
- Book a Trip
- Inspire Your Kids
- Shop Nat Geo
- Visit the D.C. Museum
- Learn About Our Impact
- Support Our Mission
- Advertise With Us
- Customer Service
- Renew Subscription
- Manage Your Subscription
- Work at Nat Geo
- Sign Up for Our Newsletters
- Contribute to Protect the Planet
Copyright © 1996-2015 National Geographic Society Copyright © 2015-2024 National Geographic Partners, LLC. All rights reserved
Find anything you save across the site in your account
Where Do Eels Come From?
By Brooke Jarvis

In the spring of 1876, a young man of nineteen arrived in the seaside city of Trieste and set about a curious task. Every morning, as the fishermen brought in their catch, he went to meet them at the port, where he bought eels by the dozens and then the hundreds. He carried them home, to a dissection table in a corner of his room, and—from eight until noon, when he broke for lunch, and then again from one until six, when he quit for the day and went to ogle the women of Trieste on the street—he diligently slashed away, in search of gonads.
“My hands are stained by the white and red blood of the sea creatures,” he wrote to a friend. “All I see when I close my eyes is the shimmering dead tissue, which haunts my dreams, and all I can think about are the big questions, the ones that go hand in hand with testicles and ovaries—the universal, pivotal questions.”
The young man, whose name was Sigmund Freud, eventually followed his evolving questions in other directions. But in Trieste, elbow-deep in slime, he hoped to be the first person to find what men of science had been seeking for thousands of years: the testicles of an eel. To see them would be to begin to solve a profound mystery, one that had stumped Aristotle and countless successors throughout the history of natural science: Where do eels come from?
The nineteenth century had brought Darwin and Mendel, Pasteur and Mendeleev, and a growing sense that scientists (a word coined only in the eighteen-thirties), with their studies and their systems and their microscopes, were at last equal to solving the great quandaries of the natural world. Questions that had befuddled mankind for centuries—where life comes from, what it is made of, how it changes, why it ends—were now seen as knowable, quantifiable, explicable. Just two years before Freud arrived in Trieste, the German biologist Max Schultze, lying on his deathbed, observed, perhaps wistfully, that he was leaving a world where “all the important questions . . . had now been settled.” All of them, that is, “except the eel question.”
What could be more ordinary than an eel? Not so long ago, European eels, Anguilla anguilla , were widely eaten. In Sweden, they might be smoked, braised in beer, or fried in butter; in Italy, boiled in tomato sauce; in England, jellied in stock, or fried with eggs into an elver cake. They were a simple and abundant food enjoyed by members of the poorer classes, like the Cockney woman described in “King Lear” who accidentally puts them in a pie still alive.
People caught eels in brooks, rivers, lakes, the sea. They also caught them, inexplicably, in ponds that dried out and refilled each year, and that had no access to other bodies of water. They couldn’t help but notice that the creatures seemed to have no ovaries, no testicles, no eggs, no milt. That they were never observed to mate. That they sometimes seemed to issue from the earth itself. Eels were unaccountable, and so, writes the Swedish journalist Patrik Svensson in “ The Book of Eels ” (Ecco), an unusual and beguiling guide to an unusual and beguiling animal, it fell to us to try our best to account for them.
The ancient Egyptians believed that eels were produced by the sun warming the Nile; Aristotle decided that eels emerged spontaneously from mud and rainwater. Pliny the Elder thought that new eels developed when old eels rubbed away parts of their bodies on rocks. As late as the eighteen-sixties, a Scottish author espoused an old belief that they began their lives as beetles. “Some believed eels were born of sea-foam,” Svensson writes, “or created when the rays of the sun fell on a certain kind of dew that covered lakeshores and riverbanks in the spring. In the English countryside, where eel fishing was popular, most people adhered to the theory that eels were born when hairs from horses’ tails fell into the water.”
The truth emerged only slowly, and was, in its own slippery way, stranger than the fiction. Careful observers discovered that what had long been taken for several different kinds of animals were in fact just one. The eel was a creature of metamorphosis, transforming itself over the course of its life into four distinct beings: a tiny gossamer larva with huge eyes, floating toward Europe in the open sea; a shimmering glass eel, known as an elver, a few inches in length with visible insides, making its way along coasts and up rivers; a yellow-brown eel, the kind you might catch in ponds, which can move across dry land, hibernate in mud until you’ve forgotten it was ever there, and live quietly for half a century in a single place; and, finally, the silver eel, a long, powerful muscle that ripples its way back to sea. When this last metamorphosis happens, the eel’s stomach dissolves—it will travel thousands of miles on its fat reserves alone—and its reproductive organs develop for the first time. In the eels of Europe, no one could find those organs because they did not yet exist.
But, even as these answers were arrived at, “the eel question,” as it was widely known, proved to be as changeable as the eel. It seemed to be forever unsolvable, for behind any eel answer there was always another eel question, shrouded by more layers of mystery.
“We know, then, that the old eels vanish from our ken into the sea, and that the sea sends us in return innumerable hosts of elvers,” a Danish searcher, Johannes Schmidt, wrote. “But whither have they wandered, these old eels, and whence have the elvers come?” Schmidt became consumed by his questions; in 1904, he left his family in Copenhagen and set out to scour the seas for the very smallest of eels. For seven years, he trawled the coasts of Europe, but found only larger larvae. For another three years, he enlisted shipping companies to net larvae as they plied the North Atlantic, and turned his own schooner west and south. Net by net, he mapped the ocean according to which parts of it contained eel larvae, and how large those larvae were, until the tiniest ones led him to their point of origin. It was a slow process, made slower by a shipwreck and a world war. Finally, nineteen years after he first set out, Schmidt announced his findings. “How long the journey lasts we cannot say,” he wrote, summoning the grandeur warranted by the occasion. “But we know now the destination sought: a certain area situated in the western Atlantic, N.E. and N. of the West Indies. Here lie the breeding grounds of the eel.”

Link copied
Schmidt had traced the Anguilla anguilla to the Sargasso Sea—a sea within a sea, a garden of seaweed bounded not by land but by great currents of water. (The American eel breeds there as well, and it is still something of a mystery how the larvae, all mixed together but genetically distinct, know which continent is their future home. The Japanese eel has its own breeding grounds, in the Pacific, and another famous freshwater eel, the electric one of South America, is not actually an eel at all; it’s a knifefish.) Schmidt’s discovery was an answer, and, in the past century, no one has successfully challenged it; that European eels come from the Sargasso Sea remains the official word of science. But, as with that sea and the animal born there, the boundaries of this knowledge are fluid and strange. Many expeditions have followed Schmidt to the breeding grounds in the decades since, each with better technology than the last. They, too, have found plenty of larvae, but, when one expedition collected and examined seven thousand fish eggs, not one of them turned out to be from an eel. Scientists have put G.P.S. trackers on silver eels beginning their migration; they’ve used hormones to bring females into heat, transported them to the breeding grounds, and attached them to buoys to use their pheromones as bait. They have dropped microphones into the water and opened the stomachs of predators. And yet no one has ever seen Anguilla anguilla mating anywhere, or so much as set eyes on a mature eel, living or dead, in the Sargasso Sea.
When Svensson was a child, in Sweden, he spent many evenings with his father, a road paver, on the banks of a stream that ran past his father’s childhood home. Together, as dusk fell, they would rig and bait their lines and throw them into the stream, then drive home to the swooping of bats. At sunrise, they checked to see which hooks had been taken by yellow-brown eels, which they collected in buckets and ate fried or boiled. (His father relished the taste, but Svensson found it nauseating; it was the fishing, and the time with his father, that he loved.) He describes his father as a thoughtful man, “fascinated by all the strange and wonderful forms life took,” but most of all by the peculiarity of eels: “ ‘They’re odd, eels,’ Dad would say. And he always seemed mildly delighted when he said it. As though he needed the mystery. As though it filled some kind of emptiness in him.”
Once, the pair tried an old Swedish fishing method that involved stringing lots of worms on a thread and then rolling them “into a quivering, stinking ball of slime and secretions and writhing bodies.” To catch the necessary worms, Svensson’s father attached electric cables to the prongs of a pitchfork sunk into a freshly watered lawn, creating a surge of electricity that brought a wave of panicked worms wriggling to the surface. With the worm ball, they caught eel after eel, more than they’d ever caught before, “like pulling carrots out of a vegetable patch.” But they didn’t do it again. There was no struggle in it, no mystery or chance or solemnity. “It didn’t tally with what we wanted the eel to be,” Svensson muses. “Maybe we had gotten too close to it.”
Svensson’s book, like its subject, is a strange beast: a creature of metamorphosis, a shape-shifter that moves among realms. It is a book of natural history, and a memoir about a son and his father. It is also an exploration of literature and religion and custom, and what it means to live in a world full of questions we can’t always answer. Svensson writes on page 1 that the eel “eludes the usual measures of the world,” and as the book progresses he begins to see other things as similarly elusive. He has seen eels that appear to be dead but are not, and eels that really are dead—chopped up and frying in butter, even—but still move as though they were alive. “To the eel, death seemed relative,” he writes. He has learned that the timing of an eel’s final transformation, the one that brings it to both its own death and the birth of the next generation, seems to be unrelated to time itself: eels might feel the pull to return to the sea after eight years inland, or after nearly sixty, or never, remaining behind in a sort of suspended animation. The eels that travel together across the ocean might be at the same stage of life, yet decades apart in age. Svensson is captivated by the implications of this. “You have to ask yourself: How does a creature like that perceive time?” he wonders.
Svensson’s grandmother was a believer in both God and gnomes. His father was not a believer, and neither is Svensson; their skepticism faltered “only where the eel was concerned.” When his grandmother was dying, she told Svensson, “I will always be with you,” and he immediately trusted her: “I didn’t need to believe in God to believe that.” Eventually, Svensson’s father became sick with cancer, likely a result of his years paving roads, breathing in the steam of asphalt. In the summers before the diagnosis, Svensson had often visited him. “We drank coffee, and talked about eels we’d caught and eels we’d lost and not much else,” he writes. As his father faded, Svensson lingered over the mysteries of time and existence, the watery border between death and life. He read Rachel Carson , who kept a tank of eels in her office and made an eel named Anguilla a protagonist of her first book, “Under the Sea-Wind.” Anguilla lived in the pleasantly warm mud of a pond, “beyond all reminders of the sea” that another of her selves once knew. Still, one day, she felt the urge to depart her current life, her current self, to transform and make her way through “frigid waters, deliberate and inexorable as time itself,” to a place where no one could follow. “No one can trace the path of the eels,” Carson wrote.
A decade later, and a decade before Carson, too, died of cancer, she published another book about the sea. She describes it as a place where man “feels the loneliness of his earth in space. And then, as never on land, he knows the truth that his world is a water world, a planet dominated by a covering mantle of ocean, in which the continents are but transient intrusions of land above the surface of the all-encompassing sea.”
The International Union for Conservation of Nature, a body charged with assessing the status of the natural world, has, unsurprisingly, had some trouble with eels. Ideally, to determine how the species is doing, the I.U.C.N. would like to know the number of “mature eels at their spawning grounds”—a number that is as knowable as the number of angels who can dance on the head of a pin.
Because ours is a world of making do, the I.U.C.N. instead counts the small glass eels that arrive in Europe each spring. For every hundred eels that showed up on the coasts in the nineteen-seventies, it’s believed that now only five come to wriggle their way inland. The formerly ordinary, everyday eel is classified as critically endangered, the last official designation on the road toward nonexistence. (Well, there is also “extinct in the wild,” but without their wild breeding grounds eels are nothing. No one has ever managed to breed them in captivity.) Svensson writes, “This is the latest and most urgent eel question: Why is it disappearing?”
There are many possible reasons, from disease to dams and locks, from fishing pressure to the warming climate, which is causing the ocean currents by which eels make their migrations to shift and change. But there may be other answers, and scientists are racing to find them—a quest that Svensson obviously supports but nonetheless considers not a little tragic. “Those of us who want to protect the eel in order to preserve something genuinely mysterious and enigmatic in a world of enlightenment will, in some ways, lose no matter how things turn out,” he writes. “Anyone who feels an eel should be allowed to remain an eel can no longer afford the luxury of also letting it remain a mystery.”
In our age of extinctions, every loss is like this: the disappearance not just of a creature from its ecosystem but of all that we might learn about it, all that we invest in it, all its layers of meaning, from our human future. To lose the eel is one grief; to lose the eel question, another. ♦
Books & Fiction
By signing up, you agree to our User Agreement and Privacy Policy & Cookie Statement . This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

By Silvia Killingsworth

By Joshua Hunt

By John J. Lennon

Where do eels come from?
European eels make an incredibly grueling, mysterious migration to spawn in the Sargasso Sea.

Critically endangered European eels ( Anguilla anguilla ) spend their adult lives in fresh water in Europe, ranging from Norway to the Mediterranean. But you won't find any baby eels in the mix. So where do all the mature eels come from?
Almost entirely across the Atlantic Ocean, as it turns out.
Adult European eels undertake a grueling 3,107 to 6,214 mile (5,000 to 10,000 kilometer) migration to the Sargasso Sea, not far from Bermuda, to spawn, lay eggs and die. Their young, transparent larvae float on the North Atlantic Drift, a current that takes them north and east across the Atlantic to Europe. The presence of small eel larvae in the Sargasso Sea — and nowhere else — revealed this far-flung spot to be their spawning ground a century ago. But only recently have researchers directly observed adult eels traveling to their final destination to lay their eggs. The findings were published October 2022 in the journal Scientific Reports .
"There [were] no records at all of any adult eels or eggs in the Sargasso Sea itself," said study co-author Ros Wright , a senior fisheries technical specialist at the United Kingdom's Environment Agency. But by tagging adult eels at the waypoint of their migration in the Azores, Wright and her team were able to trace a few to the Sargasso Sea.
Related: Can fish and other marine animals drown?
An incredible journey
European eels grow to about 2.6 feet (80 centimeters) long, but they complete one of the most dramatic migrations in the animal kingdom. The North Atlantic Drift lands them in European fresh water as "glass eels" — a transparent, wormlike stage. They gradually darken and grow larger, leaving the shelter of Europe again when they're less than 2.2 pounds (1 kilogram) in weight. Previous research had revealed that no matter where in Europe the eels hailed from, they gathered at the Azores during this migration. But no one knew how long the total migration took or what route the eels used to get from the Azores to the Sargasso Sea.

Part of the problem is that eels need to weigh at least 2.9 pounds (1.3 kg) to be fitted with a satellite tag. To find eels that were big enough, Wright and her team traveled to the Azores and set nets in rivers and pools. Some eels swim into these havens when they're small — they can climb up almost-vertical cascades, Wright told Live Science — and can't get out when they're larger. Wright and her team tagged these trapped eels and released them to continue their migration.
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.
In the October 2022 study, the researchers tracked 21 eels on a southwesterly trajectory toward the Sargasso Sea. Most of the tags popped off before the migration was done, but five of the eels made it into the Sargasso Sea while still being tracked.
The tags' data revealed why the eels are so elusive on this massive voyage. During daylight, they dive as deep as 4,921 feet (1,500 meters). At night, they swim closer to the surface, at 656 feet (200 m), still too deep to be spotted by anyone on the surface.
"That whole migration is actually quite deep and is in darkness most of the time," Wright said.
Taking it slow
The eels don't eat during this journey; their digestive systems dissolve and remake themselves as reproductive organs. But they're in no rush, Wright said. The tags showed that the eels traveled around 7.4 miles (11.9 kilometers) a day, at most. At that rate, it likely takes the eels about 18 months to make it from Europe to the Sargasso Sea, Wright said. In other words, the eels that left Europe this fall are not the same crowd that will spawn in the spring; it will be the spring of 2024 before they get their shot at reproduction.
The tracking data is "super exciting," said Caroline Durif , an ecologist at the Institute of Marine Research in Norway who was not involved in the new study but who has researched how eels navigate on this journey. The route matches what researchers suspected about the eels' journeys, she told Live Science. The animals likely navigate using Earth 's magnetic fields, she said, but exactly how this works is still not fully known.
— What's the biggest freshwater fish in the world?
— What is the oldest shark?
— What are Sea-Monkeys?
Once the eels spawn, they die. This is the natural rhythm of the life cycle for individuals, but European eels as a whole are in trouble. Their numbers have declined 95% since the early 1980s, Wright said. Some of the threats to the species may occur during the ocean-going phase of their lives, she said, which is why it's so important to understand and protect their migration routes.
"If we can establish the migratory routes, if it is really a critical area through the Azores and onwards, that has implications for things like deep sea mining," she said.
The researchers are still catching, tagging and releasing eels in the Azores, and they plan to continue reporting on the data. "We've got about another 10 to tag," Wright said.

Stephanie Pappas is a contributing writer for Live Science, covering topics ranging from geoscience to archaeology to the human brain and behavior. She was previously a senior writer for Live Science but is now a freelancer based in Denver, Colorado, and regularly contributes to Scientific American and The Monitor, the monthly magazine of the American Psychological Association. Stephanie received a bachelor's degree in psychology from the University of South Carolina and a graduate certificate in science communication from the University of California, Santa Cruz.
Ancient, 30-foot relative of great white shark unearthed in Mexico quarry
Anglerfish entered the midnight zone 55 million years ago and thrived by becoming sexual parasites
How common chemicals — including those in bed sheets — can boost eczema risk
Most Popular
- 2 James Webb telescope confirms there is something seriously wrong with our understanding of the universe
- 3 Eclipse from space: Paths of 2024 and 2017 eclipses collide over US in new satellite image
- 4 Giant, 82-foot lizard fish discovered on UK beach could be largest marine reptile ever found
- 5 Scientists discover once-in-a-billion-year event — 2 lifeforms merging to create a new cell part
- 2 Tweak to Schrödinger's cat equation could unite Einstein's relativity and quantum mechanics, study hints
- 3 New UTI vaccine wards off infection for years, early studies suggest
- 4 Lasers reveal prehistoric Irish monuments that may have been 'pathways for the dead'
- 5 Why does striking flint against steel start a fire?

IMAGES
VIDEO
COMMENTS
Scientists have unravelled a mystery surrounding one of nature's most incredible journeys. Every year, eels leave European rivers to travel in an epic migration to the Sargasso Sea in the North ...
Key Takeaways. All American and European eels are born in the Sargasso Sea — inside the Bermuda Triangle. The mystery of how eels reproduce fascinated thinkers from Aristotle to Sigmund Freud ...
Jan. 21, 2021 — European eels spawn in the subtropical Sargasso Sea but spend most of their adult life in a range of fresh- and brackish waters, across Europe and Northern Africa. Using whole ...
One single American eel reached the north west boundaries of the North Atlantic Convergence zone at > 2,000 km from the center of the Sargasso Sea 26, while a few European eels where detected at ...
Every three years, Reinhold Hanel boards a research ship and voyages to the only sea in the world that's located in the middle of an ocean. The Sargasso, bounded by currents instead of land, is ...
As an adult, the European eel undertakes the longest spawning migration of all anguillid eels, a distance of 5000 to 10,000 km across the Atlantic Ocean to the Sargasso Sea.
The reconstructed paths indicate a migration distance between ca 1,300 and 1,700 km for four eels and of ca 2,400 km for the eel that reached the Sargasso Sea (Supplementary Table 2 and Fig. 2).
This species starts life far from Europe in the Sargasso Sea, a region in the western Atlantic that is defined by the four ocean currents that form its boundaries. From December to May adult eels ...
The journey of European eels to their breeding place in the Sargasso Sea is up to 10,000km and considered one of the most impressive feats of animal migration observed in nature.
SUMMARY. One of the mysteries of the animal kingdom is the long-distance migration(5000-6000 km) of the European eel Anguilla anguilla L. from the coasts of Europe to its spawning grounds in the Sargasso Sea. The only evidence for the location of the spawning site of the European eel in the Sargasso Sea is the discovery by Johannes Schmidt at the beginning of the previous century of the ...
Between 1904 to 1922, researchers found eel larvae in the Sargasso Sea, an algae-rich patch of ocean in the North Atlantic that serves as home to a vast array of diverse marine species. For more ...
The eels did indeed make a 3,000 - 6,200 mile (5,000 - 10,000 km) migration across the Atlantic Ocean, the second longest recorded migration of a bony fish. Scientists have yet to observe a mating in the wild, which will likely be the next great eel mystery to solve, but the discovery of adult eels in the Sargasso Sea is a tremendous milestone.
1. Introduction. The European eel Anguilla anguilla (L., 1758) stands out among other fish species by its unique spawning migration. The route from European waters to the Sargasso Sea, its likely spawning ground, stretches from 5000 to 10 000 km, which is the longest distance among anguillid eels [1-3].Eels are semelparous, meaning they have a chance to spawn only once in a lifetime.
The route extended from western mainland Europe to the Azores region, more than 5000 km toward the Sargasso Sea. All eels exhibited diel vertical migrations, moving from deeper water during the day into shallower water at night. ... Meanwhile, field studies have shown that adult eels travel at between 12 and 50 km day −1 [for European eel ...
The migration of European eels ( Anguilla anguilla) between freshwater habitats in Europe and North Africa and their spawning ground in the Sargasso Sea is one of the unsolved mysteries in animal navigation. Scientists have speculated that the eels may use the Earth's magnetic field as a guide during their 6000 kilometer trip, and an article ...
That is, until now. A team of Canadian scientists used satellite tags to track an adult female eel from the coast of Nova Scotia to the northern limits of the Sargasso Sea in the middle of the ...
Schmidt had traced the Anguilla anguilla to the Sargasso Sea—a sea within a sea, a garden of seaweed bounded not by land but by great currents of water. (The American eel breeds there as well ...
Adult European eels undertake a grueling 3,107 to 6,214 mile (5,000 to 10,000 kilometer) migration to the Sargasso Sea, not far from Bermuda, to spawn, lay eggs and die. Their young, transparent ...
The collection of ≤12 mm European eel leptocephali across 2000 km of the Sargasso Sea during the two 2014 surveys provides the first clear evidence that spawning can occur across this expansive region within a short time in one spawning season. Larvae ≤12 mm were collected from 70-50° W, indicating widespread spawning several weeks ...
The European eel's singular spawning migration from European waters towards the Sargasso Sea remains elusive, including the early phase of migration at sea. During spawning migration, the movement of freshwater resident eels from river to sea has been thought to be irreversible.
Using a specially built tanker truck, the Eels on Wheels transport takes the road downstream Göta River. Below the last of the power stations, Lilla Edet, the eels are released again. From there it can travel the 7 000 kilometres to its spawning grounds in the Sargasso Sea in the western part of the Atlantic, outside Cuba and the Bahamas.