- Skip to primary navigation
- Skip to main content
High Yield Knowledge for the Ophthalmology Trainee

A guide to key oculoplastics exam techniques
Angela Chen MD, Shanlee Stevens MD, Adam Kessler, Justin Karlin MD
In this article, we will review common orbital and oculoplastic surgery examination techniques. This is intended for PGY1 and PGY2 ophthalmology residents.
When examining the eyelid, it is important to assess both structure and function.
Eyelid Examination Parameters
Other features to look for : involuntary blink dynamics (is the blink symmetric? complete?), voluntary closure (is the closure weak?), lagophthalmos (if present, look for Bell’s reflex, i.e. upgaze with eyelid closure, assess facial sensation)
Special Tests
Hertel exophthalmometry.
This is used to quantify axial globe position (the amount of proptosis or enophthalmos present).

- Place the exophthalmometer on the lateral orbital rim using the smallest base.
- Use your left eye to examine the patient’s right eye and vice versa.
- Ask the patient to direct their gaze forward, at your ipsilateral eye (your left eye)
- Line up the anterior and posterior red lines.
- Measure distance from lateral orbital rim to the most anterior part of the cornea.
- Proptosis of ≥ 22 mm or a difference of ≥ 2mm between eyes may need more evaluation.
- Repeat the same, now using your right eye to examine the patient’s left eye.
Dilation and probing
This is used to evaluate canalicular patency.
- Instill topical anesthetic
- Use a dilator to gently dilate the canaliculus
- Hold lateral traction on the eyelid
- Insert a Bowman probe into the punctum vertically ~2mm
- Slowly and gently turn the bowman probe to a horizontal position
- Advance the probe medially until you feel a hard stop against rigid lacrimal bone.
*This can be uncomfortable for patients, be sure to explain this before starting

Punctal dilator Bowman probes of various sizes Lacrimal cannula
Irrigation of the nasolacrimal system
After dilating the canaliculus, a 23 gauge lacrimal cannula on a 3 cc syringe filled with saline may be used to irrigate and evaluate patency of the lacrimal system. Gently insert the cannula into the canaliculus and flush with saline. There should be minimal resistance to irrigation in a patient with a patent nasolacrimal system.
- Patient feels saline in the back of the throat → patent
- Reflux out of the canaliculus being irrigated → obstruction at the canaliculus
- Reflux out of the opposite canaliculis → obstruction at the common canaliculus, lacrimal sac, or nasolacrimal duct.
- Note: if the patient feels saline in the back of the throat, but there is also mucopurulent reflux from the opposite canaliculus, this may indicate the presence of partial nasolacrimal duct obstruction
Phenylephrine testing
Instilling phenylephrine in patients with ptosis informs whether they would benefit from surgical excision of the Muller’s muscle (Muller’s muscle-conjunctival resection [CMMR]). Phenylephrine is an alpha-adrenergic agonist and stimulates the sympathetically innervated Muller’s muscle. Significant improvement in ptosis after instilling phenylephrine indicates the patient may be a good candidate for CMMR.
- Phenylephrine 2.5% instilled in the inferior fornix of the more ptotic eye → positive test if MRD1 increases >1.5mm in 3-5 minutes
- A single CN III subnucleus controls both levator muscles; sometimes when the more ptotic lid is elevated manually, surgically or pharmacologically, the less ptotic lid may fall
Forced duction testing
This may be used to evaluate for restrictive strabismus as well as muscle entrapment in the setting of orbital fracture. You will probably do this a lot on call! (anytime there is trauma to the orbital area)

Performing forced ductions (photo: https://surgeryreference.aofoundation.org/)
- Instill lidocaine jelly and allow it to sit for several minutes to make this as comfortable as possible.
- Grasp the conjunctiva and underlying Tenon’s capsule ~ 3 mm distal to the limbus with one or two toothed forceps (preferably 0.3 mm or 0.5 mm Castroviejo style forceps)
- Rotate the globe in the cardinal directions of gaze to assess for restriction
*Particularly helpful to assess for inferior rectus entrapment in orbital floor fracture
Reader Interactions
Dr. Neeraj is well-trained and has wide experience in the management of vitreoretinal conditions. He has vast experience in performing all types of Vitreo-Retina surgeries viz. Scleral buckling, vitrectomies, dislocated lens removal, membrane peeling, and silicone oil injection in patients with trauma, retinal detachments with proliferative vitreoretinopathy, and patients with proliferative diabetic retinopathy. Viaan Eye & Retina Centre is regarded as the Best Eye Hospital in Delhi with a highly equipped team of Eye Specialist Doctor to carry out all types of ophthalmic surgeries and diagnosis.

Copyright © 2020 EyeGuru
- Practice Modules
- Privacy Policy
- Terms of Use

- Create account
Aponeurotic Ptosis
All content on Eyewiki is protected by copyright law and the Terms of Service . This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
- 1.1 Disease
- 1.2 Etiology
- 1.3 Risk Factors
- 1.4 General Pathology
- 1.5 Histopathology
- 2.1 Symptoms
- 2.2 Physical examination
- 2.3 Diagnostic procedures
- 2.4 Differential diagnosis
- 3.1 Surgical Options
- 3.2 Complications
- 3.3 Prognosis
- 3.4 Primary prevention
- 4 References
Disease Entity
Aponeurotic Ptosis [a-pə-nu-ˈrä-tik ˈtō-səs] is recognized by the following codes as per the International Classification of Diseases (ICD) nomenclature:
- 374.3 Ptosis of eyelid
- 374.30 Ptosis of eyelid, unspecified
- H02.401 Acquired ptosis of right eyelid
- H02.402 Acquired ptosis of left eyelid
- H02.403 Acquired ptosis of bilateral eyelids

Ptosis (or Blepharoptosis) is the drooping of the upper eyelid margin. It is a common cause of reversible peripheral vision loss that affects the superior visual field first and then can go on to affect central vision. Patients may also report difficulty with reading, as certain types of ptosis can worsen when eyes are in downgaze. Patients can develop ptosis from birth (congenital) or later during life (acquired). Ptosis can also be classified by etiology: myogenic, neurogenic, mechanical, traumatic, or aponeurotic. The last is the subject of this article.
Aponeurotic ptosis is the most common type of acquired ptosis and the most common cause of ptosis overall. It is also known as senile or involutional ptosis, because it occurs most often in the elderly as an involutional disorder, meaning related to aging. This entity was first described by Jones, Quickert, and Wobig in 1975, who demonstrated that the levator aponeurosis appeared dehisced or disinserted from its normal position on the tarsus [1]
The levator aponeurosis is a fascial tissue that connects the levator palpebrae superioris muscle (levator muscle) to the tarsus, a thick plate of connective tissue that lies in the upper eyelid, as well as to the overlying skin. The levator aponeurosis transmits the force of the levator muscle to lift the upper eyelid. Any dehiscence, disinsertion, or stretching of the levator aponeurosis, either congenital or acquired, can lead to ptosis.
Common causes are involutional attenuation or repetitive traction on the eyelid, commonly seen with those that rub their eyelids frequently or in cases of contact lens use. Aponeurotic ptosis may be further worsened by eye surgery or procedures.
Congenital aponeurotic ptosis is uncommon. Most cases of aponeurotic ptosis occurring from birth are secondary to trauma during delivery.
Risk Factors
Risk factors for aponeurotic ptosis occurring from birth include forceps delivery, vacuum extraction, traumatic fetal rotation, and shoulder dystocia. Risk factors for aponeurotic ptosis occurring later in life include chronic contact lens use, inflammatory diseases, trauma, intraocular surgery, or frequent eye rubbing, as commonly seen in atopic individuals and in those with Down’s syndrome. The incidence of ptosis following cataract surgery was found to be 7.3% in one study [2] .
General Pathology
The primary changes found in acquired aponeurotic ptosis include dehiscence or disinsertion of the levator aponeurosis from the tarsus and dehiscence of the medial limb of Whitnall’s ligament from connective tissue at the medial orbital rim [3] .
A study that used ultrasound biomicroscopy to measure the thickness of the levator aponeurosis confirmed that the levator aponeurosis thickness in eyelids with aponeurotic ptosis is much thinner than that of the normal eyelid [4] .
Histopathology
Histopathological slides from the eyelids of patients with aponeurotic ptosis were evaluated, revealing that 71% of aponeuroses showed disinsertion and 12% showed attenuation of the aponeurosis (the remainder showed inconclusive changes). The remaining 17% were inconclusive. Müller’s muscle remained largely unchanged in these patients [5] .
Patients with aponeurotic ptosis may present with a spectrum of symptoms, ranging from visually asymptomatic cosmetic eyelid asymmetry to visually significant obstruction. While the superior visual field is most commonly obstructed, central vision can also be obstructed. In addition, patients may also report trouble with reading, as aponeurotic ptosis worsens in downgaze. Patients tend to compensate with overaction of the frontalis muscle, however, persistent brow elevation may lead to frontalis fatigue and even cephalgia.
If patients report a fluctuation in symptoms or eye muscle fatigue throughout the day, statin use and ocular myasthenia gravis should be ruled out [6] .
Physical examination
The physical examination of a patient with ptosis is aimed at determining etiology, eyelid muscle function through eyelid measurements, and assessment of surrounding facial structures.
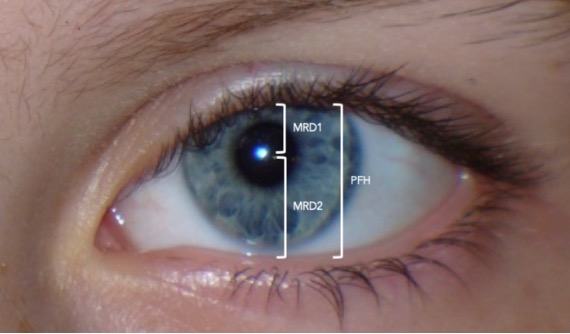
Margin reflex distance 1 (MRD1) : The distance from the upper eyelid margin to the corneal light reflex. This measurement is taken in primary position, with the patient fixating on the light source. Typically, MRD1 is 4-5 mm. In severe ptosis, the light reflex can be obstructed by the eyelid and the MRD1 is then zero or a negative value. In all cases, the more ptotic eyelid should be lifted to unmask occult contralateral ptosis (due to Hering’s law of equal innervation).
Margin reflex distance 2 (MRD2) : The distance from the corneal light reflex to the lower eyelid margin with the patient fixating on the light source. A typical MRD2 is 4-5 mm. An increased MRD2 indicates increased eye exposure and, thus, an increased risk of post-operative dry eye symptoms.
Margin to crease distance (MCD) : The distance from the upper eyelid crease to the upper eyelid margin with the patient looking down at a 45 degree angle. In Caucasians, MCD is typically 8-9 mm in males and 9-11 mm in females, while this often decreases to 2-5mm in East Asians. Aponeurotic defects characteristically have a high or an absent upper eyelid crease.
Levator function (upper eyelid excursion) : The distance from the upper eyelid margin in downgaze to upgaze with frontalis muscle function neutralized. Typically, levator function is 12-17 mm.
The levator function is classified as
- Good 8 mm or greater
- Fair 5 -7 mm
- Poor less than or equal to 4 mm
Hering’s law of equal innervation : The levator muscles obey Hering’s law of equal innervation, meaning they are innervated symmetrically. In cases of asymmetric ptosis, the levator muscles will receive an equal amount of increased central neural output to compensate for the ptosis. Therefore, the less ptotic eyelid may appear to have a normal height. However, when the more ptotic eyelid is manually elevated, the decreasing neural output to both eyelids results in descent of the contralateral eyelid. An immediate fall of the contralateral eyelid confirms the presence of bilateral, asymmetrical ptosis masked by levator “overaction.” A subclinical ptosis can thus be detected and explained to patients prior to surgery. This prevents post-operative “surprises”; if the patient decides not to do surgery on the less ptotic eye, the patient then is forewarned that the non-operated eye will appear to "develop" ptosis post-operatively.
Lagophthalmos : Lagophthalmos is the inability of the upper and lower eyelids to close completely, which leaves conjunctiva and, sometimes, a portion of the cornea unprotected. If present, the gap between the eyelids should be measured and the amount of corneal exposure documented (both in millimeters). Lagophthalmos and pre-existing signs/symptoms of dry eyes may predispose patients to postoperative exposure keratopathy.
Visual acuity and refraction : Visual acuity is always important to check prior to surgery, understanding that a patient’s refraction may change post-ptosis surgery. This can occur due to corneal contour changes secondary to eyelid pressure. Corneal topography can demonstrate an increase in against-the-rule astigmatism. These changes tend to be temporary, with a return of refractive shift towards normal by 12 months after surgery8. Ophthalmologists should avoid prescribing glasses to patients prior to and up to 3 months following ptosis surgery.
Other Physical Exam Findings :
If there are accompanying pupil or extraocular movement disorders present, entities, such as Horner's syndrome, PCOM aneurysm, and other orbital disorders should be considered. Systemic disorders causing ptosis, such as Myasthenia gravis, oculopharyngeal dystrophy, and neurological disorders, should be identified in all patients prior to surgery.
- Myasthenia gravis is notable for ptosis that fatigues, worsening at the end of the day. Levator fatigability can be assessed by asking the patient to look in extreme upgaze for up to one to two minutes and to check for improvement with the rest or ice test.
- Oculopharyngeal dystrophy, a late-onset genetic myopathy is seen as slowly progressive ptosis, dysphagia, and dysphonia (difficulty with speaking), starting around age 50.
- Oculomotor (CN 3) palsy is seen in patients with ptosis, mydriasis, and an eye positioned down and outward, causing diplopia.
- Horner Syndrome, which manifests as ptosis, miosis, and anhidrosis, results from a lesion to the sympathetic pathways.
Elderly patients, who have dermatochalasis, must be assessed carefully as the redundant upper eyelid skin may appear to cause a ptosis (pseudoptosis).
Diagnostic procedures
Visual field testing with the eyelids untaped (in the natural, ptotic state) and taped (artificially elevated) can provide objective data of the patient's level of functional visual impairment. The improvement with taped eyelids estimates the visual improvement that can be expected after surgery. This can be required by insurance companies in order to ensure coverage of treatment.
External full-face photography documents the presence and progression of the ptosis.
Pharmacologic testing is used by some to determine management of ptosis. In patients with a good levator function, some surgeons use response to topical phenylephrine testing to direct surgical management, whether by anterior levator advancement versus Müller's Muscle-Conjunctival Resection.
Differential diagnosis
- Idiopathic or genetic
- Blepharophimosis syndrome
- Congenital cranial nerve III palsy
- Congenital Horner syndrome
- Marcus Gunn jaw-winking syndrome
- Mechanical ptosis
- Traumatic ptosis
- Pseudoptosis
- Myasthenia gravis
- Chronic progressive external ophthalmoplegia
- Oculopharyngeal dystrophy
- Medication-related
- Cranial nerve III palsy
- Horner syndrome
Surgical Options
The mainstay of ptosis management relies on surgical correction, however the patient’s ocular, medical, and surgical history will determine if surgical repair is appropriate. The most important factor in surgical decision making is the levator muscle function.
When levator function is normal (greater than 10), this indicates that the levator muscle itself is strong and functioning normally. Tightening the levator muscle will elevate the eyelid margin.
- External (transcutaneous) levator advancement : Through an upper eyelid crease incision, the levator aponeurosis is surgically dissected from the tarsus and superiorly from the overlying orbital fat. A partial thickness suture is passed through the tarsus and through the levator muscle, resulting in an advancement of the levator muscle. This technique requires the patient’s cooperation to assess and adjust eyelid height after the surgical advancement.
- Internal (transconjunctival) levator/tarsus/Müller muscle resection approaches : Patients that demonstrate improvement in ptosis after instillation of topical phenylephrine may be candidates for the internal approach. These procedures focus on the removal of a part of the Müller muscle, the tarsus, or the levator aponeurosis to shorten the distance between the levator muscle and the tarsus, thus increasing its ability to elevate the upper eyelid. One advantage of this approach is the preservation of the external eyelid and lack of visible scar. The Müller muscle-conjunctival resection (MMCR) and the Fasanella-Servat ptosis repair procedures are examples of the internal approach.
When levator function is poor (less than 5mm), the levator muscle is not strong enough to lift the eyelid, no matter how it is manipulated, so the frontalis muscle is recruited.
- Frontalis muscle suspensions : Frontalis suspension surgery, commonly called a frontalis sling, suspends the eyelid directly from the frontalis muscle, allowing the patient to elevate the eyelid by recruiting the frontalis to lift the brow and eyelid. The material used for the sling may be autogenous (harvested from the patient’s tensor fascia lata), allogenic (from banked fascia lata), or synthetic (silicone or synthetic sutures).
Complications
Undercorrection is the most common complication of ptosis repair, which is seen in 10–15% of cases. As some component of post-operative undercorrection may be mechanical secondary to post-op eyelid edema, these patients should be observed until edema has resolved and the eyelid position has stabilized.
Other complications include overcorrection, unsatisfactory eyelid contour, surgical wound scarring or dehiscence, eyelid crease asymmetry, conjunctival prolapse, tarsal eversion, implant extrusion, infection, exposure keratopathy, and lagophthalmos. Cases of overcorrection should be observed until post-operative changes stabilize. Daily digital massage for several months can lower the eyelid, improving mild overcorrection.
Surgical revision is considered in patients with symptomatic over or undercorrection. Achieving symmetry between both eyelids is the most difficult aspect of ptosis repair and some surgeons use adjustable sutures and post-operative, in-office adjustments to attempt to achieve this goal.
Changes in corneal astigmatism can be seen in up to 72% of patients undergoing ptosis repair. It is generally with-the-rule and in most cases regresses back toward the pre-operative level within 1 year [7] .
The majority of ptosis procedures are successful, resulting in increased upper eyelid margin positioning.
Primary prevention
Primary prevention of acquired aponeurotic ptosis focuses on the prevention of excessive tractional forces on the eyelid, such as excessive rubbing of the eye. Patients with recurrent/seasonal allergic conjunctivitis should be advised to avoid excessive eye rubbing.
The prevention of postsurgical aponeurotic ptosis is aimed at efficient surgical time. This limits trauma to the eyelid caused by ocular inflammation and use of a lid speculum [8] .
- ↑ Jones LT, Quickert MH, Wobig JL. The Cure of Ptosis by Aponeurotic Repair. Archives of Ophthalmology. 1975;93(8):629-634. doi:10.1001/archopht.1975.01010020601008.
- ↑ Hosal B, Tekeli O, Gürsel E. Eyelid Malpositions after Cataract Surgery. European Journal of Ophthalmology . 1998;8(1):12-15. doi:10.1177/112067219800800104
- ↑ Korn, BS. 2019-2020 Basic and Clinical Science Course, Section 07: Oculofacial Plastic and Orbital Surgery eBook. https://elibrary.aao.org/epubreader/20192020-basic-clinical-science-course-section-07-oculofacial-plastic-orbital-surgery-ebook
- ↑ Hoşal BM, Ayer NG, Zilelioğlu G, Elhan AH. Ultrasound Biomicroscopy of the Levator Aponeurosis in Congenital and Aponeurotic Blepharoptosis. Ophthalmic Plastic & Reconstructive Surgery. 2004;20(4):308-311. doi:10.1097/01.iop.0000129532.33913.e7.
- ↑ Dortzbach RK. Involutional Blepharoptosis. Archives of Ophthalmology. 1980;98(11):2045. doi:10.1001/archopht.1980.01020040897022.
- ↑ Gale J, Danesh-Meyer HV. Statins can induce myasthenia gravis. Journal of Clinical Neuroscience. 2014;21(2):195-197. doi:10.1016/j.jocn.2013.11.009.
- ↑ Hoick DEE, Dutton JJ, Wehrly SR. Changes in Astigmatism After Ptosis Surgery Measured by Corneal Topography. Ophthalmic Plastic & Reconstructive Surgery. 1998;14(3):151-158. doi:10.1097/00002341-199805000-00001.
- ↑ Crum AV. Preventing & Managing Post-Surgical Ptosis. Review of Ophthalmology. https://www.reviewofophthalmology.com/article/preventing-managing-post-surgical-ptosis . Published October 9, 2010. Accessed April 19, 2020.
- Ahmad K, Wright M, Lueck CJ. Ptosis. Practical Neurology. 2011;11(6):332-340. doi:10.1136/practneurol-2011-000026.
- Boughton B. Assessing and Correcting Ptosis. American Academy of Ophthalmology. https://www.aao.org/eyenet/article/assessing-correcting-ptosis?novemberdecember-2007 . Published March 23, 2016. Accessed April 19, 2020.
- Cohen AJ, Weinberg DA. Evaluation and Management of Blepharoptosis. New York, NY: Springer New York; 2011.
- Garg A, Alió Jorge L. Surgical Techniques in Ophthalmology: Oculoplasty and Reconstructive Surgery. New Delhi: Jaypee Brothers Medical Publishers; 2010.
- Kanski JJ, Bowling B. Clinical Ophthalmology: a Systematic Approach. Edinburgh: Elsevier; 2012.
- Watanabe A, Araki B, Noso K, Kakizaki H, Kinoshita S. Histopathology of Blepharoptosis Induced by Prolonged Hard Contact Lens Wear. American Journal of Ophthalmology. 2006;141(6). doi:10.1016/j.ajo.2006.01.032.
- Yanoff M, Duker JS. Ophthalmology. St. Louis: Mosby; 2009.
- Oculoplastics/Orbit

- For Ophthalmologists
- For Practice Management
- For Clinical Teams
- For Public & Patients
Museum of the Eye
- Browse All Education
- Learning Plans
- Interactive
- Focal Points
- Wills Eye Manual
- Disease Reviews
- Clinical Webinars
- Diagnose This
- Self-Assessments
- Glaucoma Education Center
- Pediatric Ophthalmology Education Center
- Global Ophthalmology Guide
- Laser Surgery Education Center
- Redmond Ethics Center
- Ocular Trauma Resources
- Myopia Resources
- Thyroid Eye Disease Resources
- Practice Guidelines
- Drug-Resistant Pseudomonas Outbreak
- Preferred Practice Patterns
- Clinical Statements
- Ophthalmic Technology Assessments
- Patient Safety Statements
- Complementary Therapy Assessments
- Medical Information Technology
- Diagnostic Excellence
- Choosing Wisely
- Eye Care for Older Adults
- Eye Disease Statistics
- About the Hoskins Center
- Artificial Intelligence
- Premium IOLs
- Patient-Reported Outcomes with LASIK Symptoms and Satisfaction
- Multimedia Library
- 1-Minute Videos
- Presentations and Lectures
- Master Class Videos
- Basic Skills Videos
- Clinical and Surgical Videos
- Resident Lectures
- Submit a Video
- YO Video Contest
- Browse Podcast Library
- Experts InSight Podcast
- Ophthalmology Journal Podcast
- Submit an Image
- Browse Clinical News
- Editors' Choice
- Current Insight
- CME Central
- About Continuing Certification
- Claim CME Credit and View Transcript
- CME Planning Resources
- Complete Your Financial Disclosure
- LEO Continuing Education Recognition Award
- Safe ER/LA Opioid Prescribing
- Check Your Industry Payment Records
- Resident Education Home
- Flashcards and Study Presentations
- Interactive Cases and Simulations
- Diversity and Inclusion Education
- News and Advice from YO Info
- Board Prep Resources
- OKAP and Board Review Presentations
- Study Flashcards
- PGY-1 and PGY-2 Resources
- Physician Wellness
- Resident Knowledge Exchange
- Simulation in Resident Education
- Ophthalmology Job Center
- Clinical Education /
- Multimedia /
Log in to view this page

Measurement of levator excursion
- Mark Complete
Measurement of levator excursion. A, Downgaze. B, Upgaze.
All content on the Academy’s website is protected by copyright law and the Terms of Service . This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
- About the Academy
- Jobs at the Academy
- Financial Relationships with Industry
- Medical Disclaimer
- Privacy Policy
- Terms of Service
- Statement on Artificial Intelligence
- For Advertisers
FOLLOW THE ACADEMY
Medical Professionals
Public & Patients
H. Joon Kim, MD, Atlanta
A Review of Blepharoptosis Repair
How the cause of the drooping lid will guide your diagnostic investigation and eventual repair of the problem..
P tosis of the upper eyelid, or blepharoptosis, though ostensibly a straightforward problem, can actually be a challenge to diagnose and repair, thanks to its many possible causes and the strengths and weaknesses of the various surgical approaches. Here, I’ll provide advice on how to evaluate a case of acquired adult blepharoptosis, root out its cause and manage it successfully.
A review of the lid anatomy can help when planning blepharoptosis surgery. Elevation of the upper eyelid is a process controlled by three retractors. The first retractor, levator palpebrae superioris, is a striated muscle in the upper eyelid innervated by the oculomotor nerve and is primarily responsible for opening the eyelid. The second, Müller’s muscle, is a sympathetically innervated smooth muscle posterior to the levator and is responsible for about 2 mm of eyelid opening. Finally, the third and weakest retractor is the frontalis muscle in the forehead, which is innervated by the facial nerve, and can indirectly raise the upper eyelid by lifting the eyebrows. Any direct or indirect impact on these muscles can result in blepharoptosis. 1
Classification
Ptosis can result from a number of causes, some of which are quite serious and warrant investigation. The condition can be unilateral or bilateral and can occur in varying degrees of severity that lead to cosmetic and/or functional deficits.
• Aponeurotic ptosis. This is the most common form of ptosis, and is due to chronic dehiscence of the levator aponeurosis due to normal aging changes. It’s usually bilateral but is often asymmetric. The changes to the muscle can be accelerated by common events such as long-term contact lens use or intraocular surgery. Despite the severity of the ptosis, the levator function often remains normal. 2
• Myogenic ptosis. This form of ptosis stems from a myopathy, with the most common diagnoses being a chronic progressive external ophthalmoplegia (CPEO), myasthenia gravis, myotonic dystrophy or oculopharyngeal-muscular dystrophy (OPMD). The myopathy typically progresses, with worsening ptosis correlating with a decrease in levator function. The exception is myasthenia gravis, which is characterized by a fluctuating ptosis with variable levator function. These myopathies also tend to be associated with a constellation of systemic findings, such as a heart block along with CPEO in Kearns-Sayre syndrome, and dysphagia and proximal limb weakness with OPMD. 3
• Neurogenic ptosis. This variety is uncommon but can signal a serious underlying issue. It can stem from a problem of the oculomotor nerve most commonly due to ischemia from diabetes, but can also indicate an aneurysm, stroke or tumor. A congenital or acquired form of Horner syndrome can also result in mild ptosis from Müller’s muscle being affected, and can be a manifestation of a stroke, tumor (including pulmonary tumors) or vascular disease. Aberrant regeneration of the facial nerve can result in a synkinetic ptosis associated with perioral contraction. A number of supranuclear conditions can also result in neurogenic ptosis as well, such as strokes, multiple sclerosis and brain injury. 4-6
• Mechanical ptosis. This results from lesions in the lid either weighing it down or preventing it from lifting. Examples of such lesions include eyelid malignancies and symblepharon formation secondary to ocular cicatricial pemphigoid and Stevens-Johnson syndrome. 3
• Traumatic ptosis. Traumatic ptosis can result from a variety of direct or indirect mechanisms, the most obvious being direct laceration of the lid muscles and nerves. It can also arise from blunt trauma, edema or hemorrhage that causes dehiscence of the levator. 7
Evaluation
A proper evaluation of the blepharoptosis patient includes a good history, clinical exam and an appreciation for what I call the eyelid “vital signs.”
• History. Patients with ptosis often report both cosmetic and functional complaints. Ptosis can diminish the peripheral visual field and can result in difficulty with daily activities of living. The “drowsy” appearance and the aging effect of ptosis are also bothersome for patients, especially if the case is asymmetric. The age of onset and the duration of the ptosis are also important, since they can indicate a more serious underlying problem. In such cases, seek out associated symptoms, such as diplopia, diurnal variation and trouble swallowing. Take a thorough ocular and medical history of the patient and the family, and note any history of surgery or trauma.
• Clinical exam. A full ophthalmic exam is warranted, watching for anomalous head positioning, facial asymmetry, synkinesis and abnormal speech. Neutralize any frontalis excursion to eliminate raised eyebrows so you can accurately assess the eyelid position.
Evaluate the acuity and pupil, paying special attention to the presence of anisocoria. Watch for strabismus in primary gaze and perform a thorough motility exam. Note other signs, such as Cogan’s lid twitch (overshooting of the upper lid from downgaze to upgaze) and von Graefe’s sign (lagging of the upper lid on downgaze). Rule out fatigable upgaze, supplementing your exam with an ice test if there’s a high level of suspicion for myasthenia gravis. Assess any proptosis or enophthalmos with an exophthalmometer, and perform a slit lamp exam, looking particularly for signs of dry eyes. Postpone your dilated fundus exam until you’ve done a full lid exam, since dilating eye drops (i.e., phenylephrine) can temporarily raise the lid position.
• Eyelid “vital signs.” The vertical palpebral fissure (the distance between the upper and lower lid margin) should be approximately 10 mm. The normal position of the upper lid margin is about 0.5 mm to 2 mm below the superior limbus. The marginal-reflex-distance 1 (MRD1) is the distance between the center of the pupil and the upper eyelid margin and averages 4 to 5 mm (Figure 1) . The levator function is measured by the full excursion of the upper lid from downgaze to upgaze and should be between 10 to 15 mm (Figures 2A and 2B) . Deviations from these average values confirm the presence of ptosis; an evaluation of the levator function can help to narrow down the etiology. 8
• Ancillary testing. Initiate a workup if the underlying etiology is unclear. A basic workup can include a comprehensive metabolic panel, complete blood count, erythrocyte sedimentation rate and C-reactive protein. Thyroid function panel and acetylcholine receptor antibodies are common workups if thyroid disease and/or myasthenia gravis are suspected. In cases where a myopathy like CPEO is suspected, you can perform genetic testing, electromyography or even muscle biopsy. If orbital signs are present, including an abnormal pupil exam or other cranial neuropathies, neuroimaging may be in order. You can also order CTA or MRA if you suspect an aneurysm. 3 If you suspect Horner syndrome, perform pharmacological testing as well. 5
Non-surgical Treatment
Some cases of blepharoptosis don’t need surgery, and are better treated with the following approaches.
Observation is an acceptable response to entities such as traumatic ptosis or some forms of neurogenic ptosis (e.g., oculomotor palsy from ischemia), which can improve spontaneously. Observation can also be appropriate in cases of aponeurotic ptosis that don’t yet bother the patient.
Some cases respond best to a pharmacologic treatment. You should have optimal titration of systemic medication in myasthenia gravis patients and stability in thyroid patients prior to surgery. In cases of aberrant regeneration of the facial nerve resulting in synkinetic ptosis, botulinum toxin to the orbicularis oculi can improve the ptosis. 66
Ptosis repair can be classified into anterior and posterior approaches. 9 The etiology, severity of ptosis, and levator function will often determine the most appropriate method. Regardless of the technique, ptosis repair can be performed in the office setting with local infiltrative anesthesia, or in the operating suite, the latter typically involving sedation.
The anterior approaches consist of:
• External levator advancement. This is the most common procedure. Though it can address a wide range of ptosis, it relies on the presence of a functioning levator. In the procedure, the surgeon advances the attenuated or dehisced levator musculo-aponeurotic junction inferiorly onto the superior border of the tarsus. 10 Small-incision techniques can offer the benefit of minimal scarring, 11 but a traditional incision allows you to perform a simultaneous blepharoplasty (Figure 3) .
• Frontalis suspension. This procedure is a great option when there’s minimal or no levator function. 12 It allows you to bridge the frontalis muscle to the superior tarsal plate so that raising the brows will result in a more successful elevation of the lid. The bridging material can be autoplastic (i.e., autogenous tensor fascia lata) or alloplastic (i.e., silicone rods, alloderm). Alloplastic materials, especially silicone rods, are most widely used in adults due to their ease of placement and adjustability. 13
If you feel a posterior approach would be better, consider:
• Müller’s muscle conjunctival resection (MMCR). This requires excellent levator function and is ideal for mild degrees of ptosis (1 to 2 mm). It does, however, require preoperative phenylephrine testing to ensure the viability of the Müller’s muscle and the ideal candidacy for MMCR. MMCR would be indicated if you measure 1 to 2 mm of elevation of the upper lid 10 minutes after instillation of 2.5% phenylephrine. Surgical resection ranges between 6.5 and 9.5 mm, following the 4:1 rule: Perform 4 mm of resection for every 1 mm of elevation. 14-16 MMCR remains a popular choice for mild ptosis because it’s easy to perform and its results are predictable. Also, patients like that it doesn’t result in a visible scar. However, conjunctival scarring and contour issues can be problematic.
• Fasanella-Servat procedure. This procedure involves resection of the conjunctiva, Müller’s muscle and the superior border of the tarsal plate. 17 The surgeon usually performs 1 mm of lift for every 2 mm of tarsectomy or 2 mm of conjunctival-Müller resection. 18 It, too, offers the benefit of avoiding a scar. However, tarsal instability and resection of accessory lacrimal glands often lead to dry eye, so this procedure has fallen out of favor. 19,20
Both anterior and posterior approaches are very successful in elevating the lid in the appropriate setting. Eyelid swelling, bruising, and varying degrees of discomfort are to be expected during the immediate postoperative period. Common complications include overcorrection, undercorrection, asymmetry and contour issues, thus making it one of the most challenging surgeries. 3,12
In conclusion, though the cause of a patient’s blepharoptosis can be challenging to pin down, and you have to weigh the pros and cons of several surgical approaches, a thorough exam and careful surgery can usually help you achieve good results. REVIEW
Dr. Kim is an associate professor of Oculoplastics, Orbital and Cosmetic surgery at Emory University in Atlanta.
1. Sand JP, Zhu BZ, Desai SC. Surgical anatomy of the eyelids. Facial Plast Surg Clin N Am 2016; 24:89-95.
2. Godfrey KJ, Korn BS, Kikkawa DO. Blepharoptosis following ocular surgery: Identifying risk factors. Curr Opin Ophthalmol 2016;27:31-37.
3. Wong VA, Bechingsale PS, Oley CA, Sullivan TJ. Management of myogenic ptosis. Ophthalmology 2002;109:5:1023-31.
4. Averbuch-Heller L, Leigh RJ, Mermelstein V, et al. Ptosis in patients with hemispheric strokes. Neurology 2002;58:4:620-4.
5. Walton Kam, Buono LM. Horner Syndrome. Curr Opin Ophthalmol 2003;14:357-363.
6. Chen C, Malhotra R, Muecke J, et al. Aberrant facial nerve regeneration (AFR): An under-recognized cause of ptosis. Eye (Lond) 2004;18:2:159-62.
7. Jacobs SM, Tyring AJ, Amadi AJ. Traumatic ptosis: Evaluation of etiology, management, and prognosis. J Ophthalmic Vis Res 2018;13:4:447-452.
8. Neimkin MG, Holds JB. Evaluation of eyelid function and aesthetics. Facial Plast Surg Clin N Am 2016; 24:97-106.
9. Allen RC, Saylor MA, Nerad JA. Current state of ptosis repair: A comparison of internal and external approaches. Curr Opin Ophthal 2011;22:394-399.
10. Older JJ. Levator aponeurosis surgery for the correction of acquired ptosis. Analysis of 113 procedures. Ophthalmology 1983;90:1056-1059.
11. Frueh BR, Musch DC, McDonald HM. Efficacy and efficiency of a small-incision, minimal dissection procedure versus a traditional approach for correcting aponeurotic ptosis. Ophthalmology 2004;111:2158-2163.
12. Anh J, Kim NJ, Choung HK, et al. Frontalis sling operation using silicone rod for the correction of ptosis in chronic progressive external ophthalmoplegia. Br J Ophthalmol 2008;92:1685-1688.
13. Lamont M, Tyers AG. Silicone sling allows adjustable ptosis correction in children and in adults at risk of corneal exposure. Orbit 2010; 29:102-105.
14. Putterman AM, Fett DR. Muller’s muscle in the treatment of upper eyelid ptosis. Ophthalmic Surg 1986;17:354-360.
15. Weinstein GS, Buerger GF Jr. Modification of the Muller’s muscle-conjunctival resection operation for blepharoptosis. Am J Ophthalmol 1982;93:647-651.
16. Perry JD, Kadakia A, Foster JA. A new algorithm for ptosis repair using conjunctival mullerectomy with or without tarsectomy. Ophthal Plast Reconstr Surg 2002;18:426-429.
17. Fasanella RM, Servat J. Levator resection for minimal ptosis: another simplified operation. Arch Ophthalmol 1961;65:493-496.
18. North VS, Campbell AA, Callahan AB, et al. Enahanced Fasanella-Servat procedure for the graded repair of blepharoptosis. Ophthal Plast Reconstr Surg 2017;33:474-476.
19. Dailey RA, Saulny SM, Sullivan SA. Muller muscle-conjunctival resection. Ophthal Plast Reconstr Surg 2002;18:421-425.
20. Bodian M. Does conjunctival resection in ptosis surgery lead to dry-eye syndrome? Ann Ophthalmol 1989;21:213-216.
Related Articles
Trichiasis: lashes gone astray, excess baggage: lower-lid rejuvenation, the genetic basis of oculoplastic disorders, the surgical treatment of the aging brow, lower eyelid malposition: evaluation and treatment, current issue.

Table of Contents
Read digital edition, read pdf edition, subscriptions.

Copyright © 2024 Jobson Medical Information LLC unless otherwise noted.
All rights reserved. Reproduction in whole or in part without permission is prohibited.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Open access
- Published: 29 April 2021
A review of acquired blepharoptosis: prevalence, diagnosis, and current treatment options
- Jason Bacharach ORCID: orcid.org/0000-0002-7084-4327 1 ,
- Wendy W. Lee 2 ,
- Andrew R. Harrison 3 &
- Thomas F. Freddo 4
Eye volume 35 , pages 2468–2481 ( 2021 ) Cite this article
15k Accesses
33 Citations
12 Altmetric
Metrics details
- Drug therapy
- Eyelid diseases
Blepharoptosis (ptosis) is among the most common disorders of the upper eyelid encountered in both optometric and ophthalmic practice. The unilateral or bilateral drooping of the upper eyelid that characterises ptosis can affect appearance and impair visual function, both of which can negatively impact quality of life. While there are several known forms of congenital ptosis, acquired ptosis (appearing later in life, due to a variety of causes) is the predominant form of the condition. This review summarises the prevalence, causes, identification, differential diagnosis, and treatment of acquired ptosis. Particular attention is paid to the differential diagnosis of acquired ptosis and emerging treatment options, including surgical and pharmacologic approaches.
上睑下垂是视光学和眼科临床中最常见的上睑疾病之一。以上睑下垂为特征的单侧或双侧上睑下垂会影响外观和视觉功能, 这两种情况都会对生活质量产生负面影响。目前已知有几种先天性的上睑下垂, 但获得性上睑下垂 (由各种原因所导致并出现在其后的生活中) 是该病的主要形式。本文就获得性上睑下垂的发病率、病因、鉴别诊断及治疗作一综述。并特别研究获得性上睑下垂的鉴别诊断以及新的治疗方法, 包括手术和药理治疗途径。
Similar content being viewed by others

What colour are your eyes? Teaching the genetics of eye colour & colour vision. Edridge Green Lecture RCOphth Annual Congress Glasgow May 2019

Medical emergencies in the dental practice poster: revised and updated

Consensus guideline for the diagnosis and management of pituitary adenomas in childhood and adolescence: Part 2, specific diseases
Literature search notes.
Literature cited in this review was identified via a broad search of the PUBMED online database for English-language, peer-reviewed publications including search terms such as “ptosis,” “epidemiology,” “etiology,” “eyelid,” “surgical,” “pharmacologic,” “Müller’s muscle,” “adrenergic,” “visual field,” and “quality of life.” Relevant primary and review articles were reviewed and cited when providing unique primary data or a current summary of fundamental concepts. Also included, when relevant, were primary or review articles not identified via PUBMED, but cited in publications retrieved via this literature search.
Acquired ptosis overview, prevalence, and impacts
Blepharoptosis, more commonly known as “ptosis,” is an abnormal drooping of the upper eyelid with the eye in primary gaze. This drooping can affect one or both eyes, and based on time of appearance, it is broadly classified as either congenital (present at or shortly following birth) or acquired (appearing later in life). Ptosis is broadly recognised as being among the most common disorders of the eyelid encountered in the clinic, however data from large population-based studies are limited. Estimates of ptosis prevalence are largely based on data from region-specific studies, which report rates between 4.7 and 13.5% in adult populations and support the widespread nature of the condition [ 1 , 2 , 3 ]. Furthermore, these studies consistently reveal that, within adult populations, the incidence of ptosis increases with age (Table 1 and Acquired ptosis risk factors). Reports of ptosis incidence in surgical populations are consistent with those in broader patient populations. In a study evaluating a cohort of 623 patients referred for surgery in an oculoplastics department in Singapore, ptosis was the most common condition, occurring in 11.7% of patients [ 4 ].
Drooping of the upper eyelid due to ptosis can lead to the condition’s characteristic ‘sleepy’ appearance, as well as asymmetry, in both unilateral and bilateral cases [ 5 , 6 ]. Studies reveal that this can have important impacts on patient well-being, including reduced independence and increased appearance-related anxiety and depression [ 7 , 8 ]. In a study in the United Kingdom, adults referred for ptosis surgery were assessed prior to surgery using validated questionnaires addressing psychosocial factors, including appearance-related distress (the Derriford Appearance Scale (DAS 24)), anxiety and depression (the Hospital Anxiety and Depression Scale (HADS)), fearful or worrying conditions related to the perceived opinions of others (the Fear of Negative Evaluation (FNE) Scale), and self-evaluation of appearance (the Centre for Appearance Research Valence (CARVAL) scale). Patients reported levels of appearance-related distress, anxiety, and depression that were higher than typical norms in the general population and similar to levels previously reported in patients with other appearance-altering ophthalmic conditions, such as strabismus [ 8 ]. The analysis also identified significant gender differences with respect to DAS 24, HADS, FNE, and CARVAL scores, with female patients reporting higher mean scores than males [ 8 ].
From a functional perspective, obstruction of the pupil as a result of ptosis can lead to deficits in the superior visual field, detectable via visual field testing and evident even in mild cases [ 9 , 10 , 11 ]. An evaluation of the superior visual field using static perimetry testing (Humphrey Visual Field (HVF) Test) in subjects at baseline and after induction of mild or moderate ptosis using eyelid weights found that even mild ptosis was associated with significant depression of all test points along the superior hemifield, and that this worsened in the moderate ptosis condition [ 11 ]. Among more recent studies in patients with ptosis, a study validating a novel static perimetry test (the Leicester Peripheral Field Test (LPFT)) revealed that 84 of 85 ptotic eyes had a visual field deficit [ 10 ]. Visual field testing methods are described in detail the section titled Acquired ptosis identification and differential diagnosis.
The effect of ptosis goes beyond diminished performance on visual field tests. Visual field loss is associated with decreases in health-related quality of life (HRQoL) measures [ 7 ], indicating meaningful impacts on patients’ daily lives. In the Los Angeles Latino Eye Study (LALES), more than 5200 subjects underwent ophthalmic examination and visual field testing. Data from this population revealed that greater visual field loss, measured using the HVF Test, correlated with worse scores on two validated tools to assess HRQoL—the Medical Outcomes Study 12-item Short-Form Health Survey (SF-12) and the National Eye Institute Visual Function Questionnaire (NEI-VFQ-25). While bilateral moderate/severe visual field loss was associated with the greatest negative effect on HRQoL measures, decreases in HRQoL were also evident in participants with mild unilateral visual field loss [ 7 ]. The reduction in HRQoL was found to be, at least in part, due to the reduction in independence (greater difficulty driving and performing regular tasks) that arises due to visual field deficits [ 7 ]. Studies also show that improvements in subjective and objective visual performance following intervention are associated with improved HRQoL-related measures [ 12 , 13 ]. In a study of 50 patients who underwent ptosis surgery, patients showed significant improvement versus pre-surgery assessment, with respect to a range of vision-related activities and symptoms, including the ability to perform fine manual work, hang or reach objects above eye level, watch television, and read [ 12 ]. Similarly, in a study of 100 patients with unilateral or bilateral ptosis that used the same questionnaire, improvement in the superior visual field following surgery was associated with a greater functional index, and patients had significant improvement with respect to activities including performing their occupation, playing sports, and walking without assistance [ 13 ].
The upper eyelid and causes of acquired ptosis
Elevation of the upper eyelid is largely provided by two muscles—the levator palpebrae superioris (levator) and the superior tarsal (Müller’s) muscle (Fig. 1 ). The levator is a voluntary (striated) muscle that originates from the lesser wing of the sphenoid bone at the orbital apex and inserts, through its aponeurosis, onto the anterior surface of the superior tarsal plate. It also has attachments to the skin of the upper eyelid, which contribute to the formation of the lid crease. This insertion is absent or poorly formed in some Asian individuals. The levator is innervated by the superior division of the oculomotor nerve (cranial nerve III), and its contraction provides the majority (~80%) of upper eyelid elevation [ 5 , 14 , 15 , 16 ]. Müller’s muscle arises from the underside of the levator, at the level of the distal aponeurosis, and inserts onto the superior tarsal plate [ 5 , 14 , 15 ]. In contrast to the striated levator muscle, Müller’s muscle—like its analogue in the lower eyelid, the inferior tarsal muscle—is an involuntary (smooth) muscle. With contraction, Müller’s muscle helps to sustain upper eyelid elevation provided by the levator, while also supplying 1–2 mm of additional lift [ 5 , 14 ]. Similarly, in the lower eyelid, the inferior tarsal muscle assists in lowering the lid during downward gaze, though there is no striated muscle analogous to the levator. Both Müller’s muscle and the analogous inferior tarsal muscle receive sympathetic innervation from nerve fibres originating in the superior cervical ganglion [ 5 , 14 , 15 ]. A study of adrenergic receptor expression in Müller’s muscle revealed a predominance of the α 2A subtype, and lower expression of the α 1 and β 1 subtypes [ 17 ]. Further examination of receptor subtype expression in Müller’s muscle has also demonstrated expression of the α 1D , α 2C , and β 2 subtypes in patients with ptosis [ 18 , 19 ]. In contrast to Müller’s muscle, the levator predominantly expresses the β 1 -adrenergic receptor subtype, with only trace expression of the α 1 , α 2 , and β 2 subtypes [ 17 ].
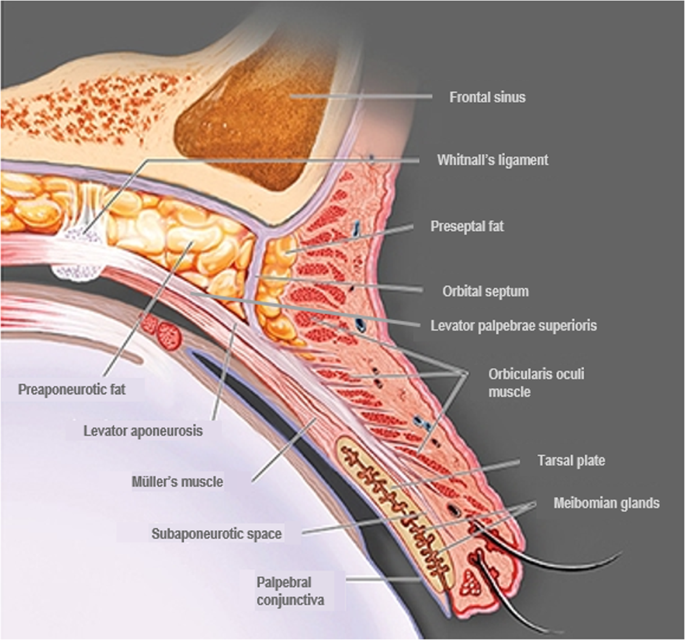
Adapted from Freddo and Chaum, 2017 [ 14 ]. The striated levator palpebrae superioris is innervated by the oculomotor nerve (cranial nerve III) and inserts, through its aponeurosis, on the anterior surface of the superior tarsal plate. Except in the eyelids of Asian individuals, the aponeurosis extends fibres through the orbicularis oculi muscle to reach the skin of the upper eyelid. The smooth Müller’s muscle arises from the underside of the levator and inserts on the superior tarsal plate. It is innervated by sympathetic fibres from the superior cervical ganglion [ 5 , 14 , 15 , 16 ].
The frontalis muscle, which inserts at the level of the eyebrows, is innervated by the facial nerve (cranial nerve VII) and its contraction raises the brow, with no direct effect on upper eyelid elevation. In patients with ptosis, however, compensatory raising of the brow via the frontalis muscle can indirectly provide slight elevation of the eyelid as well [ 15 ].
Broadly, ptosis is classified based on time of onset. Congenital ptosis (present at birth) typically has a unilateral presentation and is most often a result of developmental myopathy of the levator muscle that affects the levator’s ability to contract and raise the upper eyelid [ 20 , 21 , 22 ]. Neurogenic forms of congenital ptosis can be caused by cranial nerve III abnormalities or insufficient sympathetic innervation of Müller’s muscle. Furthermore, several craniofacial syndromes or cranial dysinnervation disorders can also underlie congenital ptosis, including Marcus Gunn jaw-winking syndrome or blepharophimosis [ 22 , 23 ].
Acquired ptosis, the predominant form of ptosis (Table 2 ), can be classified by aetiology, with cases typically defined as having an aponeurotic, myogenic, neurogenic, mechanical, or traumatic origin. Aponeurotic ptosis, the most common acquired form of the condition [ 24 ], is caused by stretching, dehiscence, or detachment of the levator aponeurosis from its insertion on the tarsus, and is typically associated with aging [ 5 , 16 , 24 ]. Myogenic ptosis is caused by primary or secondary myopathy of the levator muscle, due for example to chronic progressive external ophthalmoplegia (CPEO), oculopharyngeal muscular dystrophy (OPMD), or myotonic dystrophy [ 5 , 16 , 22 ]. Neurogenic ptosis is relatively rare and is typically caused by dysfunction or damage to the oculomotor nerve or to sympathetic nerves innervating the eyelids, or by central mechanisms [ 5 , 16 , 22 ]. Among patients with neurogenic ptosis, the most common underlying causes are oculomotor nerve (3 rd cranial nerve) palsy (35.7%), myasthenia gravis (28.6%), aberrant regeneration (14.3%), and Horner’s syndrome (7.1%) [ 24 ]. Common causes of mechanical ptosis include benign or malignant neoplasms of the eyelid, such as haemangioma, chalazion, neurofibroma, or dermoid cysts, which create excess weight that cannot be raised by the upper eyelid retractor muscles [ 5 , 22 ]. Finally, acquired ptosis can arise due to trauma to the eyelid retractor muscles, aponeurosis, or neural inputs to the eyelid. Thus, traumatic ptosis can be myogenic, aponeurotic, or neurogenic in nature [ 22 ].
Pseudoptosis does not involve pathology of the upper eyelid retractor muscles or aponeurosis, and can be due to mechanical, neurogenic, or anatomical causes. Mechanical causes include dermatochalasis (excessive upper eyelid skin that overhangs the lid margin), brow ptosis (drooping of the eyebrow), and floppy eyelid syndrome (easy eversion of the upper eyelid due to excessive lid laxity). Neurogenic causes include benign essential blepharospasm and hemifacial spasm (unilateral spasm of the upper and lower eyelids). Anatomical causes include microphthalmos (decreased size or volume of the globe) or superior sulcus deformity (deepening of the superior sulcus) [ 5 , 16 , 22 ]. Diagnostic differentiation of acquired ptosis is discussed in the section titled Acquired ptosis identification and differential diagnosis.
Acquired ptosis risk factors
Studies of adult populations consistently reveal age as a significant risk factor for the development of acquired ptosis, with reported prevalence exceeding 20% among patients aged 70 years and older (summarised in the section titled Acquired ptosis overview, prevalence, and impacts and in Table 1 ) [ 1 , 2 , 3 ]. In a 1995 study of 400 individuals ≥50 years old in the United Kingdom, 11.5% were determined to have ptosis, with the relative frequency increasing from 2.4% among individuals aged 50–59 years to 8.9% among individuals aged 60–69 years old and 20.8% among individuals aged ≥70 years [ 1 ]. A more recent study of >4700 Iranian patients 45 to 69 years old reported an incidence of 4.7%, with the lowest prevalence (3.1%) among patients aged 45–49 years and the highest prevalence (7.1%) among patients aged 60–64 years [ 2 ]. Another study, of 17,296 patients ≥40 years old in Korea, reported an overall prevalence of ptosis of 13.4%, with the lowest prevalence (5.4%) among patients 40–49 years old and the highest prevalence (32.8%) among patients ≥70 years old [ 3 ]. These two studies also reported higher rates of ptosis in patients with diabetes and hypertension [ 2 , 3 ]. Furthermore, the Korean study found an association between higher body mass index (BMI), as well as a history of cardiovascular disease, and the presence of ptosis [ 3 ]. Individuals with ptosis in this study were also found to be more likely to have hyperopia, strabismus, and cataract, in comparison to individuals without ptosis [ 3 ].
In an analysis of 251 patients referred for ptosis surgery to an ophthalmic surgery centre in Singapore, aponeurotic ptosis was the most common form of ptosis observed (60.2%). In this study, the median age among patients with aponeurotic ptosis was 62 years. The other most common forms observed in the study were traumatic (11.2%), congenital (10.4%), mechanical (8.8%), neurogenic (5.6%), and myogenic (4.0%) ptosis [ 24 ]. Similarly, an evaluation of patients presenting at an oculoplastic surgery practice in Australia revealed that involutional (aponeurotic) ptosis was the most common form among patients over 50 years of age, accounting for 17% of cases among patients aged 51–60 years, 34% of cases among patients aged 61–70 years, and 31% of cases among patients aged 71–80 years [ 25 ].
Contact lens wear, which involves repeated manipulation of the eyelid, and therefore the potential risk of microtrauma to the levator aponeurosis, has also been associated with the development of acquired ptosis, with studies linking both hard and soft contact lens wear to increased incidence (Table 2 ) [ 25 , 26 , 27 , 28 , 29 ]. A retrospective analysis of 15 patients with ptosis attributable to contact lens wear revealed that all were hard lens wearers and 13 of 15 had been wearing their lenses for >17 years. Furthermore, in 11 of the 15 patients, thinning or dehiscence of the levator aponeurosis was observed during surgery [ 25 ]. Along similar lines, an age-matched case-control study of female patients in Japan found that hard contact lens wear significantly increased the risk of ptosis versus non-wear (odds ratio 19.9 (6.32–62.9)) [ 28 ]. A retrospective analysis of 35 patients aged 18–50 years old presenting with ptosis in a hospital ophthalmology department found that 29 of the 35 patients had a history of either hard or soft contact wear (mean wear time 17.6 and 9 years, respectively) [ 26 ]. A broad analysis of environmental factors contributing to ptosis in 286 sets of adult twins (range: 18–82 years old) found a significant association between both hard and soft contact lens wear and ptosis, but no association with respect to other environmental factors evaluated, including BMI, smoking status, alcohol consumption, hours of sleep per night, or sun exposure [ 29 ]. Consistent with these individual studies, a systematic literature review found significant risk associated with both hard (odds ratio 17.38 (3.71–81.29)) and soft (odds ratio 8.12 (2.68–24.87)) contact lens wear [ 27 ].
Another known cause of ptosis is ocular surgery. A systematic literature review reported an 11.4% incidence of ptosis following ocular surgery, with the highest rate (13.4%) occurring among patients who underwent glaucoma surgery, followed by corneal (10.3%), strabismus (10.0%), cataract (9.4%), and mixed (6.5%) surgeries [ 30 ]. Postsurgical ptosis incidence also depends on surgical technique [ 28 ]. Reported rates of ptosis range from 1 to 44.4% and 0 to 12.9% among patients following extracapsular and phacoemulsification cataract surgery, respectively. Similarly, incidence after glaucoma surgery (7–19%) and vitreoretinal procedures (9.7–17%) appears to depend on the surgical technique used [ 31 ]. In glaucoma surgery, reported ptosis incidence is higher in trabeculectomy with mitomycin C (19% incidence) than when mitomycin is not used (12%) [ 31 ]. In vitreoretinal surgery, reported ptosis incidence with intravitreal steroid injection and intravitreal anti-VEGF injection with sub-Tenon’s steroid injection are reported to be 11% and 17%, respectively [ 31 ].
Ptosis following ocular surgery can be transient or persistent. Factors suspected of causing transient postsurgical ptosis include the occurrence of postsurgical oedema, haematoma, foreign body reaction, and use of neuromuscular blockade, while proposed mechanistic causes of more persistent postsurgical ptosis include the use of mitomycin C in glaucoma surgery, direct trauma to the tarsal plate, bridle suture use (with higher incidence occurring with a closed approach), and rigid eyelid speculum use, which can lead to levator aponeurosis dehiscence or detachment from the tarsal plate [ 31 , 32 , 33 , 34 , 35 ].
Similarly, transient ptosis has been reported as an adverse event following periocular neurotoxin injection [ 36 , 37 , 38 ]. A broad systematic literature review evaluated clinical safety data related to the use of botulinum toxin A for facial aesthetic treatment in >8700 total patients. Brow ptosis (3.1% incidence) was the most commonly reported adverse event in the upper face, followed by eye sensory disorders (3.0%), and eyelid ptosis (2.5%), with all events being transient and resolving spontaneously [ 36 ]. More recently, case series have described potential approaches to treating transient ptosis resulting from botulinum toxin injection, with, most notably, topical application of the adrenergic agent apraclonidine providing measurable upper eyelid elevation in some patients [ 37 , 38 ].
Ptosis can also be secondary to a range of underlying neurological or muscular conditions, including 3 rd cranial nerve palsy, CPEO, oculopharyngeal muscular dystrophy, Horner’s syndrome, and myasthenia gravis [ 5 , 16 ]. These conditions can range in severity and require different interventions than cases of primary ptosis due exclusively to upper eyelid retractor muscle or aponeurosis defects. These underlying conditions can also be emergent and potentially life-threatening, and therefore require rapid intervention. Ptosis identification and differential diagnosis are summarised in detail in Acquired ptosis identification and differential diagnosis, below.
Acquired ptosis identification and differential diagnosis
Accurately identifying ptosis, as well as its underlying aetiology and severity, is essential to successful management. Thorough clinical examination and differential diagnosis is also needed in order to rule out similar conditions or most importantly, diagnose any serious underlying cause requiring more immediate medical intervention (Table 3 ).
The initial diagnostic step is a review of patient history to understand timing of ptosis onset, as a sudden appearance may signal serious underlying pathology. If patient history suggests that ptosis may be secondary to a more serious condition, subsequent evaluation can be conducted based on the observable clinical signs. The serious neurological or muscular conditions most commonly encountered in clinical practice include Horner’s syndrome, 3 rd cranial (oculomotor) nerve palsy, myasthenia gravis, and CPEO. In a study of patients referred for ptosis surgery, 5.6% of cases had a neurogenic cause, and among these cases, the majority were due to serious underlying aetiologies (35.7% palsy of the 3 rd cranial nerve, 28.6% myasthenia gravis, 14.3% aberrant regeneration, 7.1% Horner’s syndrome). While myogenic causes (which broadly include conditions such as OPMD, CPEO, and myotonic dystrophy) were likewise uncommon in the study population (4.0% overall), 30% of the patients in this group had an underlying diagnosis of CPEO [ 24 ].
Horner’s syndrome, in its acquired form, is usually secondary to interruption of sympathetic innervation of the superior and inferior tarsal muscles due to trauma, certain tumours, or stroke. It is characterised not only by mild unilateral ptosis of the upper eyelid, but also the lower eyelid (i.e., slight elevation of the lower lid margin), ipsilateral pupillary miosis, facial anhidrosis, and a positive pupillary response (dilation) to topical phenylephrine (which can be used to differentiate between pre- and post-ganglionic Horner’s syndrome) or apraclonidine [ 5 , 16 , 39 , 40 ]. Ptosis caused by 3 rd cranial nerve palsy—which innervates, among other muscles, the levator palpebrae superioris—has a unilateral and variable presentation but is typically accompanied by diplopia and a “down and out” position of the affected eye due to partial or complete muscular paresis [ 5 , 16 , 40 ]. Like with Horner’s syndrome, 3 rd cranial nerve palsy can be secondary to an acute event such as ischaemia, aneurysm, or trauma, or to compression of the nerve by an expanding mass. Because the 3 rd cranial nerve delivers most of the parasympathetic fibres destined for the eye, dilation of the ipsilateral pupil can be observed in some cases. Pupillary involvement requires neuroimaging for the presence of an aneurysm or of a tumour that may be compressing the nerve. Lack of pupillary involvement often suggests a microvascular cause, such as diabetes mellitus [ 5 , 16 , 40 ].
Ptosis can also be an early symptom of myasthenia gravis, a condition caused by autoantibody blocking or destruction of nicotinic acetylcholine receptors, and may be accompanied by external ophthalmoplegia [ 5 , 16 , 40 ]. It can present either unilaterally or bilaterally (symmetric or asymmetric), tends to worsen with fatigue, and can be identified in-office by a positive response (upper eyelid elevation) to the rest test or ice test [ 16 , 40 , 41 , 42 ]. Furthermore, diagnosis can be confirmed via serologic testing for anti-acetylcholine receptor antibodies [ 16 ]. When myasthenia gravis is suspected, CT scanning is required, in order to identify potential thymic hyperplasia or thymoma [ 42 ]. Ptosis secondary to CPEO, a mitochondrial syndrome, can be accompanied by extraocular muscle weakness, particularly on upgaze, and reduced saccadic velocity, and requires evaluation for involvement of other systems [ 5 , 15 ].
Evaluation of the periocular skin and soft tissues is essential to identifying or excluding ptosis secondary to a mass weighing down the upper eyelid [ 5 , 16 , 22 ]. Patients should be examined for suspicious lesions, such as basal cell or squamous cell carcinoma of the skin, or unusual masses beneath the skin. A lacrimal gland mass can present as upper eyelid ptosis, and potential aetiologies for lacrimal gland masses include lymphoma, adenoid cystic carcinoma, or pleomorphic adenoma, all of which require workup prior to considering ptosis as the diagnosis. Examination should include palpation of the superolateral portion of the upper eyelid beneath the tail of the brow near the orbital rim, and if a mass is suspected, referral to a specialist is recommended.
Also essential to the clinical workup is the exclusion of “pseudoptosis” conditions, which involve no pathology of the upper eyelid retractor muscles or levator aponeurosis, but instead are due to pathologies of other structures that indirectly affect eyelid position. Pseudoptosis can arise, for example, due to a range of mechanical (dermatochalasis, brow ptosis, floppy eyelid syndrome), anatomical (globe dystopias, globe asymmetry, ocular misalignment), or neurogenic (hemifacial spasm, blepharospasm) causes, or contralateral eyelid retraction (thyroid eye disease) [ 5 , 16 , 40 ]. The pathology specific to the various forms of pseudoptosis means that treatment targeting the upper eyelid muscles or aponeurosis is unlikely to resolve the condition, so when conducting the upper eyelid exam, it is important to identify any causes of pseudoptosis. Dermatochalasis, the presence of redundant upper eyelid skin, is identified by lifting the excess eyelid skin and performing an eyelid examination. If eyelid elevation and muscle function are normal, then ptosis is excluded [ 16 ]. Evaluation of the globe can identify dystopias such as enophthalmos, hyperglobus, hypoglobus, or asymmetry caused by phthisis bulbi, microphthalmia, or other conditions affecting globe size and giving the appearance of unilateral ptosis [ 16 ]. To differentiate ptosis from contralateral eyelid retraction due to thyroid eye disease, the ptotic eyelid can be lifted and the contralateral eye observed for relaxation indicative of compensatory retraction secondary to ptosis [ 16 ]. One may also assess whether there is lid lag on downward gaze, another indication of thyroid ophthalmopathy.
After appropriate examination of the ocular and periocular structures, assessment of upper eyelid function can be performed with a few simple measurements. The distance from the central pupillary light reflex to the central margin of the upper eyelid (marginal reflex distance 1 (MRD-1)) helps define the presence and severity of ptosis. In the normal eye, MRD-1 is typically 4–5 mm, and a decrease in this measure signals the presence of ptosis [ 5 , 11 , 16 ]. Less relevant in the context of acquired ptosis is MRD-2 (the distance from the centre of the pupillary light reflex to the lower eyelid margin with the eye in primary gaze) and MRD-3 (the distance from the pupillary light reflex to the upper eyelid margin with the eye in extreme upgaze). The MRD-3 measure is used to determine the degree of levator resection required in patients with congenital ptosis and vertical strabismus [ 43 ].
Eyelid crease height, the distance from the upper eyelid crease to the eyelid margin, can likewise be informative. Normal eyelid crease height generally ranges from 7 to 8 mm in males and 9–10 mm in females, and an increase in this measure can indicate disinsertion of the levator aponeurosis [ 44 ]. Palpebral fissure height is a measure of the distance between the upper and lower eyelid margins with the eye in primary gaze, with a normal value in the range of 10–12 mm. A decrease in palpebral fissure height can be an indicator of disinsertion of the levator aponeurosis from the tarsal plate [ 16 , 44 ]. Levator function is more directly assessed using Berke’s method, in which frontalis muscle function is negated (by holding the brow) and the patient shifts from downgaze to upgaze. Levator function is classified based on the amount of upper eyelid excursion, from poor (0-4 mm lid elevation), to fair (5–11 mm), good (12–14 mm), and normal (>15 mm) [ 44 ]. Müller’s muscle function can be assessed using the phenylephrine test, in which a drop of the α-adrenergic agonist phenylephrine 2.5% is applied under the ptotic eyelid. A positive response to phenylephrine (eyelid elevation) is indicative of Müller’s muscle function and suggests that the patient is a candidate for Müller’s muscle-conjunctival resection [ 16 , 44 , 45 , 46 , 47 , 48 ].
Visual field testing is an important tool for measuring any functional deficits caused by ptosis [ 7 , 9 , 11 , 49 ]. The Goldmann Visual Field (GVF) Test is a manual kinetic perimetry test, in which the patient fixates on the centre of the testing field and indicates when they see moving illuminated targets of varying size and brightness in the peripheral field, and the visual field is mapped by the examiner [ 50 ]. The HVF Test is an automated static perimetry test using an HVF analyser, in which static illuminated targets briefly appear in the field and patients indicate when a target is seen. Most commonly, the HVF Test evaluates a 24° field (24-2 setting) using a 54-point grid [ 10 , 50 ]. The LPFT is a modified HVF Test, specifically designed to assess superior visual field deficits caused by ptosis, that demonstrates high sensitivity, specificity, and positive/negative predictive value [ 10 ]. It is an automated, observer-independent, static perimetry test that evaluates a 48° range in the superior visual field, using a 4-row, 35-point grid. The centre of fixation on the LPFT is shifted 15° inferiorly to maximise testing of the superior field, enable more natural eyelid positioning, and prevent compensatory behaviours, such as brow elevation [ 10 ].
Acquired ptosis treatment
The standard of care for ptosis management is surgical intervention. Elevation of the upper eyelid for functional or cosmetic purposes can be successfully achieved with a variety of techniques targeting the upper eyelid retractor muscles and aponeurosis [ 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 ], and the procedure (or combination of procedures) is selected based on underlying ptosis aetiology and severity (Table 4 ) [ 5 , 44 , 49 ]. Requisites for a functional indication include measurable decrease in eyelid elevation (typically defined as MRD-1 ≤ 2 mm) and accompanying superior visual field deficit, demonstrated via visual field testing [ 59 ]. Common procedures targeting Müller’s muscle include Müller’s muscle-conjunctival resection, in which Müller’s muscle and the overlying conjunctiva are excised using a posterior approach. This procedure is used for mild acquired ptosis or Horner’s syndrome with good levator function. Similarly, in the Fasanella-Servat procedure, the lower part of Müller’s muscle, overlying conjunctiva, and upper border of the tarsus are resected. This procedure is also typically reserved for mild acquired ptosis or Horner’s syndrome with good levator function, with the amount of muscle resected dependent on the degree of eyelid droop [ 5 , 6 , 44 ].
If there is dehiscence or disinsertion of the aponeurosis but levator muscle function remains good, levator muscle advancement (aponeurosis repair), using an anterior or posterior approach, can be performed. Levator resection is used in cases in which levator function is in the fair-to-good range (> 4 mm), with the amount of muscle resected dependent upon the degree of pre-surgical levator function [ 5 , 44 ]. If levator function is poor, the desired upper eyelid elevation can be provided via Whitnall’s ligament suspension, in which aponeurotic resection is followed by suturing of Whitnall’s ligament to the tarsal plate and suspension of the ligament to the periosteum of the superior orbital rim [ 44 , 60 ]. If levator function is poor and frontalis function is good, as is the case in many patients with congenital ptosis, a subcutaneous sling to connect the frontalis muscle to the upper eyelid can also be used. This procedure can also be used for acquired ptosis with a myogenic or neurogenic cause [ 5 , 44 ]. For patients with both ptosis and dermatochalasis, a combination of ptosis repair and upper lid blepharoplasty procedures may be appropriate [ 16 ].
Surgical intervention has been demonstrated to improve elevation of the upper eyelid and superior visual field deficits, and these clinical improvements can accordingly improve patients’ performance of activities of daily living and HRQoL outcomes. As noted in Acquired ptosis overview, prevalence, and impacts above, patients who undergo ptosis surgery report improved ability to perform common visual tasks and activities of daily living, leading to an improved functional index [ 12 , 13 , 49 ]. Despite these well-established benefits of surgery, however, it is not an ideal approach for some patients. In many cases, ptosis is not severe enough, with respect to appearance or functional deficit, in the view of the surgeon, patient, or payer to warrant surgical intervention. Furthermore, the potential benefits of surgical intervention must be weighed against risks of unwanted side effects or outcomes. The most common risks associated with ptosis surgery range from temporary adverse events (AEs) such as bleeding, bruising, and infection, to more persistent AEs such as scarring, eyelid crease abnormalities, over- or under-correction, and eyelid asymmetry. There are also secondary risks to over-correction, including lagophthalmos and exposure keratopathy [ 5 ]. Unilateral ptosis in particular can present unique challenges with respect to achieving desired symmetry. The levator muscles are yoke muscles bilaterally innervated by the same afferent input, which increases when one or both eyes is ptotic. In the case of unilateral ptosis, afferent input to the levator muscle of both eyes increases, resulting in elevation of the ptotic eyelid, but also the contralateral eyelid (pseudoretraction). Following unilateral ptosis surgery, the compensatory decrease in afferent input to the formerly ptotic eyelid is paralleled by a decrease in input to the contralateral eye, causing it to droop and result in secondary contralateral ptosis (Hering’s phenomenon), and the potential need for revision surgery [ 6 , 61 ]. A thorough examination for pseudoretraction is therefore essential in cases of unilateral ptosis that are candidates for surgery.
A retrospective analysis of 1519 patients who underwent ptosis surgery revealed that revision was required in 8.7% of cases, with a 6.8% revision rate in patients who underwent a posterior-approach procedure and a 9.5% revision rate in those who underwent an anterior-approach procedure [ 62 ]. Over- and under-correction were identified as the predominant reasons for revision, and the mean time to revision was 24.6 ± 25.2 weeks [ 62 ]. Among subjects who underwent unilateral ptosis surgery (355 total), 5.1% had a postoperative contralateral ptosis that prompted revision surgery [ 62 ].
Non-surgical approaches to managing ptosis—and clinical evidence supporting any of these approaches—have been comparatively limited (Table 4 ). The most conservative approaches include simple observation and (in the case of transient ptosis) waiting for self-resolution, as well as the use of mechanical interventions such as eye crutches and adhesives [ 5 ]. These mechanical interventions are, at best, temporary solutions that may present more inconvenience to patients than benefit. Reports have also described the use of scleral contact lenses for the treatment of complex ptosis [ 63 , 64 , 65 ]. While preferable to eye crutches or adhesives given the opportunity for better comfort and cosmesis, the principle of scleral contact lens use is similar, with the lens providing mechanical support to raise the ptotic upper eyelid. A retrospective analysis of scleral lens wear indications at Moorfields Eye Hospital in the United Kingdom revealed ptosis as the indication for 1.7% of eyes evaluated [ 64 ]. A case review of 10 patients with complex ptosis who were scleral contact lens wearers revealed objective clinical improvements in mean palpebral aperture and MRD-1 during lens wear, but when patients subjectively assessed cosmesis, the effect of the lenses was judged as “moderate” or “poor” for 78% of eyes assessed [ 65 ]. A subsequent study of three patients with complex ptosis similarly found objective increases in palpebral aperture and MRD-1, and scleral lens wear was reported by patients to be comfortable [ 63 ]. Still, the overall evidence for scleral lens wear is limited, as are its applications in clinical practice, at least in part because contact lens wear itself can be associated with the development of aponeurotic ptosis [ 25 , 26 , 27 , 28 , 29 ].
Topical sympathomimetic agents, including phenylephrine, apraclonidine, brimonidine, and naphazoline, have ophthalmic applications outside of ptosis, but have also been evaluated for their effects on the ptotic upper eyelid based on their potential to activate Müller’s muscle, which has been shown to express the α 1 /α 1D , α 2A , and α 2C adrenergic receptor subtypes [ 17 , 18 , 19 ]. A retrospective study of patients with dehiscence of the levator aponeurosis found that 78% of eyes instilled with a single drop of 10% phenylephrine showed a positive response (i.e., an increase in MRD-1), and that responsiveness did not depend on ptosis severity or levator function. There was an association between responsiveness and ptosis aetiology, however, with a 77% of eyes with ptosis caused by previous ocular surgery showing a ≥1.5 mm increase in MRD-1, versus 42% of eyes with aging as the only identifiable cause showing the same degree of increase [ 66 ]. While these effects on the upper eyelid are intriguing, clinically significant pupil dilation is also observed in the majority of patients following phenylephrine instillation [ 45 ], limiting its utility for ptosis treatment.
Apraclonidine demonstrates strong agonist activity at α 2 -adrenergic receptors and weaker agonist activity at α 1 -adrenergic receptors. Via its α 1 -adrenergic receptor-mediated effects, topical apraclonidine can temporarily reverse anisocoria due to Horner’s syndrome, thus supporting this diagnosis [ 16 ]. A case report also revealed elevation of the upper eyelid in three patients with Horner’s syndrome after instillation of 0.5% apraclonidine [ 67 ]. A larger evaluation of the effect of apraclonidine on upper eyelid elevation was conducted in 100 non-ptotic subjects, demonstrating small mean increases in MRD-1 at 30 and 45 min post-instillation that were hypothesised to be a result of stimulation of postsynaptic α 1 -adrenergic receptors [ 68 ].
Apraclonidine’s effect in ptotic patients has also been evaluated in a number of small-scale studies. A retrospective case series examining 7 patients with ptosis following cosmetic botulinum toxin injection revealed a mild effect of apraclonidine 0.5% on ptosis, but only when used within 4–6 weeks of ptosis self-resolution, suggesting that apraclonidine response might help predict resolution time [ 37 ]. A later case series evaluated the effect of administering two drops of apraclonidine 0.5% in a cohort of 6 patients with ptosis resulting from botulinum toxin injection and another with Horner’s syndrome, showing improvement in upper eyelid elevation 20–30 min following instillation [ 38 ]. A prospective study enroling 26 patients scheduled for ptosis surgery revealed variability in upper eyelid responsiveness to apraclonidine 0.5%, and immunohistochemical examination of resected Müller’s muscle tissue revealed higher expression of the α 1D -adrenergic receptor subtype in responsive eyelids, suggesting that the drug’s effect on the upper eyelid is mediated at least in part by agonism of α 1D receptors [ 18 ].
While studies of limited apraclonidine dosing in patients with ptosis have revealed no notable safety concerns, prospective studies of its chronic use for other ophthalmic applications (glaucoma, ocular hypertension) have reported ocular (including decreased visual acuity and allergic conjunctivitis) and non-ocular (such as dry mouth and contact dermatitis) side effects that have led to discontinuation of use [ 69 , 70 , 71 ]. In the context of treating ptosis, this side effect profile is likely undesirable to patients and practitioners.
Other adrenergic agents have also been evaluated for potential applications to ptosis. A study of 20 healthy adult volunteers found significant elevation of the upper eyelid for up to 2 h following instillation of naphazoline 0.05% (a preferential α 2 -adrenergic receptor agonist), but not brimonidine 0.2% or phenylephrine 0.12% [ 72 ]. Topical naphazoline was also reported to improve upper eyelid elevation in a cohort of 12 patients with myopathic ptosis, with limited ocular side effects, but tachyphylaxis was observed with frequent daily dosing over a period of weeks [ 73 ]. Naphazoline use has also been reported in ptotic patients with myasthenia gravis, with topical use providing observable opening of the eye in 70% of enroled patients [ 74 ]. A case study in a single patient with anterior laminectomy-induced Horner’s syndrome revealed a positive effect of twice-daily unilateral administration of brimonidine tartrate 0.1% for 3 months [ 75 ]. While suggestive of possible applications in treating some cases of ptosis, the data regarding the adrenergic agents phenylephrine, apraclonidine, brimonidine, and naphazoline are limited in scope and none of these agents are approved for the treatment of ptosis. An important consideration in the context of treating ptosis is that chronic use of some α-adrenergic agents for applications such as lowering of intraocular pressure in patients with glaucoma, can be associated with tachyphylaxis, and that this may depend on the agent’s α-adrenergic subtype selectivity [ 69 , 76 , 77 , 78 ].
More recently, the efficacy and safety of an oxymetazoline 0.1% ophthalmic solution approved for the treatment of acquired ptosis (Upneeq TM , RVL Pharmaceuticals, Inc., Bridgewater, NJ, USA) has been reported. Oxymetazoline is a direct-acting α 1 - and α 2 -adrenergic receptor agonist [ 79 , 80 ]. Like other α-adrenergic agents, oxymetazoline is thought to act by stimulating contraction of Müller’s muscle. Evidence from two phase 3 clinical trials revealed that once-daily use of oxymetazoline 0.1% for 42 days significantly improved the superior visual field and upper eyelid elevation in patients with acquired ptosis and accompanying superior visual field deficit [ 81 ]. Using the LPFT, these studies demonstrated mean 5.9 ± 6.4 and 7.1 ± 5.9 point improvements in the superior visual field on treatment days 1 (6 h post-instillation) and 14 (2 h post-instillation), respectively, both of which were statistically superior to the mean change observed with vehicle at the corresponding time points (day 1: 1.8 ± 4.1 points; day 14: 2.4 ± 5.5 points). Similarly, MRD-1 measurements showed 0.96 ± 0.89 mm and 1.16 ± 0.87 mm improvements with oxymetazoline 0.1% on treatment days 1 and 14, respectively, in comparison to 0.50 ± 0.81 mm and 0.50 ± 0.80 mm with vehicle at the same time points [ 81 ]. Importantly, these studies showed that oxymetazoline 0.1% was effective after administration of a single drop beginning on treatment day 1 (measured 6 h post-instillation) and was associated with relatively low AE rates, making it a particularly intriguing non-invasive treatment option for acquired ptosis [ 81 ]. No tachyphylaxis was reported over 42 days of once-daily use in these studies of oxymetazoline 0.1%, however longer-duration evaluation is required to more thoroughly explore the potential effects of chronic use.
Summary, conclusions, and future directions
The prevalence and wide-ranging clinical and functional implications of acquired ptosis make timely and accurate diagnosis and treatment important for eye care practitioners. Acquired ptosis is most often due to age-related changes in the upper eyelid retractor muscles [ 24 , 25 ], but underlying causes are varied, and many practices and interventions common in eye care today, such as contact lens wear and cataract and glaucoma procedures, can in fact contribute to the development of transient or more persistent forms of ptosis [ 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 ]. Along with other aetiologies discussed in this article, all warrant full examination and evaluation of treatment opportunities.
Surgery is an effective treatment option for ptosis, but non-surgical approaches have been extremely limited in both number and effectiveness. Because treatment via surgical intervention may be limited to a relatively small proportion of patients, finding ways to incorporate novel non-surgical therapeutic options into practice presents the potential to treat a wider range of patients. The evidence regarding a newly approved pharmacologic agent for the treatment of acquired ptosis [ 81 ] is therefore encouraging and suggests the opportunity to offer effective non-surgical treatment. For eye care practitioners—and indeed a range of health care professionals—the availability of an approved pharmacological option might help shift from a “detection and referral” approach to a “diagnosis and treatment” approach, with referral for surgery when appropriate. Furthermore, the expansion of therapeutic options may help to improve the patient focus of treatment, by allowing for the use of surgical and non-surgical approaches as appropriate based on underlying ptosis aetiology, severity, and patient preference.
While advances in ptosis treatment are encouraging, these remain only part of the clinical equation. To effectively treat ptosis, timely and accurate diagnosis is essential. In particular, comprehensive clinical examination and differential diagnosis are critical to understanding whether a patient’s ptosis is due to primary pathology of the upper eyelid retractor muscles—and can thus be effectively managed by surgical or pharmacological means targeting the upper eyelid—or whether the underlying cause is a more serious underlying neurological condition requiring different intervention. While in many cases ptosis might only be evaluated and treated when its onset is sudden or severity is high, examination of the upper eyelid for mild-to-moderate or progressive cases can be incorporated into the comprehensive eye exam with relative ease. Together with a focus on awareness and diagnosis, focused surgical or non-surgical treatment based on the clinical evidence offers the promise of improved ptosis treatment for more patients.
Forman WM, Leatherbarrow B, Sridharan GV, Tallis RC. A community survey of ptosis of the eyelid and pupil size of elderly people. Age Ageing. 1995;24:21–24.
Article PubMed Google Scholar
Hashemi H, Khabazkhoob M, Emamian MH, Yekta A, Jafari A, Nobovati P, et al. The prevalence of ptosis in an Iranian adult population. J Curr Ophthalmol. 2016;28:142–5.
Article PubMed PubMed Central Google Scholar
Kim MH, Cho J, Zhao D, Woo KI, Kim YD, Kim S, et al. Prevalence and associated factors of blepharoptosis in Korean adult population: the Korea National Health and Nutrition Examination Survey. Eye. 2017;31:940–6.
Article CAS PubMed PubMed Central Google Scholar
Tan MC, Young S, Amrith S, Sundar G. Epidemiology of oculoplastic conditions: the Singapore experience. Orbit. 2012;31:107–13.
Finsterer J. Ptosis: causes, presentation, and management. Aesthetic Plast Surg. 2003;27:193–204.
Zoumalan CI, Lisman RD. Evaluation and mangement of unilateral ptosis and avoiding contralateral ptosis. Aesthet Surg J. 2010;30:320–8.
McKean-Cowdin R, Varma R, Wu J, Hays RD, Azen SP, Los Angeles Latino Eye Study Group. Severity of visual field loss and health-related quality of life. Am J Ophthalmol. 2007;143:1013–23.
Richards HS, Jenkinson E, Rumsey N, White P, Garrott H, Herbert H, et al. The psychological well-being and appearance concerns of patients presenting with ptosis. Eye. 2014;28:296–302.
Article CAS PubMed Google Scholar
Alniemi ST, Pang NK, Woog JJ, Bradley EA. Comparison of automated and manual perimetry in patients with blepharoptosis. Ophthal Plast Reconstr Surg. 2013;29:361–3.
Ho SF, Morawski A, Sampath R, Burns J. Modified visual field test for ptosis surgery (Leicester Peripheral Field Test). Eye. 2011;25:365–9.
Meyer DR, Stern JH, Jarvis JM, Lininger LL. Evaluating the visual field effects of blepharoptosis using automated static perimetry. Ophthalmology. 1993;100:651–8.
Battu VK, Meyer DR, Wobig JL. Improvement in subjective visual function and quality of life outcome measures after blepharoptopsis surgery. Am J Ophthalmol. 1996;121:677–86.
Federici TJ, Meyer DR, Lininger LL. Correlation of the vision-related functional impairment associated with blepharoptosis and the impact of blepharoptosis surgery. Ophthalmology. 1999;106:1705–12.
Freddo TF, Chaum E. Anatomy of the eye and orbit: the clinical essentials. 1st edn. Philadelphia: Wolters Kluwer Health; 2018.
Hamedani AG, Gold DR. Eyelid dysfunction in neurodegenerative, neurogenetic, and neurometabolic disease. Front Neurol. 2017;8:329.
Latting MW, Huggins AB, Marx DP, Giacometti JN. Clinical evaluation of blepharoptosis: distinguishing age-related ptosis from masquerade conditions. Semin Plast Surg. 2017;31:5–16.
Esmaeli-Gutstein B, Hewlett BR, Pashby RC, Oestreicher J, Harvey JT. Distribution of adrenergic receptor subtypes in the retractor muscles of the upper eyelid. Ophthal Plast Reconstr Surg. 1999;15:92–99.
Park SJ, Jang SY, Baek JS, Chin S, Jang JW. Distribution of adrenergic receptor subtypes and responses to topical 0.5% apraclonidine in patients with blepharoptosis. Ophthalmic Plast Reconstr Surg. 2018;34:547–51.
Skibell BC, Harvey JH, Oestreicher JH, Howarth D, Gibbs A, Wegrynowski T, et al. Adrenergic receptors in the ptotic human eyelid: correlation with phenylephrine testing and surgical success in ptosis repair. Ophthalmic Plast Reconstr Surg. 2007;23:367–71.
Custer PL Blepharoptosis. In: Yanoff M, Duker JS, (eds). Ophthalmology. 3 edn. (Elsevier, St Louis, 2008) 1397–403.
Kostick DA & Bartley GB Upper eyelid malpositions: congenital ptosis. In: Albert DM, Jakobiec FA, Azar DT, Gragoudas ES, (eds). Principles and practice of ophthalmology. 1. 2 edn. (W.B. Saunders, Philadelphia, 2000) 3460–8.
Sudhakar P, Vu Q, Kosoko-Lasaki O, Palmer M. Upper eyelid ptosis revisited. Am J Clin Med. 2009;6:5–14.
Google Scholar
Mokhtarzadeh A, Harrison AR. Controversies and advances in the management of congenital ptosis. Expert Rev Ophthalmol. 2015;10:59–63.
Lim JM, Hou JH, Singa RM, Aakalu VK, Setabutr P. Relative incidence of blepharoptosis subtypes in an oculoplastics practice at a tertiary care center. Orbit. 2013;32:231–4.
Thean JHJ, McNab AA. Blepharoptosis in RGP and PMMA hard contact lens wearers. Clin Exp Optom. 2004;87:11–14.
Bleyen I, Hiemstra CA, Devogelaere T, van den Bosch WA, Wubbels RJ, Paridaens DA. Not only hard contact lens wear but also soft contact lens wear may be associated with blepharoptosis. Can J Ophthalmol. 2011;46:333–6.
Hwang K, Kim JH. The risk of blepharoptosis in contact lens wearers. J Craniofac Surg. 2015;26:e373–e374.
Kitazawa T. Hard contact lens wear and the risk of acquired blepharoptosis: a case-control study. Eplasty. 2013;13:219–24.
Satariano N, Brown MS, Zwiebel S, Guyuron B. Environmental factors that contribute to upper eyelid ptosis: a study of identical twins. Aesthet Surg J. 2015;35:235–41.
Wang Y, Lou L, Liu Z, Ye J. Incidence and risk of ptosis following ocular surgery: a systematic review and meta-analysis. Graefes Arch Clin Exp Ophthalmol. 2019;257:397–404.
Godfrey KJ, Korn BS, Kikkawa DO. Blepharoptosis following ocular surgery: identifying risk factors. Curr Opin Ophthalmol. 2016;27:31–37.
Koh V, Tatsios J, Chew PT, Amrith S. Comparison of incidence of ptosis after combined phacotrabeculectomy with mitomycin C and phacoemulsification. Indian J Ophthalmol. 2015;63:895–8.
Linberg JV, McDonald MB, Safir A, Googe JM. Ptosis followoing radial keratectomy: performed using a rigid eyelid speculum. Ophthalmology. 1986;93:1509–12.
Loeffler M, Solomon LD, Renaud M. Postcataract extraction ptosis: effect of the bridle suture. J Cataract Refract Surg. 1990;16:501–4.
Naruo-Tsuchisaka A, Maruyama K, Arimoto G, Goto H. Incidence of postoperative ptosis following trabeculectomy with mitomycin C. J Glaucoma. 2015;24:417–20.
Cavallini M, Cirillo P, Fundaro SP, Quartucci S, Sciuto C, Sito G, et al. Safety of botulinum toxin A in aesthetic treatments: a systemitc review of clinical studies. Dermatol Surg. 2014;40:525–36.
Steinsapir KD, Groth MJ, Boxrud CA. Persistence of upper blepharoptosis after cosmetic botulinum toxin type A. Dermatol Surg. 2015;41:833–40.
Wijemanne S, Vijayakumar D, Jankovic J. Apraclonidine in the treatment of ptosis. J Neurol Sci. 2017;376:129–32.
Danesh-Meyer HV, Savino P, Sergott R. The correlation of phenylephrine 1% with hydroxyamphetamine 1% in Horner’s syndrome. Br J Ophthalmol. 2004;88:592–3.
Reinhard E, Spampinato H. The OD’s guide to ptosis workup. Rev Optom. 2020;157:68–73.
Ellis FD, Hoyt CS, Ellis FJ, Jeffery AR, Sondhi N. Extraocular muscle responses to orbital cooling (ice test) for ocular myasthenia gravis diagnosis. J AAPOS. 2000;4:271–81.
Gilbert ME, Savino PJ. Ocular myasthenia gravis. Int Ophthalmol Clin. 2007;47:93–103.
Putterman AM. Margin reflex distance (MRD) 1, 2, and 3. Ophthalmic Plast Reconstr Surg. 2012;28:308–11.
Pauly M, Sruthi R. Ptosis: evaluation and management. Kerala J Ophthalmol. 2019;31:11–16.
Article Google Scholar
Barsegian A, Botwinick A, Reddy HS. The phenylephrine test revisited. Ophthalmic Plast Reconstr Surg. 2018;34:151–4.
Ben Simon GJ, Lee S, Schwarcz RM, McCann JD, Goldberg RA. Muller’s muscle-conjunctival resection for correction of upper eyelid ptosis: relationship between phenylephrine testing and the amount of tissue resected with final eyelid position. Arch Facial Plast Surg. 2007;9:413–7.
Glatt HJ, Fett DR, Putterman AM. Comparison of 2.5% and 10% phenylephrine in the elevation of upper eyelids with ptosis. Ophthalmic Surg. 1990;21:173–6.
CAS PubMed Google Scholar
Maheshwari R, Maheshwari S. Muller’s muscle resection for ptosis and relationship with levator and Muller’s muscle function. Orbit. 2011;30:150–3.
Cahill KV, Bradley EA, Meyer DR, Custer PL, Holck DE, Marcet MM, et al. Functional indications for upper eyelid ptosis and blepharoplasty surgery: a report by the American Academy of Ophthalmology. Ophthalmology. 2011;118:2510–7.
Wong SH, Plant GT. How to interpret visual fields. Pr Neurol (Fort Wash Pa). 2015;15:374–81.
Ben Simon GJ, Lee S, Schwarcz RM, McCann JD, Goldberg RA. External levator advancement vs Müller’s muscle-conjunctival resection for correction of upper eyelid involutional ptosis. Am J Ophthalmol. 2005;140:426–32.
Berlin AJ, Vestal KP. Levator aponeurosis surgery. A retrospective review. Ophthalmology. 1989;96:1033–6.
Cates CA, Tyers AG. Outcomes of anterior levator resection in congenital blepharoptosis. Eye (Lond). 2001;15:770–3.
Article CAS Google Scholar
Frueh BR, Musch DC, McDonald H. Efficacy and efficiency of a new involutional ptosis correction procedure compared to a traditional aponeurotic approach. Trans Am Ophthalmol Soc. 2004;102:199–206.
PubMed PubMed Central Google Scholar
Lucarelli MJ, Lemke BN. Small incision external levator repair: technique and early results. Am J Ophthalmol. 1999;127:637–44.
Older JJ. Levator aponeurosis surgery for the correction of acquired ptosis. Anal 113 Proced Ophthalmol. 1983;90:1056–9.
CAS Google Scholar
Pan E, Chen WL, Zhang SC, Chen Y, Yu JG. Mild to moderate blepharoptosis correction: Outcomes of levator aponeurosis posterior layer plication. Med (Baltim). 2020;99:e19038.
Putterman AM, Fett DR. Müller’s muscle in the treatment of upper eyelid ptosis: a ten-year study. Ophthalmic Surg. 1986;17:354–60.
Aetna, Inc. Eyelid surgery. Medical Policy Bulletin Number 0084. Updated 2019. http://www.aetna.com/cpb/medical/data/1_99/0084.html .
Anderson RL, Jordan DR, Dutton JJ. Whitnall’s sling for poor function ptosis. Arch Ophthalmol. 1990;108:1628–32.
Erb MH, Kersten RC, Yip CC, Hudak D, Kulwin DR, McCulley TJ. Effect of unilateral blepharoptosis repair on contralateral eyelid position. Ophthalmic Plast Reconstr Surg. 2004;20:418–22.
Chou E, Liu J, Seaworth C, Furst M, Amato MM, Blaydon SM, et al. Comparison of revision rates on anterior- and posterior-approach ptosis surgery: a retrospective review of 1519 cases. Ophthal Plast Reconstr Surg. 2018;34:246–53.
Katsoulos K, Rallatos GL, Mavrikakis I. Scleral contact lenses for the management of complicated ptosis. Orbit. 2018;37:201–7.
Pullum KW, Whiting MA, Buckley RJ. Scleral contact lenses: the expanding role. Cornea. 2005;24:269–77.
Shah-Desai SD, Aslam SA, Pullum K, Beaconsfield M, Rose GE. Scleral contact lens usage in patients with complex blepharoptosis. Ophthalmic Plast Reconstr Surg. 2011;27:95–98.
Lee GN, Lin LW, Mehta S, Freitag SK. Response to phenylephrine testing in upper eyelids with ptosis. Digit J Ophthalmol. 2015;21:1–12.
Garibaldi DC, Hindman HB, Grant MP, Iliff NT, Merbs SL. Effect of 0.5% apraclonidine on ptosis in Horner syndrome. Ophthal Plast Reconstr Surg. 2006;22:53–55.
Kirkpatrick CA, Shriver EM, Clark TE, Kardon RH. Upper eyelid response to topical 0.5% apraclonidine. Ophthal Plast Reconstr Surg. 2018;34:13–19.
Araujo SV, Bond JB, Wilson RP, Moster MR, Schmidt CM Jr., Spaeth GL. Long term effect of apraclonidine. Br J Ophthalmol. 1995;79:1098–101.
Robin AL, Ritch R, Shin D, Smythe B, Mundorf T, Lehmann RP. Topical apraclonidine hydrochloride in eyes with poorly controlled glaucoma. The Apraclonidine Maximum Tolerated Medical Therapy Study Group. Trans Am Ophthalmol Soc. 1995;93:421–38.
CAS PubMed PubMed Central Google Scholar
Stewart WC, Ritch R, Shin DH, Lehmann RP, Shrader CE, van Buskirk EM. The efficacy of apraclonidine as an adjunct to timolol therapy. Apraclonidine Adjunctive Therapy Study Group. Arch Ophthalmol. 1995;113:287–92.
Mendonça TB, Lummertz AP, Bocaccio FJ, Procianoy F. Effect of low-concentration, nonmydriatic selective alpha-adrenergic agonist eyedrops on upper eyelid position. Dermatol Surg. 2017;43:270–4.
Article PubMed CAS Google Scholar
Uncini A, De Nicola G, Di Muzio A, Rancitelli G, Colangelo L, Gambi D, et al. Topical naphazoline in treatment of myopathic ptosis. Acta Neurol Scand. 1993;87:322–4.
Nagane Y, Utsugisawa K, Suzuki S, Masuda M, Shimizu Y, Utsumi H, et al. Topical naphazoline in the treatment of myasthenic blepharoptosis. Muscle Nerve. 2011;44:41–44.
Rehmani A, Mehta I, Smith E. Treatment of ptosis using brimonidine tartrate for anterior laminectomy-induced Horner syndrome. J Neuroophthalmol. 2020;40:95–96.
Hosten LO, Snyder C. Over-the-counter ocular decongestants in the United States - mechanisms of action and clinical utility for management of ocular redness. Clin Optom. 2020;12:95–105.
McLaurin E, Cavet ME, Gomes PJ, Ciolino JB. Brimonidine Ophthalmic Solution 0.025% for Reduction of Ocular Redness: A Randomized Clinical Trial. Optom Vis Sci. 2018;95:264–71.
Schuman JS, Horwitz B, Choplin NT, David R, Albracht D, Chen K. A 1-year study of brimonidine twice daily in glaucoma and ocular hypertension. A controlled, randomized, multicenter clinical trial. Chronic Brimonidine Study Group. Arch Ophthalmol. 1997;115:847–52.
Haenisch B, Walstab J, Herberhold S, Bootz F, Tschaikin M, Ramseger R, et al. Alpha-adrenoceptor agonistic activity of oxymetazoline and xylometazoline. Fundam Clin Pharm. 2010;24:729–39.
Sugden D, Anwar N, Klein D. Rat pineal α1-adrenoceptor subtypes: studies using radioligand binding and reverse transcription-polymerase chain reaction analysis. Br J Pharm. 1996;118:1246–52.
Slonim CB, Foster S, Jaros M, Kannarr SR, Korenfeld MS, Smyth-Medina R, et al. Association of oxymetazoline hydrochloride, 0.1%, solution administration with visual field in acquired ptosis: a pooled analysis of 2 randomized clinical trials. JAMA Ophthalmol. 2020;138:1168–75.
Download references
Acknowledgements
Editorial and administrative support was provided by BioScience Communications (New York, NY, USA) through funding provided by RVL Pharmaceuticals, an affiliate of Osmotica Pharmaceuticals plc (Bridgewater, NJ, USA).
Support for manuscript preparation (see Acknowledgements) was provided by RVL Pharmaceuticals, Inc., an affiliate of Osmotica Pharmaceuticals plc.
Author information
Authors and affiliations.
North Bay Eye Associates, Petaluma, CA, USA
Jason Bacharach
Bascom Palmer Eye Institute, University of Miami Miller School of Medicine, Miami, FL, USA
Wendy W. Lee
Department of Ophthalmology and Visual Neurosciences, Department of Otolaryngology, University of Minnesota, Minneapolis, MN, USA
Andrew R. Harrison
Massachusetts College of Pharmacy and Health Sciences, Worcester, MA, USA
Thomas F. Freddo
You can also search for this author in PubMed Google Scholar
Contributions
JB, WWL, ARH, and TFF were responsible for the development of the manuscript’s concept and themes, literature review and assessment, and writing, editing, and approval of the manuscript.
Corresponding author
Correspondence to Jason Bacharach .
Ethics declarations
Competing interests.
JB reports speaker fees from Aerie Pharmaceuticals, Alcon, Allergan, Bausch & Lomb, Glaukos, New World Medical, and Sun Pharmaceutical Industries, Inc.; consultant fees from Aerie Pharmaceuticals, Alcon, Allergan, Bausch & Lomb, Injectsense, New World Medical, Optovue, and Osmotica Pharmaceuticals; personal fees from Sun Pharmaceutical Industries, Inc.; and research support from Aerie Pharmaceuticals, Allergan, Novartis, Glaukos, Optovue, and Ocular Therapeutix. WWL reports consultant fees from Allergan, Galderma, Horizon Pharmaceuticals, Mallinckrodt, Osmotica Pharmaceuticals, Revance, and Solta. ARH reports consultant fees from Osmotica Pharmaceuticals and Horizon Pharmaceuticals; and speaker fees from Horizon Pharmaceuticals. TFF reports consultant fees from Osmotica Pharmaceuticals.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Bacharach, J., Lee, W.W., Harrison, A.R. et al. A review of acquired blepharoptosis: prevalence, diagnosis, and current treatment options. Eye 35 , 2468–2481 (2021). https://doi.org/10.1038/s41433-021-01547-5
Download citation
Received : 11 February 2021
Revised : 15 March 2021
Accepted : 07 April 2021
Published : 29 April 2021
Issue Date : September 2021
DOI : https://doi.org/10.1038/s41433-021-01547-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies

- Create account
Dermatochalasis
All content on Eyewiki is protected by copyright law and the Terms of Service . This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
- 1.1 Disease
- 1.2 Etiology
- 1.3 Risk Factors
- 2.1 History
- 2.2 Physical examination
- 2.3 Differential diagnosis
- 3.1 Surgery
- 3.2 Surgical follow up
- 4 Additional Resources
- 5 References
Disease Entity
Dermatochalasis is a term used to describe the presence of loose and redundant eyelid skin. It is a common sign of periocular aging and is often seen in middle-aged and elderly people. Although more dramatically seen in the upper eyelids, dermatochalasis can also affect lower eyelids as well. It is commonly associated with orbital fat herniation, known as steatoblepharon, and drooping of the eyelids, known as blepharoptosis . Additionally, an indistinct, low or double eyelid crease may be noted.
Periocular aging from intrinsic and extrinsic factors is a common cause. Aging leads to weakening of the connective tissue and loss of skin elasticity. In addition, the skin is weighed down by the effect of gravity. The interplay of these factors leads to classic hooding of the upper lids. Weakening of the orbital septum and herniation of the orbital fat adds to the bulging appearance. Trauma, systemic disease like connective tissue disorders or thyroid eye disease , idiopathic inflammation of the eyelids known as blepharochalasis, or previous surgery can potentiate these changes.
Risk Factors
These normal anatomical changes occur in varying amounts with age. Genetic predisposition and familial inheritance are the strongest predisposing factors to dermatochalasis. Additionally, higher body mass index, male sex, lighter skin color, and current smoking may be additional risk factors. [1]
Prior trauma or facial surgery, local nerve palsies, and more rare connective tissue disorders or tumors may exaggerate this condition.
ICD9 code 374.87
ICD10 codes
Patients with dermatochalasis of the upper eyelids may report decreased peripheral vision from the interference of the drooping tissues classically known as lateral hooding. Others may complain about a heavy or tired feeling around the eyes, a dull brow ache, or interference in the central vision due to droopy lids or lashes obscuring vision. Occasionally they may describe a shadow in the upper or side vision, or skin dermatitis due to moisture within the redundant skin folds. Dermatochalasis also is a cosmetic concern, as it gives a tired and dull look to the face. Lower lid dermatochalasis is mainly a cosmetic issue but in few patients may lead to dermatitis secondary to sweat collection in the acquired folds or difficulty wearing glasses.
Physical examination

A thorough eye examination by an ophthalmologist is necessary to rule out diseases of the eye itself that may limit ameliorative options for patients bothered by dermatochalasis. Furthermore, a detailed examination to look for anatomical changes or pathology helps to formulate the appropriate management plan.
Special attention to brow position and brow contour is important to differentiate between true and pseudo dermatochalasis. Measurement of redundant eyelid skin, levator excursion, prolapsed orbital fat, presence or absence of blepharoptosis is needed to quantify the type and degree of dermatochalasis. Additionally, evaluation of the presence of eyelid retraction, amount of eyelid laxity, and changes in the surrounding bony framework and periocular tissues is necessary .
Functional disability by dermatochalasis is documented by external photography and visual field testing with and without eyelid taping or elevation.
Differential diagnosis
Blepharochalasis, Lacrimal gland herniation, Facial Nerve Palsy , Mechanical Blepharoptosis secondary to mass effect, Brow ptosis , and Floppy Eyelid Syndrome
Upper eyelid blepharoplasty to correct dermatochalasis is one of the most frequently performed procedures by the ophthalmic plastic surgeon, whether for cosmetic or functional purposes. The procedure is performed by first outlining the tissue to be excised, beginning at the upper eyelid crease. Care should be taken to ensure enough skin remains for adequate eyelid closure. Oftentimes, portions of the preseptal orbicularis will be removed with the skin. If there is significant steatoblepharon, or fat herniation, the orbital septum may be opened and preaponeurotic fat trimmed or debulked, creating a more even upper eyelid contour. Fat removal should be done conservatively, to avoid a resulting hollowed appearance. Incisions are generally well hidden within the upper eyelid crease, and sutures are used to approximate skin edges.
Lower eyelid blepharoplasty may be performed for fat herniation and excess tissue of the lower eyelids. This is generally considered a cosmetic procedure and reduces the appearance of "bags" under the eyes. Midface of face lifting can augment the result of lower lid blepharoplasty surgery.
Additional wrinkle reduction may be achieved by laser resurfacing or chemical peels. Brow lift or brow pexy to correct the associated brow ptosis are often performed along with blepharoplasty.
Risks of surgery include bleeding, bruising, scarring, asymmetry, need for additional procedures and retrobulbar hemorrhage
Surgical follow up
Blepharoplasty is generally well-tolerated with oral analgesia and cold compresses in the early post-operative period. Some ophthalmic plastic surgeons add peri-operative intravenous and post-operative oral and/or topical antibiotics to minimize the already small risk of infection. Bruising can be expected for 1-2 weeks after surgery, and swelling is most noticeable for the first several weeks. Depending on the suture used for skin closure, sutures are removed at the discretion of the surgeon usually within 1-2 weeks after surgery. External photographs are typically taken to document postoperative changes and healing process. Complete healing of the scar and tissue swelling can take several months or more.
Additional Resources
- ASOPRS Information for Patients on Blepharoplasty
- ↑ Jacobs, L.C., et al., Intrinsic and extrinsic risk factors for sagging eyelids. JAMA Dermatol, 2014. 150 (8): p. 836-43.
- Techniques in Ophthalmic Plastic Surgery with DVD: A Personal Tutorial. Jeffrey A. Nerad MD. Saunders 2010.
- Orbit, Eyelids, and Lacrimal System. BCSC Section 7. American Academy of Ophthalmology. 2008.
- http://emedicine.medscape.com/article/1212294
- Oculoplastics/Orbit
Congenital Ptosis
- Living reference work entry
- First Online: 20 August 2020
- Cite this living reference work entry

- John D. Ng 5
125 Accesses
Congenital ptosis is most commonly caused by maldevelopment of the levator palpebrae superioris muscle complex. It is less frequently associated with neurologic, myopathic, and other congenital syndromes. Congenital ptosis may cause amblyopia from visual deprivation or induced astigmatism, especially if it is unilateral or asymmetric. Therefore, once congenital ptosis is identified after birth, early evaluation and management is essential to maximize potential visual development. A complete history and examination are necessary to determine the correct etiology of the ptosis, and in cases of neurogenic ptosis or in the setting of other associated syndromic findings, neuroimaging and genetic testing may be needed to provide optimal medical and surgical management.
This is a preview of subscription content, log in via an institution to check access.

Access this chapter
Institutional subscriptions
Gregory J, Griepentrog MD, Nancy Diehl BS, Brian G, Mohney MD. Incidence and demographics of childhood ptosis. Ophthalmology. 2011;118(6):1180–3.
Google Scholar
Marenco M, Macchi I, Macchi I, Galassi E, Massaro-Giordano M, Lambiase A. Clinical presentation and management of congenital ptosis. Clin Ophthalmol. 2017;11:453–63. https://doi.org/10.2147/OPTH.S111118 . eCollection 2017.
Article CAS PubMed PubMed Central Google Scholar
Heher KL, Katowitz JA. Pediatric ptosis. Chapter 15. In: Katowitz JA, editor. Pediatric oculoplastic surgery. New York: Springer; 2002. p. 253–88.
Jubbal KT, Kania K, Braun TL, Katowitz WR, Marx DP. Pediatric blepharoptosis. Semin Plast Surg. 2017;31(1):58–64. https://doi.org/10.1055/s-0037-1598631 .
Article PubMed PubMed Central Google Scholar
Lim JM, Hou JH, Singa RM, Aakalu VK, Setabutr P. Relative incidence of blepharoptosis subtypes in an oculoplastics practice at a tertiary care center. Orbit. 2013;32(4):231–4. https://doi.org/10.3109/01676830.2013.788673 . Epub 2013 May 10.
Surve A, Sharma MC, Pushker N, Bajaj MS, Meel R, Kashyap S. A study of changes in levator muscle in congenital ptosis. Int Ophthalmol. 2019;39(6):1231–8. https://doi.org/10.1007/s10792-018-0931-1 . Epub 2018 Apr 28.
Article PubMed Google Scholar
Alshehri MD, Al-Fakey YH, Alkhalidi HM, Mubark MA, Alsuhaibani AH. Microscopic and ultrastructural changes of Müller’s muscle in patients with simple congenital ptosis. Ophthalmic Plast Reconstr Surg. 2014;30(4):337–41. https://doi.org/10.1097/IOP.0000000000000104 .
Iljin A, Zielinska A, Karasek M, Zielinski A, Omulecka A. Structural abnormalities in the levator palpebrae superioris muscle in patients with congenital blepharoptosis. Ophthalmic Surg Lasers Imaging. 2007;38(4):283–9.
PubMed Google Scholar
Wabbels B, Schroeder JA, Voll B, Siegmund H, Lorenz B. Electron microscopic findings in levator muscle biopsies of patients with isolated congenital or acquired ptosis. Graefes Arch Clin Exp Ophthalmol. 2007;245(10):1533–41. Epub 2007 May 24.
Baldwin HC, Manners RM. Congenital blepharoptosis: a literature review of the histology of levator palpebrae superioris muscle. Ophthalmic Plast Reconstr Surg. 2002;18(4):301–7.
Edmunds B, Manners RM, Weller RO, Steart P, Collin JR. Levator palpebrae superioris fibre size in normals and patients with congenital ptosis. Eye (Lond). 1998;12(Pt 1):47–50.
Bagheri A, Tavakoli M, Najmi H, Erfanian Salim R, Yazdani S. Comparison between eyelid indices of ptotic eye and normal fellow eye in patients with unilateral congenital ptosis. J Plast Reconstr Aesthet Surg. 2016;69(1):e5–9. https://doi.org/10.1016/j.bjps.2015.10.004 . Epub 2015 Oct 22.
Nagpal RC, Raj A, Maitreya A. Congenital double elevator palsy with sensory exotropia: a unique surgical management. J Ophthalmic Vis Res. 2017;12(2):222–4. https://doi.org/10.4103/2008-322X.205380 .
Ng JK, Stout AU, Aaby AA, Ng JD. Blepharophimosis syndrome with absent tear production. Ophthalmic Plast Reconstr Surg. 2015;31(3):e62. https://doi.org/10.1097/IOP.0000000000000073 .
Athappilly GK, Braverman RS. Congenital alacrima in a patient with blepharophimosis syndrome. Ophthalmic Genet. 2009;30(1):37–9. https://doi.org/10.1080/13816810802452176 .
Duarte AF, Akaishi PM, de Molfetta GA, Chodraui-Filho S, Cintra M, Toscano A, Silva WA Jr, Cruz AA. Lacrimal gland involvement in blepharophimosis-ptosis-epicanthus inversus syndrome. Ophthalmology. 2017;124(3):399–406. https://doi.org/10.1016/j.ophtha.2016.10.028 . Epub 2016 Nov 30.
Bunyan DJ, Thomas NS. Screening of a large cohort of blepharophimosis, ptosis, and epicanthus inversus syndrome patients reveals a very strong paternal inheritance bias and a wide spectrum of novel FOXL2 mutations. Eur J Med Genet. 2019. pii: S1769-7212(19)30176-4. https://doi.org/10.1016/j.ejmg.2019.05.007 . Epub ahead of print.
Lin B, Zeng B, Zhao J, Xu T, Wang Y, Hu B, Li F, Zhao Q, Liu R, Liu J, Chen JM, Huang D, Wang Y. Seven novel and three known mutations in FOXL2 in 10 Chinese families with blepharophimosis syndrome. Curr Mol Med. 2018;18(3):152–9. https://doi.org/10.2174/1566524018666180907162619 .
Article CAS PubMed Google Scholar
Bouman A, van Haelst M, van Spaendonk R. Blepharophimosis-ptosis-epicanthus inversus syndrome caused by a 54-kb microdeletion in a FOXL2 cis-regulatory element. Clin Dysmorphol. 2018;27(2):58–62. https://doi.org/10.1097/MCD.0000000000000216 .
Elzaiat M, Todeschini AL, Caburet S, Veitia RA. The genetic make-up of ovarian development and function: the focus on the transcription factor FOXL2. Clin Genet. 2017;91(2):173–82. https://doi.org/10.1111/cge.12862 . Epub 2016 Sep 29.
Nuovo S, Passeri M, Di Benedetto E, Calanchini M, Meldolesi I, Di Giacomo MC, Petruzzi D, Piemontese MR, Zelante L, Sangiuolo F, Novelli G, Fabbri A, Brancati F. Characterization of endocrine features and genotype-phenotypes correlations in blepharophimosis-ptosis-epicanthus inversus syndrome type 1. J Endocrinol Investig. 2016;39(2):227–33. https://doi.org/10.1007/s40618-015-0334-3 . Epub 2015 June 23.
Article CAS Google Scholar
Demer JL, Clark RA, Tischfield MA, Engle EC. Evidence of an asymmetrical endophenotype in congenital fibrosis of extraocular muscles type 3 resulting from TUBB3 mutations. Invest Ophthalmol Vis Sci. 2010;51(9):4600–11. https://doi.org/10.1167/iovs.10-5438 . Epub 2010 Apr 14.
Heidary G, Engle EC, Hunter DG. Congenital fibrosis of the extraocular muscles. Semin Ophthalmol. 2008;23(1):3–8. https://doi.org/10.1080/08820530701745181 .
Aubourg P, Krahn M, Bernard R, Nguyen K, Forzano O, Boccaccio I, Delague V, De Sandre-Giovannoli A, Pouget J, Depétris D, Mattei MG, Philip N, Lévy N. Assignment of a new congenital fibrosis of extraocular muscles type 3 (CFEOM3) locus, FEOM4, based on a balanced translocation t(2;13) (q37.3;q12.11) and identification of candidate genes. J Med Genet. 2005;42(3):253–9.
CAS PubMed PubMed Central Google Scholar
Demer JL, Clark RA, Engle EC. Magnetic resonance imaging evidence for widespread orbital dysinnervation in congenital fibrosis of extraocular muscles due to mutations in KIF21A. Invest Ophthalmol Vis Sci. 2005;46(2):530–9.
Yamada K, Chan WM, Andrews C, Bosley TM, Sener EC, Zwaan JT, Mullaney PB, Oztürk BT, Akarsu AN, Sabol LJ, Demer JL, Sullivan TJ, Gottlob I, Roggenkäemper P, Mackey DA, De Uzcategui CE, Uzcategui N, Ben-Zeev B, Traboulsi EI, Magli A, de Berardinis T, Gagliardi V, Awasthi-Patney S, Vogel MC, Rizzo JF 3rd, Engle EC. Identification of KIF21A mutations as a rare cause of congenital fibrosis of the extraocular muscles type 3 (CFEOM3). Invest Ophthalmol Vis Sci. 2004;45(7):2218–23.
Engle EC. The molecular basis of the congenital fibrosis syndromes. Strabismus. 2002;10(2):125–8.
Schoser BG, Pongratz D. Extraocular mitochondrial myopathies and their differential diagnoses. Strabismus. 2006;14(2):107–13.
Allen RC. Genetic diseases affecting the eyelids: what should a clinician know? Curr Opin Ophthalmol. 2013;24(5):463–77. https://doi.org/10.1097/ICU.0b013e3283638219 .
Cahill JA, Ross J. Eye on children: acute work-up for pediatric Horner’s syndrome. Case presentation and review of the literature. J Emerg Med. 2015;48(1):58–62. https://doi.org/10.1016/j.jemermed.2014.07.041 . Epub 2014 Oct 1.
Hageman G, Ippel PF, te Nijenhuis FC. Autosomal dominant congenital Horner’s syndrome in a Dutch family. J Neurol Neurosurg Psychiatry. 1992;55(1):28–30.
Zafeiriou DI, Economou M, Koliouskas D, Triantafyllou P, Kardaras P, Gombakis N. Congenital Horner’s syndrome associated with cervical neuroblastoma. Eur J Paediatr Neurol. 2006;10(2):90–2. Epub 2006 Apr 3.
Tsaloumas MD, Willshaw HE. Congenital oculomotor palsy: associated neurological and ophthalmological findings. Eye (Lond). 1997;11(Pt 4):500–3.
Ng YS, Lyons CJ. Oculomotor nerve palsy in childhood. Can J Ophthalmol. 2005;40(5):645–53.
Hamed LM. Associated neurologic and ophthalmologic findings in congenital oculomotor nerve palsy. Ophthalmology. 1991;98(5):708–14.
CAS PubMed Google Scholar
Balkan R, Hoyt CS. Associated neurologic abnormalities in congenital third nerve palsies. Am J Ophthalmol. 1984;97(3):315–9.
Demirci H, Frueh BR, Nelson CC. Marcus Gunn jaw-winking synkinesis: clinical features and management. Ophthalmology. 2010;117(7):1447–52. https://doi.org/10.1016/j.ophtha.2009.11.014 . Epub 2010 Feb 25.
Sobel RK, Allen RC. Incidence of bilateral Marcus Gunn jaw-wink. Ophthalmic Plast Reconstr Surg. 2014;30(3):e54–5. https://doi.org/10.1097/IOP.0b013e31829bb405 .
Mansukhani SA, Bothun ED, Diehl NN, Mohney BG. Incidence and ocular features of pediatric myasthenias. Am J Ophthalmol. 2019;200:242–9. https://doi.org/10.1016/j.ajo.2019.01.004 . Epub 2019 Jan 14.
Peragallo JH. Pediatric Myasthenia gravis. Semin Pediatr Neurol. 2017;24(2):116–21. https://doi.org/10.1016/j.spen.2017.04.003 . Epub 2017 Apr 7.
Pavone P, Polizzi A, Longo MR, Romano K, Vecchio M, Praticò AD, Falsaperla R. Congenital myasthenic syndromes: clinical and molecular report on 7 sicilian patients. J Pediatr Neurosci. 2013;8(1):19–21. https://doi.org/10.4103/1817-1745.111416 .
Rasiah S, Hardy TG, Elder JE, Ng CY, McNab A. Etiology of pediatric acquired blepharoptosis. J AAPOS. 2017;21(6):485–7. https://doi.org/10.1016/j.jaapos.2017.08.005 . Epub 2017 Nov 3.
Leone F, Benanti E, Marchesi A, Marcelli S, Gazzola R, Vaienti L. Surgical excision of infantile haemangiomas: a technical refinement to prevent bleeding complications. Pediatr Med Chir. 2014;36(3):7. https://doi.org/10.4081/pmc.2014.7 .
Awadein A, Fakhry MA. Evaluation of intralesional propranolol for periocular capillary hemangioma. Clin Ophthalmol. 2011;5:1135–40. https://doi.org/10.2147/OPTH.S22909 . Epub 2011 Aug 15.
Arneja JS, Mulliken JB. Resection of amblyogenic periocular hemangiomas: indications and outcomes. Plast Reconstr Surg. 2010;125(1):274–81. https://doi.org/10.1097/PRS.0b013e3181c49708 .
Slaughter K, Sullivan T, Boulton J, O’Reagan P, Gole G. Early surgical intervention as definitive treatment for ocular adnexal capillary haemangioma. Clin Exp Ophthalmol. 2003;31(5):418–23.
Walker RS, Custer PL, Nerad JA. Surgical excision of periorbital capillary hemangiomas. Ophthalmology. 1994;101(8):1333–40.
Paik JS, Kim SA, Park SH, Yang SW. Refractive error characteristics in patients with congenital blepharoptosis before and after ptosis repair surgery. BMC Ophthalmol. 2016;16(1):177.
PubMed PubMed Central Google Scholar
Anderson RL, Baumgartner SA. Amblyopia in ptosis. Arch Ophthalmol. 1980;98(6):1068–9.
Dogan AS, Acar M, Kosker M, Arslan N, Gurdal C. Alterations in corneal epithelial thickness in patients with congenital myogenic eyelid ptosis. Int Ophthalmol. 2018;38(1):53–7. https://doi.org/10.1007/s10792-016-0419-9 . Epub 2016 Dec 26.
Langford JD, Linberg JV, Blaylock WK, Chao GM. Axial myopia in congenital ptosis: an animal model. Ophthalmic Plast Reconstr Surg. 1998;14(4):261–5.
Boricean ID, Bărar A. Understanding ocular torticollis in children. Oftalmologia. 2011;55(1):10–26.
Pratt SG, Beyer CK, Johnson CC. The Marcus Gunn phenomenon. A review of 71 cases. Ophthalmology. 1984;91(1):27–30.
Wong JF, Thériault JF, Bouzouaya C, Codère F. Marcus Gunn jaw-winking phenomenon: a new supplemental test in the preoperative evaluation. Ophthalmic Plast Reconstr Surg. 2001;17(6):412–8.
Luk HM, Lo IF, Lai CW, Ma LC, Tong TM, Chan DH, Lam ST. Congenital fibrosis of extraocular muscle type 1A due to KIF21A mutation: first case report from Hong Kong. Hong Kong Med J. 2013;19(2):182–5.
Engle EC, Castro AE, Macy ME, Knoll JH, Beggs AH. A gene for isolated congenital ptosis maps to a 3-cM region within 1p32-p34.1. Am J Hum Genet. 1997;60(5):1150–7.
McMullan TF, Collins AR, Tyers AG, Robinson DO. A novel X-linked dominant condition: X-linked congenital isolated ptosis. Am J Hum Genet. 2000;66(4):1455–60. Epub 2000 Mar 14.
McMullan TW, Crolla JA, Gregory SG, Carter NP, Cooper RA, Howell GR, Robinson DO. A candidate gene for congenital bilateral isolated ptosis identified by molecular analysis of a de novo balanced translocation. Hum Genet. 2002;110(3):244–50. Epub 2002 Feb 01.
Be YS, Tsai PJ, Lin MC, Chu MY. Factors related to amblyopia in congenital ptosis after frontalis sling surgery. BMC Ophthalmol. 2018;18(1):302. https://doi.org/10.1186/s12886-018-0962-4 .
Article Google Scholar
Feldman I, Brusasco L, Malhotra R. Improving outcomes of posterior approach levatorpexy for congenital ptosis with reduced levator function. Ophthalmic Plast Reconstr Surg. 2018;34(5):460–2. https://doi.org/10.1097/IOP.0000000000001056 .
Gazit I, Gildener-Leapman J, Or L, Burkat CN, Pras E, Hartstein ME. Müller’s muscle-conjunctival resection combined with tarsectomy for treatment of congenital ptosis. Ophthalmic Plast Reconstr Surg. 2019. https://doi.org/10.1097/IOP.0000000000001410 . [Epub ahead of print].
Medel R, Molina S, Vasquez LM, Visa J, Wert A, Wolley-Dod C. Frontalis muscle flap versus maximal anterior levator resection as first option for patients with severe congenital ptosis. Ophthalmic Plast Reconstr Surg. 2018;34(6):565–9. https://doi.org/10.1097/IOP.0000000000001105 .
Gazzola R, Piozzi E, Vaienti L, Wilhelm Baruffaldi Preis F. Therapeutic algorithm for congenital ptosis repair with levator resection and frontalis suspension: results and literature review. Semin Ophthalmol. 2018;33(4):454–60. https://doi.org/10.1080/08820538.2017.1297840 . Epub 2017 Mar 15.
Lee JH, Aryasit O, Kim YD, Woo KI, Lee L, Johnson ON 3rd. Maximal levator resection in unilateral congenital ptosis with poor levator function. Br J Ophthalmol. 2017;101(6):740–6. https://doi.org/10.1136/bjophthalmol-2016-309163 . Epub 2016 Sep 6.
Daoudi C, Chahdi KO, Lezrek O, Karim A, Daoudi R. Whitnall’s ligament suspension technique in ptosis surgery. J Fr Ophtalmol. 2017;40(9):763–9.
Molinari A, Weaver DT, Goldblum TA, Silbert D, Lopez SP, Matta N. Pediatric frontalis suspension with braided polyester: a comparison of two techniques. J Pediatr Ophthalmol Strabismus. 2018;55(4):229–33. https://doi.org/10.3928/01913913-20180213-02 . Epub 2018 May 1.
Mehta A, Garg P, Naik M, Kumari A. Congenital ptosis repair with a frontalis silicon sling: comparison between Fox’s single pentagon technique and a modified Crawford double triangle technique. J AAPOS. 2017;21(5):365–9. https://doi.org/10.1016/j.jaapos.2017.05.029 . Epub 2017 July 14.
Zaky AG, Mandour SS, Zaky MA, Ebrahem AM. Two different techniques for frontalis suspension using Gore-Tex to treat severe congenital ptosis. Graefes Arch Clin Exp Ophthalmol. 2017;255(4):831–5. https://doi.org/10.1007/s00417-017-3611-3 . Epub 2017 Feb 21.
Chung HW, Seah LL. Cosmetic and functional outcomes of frontalis suspension surgery using autologous fascia lata or silicone rods in pediatric congenital ptosis. Clin Ophthalmol. 2016;10:1779–83. eCollection 2016.
Bansal RK, Sharma S. Results and complications of silicone frontalis sling surgery for ptosis. J Pediatr Ophthalmol Strabismus. 2015;52(2):93–7.
Sokol JA, Thornton IL, Lee HB, Nunery WR. Modified frontalis suspension technique with review of large series. Ophthalmic Plast Reconstr Surg. 2011;27(3):211–5. https://doi.org/10.1097/IOP.0b013e3181ef72cd .
Morris CL, Buckley EG, Enyedi LB, Stinnett S, Freedman SE. Safety and efficacy of silicone rod frontalis suspension surgery for childhood ptosis repair. J Pediatr Ophthalmol Strabismus. 2008;45(5):280–8; quiz 289–90.
Kersten RC, Bernardini FP, Khouri L, Moin M, Roumeliotis AA, Kulwin DR. Unilateral frontalis sling for the surgical correction of unilateral poor-function ptosis. Ophthalmic Plast Reconstr Surg. 2005;21(6):412–6; discussion 416–7.
Li J, Dong C, Liu X, He W. Treatment of children with congenital severe blepharoptosis by frontalis aponeurosis flap advancement under general anesthesia in a single incision. J Craniofac Surg. 2017;28(6):1495–7. https://doi.org/10.1097/SCS.0000000000003948 .
Liu H, Shao Y, Zhao Z, Zhang D. One-stage correction of blepharophimosis-ptosis-epicanthus inversus syndrome using a frontalis muscle transfer technique. J Plast Surg Hand Surg. 2014;48(1):74–9. https://doi.org/10.3109/2000656X.2013.819004 . Epub 2013 Aug 23.
Tsai CC, Lin TM, Lai CS, Lin SD. Use of the orbicularis oculi muscle flap for severe Marcus Gunn ptosis. Ann Plast Surg. 2002;48(4):431–4.
Bowyer JD, Sullivan TJ. Management of Marcus Gunn jaw winking synkinesis. Ophthalmic Plast Reconstr Surg. 2004;20(2):92–8.
Khwarg SI, Tarbet KJ, Dortzbach RK, Lucarelli MJ. Management of moderate-to-severe Marcus-Gunn jaw-winking ptosis. Ophthalmology. 1999;106(6):1191–6.
Bartkowski SB, Zapala J, Wyszyńska-Pawelec G, Krzystkowa KM. Marcus Gunn Jaw-Winking Phenomenon: management and results of treatment in 19 patients. J Craniomaxillofac Surg. 1999;27(1):25–9.
Savino G, Mandarà E, Calandriello L, Dickmann A, Petroni S. A modified one-stage early correction of blepharophimosis syndrome using tutopatch slings. Orbit. 2015;34(4):186–91. https://doi.org/10.3109/01676830.2015.1015146 . Epub 2015 June 4.
Parvizi S, Ong J, Abou Rayyah Y, Dunaway D. A novel medial canthal reconstruction technique in children with blepharophimosis syndrome. Ophthalmic Plast Reconstr Surg. 2019. https://doi.org/10.1097/IOP.0000000000001390 . [Epub ahead of print].
Yamaguchi K, Imai K, Fujimoto T, Takahashi M, Maruyama Y. Cosmetic comparison between the modified Uchida method and the Mustarde method for blepharophimosis-ptosis-epicanthus inversus syndrome. Ann Plast Surg. 2015;75(5):518–21. https://doi.org/10.1097/SAP.0000000000000198 .
Song X, Jia R, Zhu H, Zhou Y, Sun Y, Lin M, Fu Y, Li J, Li Z, Lu L, Shen Y, Ge S, Fan X. A modified staged surgical intervention for blepharophimosis-ptosis-epicanthus inversus syndrome: 125 cases with encouraging results. Ann Plast Surg. 2015;74(4):410–7. https://doi.org/10.1097/01.sap.0000437072.17014.41 .
Mandal SK, Mandal A, Fleming JC, Goecks T, Meador A, Fowler BT. Surgical outcome of epicanthus and telecanthus correction by Double Z-Plasty and Trans-Nasal fixation with prolene suture in blepharophimosis syndrome. J Clin Diagn Res. 2017;11(3):NC05–8. https://doi.org/10.7860/JCDR/2017/25651.9496 . Epub 2017 Mar.
Chen L, Pi L, Ke N, Chen X, Liu Q. The protective efficacy and safety of bandage contact lenses in children aged 5–11 after frontalis muscle flap suspension for congenital blepharoptosis: a single-center randomized controlled trial. Medicine (Baltimore). 2017;96(36):e8003. https://doi.org/10.1097/MD.0000000000008003 .
Goel R, Kishore D, Nagpal S, Jain S, Agarwal T. The relationship of amount of resection and time for recovery of Bell’s phenomenon after levator resection in congenital ptosis. Open Ophthalmol J. 2017;11:24–30. https://doi.org/10.2174/1874364101711010024 . eCollection 2017.
Kim CY, Son BJ, Son J, Hong J, Lee SY. Analysis of the causes of recurrence after frontalis suspension using silicone rods for congenital ptosis. PLoS One. 2017;12(2):e0171769. https://doi.org/10.1371/journal.pone.0171769 . eCollection 2017.
Download references
Author information
Authors and affiliations.
Departments of Ophthalmology and Otolaryngology/Head & Neck Surgery, Casey Eye Institute, Oregon Health and Science University, Portland, OR, USA
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to John D. Ng .
Editor information
Editors and affiliations.
Department of Ophthalmology, Oregon Health & Science University Department of Ophthalmology, Portland, OR, USA
Daniel Albert
Massachusetts Eye and Ear, Boston, MA, USA
Joan Miller
Department of Ophthalmology, University of Illinois at Chicago Department of Ophthalmology, Chicago, IL, USA
Dimitri Azar
Department of Ophthalmology, Harvard Medical School, Boston, MA, USA
Lucy H. Young
Section Editor information
No affiliation provided
Eric Steele
Rights and permissions
Reprints and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this entry
Cite this entry.
Ng, J.D. (2020). Congenital Ptosis. In: Albert, D., Miller, J., Azar, D., Young, L. (eds) Albert and Jakobiec's Principles and Practice of Ophthalmology. Springer, Cham. https://doi.org/10.1007/978-3-319-90495-5_83-1
Download citation
DOI : https://doi.org/10.1007/978-3-319-90495-5_83-1
Received : 22 April 2020
Accepted : 23 April 2020
Published : 20 August 2020
Publisher Name : Springer, Cham
Print ISBN : 978-3-319-90495-5
Online ISBN : 978-3-319-90495-5
eBook Packages : Springer Reference Medicine Reference Module Medicine
- Publish with us
Policies and ethics
- Find a journal
- Track your research

- Register-org
- Subscriptions
Recent Posts
- Understanding the Dynamics of EMR Spectrum with Athena and Luminello EMR
- Treatments for Hepatocellular Carcinoma
- Raising Healthy Eaters: How Puffed Baby Snacks Can Transform Mealtime Battles
- How Hearing Aids Can Transform Your Life
- Exploring Dental Implants: A Comprehensive Overview and Benefits
- Entries feed
- Comments feed
- WordPress.org
- Allergy and Immunology
- Anesthesiology
- Basic Science
- Cardiothoracic Surgery
- Cardiovascular
- Complementary Medicine
- Critical Care Medicine
- Dermatology
- Emergency Medicine
- Endocrinology, Diabetes and Metabolism
- Gastroenterology and Hepatology
- Hematology, Oncology and Palliative Medicine
- Internal Medicine
- Medical Education
- Neonatal – Perinatal Medicine
- Neurosurgery
- Nursing & Midwifery & Medical Assistant
- Obstetrics & Gynecology
- Opthalmology
- Orthopaedics
- Otolaryngology
- Physical Medicine and Rehabilitation
- Plastic Reconstructive Surgery
- Pulmolory and Respiratory
- Rheumatology
Search Engine
Ptosis surgery.
Published on 08/03/2015 by admin
Filed under Opthalmology
Last modified 08/03/2015
This article have been viewed 3653 times
CHAPTER 48 Ptosis surgery
Nicolas Uzcategui, Srinivas S. Iyengar, Steven C. Dresner
Chapter outline
Introduction
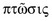
The treatment of ptosis requires accurate patient evaluation, consistent measurement of the eyelid position and function, and careful documentation of the functional deficit. Skillful use of the various surgical techniques to implement a functional and esthetic correction is required from the performing surgeon.
A practical clinical algorithm that allows the surgeon to select a reliable procedure for the management and treatment of the ptosis patient is provided. The algorithm’s goal is to help the surgeon with the selection of the best surgical procedure based on the amount of ptosis and levator function present the time of diagnosis, to achieve successful correction of the ptosis present with good postoperative results and patient satisfaction ( Fig. 48.1 ).
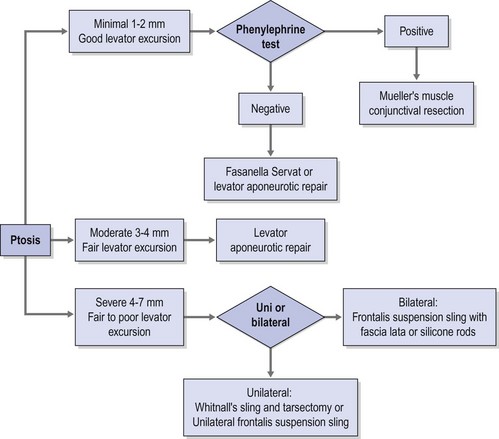
Fig. 48.1 The clinical pathway in this algorithm summarizes the surgical management of ptosis.
Epidemiologic consideration and terminology
There is a uniform paucity in the literature of studies reporting the incidence and frequency of ptosis. There is no racial or gender predilection for either involutional or congenital ptosis occurring equally among the different races and between males and females.
The most frequent cause of ptosis is the dehiscence of the levator aponeurosis; therefore the incidence of involutional ptosis increases steadily with age. Blepharoptosis has been observed more frequently in soft contact lens wearers, and increasing years of contact lens wear seem to be associated with a higher rate of blepharoptosis of involutional nature. The frequency of involutional upper eyelid ptosis is difficult to determine, mainly due to its reversible morbidity and absolute lack of mortality. However, it is increasingly recognized in the elderly population. This is particularly true in patients who have undergone cataract extraction with or without lens replacement; the etiologic factor implied is perhaps the stretching or disruption of the levator palpebrae superioris muscle when the eye is maintained open for cataract surgery using a lid speculum.
Congenital ptosis is the second most frequent cause of blepharoptosis, while other causes of ptosis are relatively infrequent. A patient who develops an acquired ptosis over a period of days or weeks can signal a serious medical problem and needs further neurologic and physical evaluation.
Congenital ptosis can affect one or both eyes; however, in approximately 70% of known cases, congenital ptosis has unilateral affectation. Congenital ptosis may be present at birth, or it may develop later in life. A droopy eyelid(s) that is present at birth, or that develops within the first year of life, is considered congenital in nature. In most cases of congenital ptosis, the problem is isolated and does not affect the vision if the eyelid doesn’t obstruct the visual axis. This holds particularly true in the critical period of visual development, corresponding to the first 3 months of life. The pupil obstruction caused by the droopy eyelid obscures the pediatric patient’s visual field, and permanent loss of vision may occur as a result of amblyopia. Congenital ptosis has no mortality and its morbidity arises from occlusion (deprivation) amblyopia, astigmatism induced from the compression of the droopy eyelid on the corneal tissue, and ocular torticollis. The astigmatism induced by congenital ptosis is generally anisometropic and can be uni or bilateral depending on whether one or both eyelids are affected by the condition. In instances where the degree of anisometropia is significant, anisometropic amblyopia can also develop. In the presence of amblyopia and/or functional limitation caused by congenital ptosis, surgery must be performed to correct the problem early in life, since recent evidence supports that the incidence of deprivation or anisometropic amblyopia in congenital ptosis can be reduced significantly 1 .
Congenital ptosis can also be seen as part of many congenital syndromes including blepharophimosis syndrome, and certain forms of congenital fibrosis of the extraocular muscles syndromes (CFEOM) 2 , Marcus Gunn jaw winking syndrome, and congenital Horner syndrome
Clinical features, diagnosis, and differential diagnosis
Since a droopy upper eyelid is an obviously visible phenomenon, patients often present for consultation mainly due to cosmetic concerns. In fewer instances patients may also be aware of, and present because of, a decrease in superior visual field limiting their activities of daily life and often express an inability to read because of severe ptosis in downgaze. Based on its etiology, blepharoptosis can be classified as true ptosis or pseudoptosis. Pseudoptosis refers to the appearance of ptosis without true eyelid margin ptosis or levator dysfunction, and can be the cause of or associated with severe dermatochalasis, orbito palpebral asymmetry, and/or changes in both ocular and orbit volume. True ptosis could be congenital or acquired, and unilateral or bilateral. Ptosis describes an abnormally low and downward displacement upper eyelid in the relaxed primary position in relationship to its physiologic position 1 or 2 mm below the superior corneoscleral limbus. Regardless of whether the ptosis is congenital or acquired it can be classified based on its pathophysiologic mechanism as:
The upper eyelids are elevated by the contraction of the levator palpebrae superioris; with time the connections between the aponeurosis of the muscle and the tarsal plate, usually in older patients, suffer thinning, lengthening, or sometimes disinsertion of the levator aponeurosis from the tarsal plate, causing the eyelid to droop by these involutional changes. Acquired ptosis secondary to levator tendon disinsertion or aponeurotic dehiscence develops spontaneously, after episodes of allergic reactions, or after minor trauma including surgical trauma in older adults, as observed in some cases post-cataract extraction. The degree of ptosis that may develop after levator aponeurosis dehiscence might be minimal to moderate, ranging from only 1–1.5 mm of ptosis, to severe, with in some instances complete ptosis in which the patient is unable to open the eye. Ptosis of the eyelids can have a subtle presentation and even go unnoticed by the patient. In some cases, patients complain of restricted visual fields. In the ptotic eyelid, the lid crease is generally present and is often slightly higher than that of the uninvolved side. Clinically the levator excursion in this kind of ptosis is relatively good, and the ptosis remains essentially unchanged in both upgaze and downgaze; this differentiates it from the inelastic levator muscle typical of congenital ptosis. Presenting signs also include: persistent wrinkles in the forehead due to contraction of the frontalis muscle (in an unconscious attempt to elevate the eyelid), and asymmetric elevation of the eyebrows, greater on the affected side. Additionally, the upper sulcus is frequently deeper than on the uninvolved side, completing the picture for clinical diagnosis.
Acquired muscular dystrophy, progressive external ophthalmoplegia, and myasthenia gravis all can be causes of non-congenital, usually late onset, ptosis of myogenic origin. In these cases the pathology lies at the level of the neuromuscular junction producing an ineffective muscular contraction that is insufficient to keep the eyelids open and elevated. Myogenic ptosis usually points to a systemic etiology that requires medical management like myasthenia gravis, and should be fully worked up, using surgery only as the option to rehabilitate the patient, since treatment is almost palliative. Ptosis in the myogenic group is generally progressive and has a high frequency of recurrence despite repeated surgery, including levator resections. When a frontalis sling procedure is performed on these cases, surgery is usually limited by the development of exposure keratopathy and lagophthalmos. Patients with an adequate tear secretion and orbicularis muscle closure are considered to be the best candidates. Whenever possible, surgery should be performed in these patients under local anesthesia to allow intraoperative adjustment of the lid position, taking into consideration the degree of postoperative lagophthalmos that the individual can tolerate. Consideration of a surgical result aiming for under-correction in these patients can benefit certain cases.
The pathologic mechanism of acquired neurogenic ptosis differs from that of myogenic ptosis based on the fact that in neurogenic ptosis there is direct damage to the nerve(s) supplying the tone and innervation for the muscular contraction to occur, without affecting the motor endplate. These examples include acquired CN III palsy and damage to the cervical sympathetic nerve chain causing Horner syndrome.
Mechanical ptosis can occur due to a solid tumor, cyst, enlarged lacrimal gland, or any other ocular adnexal neoplasm pushing down the eyelid. The surgeon’s experience and knowledge regarding these conditions will dictate surgery that will address the causative problem, thus correcting the ptosis whenever possible.
Although traumatic ptosis legitimately points to an acquired form of ptosis, this kind of ptosis varies according to the location of the direct injury to the levator muscle or its neurovascular supply. Mild degrees of trauma, whether or not associated with edema or hemorrhage, can lead to levator aponeurosis disinsertion that can be repaired using an aponeurotic approach surgery. Lacerations of the lid may sever the soft tissues, leading to scarring and secondary mechanical ptosis. When possible this problem is best managed by early and meticulous repair of the disrupted levator aponeurosis at the time of primary repair of the lid injury. When primary repair is not possible, often the eyelid and the orbit can be explored at a later time and the levator muscle identified and repaired, although the technical challenges associated with a delayed repair sometimes render suboptimal results even in the best of experienced hands. In certain instances traumatic ptosis involves damage to the nerve supply of the levator muscle. Since the levator and the superior rectus muscle share a common innervation, these injuries may affect the eye elevation, sometimes even limiting the globe’s upward excursion during Bell’s phenomenon. It is not uncommon for the patient to experience some degree of neural regeneration after trauma, so it is not a bad idea to wait at least 6 months to 1 year prior to any surgery to allow some degree of spontaneous regeneration to happen. After this period, when the degree of recovery has reached its maximum point, it is reasonable to attempt a levator resection; or a sling procedure can be performed, depending on the severity of ptosis and the degree of return of levator muscle excursion.
Although there are numerous classifications for ptosis; such as congenital versus acquired, neurogenic, myogenic, traumatic, and mechanical 3 , none of those classifications provides a complete approach to the ptosis patient, nor does any of them guide the clinician to the development of a system for adequate repair. Classifying ptosis as minimal = 0.5–1.5 mm, moderate = 2.0–3 mm, or severe >3 mm based on the amount of ptosis provides a practical framework for a logical system of repair when this system is used in conjunction with the amount of levator excursion. This last measurement is an indirect measurement of the viability of the levator palpebrae superioris and an excellent predictor of the surgical outcome. Using this matrix the appropriate choice of surgical strategy can then be applied to each of these three scenarios.
Basic considerations
The involuntary upward excursion of the eye upon attempted lid closure is denominated Bell’s phenomenon; certain surgeons feel that the presence or absence of Bell’s phenomenon is of significant importance in ptosis surgery. In most circumstances, its absence is considered a contraindication to ptosis surgery and/or if not at least an important warning sign. While the anterior statement holds true for adults, due to the risk of developing postoperative exposure keratopathy and subsequent corneal perforation after surgery in the absence of Bell’s phenomenon, it is not a contraindication to surgery in patients with congenital ptosis.
In general it is prudent to exercise extreme caution in patients when performing surgery in those with processes like thyroid myopathy, progressive external ophthalmoplegia, or dystrophies in which a poor Bell phenomenon is observed. Lagophthalmos and corneal exposure, along with decreased eye movements during REM sleep, and poor orbicularis muscle function may exist and lead to corneal perforation. Loss of the blink reflex or compromised corneal sensitivity, paralysis of the orbicularis, and significant keratitis sicca are definite contraindications to surgery.
Goals of surgery
Blepharoptosis is one of the most commonly encountered oculoplastic problems, but also at the same time one of the most challenging problems from the surgical standpoint. The goal of ptosis surgery is to obtain, as much as possible, an anatomic and physiologic result by elevating the lid or lids to an adequate position and, if necessary, to clear the pupil from the obstruction created by the eyelid’s downward displacement. Additionally, special attention must be given not to disrupt the natural contour and symmetry of the eyelids.
Thorough understanding of these goals and of the limitations of ptosis surgery is important, for both the surgeon and the patient. Also patients and their families must be fully aware of these goals and limitation before surgery is performed. It is advisable that the expectations and anticipated surgical results are discussed carefully with the patient and/or the parents preoperatively and documented in writing as part of the informed consent process. This proves particularly true in congenital ptosis, since in this instance factors inherent to the anatomic defect of the defective levator palpebrae superioris muscle impose limitations on the surgical results. Surgery cannot restore the function to a compromised muscle with an already abnormal or absent function preoperatively. In congenital ptosis with a frontalis suspension procedure or a maximal levator resection the lid level can be changed, but dynamic results will not be obtained postoperatively, and surgery may result in significant lid lag and lagophthalmos that is still more obvious on downgaze. The best result that can be hoped for in congenital ptosis surgery is a normal lid level, contour, and symmetry when the eyes are in the primary position, with adequate clearance of the visual axis.
Indications for surgery
In patients with acquired blepharoptosis, surgery is often recommended when the patient’s activities of daily life are compromised by the occlusion of the visual axis caused by the droopy eyelid, a significant superior visual field is reported or perceived by the patient, or extreme fatigability of the eyelid and occlusion of the pupil occur while in downgaze, affecting mainly activities like reading amongst others. Vision may be affected in patients regardless of the amount of ptosis. During the preoperative evaluation, visual or asthenopic symptoms secondary to the ptosis might be elicited; when these are present, they usually indicate the need for surgery. Third-party payers now require documentation of symptoms, field defects, and by clinical photographs routinely.
In most instances, the primary reason prompting the parents to seek for help in correcting congenital ptosis is cosmetic. It is generally agreed that disfiguring congenital ptosis should be repaired by age 5 years or before the child begins regular school to avoid psychosocial issues during the child’s socialization phase, but setting an arbitrary age for surgery serves no purpose. Earlier intervention is performed in children with severe bilateral ptosis that interferes with the child’s ability to learn how to walk, due to the extreme head position that they adopt with the chin-up position. Another exception arguing for early intervention is made in patients with unilateral or bilateral severe congenital ptosis where the normal visual development is compromised by total occlusion of the visual axis. In some of these instances surgical intervention may be even indicated shortly after birth. Disruption or loss of compensatory mechanisms of binocular fusion such as chin-up head position is a sign of development of amblyopia, which must be treated urgently with surgical correction of the ptosis and appropriate amblyopia management, with occlusion therapy and glasses when necessary.
Preoperative assessment
In the evaluation of the ptosis patient, history taking is one of the most important elements. If the ptosis is congenital, the physician should question the patient or family about the absence or presence of jaw winking and family history of ptosis. With acquired ptosis, the timing of onset is of extreme importance (acute vs. progressive or chronic). A history of fatigability or variable ptosis with exercise or through the day should warrant a work-up for myasthenia gravis, especially if there is noticeable improvement with rest. Associated neurologic symptoms, if any, should also be investigated. History of orbital or ocular trauma, previous ocular histories of inflammatory disorders, or contact lens wear may also be germane. The ocular exam should be complete. In addition to documenting the patient’s visual acuity and ocular motility, the importance of the pupillary function exam should not be underestimated. Anisocoria suggestive of Horner syndrome should be fully evaluated. The presence or absence of Bell’s phenomenon should be documented, as well as quantitative and qualitative properties of the tear film both by Schirmer’s testing and by study of tear break-up time. Slit-lamp examination should be used to assess the integrity of the cornea and the conjunctiva and to detect any inflammatory disease or subclinical pathology.
Children with ptosis should have full-dilated exams, retinoscopy, and assessment of ocular motility and sensory function with either fixation preference, the 10Δ base-down prism test, or a formal stereopsis test to rule out amblyopia. Careful motility examination in pediatric patients may uncover a double elevator palsy associated with the ptosis or partial third cranial nerve involvement causing the ptosis.
Assessment of ptosis and documentation
Ptosis is documented by the margin to reflex distance 1 (MRD 1 ) 4 , which is the distance from the central pupillary light reflex to the upper eyelid margin, measured in millimeters. It is important to document the amount of ptosis to the nearest 0.5 mm, if possible. The margin to reflex distance 2 (MRD 2 ) is the distance from the central pupillary light reflex to the lower eyelid margin. The MRD 1 plus the MRD 2 should equal the palpebral fissure.
The levator excursion test is the best clinical means for assessing levator function. The levator excursion is documented in millimeters, measuring the distance from extreme upgaze to downgaze with the brow immobilized by the examiner’s thumb to eliminate any contribution of the brow to lid elevation. A millimeter ruler is used vertically in the pupillary axis to assess the full excursion. Levator excursion of 10 mm or greater is considered good function; 5–9 mm of excursion is fair function; and 4 mm or less is poor function.
Patients with minimal ptosis (2 mm or less) should have a phenylephrine test performed in the involved eye or eyes after appropriate ptosis measurements have been documented. Either 2.5% or 10% phenylephrine is instilled in the affected eye or eyes. We prefer to use 2.5% phenylephrine. Usually two drops are instilled and the patient is re-examined 5 minutes later. The MRD 1 is rechecked in the affected and unaffected eyes ( Fig. 48.2 ). A rise in the MRD 1 of 1.5 mm or greater is considered a positive phenylephrine test. This indicates that Müller’s muscle is viable, and the Müller’s muscle conjunctival resection procedure can be performed, also giving the patient a reasonable prediction of the desired result.
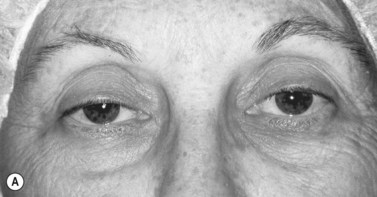
Fig. 48.2 The phenylephrine test. (A) The margin-to-reflex distance (MRD 1 ) is measured on both upper eyelids and repeated 5 minutes after instillation of 2.5–10% phenylephrine eye drops in the ptotic eye. (B) The right upper eyelid responds to the stimulating effects of the phenylephrine and shows a reduction of the ptosis (an increase in the MRD 1 ).
The contralateral eye must also be rechecked in patients with unilateral ptosis. When the ptotic eye is occluded, if the MRD 1 decreases appreciably in the opposite eye, this usually indicates that bilateral ptosis is present; this finding is consistent with Hering’s law 5 . The patient may require bilateral surgery. A negative phenylephrine test precludes the use of the Müller’s muscle conjunctival resection procedure, because the outcome of the procedure is unpredictable in this setting. Callahan and Beard 3
Share this:
- Click to share on Twitter (Opens in new window)
- Click to share on Facebook (Opens in new window)
Related posts:

Ophthalmic Surgery Principles and Practice

An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Medicine (Baltimore)
- v.97(36); 2018 Sep
Ptosis in childhood: A clinical sign of several disorders
a University-Hospital Policlinico-Vittorio Emanuele
Sung Yoon Cho
c Department of Pediatrics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
A.D. Praticò
b Department of Clinical and Experimental Medicine, Section of Pediatrics and Child Neuropsychiatry, University of Catania, Italy.
R. Falsaperla
M. ruggieri, dong-kyu jin.
Blepharoptosis (ptosis) is a common but often overlooked sign that may serve as a sign/manifestation of other conditions, ranging from a mild and purely cosmetic presentation to a severe and occasionally progressive disorder. Ptosis may show an acute onset or may manifest as a chronic disorder. Its presentation may vary: unilateral versus bilateral, progressive versus non-progressive, isolated versus complex which occurs in association with other symptoms, and congenital versus acquired (often concomitant with neuromuscular disorders).
Congenital ptosis includes the isolated type—the congenital cranial dysinnervation disorders, which are further, distinguished into different subtypes such as Horner syndrome (HS), and ptosis as a sign/manifestation of various congenital malformation syndromes.
In this article, we review the primary causes of ptosis occurring in childhood, and its various clinical presentations, including a short report on selected cases observed in our institution: a classical isolated familial ptosis comprising 14 members over 5 generations, 3 sibling with isolated congenital ptosis who in addition suffered by episodes of febrile seizures, a patient with Duane retraction syndrome who presented congenital skin and hair anomalies, and a girl with HS who showed a history of congenital imperforate hymen. A flowchart outlining the congenital and acquired type of ptosis and the clinical approach to the management and treatment of children with this anomaly is reported.
1. Introduction
Blepharoptosis or ptosis, as it is more commonly known, is a common clinical sign that may affect individuals of all ages ranging from neonates to elderly individuals. Ptosis refers to a drooping or inferior displacement of the upper eyelid with associated narrowing of the vertical palpebral fissure. The drooping may be slight or insignificant; however, in a few patients, it might be severe in that the pupils are completely covered causing visual disturbances. This disorder is caused by a dysfunction of the muscles and/or nerves that regulate elevation of the eyelid.
Two separate muscles are involved in the elevation of the eyelid—the levator palpebrae superioris, which is innervated by the superior branch of the III cranial nerve and the superior tarsal muscle (Müller's muscle), which is innervated by the cervical sympathetic system and elevates the posterior portion of the eyelid (Fig. (Fig.1 1 ).

Representation of eyelid muscles and their innervation.
Normally, the upper eyelid is positioned 1 to 2 mm below the upper corneal limbus, and the lower eyelid is placed at the sclerocorneal junction. Several methods are used to measure the inferior displacement of the upper eyelid. The most common approach in children is a measurement of the height of the palpebral fissure, which is defined as the widest distance between the upper and lower eyelid margins while the patient is in a primary gaze position (Fig. (Fig.2 2 ). [ 1 – 3 ]

Measurement of the palpebral fissure height with a ruler.
Other more sophisticated methods consist of measurement of the marginal reflex distance, upper eyelid creases, and the levator muscle function (Fig. (Fig.3 3 A and B). [ 4 , 5 ]

A&B. Eyelid measurement with a ruler using a pointing with a finger.
The incidence rate of ptosis is difficult to establish because of the diversity of disorders that are categorized within this group. The congenital type is the most common variety observed in childhood. Among the total number of patients referred for ptosis, Griepentrog et al observed congenital ptosis in 90% of 107 individuals, [ 6 ] El Essawy et al observed ptosis in 68% of 236 children, [ 7 ] and Berry-Brincat and Willshaw observed ptosis in 41% of 186 individuals (including 76 children). [ 8 ] Furthermore, congenital ptosis was reported in 0.18% of 247,389 healthy individuals in a mass screening study performed by Hu. [ 9 ]
Ptosis may show several presentations. It may be familial or sporadic, acute or chronic, unilateral or bilateral, progressive or non-progressive, may manifest as an isolated sign or occur in association with other ocular anomalies and/or other systemic disorders of varying severity, and congenital (when noticed at birth or during the first year of life) or acquired often associated with neuromuscular disorders.
When examining a child with ptosis, it is important to distinguish between ptosis and “pseudoptosis,” which resembles the former condition; however, it occurs because of a different etiology. Pseudoptosis may be related to the lack of physical support to the eyelids secondary to a defective ocular globe, as is noted with some congenital ocular malformations, such as anophthalmia and microphthalmia. [ 1 ] Dermatochalasis (redundant eyelid skin) may also cause pseudoptosis. Other known causes of pseudoptosis may be eye infections, corneal abrasions, or the presence of foreign bodies.
We review the primary causes of ptosis in childhood and present a short report on a few selected cases observed at our institution. All medical photos are presented with informed consent from patients and their parents. This study was approved by an autonomous Institutional review board of the Hospital Vittorio Emanuele of Catania.
Additionally, we discuss the clinical approaches to the management of ptosis and treatment of children affected by this anomaly.
2. Acute ptosis
Ptosis is a sign/manifestation of various disorders, and a few patients might present with an acute onset of this condition. Among these, Bell's palsy (facial nerve palsy) is the most typical example. It usually presents as an isolated entity, not associated with other cranial neuropathies or brainstem dysfunction. It results from a dysfunction of cranial nerve VII. The risk factors associated with Bell's palsy include diabetes and a recent upper respiratory tract infection. The presenting symptoms are unilateral facial weakness/paralysis of the facial muscles, with difficulty in closing or opening the eyelids associated with impaired taste and excessive tearing. [ 2 ] Prednisone is used for treatment, which should be initiated at the onset of the condition, preferably within 72 hours.
Acute botulism is an infectious disease linked to the botulinum toxin, an exotoxin produced by the gram-positive bacterium Clostridium botulinum . Three primary clinical presentations of this entity are food-borne, infant, and wound botulism. Patients presenting with wound botulism develop neurological symptoms characterized by diplopia, blurred vision, ptosis, dry mouth, dysphagia, dysphonia, and dysarthria. [ 10 , 11 ] This life-threatening disease requires administration of the human botulism immunoglobulin in the very early phases.
Miller–Fisher syndrome is considered a variant of Guillain–Barré syndrome and patients manifest with a classical triad of acute external ophthalmoplegia, ataxia, and areflexia. Similar to the presentation of Guillain–Barré syndrome, symptoms may be preceded by a viral illness. Treatment includes administration of intravenous immunoglobulins, steroids, plasmapheresis, and supportive care. [ 12 ]
Ophthalmoplegic migraine, which was previously regarded as a variant of migraine, has been recently considered a clinical expression of inflammatory cranial neuropathy, [ 13 ] which may occur before, during, or after a migraine attack.
Third cranial nerve paralysis may be caused by traumatic, inflammatory, or neurotoxic events with ptosis being a primary symptom.
3. Chronic ptosis
Chronic ptosis is classified into the congenital and acquired varieties. Each type is further classified based on the etiological factors, as well as on isolated presentation or ptosis associated with other manifestations.
4. Congenital ptosis
4.1. isolated congenital ptosis (icp).
ICP is usually noticed in patients from birth; however, it may be observed within the first year of life. This anomaly tends to remain unmodified with time (Figs. (Figs.4 4 and and5 5 ).

Image showing isolated congenital ptosis in a 2-year-old boy.

Image showing isolated congenital ptosis in a 12-year-old girl.
Histopathological studies performed in individuals with ICP have shown an involvement of the levator palpebrae superioris muscle that demonstrates muscle fibrosis, fatty tissue infiltration, and a reduction in the number of muscle fibers. [ 14 , 15 ] More recently, however, a neurological etiology has been considered for this anomaly with this condition being attributed to defective/abnormal muscle innervation. [ 16 ]
A study performed by Engle et al [ 17 ] showed that in 42 members of a family in whom 20 individuals were affected by ptosis, a 3-cM region, which was assigned the PTOS1 loci, located on the 1p32−34.1 chromosome was identified as being responsible for ptosis. McMullan et al [ 18 ] performed genetic linkage studies in a large family with an X-linked dominant congenital condition that revealed a critical region located between Xp24 and Xq27.1. [ 18 ] Additionally, McMullan et al [ 19 ] identified the ZFH4 gene, located on 8q21.12 as a candidate gene for ICP in a child with bilateral ICP, with a balanced translocation of chromosomes 8 and 10. A mutation in chromosome 8 disrupts the ZFH4 gene, which encodes a protein with a zinc-finger homeodomain that acts as a transcription factor. This protein interferes with normal muscle and nerve development causing dysfunction of the cranial nerves and the muscles they innervate. [ 19 , 20 ] Nakashima et al [ 21 ] proposed 3 candidate disease-responsible regions, 8q21.11−q22.1, 12q24.32−33, and 14q21.1−q23.2 for PTOS1, utilizing whole-genome linkage analysis performed in a Japanese family. [ 21 ]
Upon clinical examination, patients with ICP may show symmetric or asymmetric, and unilateral or bilateral involvement, with more frequent involvement of the left eyelid (approximately two-thirds of all cases reported). [ 2 ] A retrospective study performed by Griepentrog et al [ 6 ] showed that ICP was observed in 1 of 842 births, and the left eyelid was affected in 55% of the patients studied. Pavone et al reported a study involving a family comprising 14 members over 5 generations demonstrating ICP with an autosomal dominant pattern of inheritance and 70% to 90% penetrance. [ 22 ]
We have followed up this family for about 15 years. In this family, the affected individuals showed both unilateral and bilateral involvements with some of the family members presenting also synkinesia. The ptosis remained unchanged in all the individuals along this period of time. In the majority of the cases, the left eyelid was the most affected side.
Here we report on 3 siblings (2 brothers and a sister who showed ICP and all suffering in childhood by frequent episodes of febrile seizures (FS). A 5 years old boy came to our observation because of episodes of febrile seizures. He was the first child of unrelated Italian parents. The family history disclosed the presence of ICP involving the left side in the paternal line and childhood episodes of febrile seizures in the mother line. The child was born at 36 weeks of gestation by cesarean section. His birth weight was 3200 g, his height 49 cm and head circumference 36 cm, all within the normal range. Soon after birth, unilateral left side ptosis was noted. His developmental milestones were reached normally. Since the years of 2 years, the child suffered from frequent episodes of tonic-clonic generalized seizures in association with high fever lasting few minutes and not associated to postictal neurological involvement. The electroencephalography (EEG) was normal. During the previous 3 years the child presented with several episodes of the FS with frequency of 3 to 4 episodes for years. At the age of 3 years due to the high frequency of FS episodes treatment with valproate at the dosage of 20 mg/kg-day was started, but the seizures were still reported in most of the febrile episodes. Neurologic examination and psychiatric evaluation, as well as heart, thorax, abdomen, and general organs were assessed as normal, and the growth parameters were within the normal range. Routine laboratory analysis and EEG were normal while awake and during the sleep in various admissions to the hospital. The child and his siblings affected by ICP suffered in childhood by FS which were inherited from the maternal line whereas the ptosis was inherited from the paternal side. The FS episodes in the child and in his siblings disappeared from the age of 5 years.
In a case series comprising of 60 patients studied over a 5-year period, 8 patients (13%) were observed to have been affected by ICP (23) (unpublished report).
In a case series comprising 60 patients studied over a 5-year period, 8 patients (13%) were observed to have been affected by ICP. [ 23 ] (unpublished report) (Fig. (Fig.6 6 ).

Image showing a 4-year-old boy with ptosis. Isolated congenital ptosis was also observed in his sister and brother. All 3 children presented with ptosis and episodes of febrile seizures.
ICP treatment depends upon the severity of the anomaly following evaluation of the margin-reflex distance, levator excursion test, presence of amblyopia, and based on the decision of the parents and family. Surgical repair of severe ptosis is performed after 6 months of age in agreement with the family's decision. Although surgical treatment involves the use of several techniques, the most commonly recommended treatment involves anterior levator aponeurotic muscle resection or a frontalis sling or suspension procedure. [ 24 ]
4.2. Congenital cranial dysinnervation disorders (CCDDs)
CCDDs encompass a group of disorders resulting from anomalous innervation of the ocular and facial musculature. [ 25 ] These disorders include Duane retraction syndrome (DRS), blepharophimosis ptosis epicanthus inversus syndrome (BPES), congenital fibrosis of the extraocular muscles (CFEOM), and the Marcus Gunn phenomenon.
Patients diagnosed with DRS may present with abduction paresis (type 1, most common), adduction paresis (type 2, least common) or both (type 3, second most common). Contraction of the lateral rectus muscle upon attempted adduction causes retraction of the ocular globe and subsequent enophthalmos and ptosis. The 5 cardinal features of DRS type I include congenital onset, severely limited abduction, slightly limited adduction, retraction of the globe and palpebral fissure narrowing, as well as up-shoot and down-shoot on adduction. Cytogenetic abnormalities, on both, the long arm of chromosome 2 (2q31) and chromosome 8 (8q13) have been reported. [ 26 ]
We recently observed a 10-year-old boy affected by DRS type 1 who presented in association cutaneous and hair anomalies consisting of 3 to 4 café-au-lait spots measuring 3 to 5 cm in size localized to the trunk and a small patch of piebaldism on the scalp (Fig. (Fig.7 7 A–C). The boy came to our observation at the age of 3 years and 7 months for psychomotor delay and dysmorphic features. He is the third child of healthy unrelated parents. The 2 older siblings were born from the former maternal marriage and are healthy. At the time of the conception, the mother was 28 years old and the father 31 years old. During the proband's pregnancy, the mother denied having had infections or gestosis or consuming alcohol or drugs. She did not take supplementary folic acid.

Image showing a 10-year-old boy with Duane retraction syndrome. Ptosis (A) is observed to be associated with café-au-lait spots in the trunk (B), and piebaldism (C).
The boy was born at 39th week of gestation from normal delivery. The birth-weight was 3.3 Kg, length 50 cm, and head circumference 35 cm. The proband since his first months of life showed a delay in the development milestones. The first convulsive episode started at the age of 5 months, with a tonic-clonic crisis lasting a few minutes. After this episode, other seizures occurred with a frequency of 2 to 3 each month. Treatment with valproate at normal dosage yielded a noticeable reduction of the epileptic seizures. At the age of 5 years and 7 months, at the physical examination, general conditions were good. His weight was 19 Kg (50th percentile), height 105 cm (10–25th percentile) and head circumference 51 cm (50th percentile). He shows minor facial dysmorphisms consisting in large forehead with protruding metopic suture, bilateral ptosis, more evident in the left eye, hyperplasia of the eyelashes, mildly convergent in the centrum. At the neurological examination, the muscular tone and strength was normal, as was the cranial nerves, and the patellar reflexes. Hearth, thorax, internal organs were normal. Routine laboratory analyses were normal.
Results of genetic testing micro-array showed the following re-arrangement: arr[hg19] 22q11.22 (22.859.095–23.353.058 × 3). Same rearrangement was found in the father whereas the result in the mother was negative. presented also in the father. In the mother, array-CGH analysis was negative.
4.4. Blepharophimosis ptosis epicanthus inversus syndrome (BPES)
In addition to ptosis, telecanthus, and premature ovarian failure in women may be associated with BPES. In contrast to type 2 BPES, premature ovarian failure is observed only in the type 1 variant. This condition has been attributed to a mutation in the transcription factor FOXL2, resulting in the production of truncated proteins in the mesenchyme of the developing eyelid structure. [ 27 ]
4.5. Congenital fibrosis of the extraocular muscles (CFEOM)
CFEOM includes a group of disorders with non-progressive restrictive ophthalmoplegia of the extraocular muscles, which are innervated by the oculomotor, trochlear, and abducens nerves manifesting with congenital blepharoptosis. Three clinical phenotypes have been distinguished. Type 1 is inherited in an autosomal dominant fashion, and the responsible gene is KIF21A , located on chromosome 12p11.2–q12, which encodes an anterograde kinesin motor protein. [ 28 – 30 ] The position of both eyes is below the horizontal midline with severe restriction of elevation of either eye. Type 2 CFEOM is inherited in an autosomal recessive fashion, and the responsible gene is POX2A/ARIX , located on chromosome 11q13.1. [ 31 ] Type 3 CFEOM is inherited in an autosomal dominant fashion and is caused by a mutation in TUBB3 and KIF21A genes. This type is characterized by congenital bilateral exotropic ophthalmoplegia and ptosis, with pupillary abnormalities and manifests with ptosis, and ophthalmoplegia affecting the vertically acting extraocular muscles. This type could be associated with intellectual and behavioral impairment in a few patients. [ 30 , 32 – 34 ]
4.6. Marcus Gunn (MG) jaw-winking syndrome
MG jaw-winking syndrome is caused by a synkinetic anomaly secondary to aberrant innervation of the mandibular branch of the trigeminal nerve innervating the levator palpebrae muscle. Following the contraction of the pterygoid muscle, the ptotic eyelid is elevated and retracts, causing a winking movement during eating, chewing, or sucking actions. No pathogenic cause has been identified for this anomaly. [ 35 – 37 ]
4.7. Horner syndrome (HS)
HS results from a disruption in the sympathetic nervous system pathway extending between the brain and the Müller's muscle, thereby affecting the eye and ipsilateral side of the face. Patients with HS manifest in ipsilateral ptosis, miosis, and anhidrosis. The disorder may be congenital and could be associated with a lighter color of the iris of the affected eye. HS may present in association with contralateral hemifacial flushing and ipsilateral hypohidrosis (Harlequin syndrome). Dystocic delivery, often associated with brachial plexus injury, is considered a possible cause of HS. [ 38 , 39 ] .
Recently, we observed a young girl with HS (miosis and mild ptosis in the absence of anhidrosis) who presented with a history of congenital imperforate hymen. This 4 years old girl was admitted as outpatient to our institution with the diagnoses of HS. The neonatal history displayed a surgical intervention for imperforate hymen with the absence of a vaginal opening and bulging of vagina. The subsequent period and the developmental stages were normal. The anomaly was initially overlooked, at the age of 3 years old due to the presence of miosis, partial ptosis, and mild hypohydrosis the diagnosis of congenital HS was performed. Physical and laboratory examination and neuroimaging were normal. Cocaine test confirmed the diagnosis of HS. At the present age of 8 years old the girls leads a normal life and good scholastic performances.
Although HS may also be an acquired entity, this variant is less commonly reported in children compared with congenital HS. The disorder may appear as a consequence of cerebral lesions, with lesions located between the hypothalamus and the fibers moving from the spinal cord or a peripheral location in the superior cervical ganglia or cervical sympathetic chain. Tumors, neuromyelitis optica, postviral damage, internal carotid artery agenesis, cervical disc herniation, as well as neuroblastomas and other associated disorders may be the possible etiological factors contributing to the development of this disease entity. [ 38 – 40 ]
4.8. Congenital facial palsy (CFP)
CFP is usually traumatic and rarely developmental in origin. In the latter case, patients may present with microtia and atresia of the external auditory canals as anomalies associated with the disorder. [ 2 , 3 ]
4.9. Congenital myasthenic syndromes (CMS)
CMS are a heterogeneous group of disorders affecting neuromuscular transmission. These disorders are distinguished by molecular defects and the localization of the dysfunction at the presynaptic, postsynaptic, and neuromuscular junction. Mild ptosis is the most common sign in patients, although the severity and course of the disorder could vary, involving ocular or bulbar and limb muscle impairment. Patients may present in the neonatal period with respiratory failure with episodes of apnea, cyanosis, and generalized weakness. The genes most commonly associated with this condition are CHAT , CHRNE , COLQ , DOK7 , GFPT1, and RAPSN . [ 41 – 43 ] Treatment consists of the administration of acetylcholinesterase inhibitors and/or 3,4-diaminopyridine, a potent potassium channel blocker.
4.10. Congenital ptosis in congenital malformation syndromes
4.10.1. turner syndrome (ts).
This syndrome is the most common sex chromosome abnormality observed in females, with an incidence of 1 in 2500 live female births. The primary features of TS consist of growth retardation, gonadal dysgenesis, congenital and acquired cardiovascular anomalies, and a specific cognitive and psychosocial phenotype. Other signs observed in patients include a webbed neck, shield chest, epicanthal folds, and ptosis. The presence of edematous hands and feet, related to lymphedema in a newborn are suggestive of the diagnosis. The classic phenotypic presentation is associated with the 45, X karyotype. [ 44 , 45 ]
4.10.2. Noonan syndrome (NS)
NS is an autosomal dominant disorder in which patients show a broad spectrum of clinical presentations including unusual facial features, congenital heart abnormalities, increased rate of tumor incidence, a webbed neck, as well as characteristic chest anomalies such as superior pectus carinatum and inferior pectus excavatum. Other characteristic features/signs are related to coagulation defects, lymphatic dysplasia, and ocular anomalies including ptosis and hypertelorism. NS is the result of germline mutations in the genes that encode protein components of the intracellular RAS/MAPK pathway. [ 46 ]
4.10.3. Smith–Lemli–Opitz syndrome (SLOS)
SLOS is one of the multiple congenital malformation syndromes showing an autosomal recessive pattern of inheritance. It occurs secondary to an inborn error of cholesterol metabolism linked to a deficiency of the enzyme 7-dehydrocholesterol reductase. Patients with SLOS type 1 present with bitemporal narrowing, growth retardation, pre- and postnatal microcephaly, and moderate-to-severe intellectual disability. Facial and systemic malformations include ptosis, cleft palate, cardiac defects, underdeveloped external genitalia, post-axial polydactyly, and 2 to 3 toe syndactyly. [ 47 ]
4.10.4. Rubinstein–Taybi syndrome (RSTS)
RSTS is a congenital malformation in which patients present with distinctive facial features, broad and short thumbs, and first toes. The latter feature is considered typically suggestive of the diagnosis. The craniofacial dysmorphism in patients diagnosed with this condition consists of microcephaly, a low anterior hair line, downslanting palpebral fissures, ocular signs (including ptosis, epicanthus, and strabismus), a broad nasal bridge, a beaked nose, and a prominent columella. Mutations in 2 functionally related genes, CREBBP and EP300 , have been reported as the causative factors in 55% to 78% of patients. [ 48 , 49 ]
5. Acquired ptosis
As is noted with the congenital variety, ptosis is a sign/manifestation of various acquired disorders. However, in contrast to the congenital form, acquired ptosis is usually characterized by a progressive and severe/serious course. Several metabolic, neuromuscular, muscular, and/or traumatic conditions may clinically present with ptosis.
5.1. Chronic progressive external ophthalmoplegia (CPEO)
CPEO is a mitochondrial disease with gradually progressive, usually bilateral ptosis, and limited ocular mobility in all directions of gaze. [ 50 , 51 ] Mitochondria are ubiquitously distributed cellular organelles; therefore, mutations in mitochondrial DNA or associated nuclear mutations present with a vast spectrum of systemic disorders. CPEO encompasses a heterogeneous and wide-ranging group of disorders including the above-mentioned CPEO syndrome in which patients might show mild clinical features restricted to bilateral ptosis. [ 51 , 52 ] Other examples are the Kearns–Sayre syndrome, in which patients manifest with progressive external ophthalmoplegia, retinitis pigmentosa, and heart blocks. Cerebellar ataxia, sensorineural hearing loss, and increased protein content of the cerebrospinal fluid have also been reported. Muscle biopsy evaluation and molecular genetic analysis may help to confirm the diagnosis. Histopathological analysis of muscle tissue shows ragged-red, and ragged-blue fibers, or cytochrome C oxidase-negative fibers. [ 53 ]
Patients diagnosed with Pearson marrow-pancreas syndrome present with sideroblastic anemia with vacuolization of marrow precursors and exocrine pancreatic dysfunction. [ 54 ] Several other diseases may present with CPEO such as mitochondrial encephalomyopathy lactic acidosis and stroke-like episodes (MELAS), mitochondrial neurogastrointestinal encephalomyopathy (MNGIE) caused by mutations in the nuclear gene TYMP , as well as sensory ataxic neuropathy, dysarthria/dysphagia, and external ophthalmoplegia (SANDO). [ 55 – 57 ] Other disorders such as spinocerebellar degeneration, Refsum's disease, and abetalipoproteinemia may also be included in the CPEO category of disorders. [ 1 ]
5.2. Oculopharyngeal muscular dystrophy (OPMD)
OPMD is an autosomal dominant disorder in which patients present with ptosis, swallowing anomalies, proximal limb weakness, and presence of nuclear aggregates in muscles. The disorder is caused by a trinucleotide repeat expansion in the PABPN1 gene. Presently animal research is underway to identify an optimal treatment strategy for patients with OPMD. [ 58 ]
5.3. Myotonic dystrophy (dystrophia myotonica [DM])
DM is a genetic disorder showing an autosomal dominant pattern of inheritance that affects muscles and other organs including the heart and the brain. The primary clinical features observed in patients include progressive muscular weakness, atrophy, and myotonia in addition to ophthalmologic involvement in the form of ptosis, cataracts, and pigmentary retinopathy. The disorder runs a progressive course with patients showing accelerated aging and rapidly worsening muscular weakness, cognitive decline, and metabolic dysfunction. Myotonia is exacerbated by fatigue, cold, and excitement. Two types of DM have been recognized—type 1 is caused by an expansion of a CTG triplet repeat in the DMPK gene, whereas type 2 is caused by an expansion of a CCTG tetramer repeat in CNBP . DM mutations lead to the expression of dominant-acting RNAs in all patients. [ 59 , 60 ]
Diagnostic tests used are: progressively worsening ptosis with sustained upward gaze for 1 or 2 minutes, and rapid opening and closing of fists causes fatigue of the hand muscles and difficulty in elevation of the arms for greater than 1 to 2 minutes. The ice and the Tensilon test demonstrate good sensitivity in diagnosing this condition. Administration of Tensilon (a short-acting acetylcholinesterase inhibitor) demonstrates an improvement in ptosis and ophthalmoplegia. Treatment with acetylcholinesterase inhibitors is widely utilized. [ 2 ]
5.4. Myasthenia gravis
Acquired autoimmune myasthenia gravis (AMG) is a postsynaptic neuromuscular disorder in which patients present with heterogeneous clinical signs and the presence of antibodies. These antibodies are directed against a component of the neuromuscular junction, most commonly, the acetylcholine receptors. [ 61 ] Reportedly, an antibody directed against the nicotinic acetylcholine receptor has been demonstrated in 85% of the patients diagnosed with generalized AMG and 50% of patients with ocular myasthenia gravis (OMG). Patients with OMG present with a history of painless weakness or fatigability of the extraocular muscles, and ptosis. A progressive decrement in the muscle action potential response to repetitive nerve stimulation at 2 to 3 Hz confirms the diagnosis of this disorder. In patients showing a negative response, diagnosis may be confirmed through single fiber electromyography. Treatment with acetylcholinesterase inhibitors may show a good effect in managing ptosis. [ 62 , 63 ]
6. Surgical treatment of ptosis
The primary objective of surgery in the management of ptosis is to restore normal eyelid functions, essentially by elevating the position of 1 or both eyelids and by creating a lid fold, if necessary. Moreover, special attention is needed for maintenance of proper contour and symmetry of the lids.
Patients or their caregivers should be aware of the several limitations associated with this condition and that the results may not be conclusive—particularly in those with congenital ptosis because factors inherent to the anatomic defect can limit the surgical results. The expectations and goals of the surgery for children being performed must be discussed carefully with either the parents and caregivers or both, preoperatively. A defective levator muscle showing abnormal and/or absent function preoperatively, cannot be restored surgically. The lid level can be changed through a modified Crawford technique, although dynamic limitations in the affected muscle are known to persist postoperatively, causing significant lid lag or lagophthalmos. Other complications may be inappropriate eyelid closure, an exacerbation of a pre-existing tear deficiency, and secondary exposure keratopathy. [ 64 ]
7. Conclusions
In conclusion, ptosis is a noticeable sign associated with various diseases, ranging from a mild to a very severe type, with the involvement of various body organs. Usually, ptosis can be easily identified clinically; however, diagnosis might be difficult in patients presenting with complex types that are associated with diverse manifestations. A flowchart outlining the clinical diagnosis of ptosis has been summarized in Figure Figure8. 8 . Although a few of these disorders are amenable to treatment, there is no definitive therapy available for many of these conditions.

Flow chart outlining ptosis: congenital (A) and acquired (B) types.
Acknowledgments
We thank all the individuals who have been diagnosed with the afore-mentioned rare diseases and their families and all the clinical and research laboratory staff who have been instrumental in the successful completion of our work. This study was supported by a grant from Samsung Medical Center (#GFO2170061).
Author contributions
Data curation and flow chart compilation: AD Praticò.
Formal analysis: AD Praticò, R Falsaperla.
Methodology: M Ruggieri.
Supervision: Dong-Kyu Jin.
Validation: R Falsaperla.
Visualization: M Ruggieri.
Writing – original draft: P Pavone.
Writing – review & editing: Sung Yoon Cho.
Abbreviations: AMG = acquired autoimmune myasthenia gravis, BPES = blepharophimosis ptosis epicanthus inversus syndrome, CCDDs = congenital cranial dysinnervation disorders, CFP = congenital facial palsy, CMS = congenital myasthenic syndromes, CPEO = chronic progressive external ophthalmoplegia, CFEOM = congenital fibrosis of the extraocular muscles, DM = dystrophia myotonica, DRS = duane retraction syndrome, HS = Horner syndrome, ICP = isolated congenital ptosis, NS = Noonan syndrome, OPMD = oculopharyngeal muscular dystrophy, RSTS = Rubinstein–Taybi syndrome, SLOS = Smith–Lemli–Opitz syndrome, TS = Turner syndrome.
This study was supported by a grant from the Samsung Medical Center (#GFO217006).
The authors declare that they have no competing interests.
The authors have no conflicts of interest to disclose.
Tank ride & bazooka Moscow
Only in Russia!
Ask us for availabilities
Starts at 9am
Duration: 1 day
Tour available in
Want to ride a tank? Of course you do.
Welcome to Russia, where everything and anything is possible—especially riding on a tank, shooting an AK-47 and a bazooka, all while drinking vodka.
At Put-in tours, we have put together the ultimate excursion for men (and their girlfriends). Join us in our Soviet van for an entire afternoon of all-inclusive Russian fun on the battlefield!
Start by visiting Park Patriot, the impressive Russian army exhibition center and museum. There, try your driving skills at the T-80 tank official simulator , before you ride on two iconic Soviet armored vehicles (BMP-1 and/or BTR-80) on an off-road training path. Along the way, hone your skills while a drill sergeant shouts commands at you. You may not understand, but impress him anyway with your grenade launching technique and AK-47 assembling speed. Or suffer the consequences.
After that, take another shot of vodka , enjoy the army field lunch provided, and move on to the next activity…

Honey, I shot a bazooka!
Phase two brings a variety of iconic Russian war weapons to the tip of your trigger finger. Learn about and test the Makarov PM, PPSH-41, Kalashnikov AK-47, RPK, DP28 machine gun, several semi-auto or auto carbines and more… Firing off a bazooka is the grand finale, sure to leave you breathless—just before you send the photos to your mom.
To prevent you from inadvertently blowing up your best friend or transforming your girlfriend into a strainer, we only use non-lethal (but real!) ammo.
And because you’ll want to keep the good times rolling, we also offer a 10% discount on pub-crawl tickets.
Hotel pick-up (when possible – please contact us)
Transport in our classic Soviet van
Army field lunch, drinks and vodka
Visit Park Patriot & try the T-80 tank simulator
Ride on a iconic Soviet armoured vehicle: BMP-2
Shoot 10 Soviet rifle rounds and 1 bazooka shell
Adults : 28 100 RUB / passenger
Good to know
Please contact us before the tour to make an appointment
No minimum age
Shooting and bazooka are blank for security reasons
Let us know if you have any food allergies or special dietary needs
Don’t wear your best shoes
Join the experience, book your seat now!
* we run this excursion for groups > 5 persons. If you are less then 5 people, please contact us by email to know about availabilities.
Generally, hotel pick-up is possible . Contact us beforehand to make an appointment.
Otherwise, we will pick you up at 9:00 in front of St Basil’s Cathedral , on the red square.
Contact us for more details
Follow us on Social Media...
Our partners
© Copyright 2021 - Put-in tours Designed by SD Marketing & Design
Put-in tours
At Put-in tours, we put you in our classic Soviet vans to go explore Moscow, Saint Petersburg and Russian culture off the beaten path. Discover our Moscow city guided tour, visit Moscow by night, join our banya & Sergiyev Posad excursion, visit and dine in one of Moscow's oldest monastery or even Luzhniki stadium, before you party on our famous pubcrawl! Original and atypical tours : Shoot AK47 and a bazooka after riding on a tank with our tank & bazooka excursion ! Extreme tours: Fly a fighter jet in Moscow onboard a L-29 or L-39 aircraft!
© Copyright 2021 – Put-in tours
Design web: SD Marketing & Design
Home About us Videos Moscow Saint-Petersburg Contact Online booking Blog Disclaimer Privacy Policy
WhatsApp us

IMAGES
VIDEO
COMMENTS
Levator Palpebrae Superioris Function (Excursion) Levator. function test. The position of the upper eyelid margin is noted in downgaze by the 1 cm hash (A), and then in upgaze without activation of the frontalis muscle (B). In this patient with ptosis, the levator excursion is approximately 5 mm. Normal levator function is approximately 15 mm. ...
Levator excursion or levator function: Distance upper lid elevates from 1) downgaze with hand on patient's forehead followed by 2) upgaze without change in head position: Good: 12 + ... Phenylephrine 2.5% instilled in the inferior fornix of the more ptotic eye → positive test if MRD1 increases >1.5mm in 3-5 minutes ;
Levator function (upper eyelid excursion): The distance from the upper eyelid margin in downgaze to upgaze with frontalis muscle function neutralized. Typically, levator function is 12-17 mm. ... the patient to look in extreme upgaze for up to one to two minutes and to check for improvement with the rest or ice test. ...
Measurement of levator excursion. File Size: 676 KB. Related: Measurement of levator excursion, Eyelid malposition. View Full Image. Image License and Citation Guidelines. Add to My Bookmarks. Comments. Views 229. Measurement of levator excursion.
The levator function is measured by the full excursion of the upper lid from downgaze to upgaze and should be between 10 to 15 mm (Figures 2A and 2B). Deviations from these average values confirm the presence of ptosis; an evaluation of the levator function can help to narrow down the etiology. 8 • Ancillary testing.
Levator action: It is the amount of excursion measured with a millimeter scale when the eyelid moves from extreme downgaze to extreme upgaze with frontalis action negated. Normal levator action is greater than 15mm. ... Ice test: An ice pack is placed over the closed ptotic eyelid for 2 minutes. Ptotic measurements are repeated after 2 minutes.
Care should be taken to negate flexion of the forehead frontalis muscle which could falsely elevate the levator muscle excursion. Normal levator muscle function is 15 mm. Levator function may not present below normal levels in all cases of myogenic ptosis. Measuring the velocity of the patient's eyelid movement from a downward to an upward ...
Levator function: Berke's method estimated by measuring the upper eyelid excursion, from downgaze to upgaze with frontalis muscle function negated and with the head positioned in the frontal or Frankfort plane. ... Iliff test: It is used to assess levator function in infants. Upper eyelid of the child is everted as the child looks down. If the ...
Levator function is classified based on the amount of upper eyelid excursion, from poor (0-4 mm lid elevation), to fair (5-11 mm), good (12-14 mm), and normal (>15 mm) . Müller's muscle ...
Levator muscle function: ... Then patient looks upward, and the amount of excursion is measured with a scale which can be graded as normal (15 mm), excellent (over 12 mm), good (9 to 11 mm), fair (5 to 9 mm) or poor (less than 4 mm). This test is very important in determining the surgical procedure of choice for ptosis correction.
Measurement of redundant eyelid skin, levator excursion, prolapsed orbital fat, presence or absence of blepharoptosis is needed to quantify the type and degree of dermatochalasis. Additionally, evaluation of the presence of eyelid retraction, amount of eyelid laxity, and changes in the surrounding bony framework and periocular tissues is ...
• Upper eyelid excursion upon shift from downgaze to upgaze, ... decreased excursion indicates greater degree of levator functional impairment (0-4 mm lid elevation = poor; 5-11 mm = fair; 12-14 mm = good; >15 mm = normal) ... The HVF Test is an automated static perimetry test using an HVF analyser, in which static illuminated targets briefly ...
Levator excursion informs the surgeon of the extent of lid movement and is the most important measurement in determining which surgical procedures are most appropriate for correcting the congenital ptosis. ... The ice and rest test are difficult to perform in small children, and observation and family history will need to be relied on in ...
The Tensilon test at Childrens Hospital-Los Angeles was performed by an experienced pediatric neuro-ophthalmologist. Patient 4 was French-Canadian, had a positive family history of progressive ptosis and pharyngeal signs (trouble swallowing), and was diagnosed with oculopharyngeal dystrophy. ... Levator excursion was measured as the distance of ...
Levator function measures the distance of excursion of the upper eyelid margin from far downgaze to upgaze while the frontalis muscle is held still with the examiner's hand (normal is 14 mm or ...
Introduction. Frontalis suspension is a technique used for the correction of severe ptosis with poor or absent levator muscle function. Citation 1 - Citation 3 Poor levator function is identified by the upper lid excursion test when levator induced upper eyelid movement is 4 mm or less. Citation 4, Citation 5 This suspension technique involves the use of a sling to attach the tarsal plate to ...
The levator excursion test is the best clinical means for assessing levator function. The levator excursion is documented in millimeters, measuring the distance from extreme upgaze to downgaze with the brow immobilized by the examiner's thumb to eliminate any contribution of the brow to lid elevation. A millimeter ruler is used vertically in ...
Margin-to-reflex distance 1 (MRD1) and levator excursion were recorded and photography of lid height was performed both pre- and 5 minutes post-phenylephrine testing. Results. A total of 77 ptotic upper eyelids of 47 patients were included. In 22% of lids, phenylephrine testing produced no response; in 18%, lid elevation of 0.5-1 mm; in 35% ...
t was video about nothing and we didn't want to publish it but since some of the fans are interested in Moscow we put the clip together although it's been al...
🎧 Wear headphones for the best experience.In this video, we will walk along the famous tourist routes of Moscow, take a walk along the renovated embankments...
Walking tour around Alexander Garden. Alexander Gardens (Russian: Александровский сад) was one of the first urban public parks in Moscow, Russia. June 11, 20...
ICP treatment depends upon the severity of the anomaly following evaluation of the margin-reflex distance, levator excursion test, presence of amblyopia, and based on the decision of the parents and family. Surgical repair of severe ptosis is performed after 6 months of age in agreement with the family's decision. Although surgical treatment ...
Welcome to Russia, where everything and anything is possible—especially riding on a tank, shooting an AK-47 and a bazooka, all while drinking vodka. At Put-in tours, we have put together the ultimate excursion for men (and their girlfriends). Join us in our Soviet van for an entire afternoon of all-inclusive Russian fun on the battlefield!