Albatrosses are threatened with extinction – and climate change could put their nesting sites at risk
Postdoctoral research fellow, Department of Plant and Soil Science, University of Pretoria

Disclosure statement
Mia Momberg does not work for, consult, own shares in or receive funding from any company or organisation that would benefit from this article, and has disclosed no relevant affiliations beyond their academic appointment.
University of Pretoria provides funding as a partner of The Conversation AFRICA.
View all partners

The wandering albatross ( Diomedea exulans ) is the world’s largest flying bird , with a wingspan reaching an incredible 3.5 metres. These birds are oceanic nomads: they spend most of their 60 years of life at sea and only come to land to breed approximately every two years once they have reached sexual maturity.
Their playground is the vast Southern Ocean – the region between the latitude of 60 degrees south and the continent of Antarctica – and the scattered islands within this ocean where they make their nests.
Marion Island and Prince Edward Island , about 2,300km south of South Africa, are some of the only land masses for thousands of kilometres in the Southern Ocean.
Together, these two islands support about half of the entire world’s wandering albatross breeding population, estimated at around 20,000 mature individuals . Every year scientists from South African universities survey Marion Island to locate and record each wandering albatross nest.
The species, listed as vulnerable by the International Union for Conservation of Nature , faces huge risks while in the open ocean, in particular due to bycatch from longline fishing trawlers. This makes it important to understand their breeding ecology to ensure that the population remains stable.

I was part of a study during 2021 to investigate which environmental variables affect the birds’ choice of nest site on Marion Island. The birds make their nests – a mound of soil and vegetation – on the ground. We looked at wind characteristics, vegetation and geological characteristics at nest locations from three breeding seasons.
Elevation turned out to be the most important variable – the albatrosses preferred a low (warmer) site and coastal vegetation. But these preferences also point to dangers for the birds from climate change. The greatest risk to the availability of nesting sites will be a much smaller suitable nesting range in future than at present. This could be devastating to the population.
Variables influencing nest site selection
Marion Island is of volcanic origin and has a rough terrain. Some areas are covered in sharp rock and others are boggy, with very wet vegetation. There is rain and strong wind on most days. Conducting research here requires walking long distances in all weathers – but the island is ideal for studying climate change, because the Southern Ocean is experiencing some of the largest global changes in climate and it is relatively undisturbed by humans.
Using GPS coordinate nest data from the entire breeding population on Marion Island, we aimed to determine which factors affected where the birds breed. With more than 1,900 nests, and 10,000 randomly generated points where nests are not present, we extracted:
elevation (which on this island is also a proxy for temperature)
terrain ruggedness
distance to the coast
vegetation type
wind turbulence
underlying geology.

The variables were ranked according to their influence on the statistical model predicting the likelihood of a nest being present under the conditions found at a certain point.
The most important variable was elevation. The majority of the nests were found close to the coast, where the elevation is lower. These areas are warmer, which means that the chicks would be less exposed to very cold temperatures on their open nests.
The probability of nests being present also declined with distance from the coast, probably because there are more suitable habitats closer to the coast.
Vegetation type was strongly determined by elevation and distance from the coast. This was an important factor, as the birds use vegetation to build their nests. In addition, dead vegetation contributes to the soil formation on the island, which is also used in nest construction.

The probability of encountering nests is lower as the terrain ruggedness increases since these birds need a runway of flat space to use for take-off and landing. During incubation, the adults take turns to remain on the nest. Later they will leave the chick on its own for up to 10 days at a time. They continue to feed the chick for up to 300 days.
Areas with intermediate wind speeds were those most likely to have a nest. At least some wind is needed for flight, but too much wind may cause chicks to blow off the nests or become too cold.
Delicate balance
Changing climates may upset this delicate balance. Human-driven changes will have impacts on temperature, rainfall and wind speeds, which in turn affect vegetation and other species distribution patterns .
By 2003, Marion Island’s temperature had increased by 1.2°C compared to 50 years before. Precipitation had decreased by 25% and cloud cover also decreased, leading to an increase in sunshine hours . The permanent snowline which was present in the 1950s no longer exists . These changes have continued in the 20 years since their initial documentation, and are likely to continue.
Strong vegetation shifts were already documented in the sub-Antarctic years ago. Over 40 years, many species have shifted their ranges to higher elevations where the temperatures remain cooler. Wind speeds have also already increased in the Southern Ocean and are predicted to continue doing so, which may have effects on the size of areas suitable for nesting.
If nesting sites move to higher elevations on Marion Island as temperatures warm, and some areas become unsuitable due to changes in vegetation or wind speeds, it is likely that the suitable nesting area on the island will shrink considerably.
Our study adds to what is known about the elements affecting nest-site selection in birds. Notably, we add knowledge of wind, an underexplored element, influencing nest-site selection in a large oceanic bird. The results could also provide insights that apply to other surface-nesting seabirds.
- Climate change
- Southern ocean
- Natural world

Faculty of Law - Academic Appointment Opportunities

Operations Manager

Senior Education Technologist

Audience Development Coordinator (fixed-term maternity cover)

Lecturer (Hindi-Urdu)
The Wandering Albatross and Global Warming
The giant oceanic birds are producing more and plumper chicks, at least for now
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/accounts/headshot/greg-laden-240.jpg)
Weather changes not just from season to season, but also from year to year. Where I live in Minnesota, we had only a few days of frost before the year’s end, and January, normally the coldest month of the year, was relatively balmy. But in another year we might have days on end of sub-zero weather during the winter. It is hard for a person to detect climate change at this scale, even though global temperature measurements clearly show that the planet has warmed.
But every now and then something comes along that demonstrates a longer term trend that we can see and measure more directly. For instance, the USDA recently released a new version of its “ Plant Hardiness Zone Map .” If you are a gardener in the United States, you probably already know about this map; its zones are used to determine what kinds of plants can be grown outdoors in your area, the estimated dates of the last killing frost in the spring and the first killing frost in the fall. This is at least the second time in my memory that this map has been redrawn with all the zones moved to the north, reflecting a warming planet in a way that every gardener can observe and understand.
Not all global climate changes are simple warming, however. Global warming causes changes in ocean and atmospheric circulation as well. Westerly winds in the southern Pacific Ocean have shifted south towards the pole and have become more intense. A recent study in Science shows that the foraging patterns of breeding Wandering Albatross ( Diomedea exulans ) on the Crozet Islands has been changed by global warming in a way that seems to benefit them now, but that will likely harm them in the future.
Albatross are members of the bird order Procellariiformes, also known as the “tubenoses” because of the tube-like “nostrils” on their beaks. There are about 170 species of this kind of bird, including the petrels, shearwaters, storm petrels, diving petrels, and albatrosses. It is commonly said that the ocean is the last great frontier on earth, and this is probably true. It should not come as a surprise, then, that the Procellariiformes are among the “last great frontiers” of birding and bird research. Since the tubenoses spend almost all of their time at sea, they are hard to study. They come to land only to breed, and even then, usually on remote islands. They are so committed to being in the air over the ocean or floating on the surface of the sea that most members of this order are unable to walk at all. One group of tubenoses has the capacity to shoot a stream of noxious liquid (from its gut) at potential predators, which is an interesting adaptation to being unable to stand up and peck at intruders attempting to eat one’s egg or chick. (See this post for more information on tubenoses and a review of an excellent recent book on the tubenoses of North America.)
For all these reasons, foraging during nesting is a stress point in the life history of albatross. The birds forage by soaring around over the ocean, using wind as their main form of propulsion, literally sniffing out food sources (they have excellent smelling abilities). Therefore, the pattern of oceanic winds should matter a lot to their survival, especially during breeding season.
Which brings us back to changes in wind patterns due to global warming. The study by Henri Weimerskirch, Maite Louzao, Sophie de Grissac and Karine Delord is destined to become a classic because it touches on a sequence of logically connected observations to tell a compelling story. For my part, I’m going to use this in a classroom to demonstrate interesting science at my next opportunity. Let’s go over it step by step.
Albatross breeding is clearly difficult, and failure is likely common. One indicator of this is the fact that wandering albatross lay only one egg per season. Most coastal and terrestrial birds lay more than one, and in many species the number they lay varies from year to year depending on conditions. If wandering albatross lay only one egg, ever, there is a sort of underlying biological expectation of a low success rate.
For most birds, size matters. Within the normal range for a species, individual birds grow larger when conditions are good, and those birds do better in periods of difficulty because a large body stores more reserves and provides for more effective competition with other birds. A bird can grow large and bring lots of food back to the nest only if foraging is good, and the amount of food a bird obtains in a day is a combination of time (how long one forages) and the amount of food available in the environment.
The amount of food an albatross can obtain depends in part on the total area of the ocean that is searched each day, which in turn depends on how fast the bird flies. Since the albatross soars on the wind most of the time, this means that everything depends on factors such as the speed and direction of the wind. The study we are looking at today combines all of these things in an elegant exposé of the link between climate and the difficult job of producing baby albatrosses.
The wandering albatross travel enormous distances from their breeding grounds, often going more than 1,000 miles before returning to the nest to relieve their mate from guard duty. Males forage more widely and more to the south than females, who prefer northern waters. During this time, the birds use the wind as their primary form of locomotion. The researchers have shown that the winds in this region have increased in strength by a measurable amount, owing to shifts related to global warming. The average wind speed has gone up by about 10 percent from the 1990s to the present day. This allows the birds to move from foraging area to foraging area more swiftly than otherwise possible.
The total amount of time it takes both male and female albatross to complete a full journey of a given distance has decreased by between 20 percent and 40 percent from the 1990s to the present, and the speed at which the birds are observed to fly has gone up about the same for females, though the observed speed increase for males is not statistically significant. This is direct evidence that the amount of time spent foraging is less under present conditions than it was in the recent past, and it can be inferred that this is caused by the correlated increases in wind speed.
During the same period of time, the birds have gotten bigger. In 1990 the average female was about 7,500 grams and by 2010 females were about 8,500 grams. Males increased by about the same percentage, going from the mid-9,000 range to about 10,500 grams. These differences in mass are not reflected in the overall dimensions of the bird, just their weight. This indicates that during periods when the birds are on average smaller, many are underfed.
Breeding success for albatross varies considerably. The chance of successfully launching a baby albatross from the nest for the 350 pairs studied ranges from about 50 percent to just over 80 percent depending on the year (I’m leaving out one really bad year when the success rate was only 25 percent). During the past 40 years, over which it is thought the wind patterns have changed as described above, the “moving average” of breeding success (taking a few years together into account to dampen natural variation) has changed from about 65 percent to about 75 percent. These birds indeed seem to be benefiting from changes in wind pattern caused by global warming.
Most changes in weather, patterns of wind and rain and other effects of global warming are negative, as any review of the literature on this topic over the past decade will show. The benefits being experienced by these birds is unusual. But it may also be temporary. The researchers who produced this result say that the shift of winds towards the poles that brought higher energy patterns to these islands is likely to continue. As wind speeds increase, the benefit the birds will receive will at first level off then start to decrease, as overly windy conditions are bad for the albatross. The shift of westerly winds to the south of the islands will probably decrease the viability of foraging over the next few decades because it will make it easier for the birds to get to places with lower quality forage and thus decrease the rate of obtaining food. So, if the current changes in wind patterns are a gravy train for the Crozet Island wandering albatross, the train may eventually leave the station without them.
Weimerskirch, H., Louzao, M., de Grissac, S., & Delord, K. (2012). Changes in Wind Pattern Alter Albatross Distribution and Life-History Traits Science, 335 (6065), 211-214 DOI: 10.1126/science.1210270
Get the latest Science stories in your inbox.
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/accounts/headshot/greg-laden-240.jpg)
Greg Laden | | READ MORE
Greg Laden is a freelance science writer.
Smithsonian Ocean
Wandering albatross.

A wandering albatross has the largest wingspan of any bird, 3.5 meters (11.5 feet) tip to wing tip.
- Make Way for Whales
- Sharks & Rays
- Invertebrates
- Plants & Algae
- Coral Reefs
- Coasts & Shallow Water
- Census of Marine Life
- Tides & Currents
- Waves, Storms & Tsunamis
- The Seafloor
- Temperature & Chemistry
- Ancient Seas
- Extinctions
- The Anthropocene
- Habitat Destruction
- Invasive Species
- Acidification
- Climate Change
- Gulf Oil Spill
- Solutions & Success Stories
- Get Involved
- Books, Film & The Arts
- Exploration
- History & Cultures
- At The Museum
Search Smithsonian Ocean
Albatross: Lifetime at Sea
When hearing the word albatross , some might think of a really good round of golf (three under par). Like scoring an albatross in golf, sighting a long-lived master of flight in the Albatross family is a special treat. Chances are you haven’t seen one in person, but to put a name to this special type of seabird opens the door to their world.
Masters of Efficient Flight
There are 22 species of albatross that share the gift of efficient long-distance gliding flight. They are famously recognized by their lengthy wingspans with the Wandering Albatross holding the record at nearly 12 feet. These remarkable wingspans are vital for a lifetime at sea. With the help of air currents and temperature changes, these wings are able to provide enormous amounts of lift; albatross can spend hours in flight without rest or a single flap. Their flying abilities allow albatross to journey thousands of miles across open oceans.

Many people view their elders and put some thought into what those eyes have seen over a lifetime; what experiences that person has had, or wisdom and knowledge they’ve picked up through the years. These same thoughts could be applied when looking into the eyes of an albatross. Albatross can live decades and spend most of their long lives at sea. When an albatross encounters a fishing vessel or is counted on the breeding grounds, these birds may be decades older than the people studying these magnificent gliders. It could be safe to assume that an adult albatross knows their way around the seas better than the career fisherman or woman they are following.
Throughout history, humans have shared the seas with these seabirds. Many sailors recognize that albatross will follow their vessels, looking for an easy meal. Interactions, intentional or accidental, have resulted in the near-extinction of some species of albatross. Conservation efforts have been put in place by multi-nation partnerships, which have contributed to success in rising numbers of albatross seen in the Pacific Ocean.
Albatrosses in Alaska
Alaska is within the range of Short-tailed, Laysan and Black-footed Albatross which are commonly seen at-sea. These birds take to land to breed on ocean islands, including the world’s largest albatross colony on Midway Atoll National Wildlife Refuge .
Short-tailed Albatrosses
This endangered species breeds primarily on two remote islands in the western Pacific with the majority (~85%) breeding on Torishima, Japan (an active volcano in the Izu Island Group, northwest of Taiwan). From 2008 to 2012 the U.S. Fish and Wildlife Service and Japanese partners at the Yamashina Institute for Ornithology worked together to establish a third breeding colony by translocating chicks from Torishima to a historic breeding location on the island of Mukojima. Recently, short-tailed albatrosses have also successfully bred on Midway Atoll.
Short-tailed Albatrosses generally head toward their feeding grounds around April and May, but have been known to make the long journey into Alaskan waters just to feed and return to their nest. They have been seen feeding along shelf breaks in the Bering Sea and Gulf of Alaska, along the Aleutian Islands, and southeast Alaska. They also occur along the Pacific coasts of Canada and the United States including waters along Washington, Oregon, and California.
Short-tailed Albatross follow fishing vessels and are sometimes hooked or entangled in longline fishing gear and drowned. The U.S. Fish and Wildlife Service has been working with the commercial fishing industry, Washington Sea Grant, and National Marine Fisheries Service to minimize take of this endangered seabird. Through this collaborative conservation effort, a type of seabird avoidance technology called “streamerlines” was developed to reduce the bycatch of albatrosses.
Streamerlines create a visual barrier that keeps seabirds away from the baited hooks. In Alaska, streamerlines deployed on fishing vessels has led to a major reduction in the bycatch of albatrosses. Fishermen who have used streamerlines to ward off seabirds say there is also a financial benefit: the streamer lines keep seabirds from swiping their bait, saving them money in the long run.
From near extinction at the turn of the 20th century, to being listed as endangered throughout its range in 2000, the population of short-tailed albatross continues to grow with a current estimate of 7,365 individuals and a population growth rate of 8.9%. This is something to celebrate.
Black-footed Albatross
Unlike the Short-Tailed Albatross, the Black-footed Albatross is not currently listed as threatened or endangered under the Endangered Species Act (ESA). Black-Footed Albatross are only found in the Pacific Ocean with breeding populations located on the Hawaiian and Japanese islands. Breeding occurs from late fall to mid-summer and involves a colorful display of head bobs, wing flaps, and foot stomps. If you have not witnessed a Black-Footed Albatross mating dance, that should be your next internet search as it is a sight to see. Black-footed Albatross, like other albatross species, are thought to mate for life but will find a new mate if their partner disappears or passes away.
After breeding these seabirds can be seen in the North Pacific where they feed on fish, squid, and crustaceans. Like other albatross species, these birds can also be seen tailing ships for easy meals and have sometimes become victims to accidental entanglement into fishing equipment at sea. They too have benefited from Short-tailed Albatross conservation efforts via reduced accidental bycatch.

Laysan Albatross
One of the easier identifiable albatross seen in the seas surrounding Alaska is the Layson Albatross. These seabirds are generally smaller in size when compared to other albatross sharing its range, but is most noticeably different by its white belly and head that is often referred to as “gull-like”. Add in a gray-brown wings with white undersides and a dark tail and you’ve got yourself a Laysan Albatross.

Laysan Albatross are more commonly seen out at sea away from North American shores. 97.7% of the population call the northwestern Hawaiian Islands home during the breeding season (late fall to mid-summer) before moving north through the Pacific eventually making their way to Alaskan fishing regions. For those of you traveling through the southwest, don’t be too surprised to see one of these seabirds overhead, they’ve been known to wander inland during their migration north.
These seabird’s have a diet consisting of squid, fish, crustaceans and flying fish eggs. They primarily feed at night. In regards to fishing bycatch, this could be beneficial or negative depending on fishing operation times and the effectiveness and use of mitigating equipment such as streamers at night. Like other albatross, Laysan Albatross sometimes fall victim to fishing equipment such as baited lines and driftnets. They have also benefited from conservation efforts to reduce seabird bycatch during fishing operations. Fishing bycatch, however, is not the only issue that Laysans and other sea life must face, plastics and debris scattered through the world’s oceans are also part of this seabird’s diet, which in many cases can prove to be fatal.
A note on plastic pollution: Be Part of the Solution
Like many birds, albatross can fall victim to plastic pollution that makes its way to sea. Because they feed along the surface on squid, krill, fish eggs and other items, albatrosses often accidentally swallow floating plastic. This becomes a problem when their stomach becomes impacted and full of plastic resulting in lack of nutrition from natural prey. On the breeding grounds, baby albatrosses suffer from a diet of this plastic trash brought in by their parents from the ocean. Parents feed their chicks by regurgitating what they’ve found out at sea. It’s estimated that adult albatrosses unwittingly bring back thousands of pounds of marine debris back to places like Midway atoll every year. Dead chicks that have starved due to plastic ingestion can be found on the breeding grounds and are testament to this global problem.One way you can make a small difference is picking up plastic trash before it makes its way into rivers and eventually to sea.

You can help albatross and all seabirds by recycling as much or your plastic as possible, saying ‘no’ to single use plastic, using a re-usable water bottle, bringing re-usable bags to the grocery store. We can all do our part to help make the oceans safe for all birds and ensure that the graceful flight of the albatross can be witnessed by generations to come.
In Alaska we are shared stewards of world renowned natural resources and our nation’s last true wild places. Our hope is that each generation has the opportunity to live with, live from, discover and enjoy the wildness of this awe-inspiring land and the people who love and depend on it. Compiled by Kristopher Pacheco, Alaska Digital Media Assistant for the U.S. Fish and Wildlife Service, with Katrina Liebich and staff from Migratory Birds Management and Ecological Services. For this article and others, follow us on Medium .
Recreational Activities
Related stories.

Latest Stories

You are exiting the U.S. Fish and Wildlife Service website
You are being directed to
We do not guarantee that the websites we link to comply with Section 508 (Accessibility Requirements) of the Rehabilitation Act. Links also do not constitute endorsement, recommendation, or favoring by the U.S. Fish and Wildlife Service.

Northeast India News, Assam News, Breaking News of Northeast | Latest News Live | EastMojo

Albatrosses are threatened with extinction; climate change could put their nesting sites at risk

The wandering albatross ( Diomedea exulans ) is the world’s largest flying bird , with a wingspan reaching an incredible 3.5 metres. These birds are oceanic nomads: they spend most of their 60 years of life at sea and only come to land to breed approximately every two years once they have reached sexual maturity.
Trending Stories

Latest Stories

Leave a comment
Leave a comment cancel reply.
Disclaimer: Stories/articles published under EM Buzz (eastmojo.com/em-buzz) are provided by third parties and EastMojo.com has no direct relation with these articles.

Animal encyclopedia
Exploring the magnificent wandering albatross.
September 4, 2023

John Brooks
September 4, 2023 / Reading time: 6 minutes

Sophie Hodgson
We adhere to editorial integrity are independent and thus not for sale. The article may contain references to products of our partners. Here's an explanation of how we make money .
Why you can trust us
Wild Explained was founded in 2021 and has a long track record of helping people make smart decisions. We have built this reputation for many years by helping our readers with everyday questions and decisions. We have helped thousands of readers find answers.
Wild Explained follows an established editorial policy . Therefore, you can assume that your interests are our top priority. Our editorial team is composed of qualified professional editors and our articles are edited by subject matter experts who verify that our publications, are objective, independent and trustworthy.
Our content deals with topics that are particularly relevant to you as a recipient - we are always on the lookout for the best comparisons, tips and advice for you.
Editorial integrity
Wild Explained operates according to an established editorial policy . Therefore, you can be sure that your interests are our top priority. The authors of Wild Explained research independent content to help you with everyday problems and make purchasing decisions easier.
Our principles
Your trust is important to us. That is why we work independently. We want to provide our readers with objective information that keeps them fully informed. Therefore, we have set editorial standards based on our experience to ensure our desired quality. Editorial content is vetted by our journalists and editors to ensure our independence. We draw a clear line between our advertisers and editorial staff. Therefore, our specialist editorial team does not receive any direct remuneration from advertisers on our pages.
Editorial independence
You as a reader are the focus of our editorial work. The best advice for you - that is our greatest goal. We want to help you solve everyday problems and make the right decisions. To ensure that our editorial standards are not influenced by advertisers, we have established clear rules. Our authors do not receive any direct remuneration from the advertisers on our pages. You can therefore rely on the independence of our editorial team.
How we earn money
How can we earn money and stay independent, you ask? We'll show you. Our editors and experts have years of experience in researching and writing reader-oriented content. Our primary goal is to provide you, our reader, with added value and to assist you with your everyday questions and purchasing decisions. You are wondering how we make money and stay independent. We have the answers. Our experts, journalists and editors have been helping our readers with everyday questions and decisions for over many years. We constantly strive to provide our readers and consumers with the expert advice and tools they need to succeed throughout their life journey.
Wild Explained follows a strict editorial policy , so you can trust that our content is honest and independent. Our editors, journalists and reporters create independent and accurate content to help you make the right decisions. The content created by our editorial team is therefore objective, factual and not influenced by our advertisers.
We make it transparent how we can offer you high-quality content, competitive prices and useful tools by explaining how each comparison came about. This gives you the best possible assessment of the criteria used to compile the comparisons and what to look out for when reading them. Our comparisons are created independently of paid advertising.
Wild Explained is an independent, advertising-financed publisher and comparison service. We compare different products with each other based on various independent criteria.
If you click on one of these products and then buy something, for example, we may receive a commission from the respective provider. However, this does not make the product more expensive for you. We also do not receive any personal data from you, as we do not track you at all via cookies. The commission allows us to continue to offer our platform free of charge without having to compromise our independence.
Whether we get money or not has no influence on the order of the products in our comparisons, because we want to offer you the best possible content. Independent and always up to date. Although we strive to provide a wide range of offers, sometimes our products do not contain all information about all products or services available on the market. However, we do our best to improve our content for you every day.
Table of Contents
The Wandering Albatross is a truly remarkable bird that captivates the imagination of wildlife enthusiasts and researchers alike. With its impressive wingspan and majestic flight, this magnificent creature has a unique story to tell. In this article, we will delve into the world of the Wandering Albatross, exploring its characteristics, habitat, life cycle, diet, threats, conservation efforts, and even its role in culture and literature.
Understanding the Wandering Albatross
The Wandering Albatross, a majestic seabird, is a fascinating creature that captures the imagination with its impressive size and unique characteristics . Let’s delve deeper into the defining features and habitat of this remarkable bird.
Defining Characteristics of the Wandering Albatross
With a wingspan of up to 11 feet, the Wandering Albatross boasts the largest wingspan of any bird in the world. This remarkable wingspan allows it to glide effortlessly over the vast open oceans it calls home. As it soars through the air, its wingspan creates a mesmerizing spectacle, showcasing the bird’s incredible adaptability to its environment.
The Wandering Albatross is easily recognizable by its distinctive white feathers , sleek body, and long, slender wings . These defining features not only contribute to its graceful appearance but also serve a purpose in its survival. The white feathers help camouflage the bird against the bright sunlight reflecting off the ocean’s surface, while the sleek body and long wings enable it to navigate the winds with precision.
The Albatross’s Unique Habitat
These graceful birds are found primarily in the southern oceans, particularly around the Antarctic region. The vast expanse of the Southern Ocean provides an ideal environment for the Wandering Albatross to thrive. With its ability to cover immense distances, the bird utilizes the strong winds to its advantage, effortlessly gliding across the ocean in search of food and suitable breeding grounds.
During their long journeys, Wandering Albatrosses traverse various oceanic regions, from the sub-Antarctic to as far as the coast of South America. Their nomadic lifestyle allows them to explore different ecosystems , adapting to the ever-changing conditions of the open ocean.
When on land, the Wandering Albatross prefers remote and isolated islands for nesting. These islands provide the perfect breeding environment, away from human disturbance and terrestrial predators. Here, amidst the rugged cliffs and pristine beaches, the albatrosses establish their colonies, creating a spectacle of life in the midst of the vast ocean.
These incredible birds are known to return to the same nesting sites year after year, demonstrating their strong site fidelity . The remote islands become their sanctuary, where they engage in courtship rituals, build nests, and raise their young. It is a testament to their resilience and adaptability that they have managed to maintain these nesting sites for generations, despite the challenges they face in the ever-changing world.
As we continue to explore and understand the Wandering Albatross, we uncover more about its remarkable adaptations, behaviors, and interactions with its environment. The more we learn, the more we appreciate the intricate web of life that exists in the vast oceans, where these magnificent birds reign supreme.
The Life Cycle of the Wandering Albatross
Breeding and nesting patterns.
The breeding season for the Wandering Albatross begins in the austral summer months, with courtship rituals that involve intricate displays of dance and vocalizations . These courtship displays are not only a way for the albatrosses to attract a mate but also a means of establishing dominance within their colony. The males showcase their impressive wingspan and perform elaborate dances, while the females respond with their own graceful movements.
Once a pair bonds, they establish a nest on the chosen island and begin the process of reproduction. The nests are carefully constructed using a combination of soil, grass, and other materials found on the island. The albatrosses take great care in selecting the perfect location for their nest, ensuring it is protected from the harsh elements and predators.
The female typically lays a single egg, which both parents take turns incubating. Incubation lasts for approximately 60 days, during which the parents rotate shifts to keep the egg warm and protected. This shared responsibility is a testament to the strong bonds formed between Wandering Albatross pairs. The parents take turns leaving the nest to search for food, returning to regurgitate the nutrient-rich meal for their growing chick.
During the incubation period, the albatrosses face numerous challenges. They must withstand strong winds, freezing temperatures, and potential threats from predators . Despite these difficulties, the dedicated parents remain vigilant, ensuring the survival of their offspring.
Growth and Development Stages
After hatching, the chicks are cared for and fed by both parents. The parents regurgitate a nutrient-rich oil that provides essential nourishment for the growing chick. This feeding process continues for several months until the chick becomes independent enough to forage on its own. The oil not only provides the necessary nutrients but also helps to strengthen the chick’s immune system, protecting it from potential diseases.
As the chick grows, it undergoes various developmental stages. Its downy feathers gradually give way to juvenile plumage, which is darker in coloration. The chick’s beak also undergoes changes, becoming stronger and more adapted to catching prey. During this time, the parents continue to provide guidance and protection, teaching the chick essential survival skills.
It takes years for a Wandering Albatross chick to reach maturity. During this time, they undergo a remarkable transformation, gradually developing their characteristic white plumage and mastering their flight skills. The albatrosses spend a significant portion of their juvenile years at sea, honing their flying abilities and exploring vast oceanic territories. It is during this period that they face various challenges, including encounters with other seabirds and potential threats from human activities.
It is this lengthy growth period that contributes to the vulnerability of this species and its slow population recovery. The Wandering Albatross faces numerous threats, including habitat loss, climate change, and accidental capture in fishing gear. Conservation efforts are crucial to ensure the survival of these magnificent birds and their unique life cycle.
The Wandering Albatross’s Diet and Hunting Techniques
Preferred prey and hunting grounds.
The Wandering Albatross is primarily a scavenger, feeding on a variety of marine organisms, including squid, fish, and crustaceans. They use their keen eyesight to spot potential prey items floating on the ocean surface, and once sighted, they plunge-dived from great heights to capture their meal. Additionally, these birds are known to scavenge carrion and exploit fishing vessels for an easy meal.
Adaptations for Hunting in the Open Ocean
Surviving in the harsh oceanic environment requires specialized adaptations, and the Wandering Albatross is well-equipped for the task. Its long wings enable it to glide effortlessly for long periods, conserving energy during hours of flight. The bird’s keen sense of smell allows it to locate food sources, even from great distances. These adaptations make the Wandering Albatross a formidable hunter and a vital component of the oceanic ecosystem.
Threats and Conservation Efforts
Human impact on the wandering albatross.
Despite their grace and beauty, Wandering Albatrosses face numerous threats that have contributed to their decline. One of the main challenges is the destructive impact of longline fishing operations, where the birds mistakenly become hooked or tangled in the fishing gear. Additionally, pollution, habitat degradation, and climate change further jeopardize the survival of these birds.
Current Conservation Strategies and Their Effectiveness
To safeguard the future of the Wandering Albatross, concerted conservation efforts are underway. Several measures have been implemented, including the establishment of protected areas and marine reserves, the development of guidelines for responsible fishing practices, and public awareness campaigns to promote the importance of nurturing this iconic species. While progress has been made, continued efforts are required to ensure the recovery and long-term survival of the Wandering Albatross.
The Role of the Wandering Albatross in Culture and Literature
Symbolism and significance in various cultures.
Throughout history, the Wandering Albatross has held deep cultural significance in many communities. In some cultures, these birds are considered symbols of loyalty, freedom, and endurance. They are often associated with seafaring traditions and are believed to bring good fortune to sailors.
The Albatross in Classic and Contemporary Literature
The haunting imagery of the Wandering Albatross has inspired numerous works of literature. Perhaps the most famous reference is found in Samuel Taylor Coleridge’s poem, “The Rime of the Ancient Mariner,” where an albatross is depicted as a harbinger of both good and ill fortune. Furthermore, many modern authors have woven the essence of the Wandering Albatross into their stories, capturing its mystique and its role as a symbol of the natural world.
In conclusion, the Wandering Albatross is a remarkable bird with a captivating presence. From its unique characteristics to its adaptations for survival in the open ocean , this magnificent creature enthralls all who encounter it. However, its existence is threatened by human activities and environmental changes. Through ongoing conservation efforts and a deeper appreciation of its cultural significance, we can work towards ensuring a future where the Wandering Albatross continues to grace the skies above the vast southern oceans.
Related articles
- Fresh Food for Cats – The 15 best products compared
- The Adorable Zuchon: A Guide to This Cute Hybrid Dog
- Exploring the Unique Characteristics of the Zorse
- Meet the Zonkey: A Unique Hybrid Animal
- Uncovering the Secrets of the Zokor: A Comprehensive Overview
- Understanding the Zebu: An Overview of the Ancient Cattle Breed
- Uncovering the Fascinating World of Zebrafish
- Watch Out! The Zebra Spitting Cobra is Here
- The Fascinating Zebra Tarantula: A Guide to Care and Maintenance
- The Yellow-Bellied Sapsucker: A Closer Look
- Uncovering the Mystery of the Zebra Snake
- The Amazing Zebra Pleco: All You Need to Know
- Discovering the Fascinating Zebra Shark
- Understanding the Impact of Zebra Mussels on Freshwater Ecosystems
- Caring for Your Zebra Finch: A Comprehensive Guide
- The Fascinating World of Zebras
- The Adorable Yorkshire Terrier: A Guide to Owning This Lovable Breed
- The Adorable Yorkie Poo: A Guide to This Popular Dog Breed
- The Adorable Yorkie Bichon: A Perfect Pet for Any Home
- The Adorable Yoranian: A Guide to This Sweet Breed
- Discover the Deliciousness of Yokohama Chicken
- Uncovering the Mystery of the Yeti Crab
- Catching Yellowtail Snapper: A Guide to the Best Fishing Spots
- The Brightly Colored Yellowthroat: A Guide to Identification
- Identifying and Dealing with Yellowjacket Yellow Jackets
- The Yellowish Cuckoo Bumblebee: A Formerly Endangered Species
- The Yellowhammer: A Symbol of Alabama’s Pride
- The Benefits of Eating Yellowfin Tuna
- The Yellow-Faced Bee: An Overview
- The Majestic Yellow-Eyed Penguin
- The Yellow-Bellied Sea Snake: A Fascinating Creature
- The Benefits of Keeping a Yellow Tang in Your Saltwater Aquarium
- The Beautiful Black and Yellow Tanager: A Closer Look at the Yellow Tanager
- The Fascinating Yellow Spotted Lizard
- What You Need to Know About the Yellow Sac Spider
- Catching Yellow Perch: Tips for a Successful Fishing Trip
- The Growing Problem of Yellow Crazy Ants
- The Rare and Beautiful Yellow Cobra
- The Yellow Bullhead Catfish: An Overview
- Caring for a Yellow Belly Ball Python
- The Impact of Yellow Aphids on Agriculture
- Catching Yellow Bass: Tips and Techniques for Success
- The Striking Beauty of the Yellow Anaconda
- Understanding the Yarara: A Guide to This Unique Reptile
- The Yakutian Laika: An Overview of the Ancient Arctic Dog Breed
- The Fascinating World of Yaks: An Introduction
- Everything You Need to Know About Yabbies
- The Xoloitzcuintli: A Unique Breed of Dog
- Uncovering the Mystery of Xiongguanlong: A Newly Discovered Dinosaur Species
- Uncovering the Mysteries of the Xiphactinus Fish
- Camp Kitchen
- Camping Bags
- Camping Coolers
- Camping Tents
- Chair Rockers
- Emergency Sets
- Flashlights & Lanterns
- Grills & Picnic
- Insect Control
- Outdoor Electrical
- Sleeping Bags & Air Beds
- Wagons & Carts
- Beds and furniture
- Bowls and feeders
- Cleaning and repellents
- Collars, harnesses and leashes
- Crates, gates and containment
- Dental care and wellness
- Flea and tick
- Food and treats
- Grooming supplies
- Health and wellness
- Litter and waste disposal
- Toys for cats
- Vitamins and supplements
- Dog apparel
- Dog beds and pads
- Dog collars and leashes
- Dog harnesses
- Dog life jackets
- Dog travel gear
- Small dog gear
- Winter dog gear
© Copyright 2024 | Imprint | Privacy Policy | About us | How we work | Editors | Advertising opportunities
Certain content displayed on this website originates from Amazon. This content is provided "as is" and may be changed or removed at any time. The publisher receives affiliate commissions from Amazon on eligible purchases.

Woods Hole, Mass. — Wandering albatrosses, which are an iconic sight in the Southern Ocean, are highly adapted to long-distance soaring flight. Their wingspan of up to 11 feet is the largest known of any living bird, and yet wandering albatrosses fly while hardly flapping their wings. Instead, they depend on dynamic soaring—which exploits wind shear near the ocean surface to gain energy—in addition to updrafts and turbulence.
Now researchers, including Philip Richardson , a senior scientist emeritus in Physical Oceanography Department at the Woods Hole Oceanographic Institution (WHOI), are unlocking more clues about exactly how wandering albatrosses are such amazing flyers.
In a new paper analyzing GPS tracks of wandering albatrosses, researchers have found that the birds’ airspeed increases with wind speed up to a maximum airspeed of 20 meters per second (m/s; 45 mph). Researchers developed a model of dynamic soaring, which predicts that the birds could fly much faster than 20 m/s. The paper concludes that the birds limit their airspeed by adjusting the turns in their trajectories to be around 60°, and that in low winds the birds exploit updrafts over waves to supplement dynamic soaring.
“We hypothesize that wandering albatrosses limit their maximum across-wind airspeeds to ~ 20 m/s in higher wind speeds (and greater wind turbulence), probably to keep the aerodynamic force on their wings during dynamic soaring well below the mechanically-tolerable limits of wing strength,” according to the paper, “Observations and Models of Across-wind Flight Speed of the Wandering Albatross,” published in the journal Royal Society Open Science .
The paper adds that, given the complex field of wind waves and swell waves often present in the Southern Ocean, “it is also possible that birds find it increasingly difficult to coordinate dynamic soaring maneuvers at faster speeds.”
Regarding low flight speeds by albatrosses, the paper notes that a theoretical model predicted that the minimum wind speed necessary to support dynamic soaring is greater than 3 m/s. “Despite this, tracked albatrosses were observed in flight at wind speeds as low as 2 m/s. We hypothesize at these very low wind speeds, wandering albatrosses fly by obtaining additional energy from updrafts over water waves,” according to the paper.
“We tried to figure out how these birds are using the winds to go long distances—without overstressing their wings—for foraging for food and returning to feed their chicks. To do that, we modeled dynamic soaring and what different turn angles would do to stress on the birds’ wings and speed over the water,” said journal paper co-author Richardson. A dynamic soaring trajectory is an s-shaped maneuver consisting of a series of connected turns, he noted.
“This research is a step in the direction of understanding how wandering albatrosses are able to do these foraging trips and maintain a fairly large population. These birds figured out an amazing way to use the wind to almost effortlessly soar for thousands of miles over the ocean. We wanted to find out exactly how they did it,” he said.
In addition to learning more about albatrosses, the study could have broader implications for helping researchers better understand how to use dynamic soaring to power potential albatross-type gliders to observe ocean conditions, Richardson added.
Trajectories of breeding wandering albatrosses nesting on South Georgia Island in the South Atlantic. These birds are highly adapted to long-distance soaring flight assisted by a wingspan of up to 11 feet--the largest known of any living bird. They use the winds to soar thousands of miles seeking food to bring back to nourish their chicks. (Map by Natalie Renier, ©Woods Hole Oceanographic Institution)
For the study, researchers used GPS to track 46 wandering albatrosses during foraging trips the birds made between February to September 2004. The birds were breeding on Bird Island, which is off the northwest tip of South Georgia in the Southern Atlantic Ocean. Wandering albatrosses lack sufficient musculature to sustain continuous flapping flight for long periods of time; however they have a shoulder lock that mechanically holds their wings outstretched so that little energy is expended while soaring, according to the paper.
Since the earliest days of scientific inquiry, the way that many birds are able soar—that is, fly without flapping their wings—has fascinated and perplexed observers, said paper co-author Ewan D. Wakefield , affiliate researcher at the University of Glasgow and postdoctoral research associate at the University of Durham, UK. Wandering albatrosses are particularly remarkable for their ability to soar over the surface of the sea for long periods, covering vast distances, Wakefield said. He added that the physical principles explaining dynamic soaring flight were established over a century ago: Basically, albatrosses swoop up and down between layers of fast and slow moving air near the surface of the sea, gaining airspeed each time they do so.
“However, as our study shows, real-world albatross flight differs considerably from the predictions of simple physical models,” Wakefield said. “On the one hand, our GPS-tracking data show that they can and do fly in lighter winds than dynamic soaring models say should be possible. We suspect that this is because they can also fly by surfing updrafts created by the large waves that constantly surge around their Southern Ocean home. On the other hand, the upper limit of albatrosses' airspeed that we measured is much slower than physics predicts. We think that this is because albatrosses need to keep the forces on their wings within tolerable limits. After all, they're made from bone and muscle, not aluminum and titanium. Our study therefore points to ways in which theoretical models need to be refined to capture more faithfully the amazing complexity and beauty of albatross flight.”
Richardson recalled being entranced by wandering albatrosses ever since he observed them during a 1997 oceanographic cruise in the South Atlantic Ocean. “We were steaming upwind at 15 knots, pounding into waves, and these albatrosses caught up to us from astern and were cruising around and having a grand old time,” Richardson said. “I sat there for hours watching these birds in amazement, and wondering how they could fly like that. Now we are learning more about how they do it.”
Funding for this research was provided by the Woods Hole Oceanographic Institution emeritus fund and the UK Natural Environment Research Council.
Authors: Philip L. Richardson 1 and Ewan D. Wakefield 2
Affiliations:
1 Department of Physical Oceanography, Woods Hole Oceanographic Institution, Woods Hole, MA, USA
2 Institute of Biodiversity, Animal Health and Comparative Medicine, University of Glasgow, Glasgow, UK
About Woods Hole Oceanographic Institution
The Woods Hole Oceanographic Institution (WHOI) is a private, non-profit organization on Cape Cod, Massachusetts, dedicated to marine research, engineering, and higher education. Established in 1930, its primary mission is to understand the ocean and its interaction with the Earth as a whole, and to communicate an understanding of the ocean’s role in the changing global environment. WHOI’s pioneering discoveries stem from an ideal combination of science and engineering—one that has made it one of the most trusted and technically advanced leaders in basic and applied ocean research and exploration anywhere. WHOI is known for its multidisciplinary approach, superior ship operations, and unparalleled deep-sea robotics capabilities. We play a leading role in ocean observation and operate the most extensive suite of data-gathering platforms in the world. Top scientists, engineers, and students collaborate on more than 800 concurrent projects worldwide—both above and below the waves—pushing the boundaries of knowledge and possibility. For more information, please visit www.whoi.edu
Key takeaways:
- By analyzing GPS tracks of wandering albatrosses, researchers have found that the birds’ airspeed increases with wind speed up to a maximum of 20 meters per second (45 miles per hour).
- Researchers developed a model of dynamic soaring, which predicts that the birds could fly much faster than 20 meters per second (m/s). However, researchers hypothesize that the birds limit their maximum across-wind airspeeds to about 20 m/s in higher wind speeds (and greater wind turbulence), probably to keep the aerodynamic force on their wings during dynamic soaring well below the mechanically-tolerable limits of wing strength.
- The paper concludes that the birds limit airspeed by adjusting the turns in their trajectories to be around 60° and that in low winds the birds exploit updrafts over waves to supplement dynamic soaring.
- Although a theoretical model predicted that the minimum wind speed necessary to support dynamic soaring is greater than 3 meters per second (m/s), GPS-tracked albatrosses were observed in flight at wind speeds as low as 2 m/s. Researchers hypothesize at these very low wind speeds, wandering albatrosses fly by obtaining additional energy from updrafts over water waves.
- The study points to ways in which theoretical models need to be refined to capture more faithfully the amazing complexity and beauty of albatross flight.
Wandering Albatross
These remarkably efficient gliders, named after the Greek hero Diomedes, have the largest wingspan of any bird on the planet

Region: Antarctica
Destinations: Bouvet Island, Antarctic Peninsula, South Georgia
Name : Wandering Albatross, Snowy Albatross, White-winged Albatross ( Diomedea exulans )
Length: Up to 135 cm.
Weight : 6 to 12kg.
Location : All oceans except in the North Atlantic.
Conservation status : Vulnerable.
Diet : Cephalopods, small fish, crustaceans.
Appearance : White with grey-black wings, hooked bill.
How do Wandering Albatrosses feed?
Wandering Albatrosses make shallow dives when hunting. They’ll also attempt to eat almost anything they come across and will follow ships in the hopes of feeding on its garbage. They can gorge themselves so much that they become unable to fly and just have to float on the water.
How fast do Wandering Albatrosses fly?
Wandering Albatrosses can fly up to 40 km per hour.

What are Wandering Albatross mating rituals like?
Wandering Albatrosses mature sexually around 11 years of age. When courting, the male Wandering Albatross will spread his wings, wave his head around, and rap his bills against that of the female while making a braying noise. The pair will mate for life, breeding every 2 years. Mating season starts in early November with the Albatrosses creating nests of mud and grass on one of the Sub-Antarctic islands. The female will lay 1 egg about 10 cm long, sometime between the middle of December and early January. Incubation takes around 11 weeks, the parents taking turns. Once the chick is born the adults switch off between hunting and staying to care for the chick. The hunting parent returns to regurgitate stomach oil for the chick to feed on. Eventually both parents will start to hunt at the same time, visiting with the chick at widening intervals.

How long do Wandering Albatrosses live?
Wandering Albatrosses can live for over 50 years.
How many Wandering Albatrosses are there today?
There are about 25.200 adult Wandering Albatrosses in the world today.
Do Wandering Albatrosses have any natural predators?
Because they’re so big and spend almost all of their lives in flight, Wandering Albatrosses have almost no natural predators.
7 Wonderful Wandering Albatross Facts
- The Wandering Albatross is the largest member of its genus ( Diomedea ) and is one of the largest birds in the world.
- Wandering Albatrosses are also one of the best known and most studied species of birds.
- Diomedea refers to Diomedes, a hero in Greek mythology; of all the Acheaens he and Ajax were 2 nd only to Achilles in prowess. In mythology all of his companions turned into birds. Exulans is Latin for “exile” or “wanderer.”
- Wandering Albatrosses have the largest wingspan of any bird in the world today, stretching up to 3.5 metres across.
- Wandering Albatrosses are great gliders – they can soar through the sky without flapping their wings for several hours at a time. They’re so efficient at flying that they can actually use up less energy in the air than they would while sitting in a nest.
- Wandering Albatrosses have a special gland above their nasal passage that excretes a high saline solution. This helps keep salt level in their body, combating all the salt water they take in.
- Wandering Albatrosses get whiter the older they get.

Related cruises

Falkland Islands - South Georgia - Antarctica
Meet at least six penguin species!
PLA20-24 A cruise to the Falkland Islands, South Georgia & the Antarctic Peninsula. Visit some of the most beautiful arrays of wildlife on Earth. This journey will introduce you to at least 6 species of penguin and a whole lot of Antarctic fur seals!
m/v Plancius
Cruise date:
18 Oct - 7 Nov, 2024
Berths start from:

Antarctica - Basecamp - free camping, kayaking, snowshoe/hiking, photo workshop, mountaineering
The best activity voyage in Antarctica
HDS21a24 The Antarctic Peninsula Basecamp cruise offers you a myriad of ways to explore and enjoy the Antarctic Region. This expedition allows you to hike, snowshoe, kayak, go mountaineering, and even camp out under the Southern Polar skies.
m/v Hondius
1 Nov - 13 Nov, 2024

Weddell Sea – In search of the Emperor Penguin, incl. helicopters
Searching for the Elusive Emperor Penguins
OTL22-24 A true expedition, our Weddell Sea cruise sets out to explore the range of the Emperor Penguins near Snow Hill Island. We will visit the area via helicopter and see a variety of other birds and penguins including Adélies and Gentoos.
m/v Ortelius
10 Nov - 20 Nov, 2024

OTL23-24 A true expedition, our Weddell Sea cruise sets out to explore the range of the Emperor Penguins near Snow Hill Island. We will visit the area via helicopter and see a variety of other birds and penguins including Adélies and Gentoos.
20 Nov - 30 Nov, 2024

Antarctica - Basecamp - free camping, kayaking, snowshoe/hiking, mountaineering, photo workshop
HDS23-24 The Antarctic Peninsula Basecamp cruise offers you a myriad of ways to explore and enjoy the Antarctic Region. This expedition allows you to hike, snowshoe, kayak, go mountaineering, and even camp out under the Southern Polar skies.
23 Nov - 5 Dec, 2024
We have a total of 62 cruises

An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List

Vultures of the Seas: Hyperacidic Stomachs in Wandering Albatrosses as an Adaptation to Dispersed Food Resources, including Fishery Wastes
David grémillet.
1 CEFE-CNRS, UMR5175, Montpellier, France
2 Percy FitzPatrick Institute and DST-NRF Centre of Excellence at the University of Cape Town, Rondebosch, South Africa
Aurélien Prudor
3 CEBC-CNRS, UPR1934, Villiers en bois, France
Yvon le Maho
4 Institut Pluridisciplinaire Hubert Curien, Université de Strasbourg and CNRS UMR7178, Strasbourg, France
Henri Weimerskirch
Conceived and designed the experiments: DG HW YLM. Performed the experiments: DG AP. Analyzed the data: DG AP. Contributed reagents/materials/analysis tools: YLM HW DG. Wrote the paper: DG.
Animals are primarily limited by their capacity to acquire food, yet digestive performance also conditions energy acquisition, and ultimately fitness. Optimal foraging theory predicts that organisms feeding on patchy resources should maximize their food loads within each patch, and should digest these loads quickly to minimize travelling costs between food patches. We tested the prediction of high digestive performance in wandering albatrosses, which can ingest prey of up to 3 kg, and feed on highly dispersed food resources across the southern ocean. GPS-tracking of 40 wandering albatrosses from the Crozet archipelago during the incubation phase confirmed foraging movements of between 475–4705 km, which give birds access to a variety of prey, including fishery wastes. Moreover, using miniaturized, autonomous data recorders placed in the stomach of three birds, we performed the first-ever measurements of gastric pH and temperature in procellariformes. These revealed surprisingly low pH levels (average 1.50±0.13), markedly lower than in other seabirds, and comparable to those of vultures feeding on carrion. Such low stomach pH gives wandering albatrosses a strategic advantage since it allows them a rapid chemical breakdown of ingested food and therefore a rapid digestion. This is useful for feeding on patchy, natural prey, but also on fishery wastes, which might be an important additional food resource for wandering albatrosses.
Introduction
The capacity of animals to survive and reproduce in a given environment is often seen as primarily limited by energy acquisition (the metabolic theory of ecology [1] ). Yet two additional bottlenecks occur: (a) their ability to shed excess heat generated by muscle activity (heat dissipation limit theory [2] ), and (b) their capacity to digest food. This latter alternative has long been neglected, yet Karasov, Diamond and colleagues demonstrated the existence of digestive bottlenecks in a series of species, hummingbirds (e.g. Selasphorus rufus ) being the classic example [3] , [4] . Ecologically, digestion is a fundamental process since it does not only condition the fate of individual organisms, but also the flow of matter and energy across food webs [5] .
Biologically, digestion serves the purpose of breaking down and assimilating ingested food. In the digestive tract it is aided by mechanical churning, low pH, digestive enzymes, and the occasional symbiont [6] . The severity of this process largely depends upon the texture and hardiness of the food: when the aforementioned hummingbird feeds, nectar is easy to break down. At the other extreme, ostrich ( Struthio struthio ) food is proverbially tough.
In particular, generalists and/or scavengers need to be able to digest a broad diet, including hardy food [7] . Moreover, foraging theory predicts that animals feeding on patchy food should be capable of ingesting large amounts, and to digest them as quickly as possible [8] . This is particularly marked in birds which need to become airborne, even after the largest meals. A prime example of this strategy is found in vultures feeding on carrion. These species have large stomachs, and also very low stomach pH (1.5) which plays a crucial role in chemically dissolving hard parts, especially bones [9] . A pH of 1 to 2 is also optimal for proteolytic enzymes that play a crucial role in the breakdown of food [10] .
In the Southern Ocean, series of studies have addressed the capacity of marine predators to acquire food [11] , but little is known about their digestive physiology and potential digestive bottlenecks. In seabirds, pioneering work demonstrated that some prey, in particular squid, are more difficult to digest than others, that feeding on squid leads to delayed gastric emptying [12] , and that birds eating squid tend to have longer digestive tracts [13] .
Wandering albatrosses ( Diomedea exulans ), the largest extant seabird species, primarily feed on squid caught at the ocean’s surface [14] . However their diet is not restricted to squid, but shows a large variety of other prey such as fishes, carrion of seabirds and marine mammals, as well as fishery wastes, whose proportion vary according to sites or stages of the breeding season [15] – [18] . Wandering albatross food occurs in discrete and unpredictable patches; birds fly for extended periods before ingesting large squid or other prey at irregular intervals [19] . The most profitable predatory strategy is therefore to ingest as much food as possible whenever available and to move to another patch [20] . Albatross stomach morphology reflects this evolutionary constraint, with an estimated volume of 3–4 L [21] , which allows birds to ingest large single prey items of up to 3.2 kg [19] , i.e. over 30% of their own body mass. After such large meals, wandering albatrosses may have difficulties to take off if wind conditions are not favourable, which explains why they often remain at the ocean surface for several hours [22] . If they do manage to take off rapidly (in strong winds), such additional food load may increase their flight costs by increasing wing loading [23] . Wandering albatrosses therefore clearly should process large meals as quickly as possible, a strategy that they theoretically share with vultures that face similar foraging and flight constraints.
In this context, we tested the hypothesis that wandering albatrosses are vultures of the seas, designed to rapidly digest large volumes of hardy food such as squid, and are therefore pre-adapted to rapidly process fishery waste, a recently occurring resource that provides large quantities of food during a short period of time. To address this issue, we performed GPS-tracking of wandering albatrosses at sea, and recorded their stomach pH during, and in-between meals. These pH levels were then compared with those of other seabird species feeding on a variety of food types and with vulture stomach pH to test the prediction that wandering albatross stomach pH is as low as that of vultures.
Ethics Statement
All scientific procedures were validated by the ethics committee of the French Polar Institute (IPEV), were conducted according to its guidelines and under permits of the Réserve Naturelle des Terres Australes and of the Comité de l’Environnement Polaire.
The study was conducted in January – March 2011 on Possession Island (46°S, 51°E), Crozet Archipelago, Southern Ocean. Wandering albatrosses were studied while incubating, a period during which parents take shifts at the nest while a partner forages at sea for periods of a few days to a month [24] . Birds were caught at the nest within the framework of a long-term monitoring program of their foraging behaviour. Great care was taken to minimize stress while handling, which lasted <10 min in all cases. Birds were either fitted with a GPS data logger to record their movements at sea, or with a pH data logger to record stomach pH.
GPS Positioning
We used miniaturized GPS recorders (i-gotU, Mobile Action Technology Inc, New Taipei City, Taiwan; 44.5×28.5×13 mm, 20 g i.e. 0.2% bird body mass) attached with waterproof tape to feathers. Birds were captured and fitted with the GPS after they have been relieved by their partner and were about to leave for a foraging trip at sea. Device and tape were removed upon return to the colony after a single foraging trip. This technique has been successfully used on this species for nearly two decades [25] , with no measurable effects on behaviour, reproductive output or survival [26] . Devices were programmed to record a GPS position every 15 min across the foraging trip. Stored data were mapped on Google Earth® to illustrate wandering albatross at-sea home range.
Stomach pH and Temperature Recordings
We studied stomach pH and temperature using autonomous, miniaturized recorders enclosed in a titanium housing that was swallowed by the birds and remained in the stomach for the time of the measurement. The devices used (pH-meter, Earth & Ocean Technologies, Kiel, Germany, 11 cm long, 2 cm in diameter, mass 80 g i.e. 0.9% of bird body mass) are fully described in [27] , which also provide all necessary details about preparation, calibration procedures and data handling. Devices were set to record pH (accuracy 0.02 pH units) and temperature (accuracy <0.1°C) every ten seconds. Temperature data were analysed following [21] and [28] so as to estimate the mass of prey caught at sea using the amplitude and the duration of the temperature drop recorded in the stomach after prey ingestion.
The deployment procedure in the field closely followed previous investigations conducted in the same species [28] , using devices of the same mass and size, which nonetheless only recorded stomach temperature: Birds were induced to swallow the pH-meter at the beginning of the experiment, and it was recovered at the end of the measurement by stomach flushing, a technique which has been routinely used to gather stomach contents of seabirds for the purpose of dietary studies [29] .
GPS-tracking
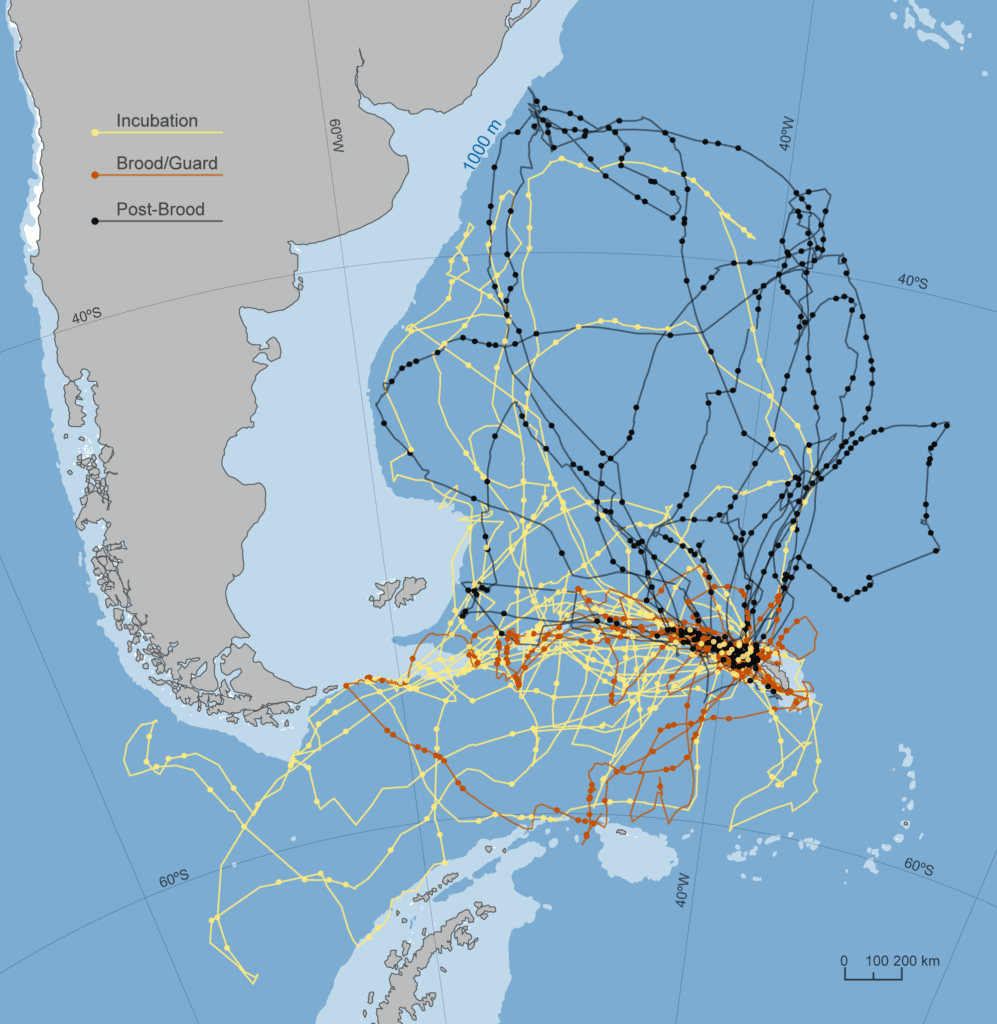
We equipped a total of 43 birds with GPS recorders. One device did not collect data, a second was lost at sea, and a third only collected data for 12 hours. Therefore a total of 40 complete tracks were collected, for at-sea journeys of between 3.6 and 21.1 days (mean 9.3±4.9), during which birds travelled between 475 and 4507 km (mean 3511±2718). As demonstrated in previous work, the duration of trips was very variable, with trips occurring over oceanic waters, as well as over the shelf edge ( Fig. 1 ).

(A). Five birds performed long trips towards northwest, three performed long trips towards southeast, five birds performed intermediate trips, nine birds remained between the Crozet Archipelago and the westward Prince Edward Islands, and 18 birds remained on the Crozet plateau (B), extensively foraging along its edge; suggesting local interactions with fishing vessels.
Stomach Temperature and pH Patterns
We equipped a total of 5 birds with pH-meters. Two individuals were equipped for a few hours at the nest during an initial test phase, while three were equipped before going out to sea. Within the latter group, only one bird came back to the nest with its pH-meter, the two others regurgitated the device at sea, something which had already occurred in previous studies using similar stomach loggers [28] , as it is the natural mechanisms by which wandering albatrosses and other seabirds evacuate indigestible food parts, such as squid beaks.
We therefore analyzed stomach pH and temperature recordings for three birds. In the bird that went out to sea (for a period of 7 days, Fig. 2 ), basal stomach pH was extremely low (pH 1.35±0.14), occasionally decreasing to pH 0.51. Parallel temperature recordings indicated ingestion of cold prey ( Fig. 2 ), who’s estimated mass was on average 110±280 g. Prey items were occasionally large, up to an estimated 1160 g. After the intake of such large items, stomach pH rose sharply (up to pH 4.88), and re-acidification to baseline levels only occurred within several hours to one day ( Fig. 2 ). The two birds that stayed on the nest and did not feed showed stable, very low stomach pH levels (average pH 1.50±0.13 and 1.65±0.10, respectively). These values are in line with the ground pH level recorded in the bird that went out to sea, and the average baseline pH was therefore pH 1.50±0.13 across all three birds.

Using the first stomach pH recording ever conducted in a foraging petrel, we validate our prediction that the stomach pH of wandering albatrosses is extremely low ( Fig. 2 ). Such low pH is very close to the baseline stomach pH recorded in white-backed griffon vultures ( Fig. 3 , [30] ), and is significantly lower than pH levels recorded in a variety of other seabird species that mainly feed on fish and were previously studied using the same miniaturised, autonomous pH-meters ( Fig. 3 ).

Our findings are based upon a very limited sample size, consisting of only one recording at sea and two for birds at the nest. They should be complemented by further recordings on a larger number of birds across different stages of the breeding cycle and also across different petrel species showing contrasting dietary preferences. However, our three recordings show consistent, extremely low baseline pH levels of 1.5 on average. Such physiological parameters are unlikely to show strong inter-individual variability, and indeed standard deviations for stomach pH measurements conducted in other bird species are within the same pH unit. We are therefore confident that our recordings demonstrate highly acidic (<2) stomach pH in wandering albatrosses.
Such low pH favours rapid chemical digestion of the food and is also optimal for proteolytic enzyme kinetic [10] . It is likely that this physiological characteristic evolved as a response to a diet largely composed of squid, and to a patchy distribution of this food resource resulting in large, infrequent meals. The strategy of wandering albatrosses is indeed to cover long distances rapidly and at low costs, to increase the probability of encountering dispersed prey patches whose distribution is unpredictable [22] , [31] . They catch on average one prey every 200 km, and some prey can be as heavy as 3.2 kg [22] , an additional load that increases wing loading and reduce optimality of flight [23] , [32] . As indicated above, they often remain at the sea surface for several hours after having swallowed large prey items [22] . This time spent on the sea surface without capturing additional prey probably corresponds to their digestion time, a period during which low stomach pH allows them to process food quickly, to become airborne again and fly at the lowest-possible energetic costs [31] . Being able to digest rapidly large meals represents an important advantage by reducing time spent on the water, or flight costs. This strategy is the marine equivalent to that of foraging vultures, which also remain on the ground after large meals.
However, low stomach pH represents also a strategic advantage for seabirds feeding upon fishery wastes: they can absorb large volumes of this patchy resource, and digest them rapidly. Direct observations around the Crozet-Kerguelen islands conducted from long-liners producing wastes (A. Prudor, unpubl data) show that wandering albatrosses are the dominant species within multi-species flocks attending fishing vessels because of their large body size and aggressive behaviour [31] . They also have sufficient stomach volume to ingest large volumes of these wastes, yet after a large meal they typically stay at the ocean’s surface for several hours.
Wandering albatrosses from the Crozet islands are thought to feed to some extent on wastes from long liners harvesting Patagonian toothfish ( Dissostichus eleginoides ), yet the amount of fishery waste that they actually consume remains to be determined, as well as the incidence of this artificial food resource upon seabird apparent fitness. Indeed, fishery wastes are generally beneficial to scavenging seabirds [33] , yet in certain cases they set ecological traps and diminish reproductive success [34] .
Acknowledgments
We are grateful to all participants of the 48th Crozet overwintering team, in particular Maxime Loubon, Anaëlle Atamaniuk, Simon-Pierre Babski and Jérémy Tornos for their dedicated help during fieldwork. Many thanks also to Emilie Tew Kai and Bénédicte Martin for computing and illustrative assistance.
Competing Interests: The authors have declared that no competing interests exist.
Funding: This study was funded by Centre National de la Recherche Scientifique and by the French Polar Institute Paul-Emile Victor (programme 109). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.

Wandering Albatross
Diomedea exulans.
The snowy albatross, also known as the white-winged albatross or goonie, is a majestic seabird belonging to the Diomedeidae family. It is recognized for its impressive wingspan, which is the largest of any living bird, and its predominantly white plumage that becomes whiter with age. The snowy albatross is distinguished by its large pink bill and feet, and the males exhibit whiter wings than females.
Identification Tips
Adult snowy albatrosses have white bodies contrasted with black and white wings. The wings of males are predominantly white, with only the tips and trailing edges presenting as black. This species is the whitest within its complex, with others showing more brown and black on the wings and body. A salt gland above their nasal passage helps them excrete excess salt due to their oceanic diet.
The snowy albatross boasts a wingspan that can exceed 3.5 meters (11 feet), with an average span of around 3.1 meters (10 feet 2 inches). Body length ranges from 107 to 135 cm (3 feet 6 inches to 4 feet 5 inches), with females being slightly smaller than males. Adults typically weigh between 5.9 to 12.7 kg (13 to 28 lb).
Distribution and Habitat
This bird has a circumpolar range in the Southern Ocean and breeds on islands such as South Georgia, Crozet, Kerguelen, Prince Edward, and Macquarie. It is also seen feeding year-round off the coast of New Zealand and is known for its extensive flights, sometimes circumnavigating the Southern Ocean three times in a year.
The snowy albatross is a far-ranging bird, spending most of its life in flight and landing only to breed and feed. It is capable of gliding for hours without flapping its wings, thanks to its large wingspan.
Song & Calls
During courtship, snowy albatrosses engage in a variety of displays, including spreading their wings, head-waving, bill-rapping, and producing a range of vocalizations from screams and whistles to grunts and bill clapping.
Snowy albatrosses are monogamous, often mating for life, and breed biennially. They lay a single white egg with a few spots in a large grassy nest. Incubation takes about 11 weeks, with both parents sharing the responsibility. The chicks are nurtured by both parents, who take turns foraging for food.
Similar Species
The snowy albatross is part of the wandering albatross species complex, which includes the Tristan albatross and the Antipodean albatross. It can be distinguished from its relatives by its whiter plumage and larger size.
Diet and Feeding
These birds feed on cephalopods, small fish, and crustaceans, often foraging further out in the open ocean than other albatross species. They are known to follow ships and can make shallow dives to capture their prey.
Conservation Status
The IUCN lists the snowy albatross as vulnerable. Threats include longline fishing and pollution. Conservation measures have been implemented in some regions to reduce bycatch and protect their breeding grounds.

.css-1cn5y0j{border-radius:0.25rem;font-size:0.875rem;line-height:1.25rem;font-weight:650;letter-spacing:0em;--tw-text-opacity:1;color:rgb(45 49 66 / var(--tw-text-opacity));font-style:normal;font-weight:650;margin-left:2rem;margin-right:2rem;margin-bottom:1.5rem;font-size:1.875rem;line-height:2.25rem;}@media (min-width: 768px){.css-1cn5y0j{margin-left:3rem;margin-right:3rem;}} Wandering Albatrosses on Birda
More albatrosses, amsterdam albatross, antipodean albatross, tristan albatross, southern royal albatross, northern royal albatross, short-tailed albatross, laysan albatross, waved albatross, black-footed albatross, sooty albatross, light-mantled albatross, buller's albatross, indian yellow-nosed albatross, shy albatross, atlantic yellow-nosed albatross, grey-headed albatross, chatham albatross, campbell albatross, black-browed albatross, salvin's albatross, your birdwatching journey like never before, connect with nature in minutes, discover the joy of birding, play your part in saving nature.

What Our Birders Say
Simply fantastic, fun way to add to your birdwatching experience, great app for learning birds, ideal birdwatch companion, a great app, awesome birding community, very good database, a mordern game changer, the best bird logging app.


Meet the Largest Flying Bird in the World: The Wandering Albatross
Published: July 15, 2023
The animal kingdom is filled with diverse incredible creatures, each with unique characteristics and abilities. Among them, the wandering albatross stands out as one of the most fascinating birds on the planet. With a wingspan of over three meters, it proudly holds the title of the largest flying bird in the world. These majestic creatures are known for their long-distance flights over the open ocean and remarkable resilience in surviving harsh weather conditions. Get ready to be amazed by this remarkable bird’s incredible abilities and features!

Soar to any section below!
Physical Characteristics
The wandering albatross can span over three meters, making it the largest flying bird in the world. This feature sets the wandering albatross apart from all other birds, giving it a unique and majestic appearance. As for their weight, albatrosses are relatively light despite their size, weighing in at around 7-11 kilograms.
The wandering albatross’s wingspan is a marvel of nature. Its wings are incredibly long and broad, specifically suited to gliding over long distances. Although they may look cumbersome, these wings are perfectly designed to give the bird maximum lift while minimizing drag during flight. This allows the wandering albatross to fly great distances without too much energy.
Feather Colors
The wandering albatross is mainly white, with black feathers on its back and wings. The color of its feathers gives the wandering albatross a striking appearance and serves a practical purpose. The white feathers help the bird blend with its surroundings, making it less visible to potential predators. On the other hand, the black feathers on its back help absorb heat, which is important when flying over the open ocean.
The wandering albatross’ beak is distinctive, with a hooked shape perfectly suited to its diet. These birds are primarily scavengers and will eat anything from squid to fish, with the occasional seal carcass thrown in. Their hooked beak helps them rip apart tough materials, such as fish skin, which they swallow whole.
Check out: Unearth the Reality of Georgia’s Brown Recluse Spiders .
Behavior And Lifestyle Of The Wandering Albatross

The wandering albatross is not just a remarkable bird because of its physical characteristics, it also showcases fascinating behaviors that have captivated researchers and bird enthusiasts alike. In this section, we delve into the distinct behaviors of the wandering albatross, including its breeding habits, migration patterns, hunting techniques, and socialization within flocks.
Breeding Habits
Breeding is a crucial part of the wandering albatross’s life cycle, and they typically breed on remote sub-Antarctic islands. These islands provide a haven for the birds to mate and rear their young without the threat of predators. Breeding pairs will mate for life; every breeding season, they will mate and produce a single egg that they take turns incubating. During incubation, the male and female albatrosses stay in the nest to keep the egg warm. Once the egg hatches, the parents feed the chick, regurgitating food from their stomachs to feed their young.

Migration Patterns
One of the most unusual behaviors of the wandering albatross is its long-distance migration patterns. These birds can fly thousands of kilometers over the open ocean, often without resting, for months. The albatrosses do this to find food, as their main source of nutrition is squid and fish, which they hunt in the open ocean. The wandering albatross also has a unique way of navigating their migrations. They use the Earth’s magnetic field as a guide, using their ability to sense the Earth’s magnetic field to orient themselves and navigate their journeys.
Hunting Techniques
When it comes to hunting, the wandering albatross has developed unique techniques that allow them to thrive in the harsh and challenging conditions of open ocean hunting. They use their incredible eyesight and sense of smell to locate squid and fish in the water. Once they spot their prey, they use their long, powerful wings to fly just above the water’s surface, dipping their beaks into the water to snatch up their meal.
Check out: Surviving the Realm of Tiger Snake Bites .
Socialization Within Flocks
The wandering albatross is a highly social bird, often forming large flocks when not breeding. These flocks provide safety and companionship for the birds while on their long journeys. They also perform elaborate courtship rituals within these flocks, using intricate dance moves and calls to attract potential mates.
Check out: Lost Cat’s Remarkable Cross-Country Journey Home .
Conservation Status Of The Wandering Albatross

The wandering albatross is undoubtedly one of the most striking birds on the planet. Unfortunately, it is one of the most vulnerable species and is listed as “endangered” under the IUCN Red List , meaning it is at risk of extinction. The wandering albatross faces numerous threats to its population, including climate change, habitat loss, and human activities such as fishing, pollution, and plastic waste.
Threats To Wandering Albatross Population
Climate change has caused a significant impact on the wandering albatross population. Changes in water temperature and ice cover affect the bird’s food supply, which can result in lower breeding success rates. The increase in plastic waste has also led to many albatrosses suffering entanglement and ingestion of plastic debris, resulting in death. The longline fishing industry is another serious threat to their population, with these birds accidentally killed by fishing hooks and nets.
Conservation Efforts
Several conservation efforts have been implemented to combat these threats to the wandering albatross population. The Agreement on the Conservation of Albatrosses and Petrels (ACAP) is an international agreement aimed to conserve albatross and petrel species and reduce the impact of harmful fishing practices.
The ACAP framework has implemented measures such as using bird-scaring streamers and setting longline fishing at night to avoid seabirds. There are also efforts to reduce plastic pollution through cleanup projects and recycling campaigns.
Success Stories
Despite the threats, there are some success stories. For example, in Macquarie Island, a designated United Nations Educational, Scientific, and Cultural Organization ( UNESCO ) World Heritage Site, the wandering albatross population is thriving due to strict conservation measures, including removing introduced animals such as rats and rabbits, which prey on the bird’s eggs and chicks.
Further efforts have led to the reduction of bird deaths due to fishing hooks. In South Africa, using small circle hooks has reduced the number of albatrosses caught in fishing gear by over 90%. These hooks do not harm the birds and can be easily removed if caught.
The wingspan of a Wandering Albatross can reach up to 11 feet, the largest of any bird in the world.
Wandering Albatross mainly feeds on fish and squid and can travel up to 600 miles daily to find food.
Wandering Albatross can live for up to 50 years and are known for their lifelong monogamous breeding pairs and unique courtship rituals.

The wandering albatross is an extraordinary bird that continues to capture the hearts and minds of scientists, birdwatchers, and nature enthusiasts worldwide. Its remarkable wingspan, ability to fly long distances over the open ocean, and resilience in harsh weather conditions are just a few qualities that set this bird apart from its peers. It’s no wonder that the wandering albatross is the world’s largest flying bird. With all its fantastic abilities and characteristics, it’s an animal kingdom marvel that deserves all the admiration and respect it gets.
Thanks for reading along! See below for related article links.
- Great White Shark Vs. Bull Shark
- The World’s Largest Land Predator: The Polar Bear
- Gorilla Vs. African Forest Buffalo
- Unveiling The Longest Snake In the World
- Discover Pennsylvania’s Hidden Threat: The Timber Rattlesnake
- Latest Posts
- Watch: Why You Should Never Approach A Bison in Yellowstone National Park (Video) - April 8, 2024
- Watch: Python Swallows A Full-Grown Crocodile - April 8, 2024
- Watch: Magpie Helps Hedgehog Cross The Street - April 8, 2024
Wandering albatrosses exert high take-off effort only when both wind and waves are gentle
- Leo Uesaka author has email address
Yusuke Goto
Masaru naruoka, henri weimerskirch, katsufumi sato, kentaro q. sakamoto.
- Atmosphere and Ocean Research Institute, The University of Tokyo, Kashiwa, Chiba 277-8564, Japan
- Information and Technology Center, The University of Tokyo, Kashiwa, Chiba, 277-0882, Japan
- Graduate School of Environmental Studies, Nagoya University, Furo, Chikusa, Nagoya, 464-8601, Japan
- Centre d’Etudes Biologiques de Chize (CEBC), UMR 7372 CNRS, Université de La Rochelle, 79360 Villiers-en-Bois, France
- Aeronautical Technology Directorate, Japan Aerospace Exploration Agency (JAXA), Chofu, Japan
- https://doi.org/ 10.7554/eLife.87016.2
- Open access
- Copyright information
The relationship between the environment and marine animal small-scale behavior is not fully understood. This is largely due to the difficulty in obtaining environmental datasets with a high spatiotemporal precision. The problem is particularly pertinent in assessing the influence of environmental factors in rapid, high energy consuming behavior such as seabird take-off. To fill the gaps in the existing environmental datasets, we employed novel techniques using animal-borne sensors with motion records to estimate wind and ocean wave parameters and evaluated their influence on wandering albatross take-off patterns. Measurements revealed that wind speed and wave heights experienced by wandering albatrosses during take-off ranged from 0.7 ∼ 15.4 m/s and 1.6 ∼ 6.4 m, respectively. The four indices measured (flapping number, frequency, sea surface running speed, and duration) also varied with the environmental conditions (e.g., flapping number varied from 0 to over 20). Importantly, take-off was easier under higher wave conditions than under lower wave conditions at a constant wind speed, and take-off effort increased only when both wind and waves were gentle. Our data suggests that both ocean waves and winds play important roles for albatross take-off and advances our current understanding of albatross flight mechanisms.
Impact statement
Wind and ocean wave conditions experienced by albatrosses were estimated using an animal-borne recorder and revealed that take-off was easier under higher wave conditions.
eLife assessment
This fundamental study advances our understanding of seabird responses to environmental conditions, with implications for movement ecology, flight biomechanics, animal foraging, and bio-energetics. Animal-borne data-loggers are used to generate a compelling high quality dataset on animal movement and environmental conditions. The study will interest ornithologists, comparative bio-mechanists, ocean ecologists and those interested in technological advances in animal sensors.
- https://doi.org/ 10.7554/eLife.87016.2.sa3
- Read the peer reviews
- About eLife assessments
Introduction
Various oceanic environmental factors affect the ecology of marine animals. Predicted climate changes suggest increases in extreme climatic events (such as cyclones). Thus, evaluating individual relationships between each environmental factor and marine animal behaviors is urgent for marine ecological conservation, especially for top predators that significantly impact the entire ecosystem. However, there are potential limitations: direct measures of marine animals empirical environmental data is nearly impossible due to the spatiotemporal gaps in the observation network of the open ocean ( Ardhuin et al., 2019 ; Villas Bôas et al., 2019). Various environmental parameters (such as ocean wind, waves, and sea surface temperature) are assumed to be important factors affecting the movement and foraging of flying seabirds ( Dunn, 1973 ; Haney et al., 1992 ; Adams and Navarro, 2005 ; Nevitt et al., 2008 ; Suryan et al., 2008 ). Previous research has revealed that many interesting seabird behaviors correlate with the ocean environment. However, the environmental data largely relies on ocean climatic models ( Padget et al., 2019 ; Weimerskirch and Prudor, 2019 ; Clay et al., 2020 ; Clairbaux et al., 2021 ), as in situ observation data is limited and often collected a long distance from the bird. For example, records are collected at the colony island or using the nearest government observation point ( Kogure et al., 2016 ; Yamamoto et al., 2017 ). Therefore, interpreting the data is difficult when doubts exist on whether birds actually experienced the same environmental conditions, making any conclusions conservative estimates only ( Clay et al., 2020 ; Clairbaux et al., 2021 ). For instance, although winter cyclones in the North Atlantic can induce mass seabird mortality, revealing the small-scale behavioral responses which lead to mortality is almost impossible with the spatiotemporal limits of thermodynamic modelling data ( Clairbaux et al., 2021 ).
Seabird take-off may be affected by the surrounding environment ( Clay et al., 2020 ) but has never been effectively investigated. Notably, behaviors with short timeframes (such as take-offs) require localized environmental data on spatiotemporally small scales, which is difficult to obtain, even using mathematical weather models. Many procellariiformes have special flight techniques that use vertical wind shear, called dynamic soaring ( Rayleigh, 1883 ; Richardson, 2011 ), while take-off requires a large amount of energy ( Sakamoto et al., 2013 ) owing to vigorous flapping ( Sato et al., 2009 ; Sakamoto et al., 2013 ) and sea surface running to reach the velocity to initiate take-off ( Sato et al., 2009 ). Previous research revealed the heart rate of the largest seabird, wandering albatross ( Diomedea exulans ), drastically increases at the moment of take-off reaching 3 – 4 times the basal heart rate ( Weimerskirch et al., 2000 ). After take-off, the tachycardia progressively decreases during flight ( Weimerskirch et al., 2000 ), the flying heart rate is close to the basal rate of a resting bird on the nest. Therefore, the high energy expenditure associated with take-off strongly influences the total energy expenditure of wandering albatross during the foraging trip, unlike flight duration or distance ( Shaffer et al., 2001a ). Thus, take-off is one of the most important behaviors in the daily energy budget of flying seabirds in the open ocean. Understanding the relationship between take-off and the ocean environment is critical for estimating the future climate change effects on the life history of seabirds ( Weimerskirch et al., 2012 ).
Previous research has partially identified the role of wind conditions on take-off when investigating general flight tactics of seabirds ( Kogure et al., 2016 ; Clay et al., 2020 ). For example, the flapping effort of the European shag ( Gulosus aristotelis ) at take-off decreases as wind speed increases ( Kogure et al., 2016 ). However, a comprehensive understanding of take-off has not been achieved as other environmental parameters, such as waves, have not been investigated. Ocean waves potentially affect take-off efforts because seabirds usually run on the ocean surface as they take-off ( Norberg and Norberg, 1971 ; Sato et al., 2009 ). Additionally, the ocean surface slope is a key factor in creating complicated wind patterns immediately above the surface, which may affect the flight tactics of procellariiformes ( Bousquet et al., 2017 ).
In this study, we devised a new approach to estimate the empirical local environmental conditions using seabird dynamic motion records without the aid of either mathematical weather models or observational data. The recent development of animal-borne recorders has been remarkable ( Wilmers et al., 2015 ). It is now possible to deploy various sensors on animal-borne recorders, to generate a new field of oceanography: ocean observations using animal-borne sensors ( Harcourt et al., 2019 ; McMahon et al., 2021 ). Many studies have reported that marine environmental data can be collected using highly mobile marine animals such as pinnipeds, sea turtles, and seabirds ( Charrassin et al., 2002 , 2008 ; Roquet et al., 2014 ; Doi et al., 2019 ). Furthermore, unlike direct measurement by deploying sensors (e.g., thermometers), indirect techniques to observe the physical environment via the dynamic animal motion records ( Yoda et al., 2014 ; Yonehara et al., 2016 ; Goto et al., 2017 ; Sánchez-Román et al., 2019 ; Uesaka et al., 2022 ) generated using the Global Navigation Satellite System (GNSS) have been developed recently. GNSS is now able to record animal position and movement every second (or even in sub-second scales) using very small animal-borne recorders. These newly developed techniques using animal-borne recorders should compensate for previous observational gaps in oceanic data, especially in the open ocean and polar regions where our access and deployment of observation equipment is complicated. The environmental variables obtained directly from free ranging animals provide the localized environmental conditions they experience. Seabirds are one of the most enthusiastically studied oceanic species because of their high mobility and adaptability to both air and water. Methods involving wind and wave observation, using GNSS data regarding the flight paths and floating motions of seabirds on the sea surface, are well-developed and have potential applicability in future studies ( Yonehara et al., 2016 ; Goto et al., 2017 ; Uesaka et al., 2022 ).
Wandering albatrosses were investigated because their habitat includes the Subantarctic (30 °S – 60 °S), where the ocean is annually rough ( Suryan et al., 2008 ) causing their flight behaviors to be largely influenced by the ocean conditions ( Richardson, 2011 ; Weimerskirch et al., 2012 ). Furthermore, previous studies have revealed the foraging area has shifted southward annually with the polar shift of the westerly wind pattern ( Weimerskirch et al., 2012 ). Considering the enormous cost of take-off ( Weimerskirch et al., 2000 ; Shaffer et al., 2001a ), studying their response to various environmental conditions is essential for us to estimate the impacts of climate change on the life history of seabirds. We aim to estimate the physical environmental conditions (ocean winds and wave heights) experienced by wandering albatrosses as they take-off by utilizing the dynamic motion records to evaluate the effects of wind and wave conditions on take-off dynamics ( Fig. 1 ). Procellariiformes, like many seabird species, require extensive limb motion for take-off which is not limited to flapping behavior. Therefore, the evaluation of take-off effort involves both surface running and flapping behaviors. The wandering albatross individuals were tagged using recorders that include both GPS and acceleration sensors with high time resolutions.

Conceptual framework of the study estimating environmental conditions experienced by studied individual.
We obtained 1477 h from 44 wandering albatrosses in 2019 (N = 21, 623 h) and 2020 (N = 23, 854 h). Two types of recorders with different battery sizes were used. The mean recording time of the trip data and the standard deviation (SD) was 9.5 ± 1.3 h for the small battery recorders and 59.7 ± 9.6 h for the large battery recorders. The albatross sex ratio was balanced between years and recorder type (Table. S1).
The absolute value of the GPS horizontal velocity revealed 703 take-offs from 1477 h of trips. A total of 453 out of 703 take-offs were followed by more than 5 min of flight. For each flight, the wind speed and direction were estimated using the flight path ( Yonehara et al., 2016 ). A total of 299 take-offs occurred after more than 15 min of floating time. Wave heights were estimated for each of the 299 take-offs using the floating motions ( Uesaka et al., 2022 ). For 185 take-offs, we estimated the wind and wave conditions in combination.
Environmental conditions at the take-off moment
Of the 453 estimated wind parameters, 26 were unreliable based on the Akaike information criterion (AIC) comparison and were not included in the analysis. The remaining 427 results revealed wind speeds of 6 – 8 m/s were most frequently experienced by taking-off wandering albatrosses (Fig. S1A). Mean ± SD of the estimated wind speed was 6.5 ± 2.7 m/s, and the maximum and minimum wind speeds were 15.4 and 0.7 m/s, respectively. Winds blowing from west to east were frequently observed (Fig. S1B). This result is consistent with the prevalence of westerlies around the wandering albatross breeding colony ( Nicholls et al., 1997 ; Weimerskirch et al., 2015 ).
Ocean waves were estimated using all 299 take-offs after more than 15 min of floating time to calculate the significant wave height. The most frequently experienced wave heights ranged from 2.5 ∼ 3.0 m at the take-off moment (Fig. S1C) and the mean ± SD was 3.0 ± 0.8 m. The minimum and maximum wave heights were 1.6 and 6.4 m, respectively. Like wind direction, the wave direction (coming from) had a west bias due to the westerlies (Fig. S1D).
Take-off properties
To quantify the take-off effort, we calculated four parameters: running duration, running speed, flapping number, and flapping frequency from the acceleration records obtained at the moment of take-off. Mean ± SD running duration of wandering albatross was 5.1 ± 1.5 s with a range from 1.1 to 11.7 s (Fig. S2A). The mean value for males was slightly lower than that for females (Fig. S3A), however, the difference was not significant (M: 5.0 ± 1.5 s, F:5.2 ± 1.5 s, p = 0.10, Mann-Whitney U test). The albatross running speed mean value ± SD was 6.5 ± 1.6 m/s (Fig. S2B). Male birds had slightly higher speeds than females (M: 6.7 ± 1.5 m/s, F:6.3 ± 1.6, p < 0.01, Fig. S3B). Running duration and speed significantly correlated (Pearson’s r = 0.57, p < 0.01). The linear regression slope (with a fixed intercept of zero) was 1.23 m/s 2 (Fig. S4). We interpret the slope as the running wandering albatross acceleration.
The flapping number, i.e., the number of wing flaps after the running phase, was estimated using the dorsoventral acceleration. The mean flapping number was 4.3 times with a range from zero to over 20 times (Fig. S2C). Take-offs without flapping after the running phase were frequently observed (33.3%). Conversely, continuous flapping above 20 times were also occasionally observed, which corresponds to a lengthy flapping duration (8 s ∼) after take-off, considering the flapping frequency of wandering albatross (2.5 ∼ 3.0 Hz). There was no significant difference in flapping number between the sexes (p = 0.22, Mann-Whitney U test, Fig. S3C). The mean ± SD flapping frequency was 2.55 ± 0.29 Hz, and most ranged from 2 to 3 Hz (Fig. S2D). However, some flapping frequency results were outside the detection range (1.8 ∼ 4 Hz) and not included in our analysis. Therefore, the sample size of the flapping frequency used in our analysis was 669. There was no significant difference in flapping frequency between the sexes (p = 0.18, Mann-Whitney U test, Fig. S3D).
Environmental effects on take-off parameters
The take-off directions were compared with the wind direction estimated from the flight path after take-off. Wandering albatrosses tended to take-off with headwinds (p < 0.01, v-test) ( Fig. 2 ). However, the cruising direction (moving direction from the take-off point to the bird location after 5 min) did not correlate with headwind direction. The mean ± SD air speed of wandering albatrosses at the end of the running phase (lift-off moment from the sea surface) calculated using the running speed, wind speed, and relative take-off direction was 12.2 ± 3.1 m/s.

Effect of wind direction on wandering albatross take-off. Relative take-off direction to wind direction (black circles, n = 427) significantly distributed around 0° (head-wind), in contrast to cruising direction relative to the wind (gray x-mark, n = 427). The radial axis represents the wind speed.
The relationships between each take-off parameter (running duration, running speed, flapping number, and flapping frequency) with environmental conditions (wind speed and wave height) were tested using linear mixed models (LMMs). The running duration required for wandering albatross take-off significantly decreased as wind speed and wave height increased ( Fig. 3 ). Similarly, the running speed was significantly lower under stronger wind and higher wave conditions. Wandering albatrosses tend to flap fewer times under stronger wind conditions. Conversely, wandering albatrosses can flap over 20 times in weak wind conditions, although the flapping number in weak wind conditions varies greatly. There is also a declining trend in the flapping number with wave height. Albatross take-offs in wave heights below 2 m always require flapping. The flapping frequency was lower as the wind speed and wave height increased, however, the trend with higher wave heights remains unclear. The LMMs results are provided in Table S2 of the supplementary information.

Environmental effect on take-off. Effort for the take-off (running duration, running speed, flapping number, and flapping frequency) significantly decreased as wave height and wind speed increased (p < 0.01) except the relationship between flapping frequency and wave height (p = 0.026). Solid line shows the linear regression line determined from the LMMs and the number at the right top corner on each graph shows the sample sizes.
Independent effect of wind and waves on take-off
Although some ocean wave components are generated by ocean winds, the correlation between the wind speed and wave height is not consistent. Some of the albatross take-offs involved information on both wind speed and wave height. Therefore, we evaluated the respective effects of wind and waves on wandering albatross take-offs. The correlation between wind speed and wave height was not strong (r = 0.27, p < 0.01). Some take-offs were performed in weak winds but high wave conditions or the opposite conditions ( Fig. 4A ). Take-off conditions were divided into four environmental categories using the peak value, which were 6.0 m/s (wind speed) and 2.8 m (wave height). The categories comprised: 48 samples (weak wind low wave: WL), 33 samples (weak wind high wave: WH), 27 samples (strong wind low wave: SL), and 77 samples (strong wind high wave: SH). The running duration varied significantly between the four categories (p < 0.01, Kruskal-Wallis test). The mean running duration in the WL conditions was 6.0, which was the longest of the four categories ( Fig. 4B ). Relatively long running (of over 6 s) mainly occurred in WL conditions, and the running duration decreased with the wind speed or wave height (Fig. S5). Similar results were obtained for both running speed and flapping number. However, flapping frequency did not significantly vary between the four categories (p = 0.06). Take-offs involving over 30 flaps mainly occurred in WL conditions.

(A) Correlation between wind speed and wave height was weak (r = 0.27, n = 185). Bar charts and solid lines written above and right of the scatter plot are normed histograms of wind speed, wave height and curve fitted lines. Based on the peak value of fitted lines scatter plots were divided into four groups, WL: weak wind low wave (open square), WH: weak wind high wave (filled square), SL: strong wind low wave (open circle), and SH: strong wind high wave (filled circle). (B) Take-off effort comparison among four groups (a: running duration, b: running speed, c: flapping number, and d: flapping frequency). Cross mark indicates the mean value.
The variance inflation factor (VIF) of wind speed and wave height was 6.86, which did not exceed the general threshold of 10 ( Dormann et al., 2013 ). Among the LMMs, models including wind speed, wave height, and the interaction used the smallest AIC for all take-off parameters (Table S3). However, the difference between the lowest and the second lowest AIC was below two for running speed, flapping number, and flapping frequency. The running duration simulation using the estimated coefficient shows that even under weak wind conditions (2 m/s), running duration decreases from 8 to 4 s as the wave height increases. Conversely, low values were maintained under strong wind conditions (8 m/s) regardless of the wave height ( Fig. 5 ). Similarly, the running speed decreased from 9 to 6 m/s as the wave height increased, regardless of the wind strength. The flapping number followed the same trend. Conversely, the flapping frequency did not decrease as the wave height increased.

(A) running duration, (B) running speed, (C) flapping number, and (D) flapping frequency in response to the wave height change under weak wind (dashed line, 2 m/s) and strong wind (solid line, 8 m/s) conditions estimated from the LMM results. Gray area represents 99% CI.
Although observational networks in the ocean are under development and mathematical weather modelling accuracy is increasing, they remain unable to accurately estimate the surrounding environment of marine animals at small scales. Here, we demonstrated that environmental variables estimated using individual animal recorders provide valuable new insight into locomotor behavior when spatiotemporal scale and accuracy of mathematical weather models and observational networks are too broad for the research. In this study, we provided details on how seabird take-offs are affected by wind and waves.
Seabird take-offs using accelerometers
We quantified the running behavior of seabirds at the moment of take-off, which is the most energy-consuming behavior for soaring seabirds ( Weimerskirch et al., 2000 ; Shaffer et al., 2001a ; Sakamoto et al., 2013 ). Previous studies have ascribed this large energy expenditure to the vigorous flapping required for take-off ( Shaffer et al., 2001a ; Sato et al., 2009 ; Clay et al., 2020 ). Indeed, the continuous flapping behavior, which is rare in cruising flight, was recorded even after the running phase of take-off in this study. However, we suggest that the running behavior should also entail a large cost in take-off because albatrosses have to reach a fast initial speed to lift off the sea surface by rapidly moving their hindlimbs for up to ∼ 10 s in unfavorable conditions (as demonstrated in this study).
We provide a first attempt at detecting the running signal of seabird take-offs and construct a relatively simple algorithm (which can be easily applied to other species) using lateral acceleration. The running duration may increase or decrease by approximately 0.5 s depending on the algorithm configuration, such as smoothing parameters and threshold values. However, we focused on the relative changes in the running behavior in association with wind speed and wave height, and absolute value error is not a serious problem.
The flapping characteristics of wandering albatross during the running phase were also researched. However, the dorsoventral acceleration signal fluctuates during the running phase making it difficult to identify each flapping signal, even after applying the band-pass filter. Therefore, counting the number of flaps immediately after the running phase was the only reliable parameter for evaluating flapping effort. Sato et al. (2009) reported that the flapping frequency of wandering albatrosses at the moment of take-off is higher (2.9 ∼ 3.4 Hz) than that of cruising flight (2.5 ∼ 2.7 Hz). In our study, the flapping frequency after the running phase was not as high as Sato et al. (2009) reported. Therefore, it is likely that wandering albatrosses undertake high frequency flapping only during the running phase, as they lift off the sea surface. After lift-off (i.e., the running phase is completed), wandering albatrosses continue with a moderate flapping frequency until they reach a certain degree of flight stability. Simultaneous video records of flapping and running motions with acceleration records are required to separate the parameter estimates.
In-depth studies on seabird take-offs are just beginning with the aid of miniaturized animal-borne recorders with the main aim of understanding how seabirds flap their wings. However, in land birds (e.g., finches and doves) take-off requires a large contribution by hindlimbs ( Provini et al., 2012 ) and the role of the hindlimb in take-off kinematics is as important as that of the wing ( Provini and Abourachid, 2018 ). Therefore, it is highly likely that seabird take-offs also require a substantial contribution by the hindlimbs, and thus, further seabird hindlimb research is required. Our study provides the basic characteristics of wandering albatross running behavior, including running duration and speed.
Take-off effort with environmental conditions
Our results demonstrate that wandering albatrosses can take-off in a variety of environmental conditions (wind speed: 0.7 ∼ 15.4 m/s, wave height: 1.6 ∼ 6.4 m). A previous study on wandering albatrosses identified the transition state from resting to flying tended to increase as the wind speed increased ( Clay et al., 2020 ). Our results found some take-offs were performed under weak wind (2 – 4 m/s) conditions, suggesting wind speed is not the only parameter influencing flight decisions of wandering albatross, and that wave height should be included in future studies.
The results showed that the running and flapping behavior tended to decrease as the wind or wave conditions increased. Running duration decreased as either the wind speed or the wave height increased and peaked when both the wind and wave conditions were weak. The same trends existed in running speed and flapping number. Although optimum statistical models for each take-off parameter were determined using the AIC value, some models provided similar results to this model. For instance, the AIC difference in running speed between the best model and the second-lowest AIC model was only 0.27. However, both models included wind speed and wave height as the explanatory variables, similar to the other take-off parameters, except flapping frequency. The purpose of constructing a linear model was to clarify whether the effects of wind and waves are independent. As long as both wind speed and wave height were included as explanatory variables in the model, they reduced the running and flapping behavior requirement. Therefore, we can conclude that both strong winds and high waves aid wandering albatross take-offs. The flapping frequency after the running phase was the only parameter that did not correlate with wave height (as identified using the LMMs model). However, we assume the flapping frequency during the running phase is more important. Future research needs to investigate the effects of wave height as wandering albatrosses need to climb up or run down the wave slope. Therefore, the flapping frequency during the running phase should be highly influenced by wave height.
Contribution of strong wind and high waves to seabird take-off
The reduced running behavior and flapping times under strong wind conditions is simply described by the lift force mechanism which has been predicted by previous studies ( Kogure et al., 2016 ; Clay et al., 2020 ). Seabirds need to gain lift force before take-off, and the magnitude of force is proportional to the square of the relative speed of the wings to the surrounding air (air speed) ( Vogel, 1983 ). It has been anecdotally suggested that seabirds take-off into the wind (i.e., headwind), because stronger winds can produce a sufficiently large lift even before the ground speed of the seabird reaches the value required for flight. As a partial demonstration of this theory, a study on the European shag ( Gulosus aristotelis ) ( Kogure et al., 2016 ) found the take-off direction was significantly biased toward headwinds. Regarding soaring seabirds, only one study ( Clay et al., 2020 ) on wandering albatrosses has confirmed a bias in take-off direction with wind direction. However, the authors acknowledge the limits in the mathematical weather model and GPS sampling resolutions and recognize the unreliability of small-scale responses to in situ variation in the atmosphere. Our study reveals wandering albatrosses significantly tend to take-off into the wind, using robust fine scale data estimated from the flight records of wandering albatross. Moreover, there was no correlation between cruising and headwind direction, indicating that wandering albatrosses face the wind on take-off regardless of their destination. Our data is reliable as the empirical value provided is actually experienced by the albatross. Furthermore, by quantitatively evaluating the flapping and running effort, we demonstrate the theory of effortless take-offs by soaring seabirds in stronger wind conditions.
The mean air speed of wandering albatrosses at the end of the running phase was close to the average flight speed (approximately 15 m/s) ( Weimerskirch et al., 2002 ), and similar to predicted best glide speeds, ( Shaffer et al., 2001b ) indicating that wandering albatrosses gain sufficient lift at the end of the running phase and efficiently utilize ocean wind. Wind speed varies with altitude, therefore the wind blowing on the ocean surface must be smaller than the values estimated from the flight records of wandering albatross as they usually fly 3 – 12 m above the ocean surface ( Pennycuick, 1982 ). Therefore, the calculated air speed is probably an overestimate when compared with the ocean surface. To compensate for the insufficient lift force gained during the running phase, wandering albatrosses flap their wings several times after the running phase. Therefore, the flapping number in weak wind conditions can exceed dozens before reaching stable flight.
The most important finding of our study is that the take-off effort estimated by the running behavior and number of flaps decreased not only with stronger winds but also with higher waves. While the role of ocean wind on flying seabirds has been well described ( Pennycuick, 2008 ), how ocean waves influence the flight of seabirds remains largely unknown. However, many studies have reported the characteristic flight of soaring seabirds by tracking the ocean wave surface over long distances ( Pennycuick, 1982 , 2008 ; Richardson, 2011 ; Stokes and Lucas, 2021 ), which even occurs in weak or no wind conditions ( Pennycuick, 1982 ). Seabirds seem to be aided by atmospheric forces above the slope-like wave topography; the flight method using the shape of wave is called wave-slope soaring ( Richardson, 2011 ). It is well recognized that air flows occur above ocean waves ( Buckley and Veron, 2016 ; Bousquet et al., 2017 ). Richardson (2011) described the theoretical model of wave-slope soaring, where the flight mechanism of albatross is a combination of both dynamic soaring, which uses vertical wind shear above the ocean surface (∼ 15 m), and wave-slope soaring, which uses the updraft caused by the wave topography. Thus, seabirds can continue to soar in weak wind conditions. Further, mathematical analysis has revealed that the wave-induced updraft (even in windless conditions) can provide 60% of the transportation cost of a brown pelican ( Pelecanus occidentalis , 2 ∼ 3 kg), which is a wave-slope soarer ( Stokes and Lucas, 2021 ). Thus, it is possible that the take-off effort by wandering albatross is also reduced by high waves. While qualitative field observations and mathematical demonstrations provide the only previous research on the role of waves on soaring seabirds, we experimentally demonstrated that ocean waves aid the most energy-consuming behavior, take-off. This finding helps future discussions on ocean topographical mechanisms affecting seabird flight.
The mechanism by which high waves aid wandering albatross take-off is not entirely clear. It is difficult to conclude a certain updraft is producing additional lift for wandering albatrosses, and it is also possible that there are other unresolved mechanisms. For example, a rough topographic surface can provide a favorable bump, like a slope or cliff to jump off into the air. Our results were restricted to wave height as the parameter of the ocean surface. Future research involving ocean surface steepness or wave frequency components will reveal the detailed mechanism of how waves facilitate seabird take-off behavior. In particular, ocean surface topography relies heavily on whether the dominant wave component is due to a swell (low-frequency waves propagated from a distance) or wind waves (high-frequency waves generated by local wind); moreover, this topography affects the wind pattern on the sea surface.
In conclusion, we revealed how the take-off effort of wandering albatross changes in various oceanic conditions. As take-off is one of the most energy-consuming behaviors that can dominate the total energy expenditure of a wandering albatross journey, this data will be of great value for considering how climate changes can alter the life of albatrosses. Future research, especially on albatrosses, should quantitatively evaluate the energy consumption of take-off with the wind and wave conditions. Currently, there is no major barrier to accomplishing this goal, it would require utilizing motion records to estimate the surrounding environment with additional methods to estimate energy consumption, such as cardiograms. Recognizing the negative effect of the changing oceanic environment on seabirds ( Sydeman et al., 2015 ), revealing the direct small-scale mechanisms of environmental factors (such as wind, wave, tide, current, and sea surface temperature) effects on animal behavior, especially in take-off is urgently required. The concept of estimating the surrounding environment using motion records is a novel solution with great potential to unravel the small spatiotemporal uncertainties in seabird research.
Materials and Methods
Field experiment.
The recorders, Ninja-scan (Little Leonardo, Tokyo, Japan), record triaxial acceleration at a very high time resolution (100 Hz). Ninja-scan also records 3D GPS positions (5 Hz), Doppler velocity (5 Hz), temperature (6 Hz), pressure (6 Hz), geomagnetism (6 Hz), and angular velocity (100 Hz). There are two types of Ninja-scans with different battery masses ( Naruoka et al., 2021 ). Small Ninja-scans weighed 28 g, which is 0.3 ∼ 0.4% of wandering albatross body mass, and are expected to record for 7 h. Large Ninja-scans weighed 91 g, which corresponds to 0.8 ∼ 1.3% of wandering albatross body mass, and are expected to record for 65 h.
Ninja scans were attached to breeding wandering albatrosses at Possession Island, Crozet archipelago (46°25 S, 51°44 E) in the South Indian Ocean in 2019 and 2020. In 2019, 12 small Ninja scans were attached (in tandem) to 6 individuals. On each bird, one recorder had a delay timer so that the two recording periods did not overlap. Additionally, 15 birds had individual Ninja-scans attached, of which 8 were small Ninja-scans and 7 were large Ninja scans. In 2020, 10 small Ninja-scans were attached in tandem to 5 individuals. Additionally, 19 birds had individual Ninja-scans attached, of which 7 were small Ninja-scans and 12 were large Ninja-scans. In summary, 21 and 24 wandering albatrosses were tagged in 2019 and 2020, respectively. All experiments were performed from late January to early March of each year, which corresponds to the incubation period of wandering albatrosses. Recorders were attached to the back of each bird with waterproof tape (Tesa, Hamburg, Germany) and glue (Loctite; Henkel, Dusseldorf, Germany). All recorders were retrieved within 35 days. One small Ninja-scan which had been attached in isolation in 2020 did not work correctly. The effects of the attached recorders on wandering albatrosses were previously assessed ( Phillips et al., 2003 ; Barbraud and Weimerskirch, 2012 ) and revealed that small recorders (less than 3% of their body mass) do not negatively impact breeding or foraging behaviors. The experiment was conducted as part of Program 109 of the Institut Polaire Paul Emile Victor with permission from the Préfet des Terrs Australes et Antarctiques Françaises, France.
Take-off identification
First, data recorded on the colony island was eliminated based on the GPS position. Then, take-off was determined using the absolute value of the GPS horizontal velocity. When wandering albatrosses float on the sea surface (i.e., before take-off), a relatively low speed which is generally below 2.5 m/s, is recorded, while the flying speed exceeds 5 m/s ( Weimerskirch et al., 2002 ). Take-off was defined as the moment when the horizontal speed exceeds 4 m/s and rises to a higher speed. The soaring (flying) speed occasionally meets this criteria. Therefore, the horizontal speed was smoothed using the moving average (20 points : 0.4 s). If the horizontal speed crossed the 4 m/s line several times within a short period, they were classed as take-offs for very short flights and were not used in our investigation. Therefore, we selected only take-offs that included over 30 s of floating followed by over 30 s of flying.
Wind estimation
Yonehara et al., (2016) proposed estimating the wind speed and direction of seabird flight paths using the sinusoidal curve relationship between flight speed and flight direction. When seabirds fly in the air, their flight speed against the ground (ground speed) is mainly affected by the wind speed, which is maximized in tail winds and minimized in head winds. The maximum speed is the sum of the flight speed against air (air speed) and wind speed, whereas the minimum speed is the difference between the air speed and wind speed. The relationship between the flight speed (ground speed) and flight direction recorded by the GPS are fitted using a sinusoidal curve ( Shimatani et al., 2012 ). We followed the methodology in Yonehara et al. (2016) to collate the flight speed 𝑉 and flight direction 𝜃 data for 5 min after take-off and the curve was fitted using the following equation:
where 𝑉 𝑎 is the air speed, 𝑉 𝑤 is the wind speed, and 𝜙 𝑤 is the wind direction. Ten seconds immediately after the take-off moment was not included in the estimation. Following Yonehara et al. (2016) , the AIC of the sinusoidal fitting was compared to the linear fitting with a fixed slope of zero. When the AIC difference between the linear and sinusoidal fitting was below two, the estimated results were considered unreliable and discarded. Wind speeds and directions were not calculated when take-offs were not followed by over five minutes of flight. The sinusoidal fitting was performed using Igor Pro version 8.04 (Wavemetrics, Portland, OR, USA).
Wave estimation
The ocean wave properties experienced by seabirds before take-off were estimated by analyzing the floating motion at the sea surface ( Uesaka et al., 2022 ). The wave height was estimated from the vertical GPS displacement records before take-off. The estimate requires sufficiently long records of vertical displacement. Therefore, the wave height was not calculated for take-offs that did not follow a surface floating time of over 15 min. The sampling period of 15 min ensured the reliability of the wave statistics ( Whitford et al., 2001 ) and provided a large volume of estimated wave data. The estimate did not include the 10 s before the detected take-off moment. We followed the methodology of Uesaka et al. (2022) . The vertical GPS displacement records were high-pass filtered using a cut-off frequency of 0.07 Hz to eliminate the GPS derived error ( Olynik et al., 2002 ). We separated the time series record of the vertical displacement into individual waves by applying the zero-up-crossing method. The mean wave height of the highest third of all individual waves was calculated to provide the significant wave height, which is the most widely used statistical wave parameter ( Whitford et al., 2001 ).
Sea surface running by seabirds
Many procellariiformes require a running phase before take-off from the sea surface ( Sato et al., 2009 ). However, studies using accelerometers have not focused on the acceleration signal of this behavior. Surface running involves asymmetrical leg movements. Therefore, the lateral acceleration obtained from the recorder (attached to the back of the seabirds) provided signals derived from the running motion ( Fig. 6 ). We confirmed that running signals appear in the lateral acceleration records at the moment of take-off by streaked shearwaters ( Calonectris leucomelas ), which are phylogenetically similar to wandering albatrosses (see Supplementary Information Text and Fig. S6).

Time series data of horizontal speed (top), lateral acceleration (middle), and dorsoventral acceleration (bottom) signals of the wandering albatross at the moment of take-off. Horizontal speed starts increasing from the beginning of the take-off. Red square shows the detected running phase based on the variance of the lateral acceleration signal. Red bars show the detected flapping behavior after the running phase based on the dorsoventral acceleration signal. Dorsoventral signal during the running phase fluctuates, probably due to the shaking body derived from the running motion, and thus it is not easy to judge the existence of flapping behavior.
To explore the running duration of wandering albatross, we constructed an algorithm to detect the running phase from the lateral acceleration around take-off. The lateral acceleration signal is composed of a dominant component (0.25 ∼ 0.4 s) and a high-frequency fluctuation component (< 0.2 s period). Although the dominant component is the lateral movement derived from surface running, the flapping period of wandering albatross appears around this period (0.3 ∼ 0.4 s). The flapping behavior is laterally symmetrical and does not appear in the lateral acceleration records. However, this is not always the case, when (occasionally) recorders are attached to the back of the seabird in a slightly tilted position. To avoid confusion between running and flapping behavior, a high-frequency fluctuation component in the lateral acceleration signal was used to detect the running phase. A band-pass filter was designed to extract the high-frequency fluctuation component from the acceleration records, and then the variance per unit time (0.6 s) was calculated at each point. Running phase was defined as when the acceleration variance exceeded the threshold value (2% of the peak value). This algorithm reasonably detects the running phase regardless of the running duration. If there is a signal gap in the middle of the running phase, the algorithm regards the gap as the end of the running phase, underestimating the running duration. However, these cases are rare, and we assume it does not affect our evaluation of the running characteristics of wandering albatross. The horizontal speed at the end of the running phase and take-off direction were calculated using the GPS velocity. The take-off direction was defined as the vectoral average direction during the running phase. We also calculated the cruising direction which was defined as the moving direction 5 min after take-off. All procedures were performed using Igor Pro version 8.04 (Wavemetrics, Portland, OR, USA).
Flapping behavior after the running phase
Dorsoventral acceleration records include signals derived from seabird wing flapping behavior ( Fig. 6 ). The flapping signals during the running phase fluctuate, which is assumed to be caused by the leg-derived dorsoventral motion. This caused the flapping data to be unclear in identifying the flapping number and frequency. Therefore, we only focused on the wing flapping signals after the running phase. A bandpass filter extracted the clearest flapping signals (1.8 ∼ 4.0 Hz). The number of continuous flapping signals after the running phase were counted. The flapping period of the wandering albatross is approximately 0.3 ∼ 0.4 s, therefore we defined the end point when the flapping interval exceeded 0.5 s. The flapping frequency after the running phase was calculated using the spectral peak value of the continuous wavelet-transformed dorsoventral acceleration. All procedures were performed using Igor Pro version 8.04 (Wavemetrics, Portland, OR, USA).
Comparison of the take-off parameters with environmental conditions
The wind directional bias of the take-off direction was tested using the v-test (modified Rayleigh test). The air speed 𝑉 𝑎 at the end of the running phase was estimated using the following equation based on the parameters obtained from this study:
where 𝑉 𝑟 is the running speed at the end of the running phase, 𝑉 𝑤 is the wind speed, 𝜃 𝑡 is the take-off direction, and 𝜙 𝑤 is the wind direction. The effects of wind speed and wave height on each take-off parameter (running duration, running speed, flapping number, and flapping frequency) were evaluated using linear mixed models (LMMs) with individuals treated as random effects. To identify significance levels, the models were compared to null models based on the AIC value.
To evaluate the combined effect of wind and waves, we categorized take-off conditions into four groups, “WL conditions,” “WH conditions,” “SL conditions,” and “SH conditions”.
Threshold values were decided based on the peak in the curve of the fitted probability density distribution (wind speed: 6.0 m/s, wave height 2.8 m). Weibull distribution and log normal distribution were used as the fitting function for wind speed and wave height, respectively ( Ferreira and Guedes Soares, 2000 ; Carta et al., 2009 ). The values of each take-off parameter were compared between the four groups by Kruskal-Wallis test. Furthermore, the independent effects of wind and waves on take-off parameters were evaluated using LMMs, including wind speed, wave height, and their interaction as explanatory parameters with individuals as random effects. VIF was also calculated before the LMMs analysis to assess whether the multicollinearity effect could be dismissed. The V-test was performed using Igor Pro version 8.04 (Wavemetrics, Portland, OR, USA). Statistical test and LMMs calculations were performed using the Python 3.0 and PypeR package.
Acknowledgements
We thank Yoshinari Yonehara, Julien Collet, Timothée Poupart, and all the members of the research station in the Crozet Islands for their support during the fieldwork. We appreciate the fruitful comments of Kagari Aoki, Chihiro Kinoshita, Laxmi Kumar Parajuli, and Aran Garrod. We are grateful to Taichi Sakamoto and Takashi Mukai (ATTACCATO) for providing customized rechargeable Li-ion batteries for the Ninja-scans. We also thank Michihiko Suzuki and Koichiro Ikeda (Little Leonardo) for molding the Ninja-scans. The field studies were financially supported by a research project entitled “Cyber ocean: next generation navigation system on the sea” funded by the CREST program (JPMJCR1685) of Japan Science and Technology Agency; Grants-in-Aid for Scientific Research from JSPS (17H00776 to K. Sato); the Tohoku Ecosystem-Associated Marine Science; and Institut Polaire Français Paul-Émile Victor Program 109.
- Johannessen J
- Weimerskirch H
- Bousquet GD
- Triantafyllou MS
- Slotine JJE
- Velázquez S
- Charrassin JB
- Timmermann R
- Clairbaux M
- Mathewson P
- Helgason HH
- Anker-Nilssen T
- Bringsvor IS
- Christensen-Dalsgaard S
- Danielsen J
- Erikstad KE
- Lorentsen SH
- Reiertsen TK
- Thórarinsson TL
- Fitzsimmons MG
- Frederiksen M
- Gilchrist HG
- Huffeldt NP
- Johansen KL
- Kouwenberg AL
- Linnebjerg JF
- Tranquilla LMF
- Montevecchi W
- Grémillet D.
- Phillips RA
- den Ouden O
- Clusella-Trullas S
- Patrick SC.
- Marquéz JRG
- Lafourcade B
- Münkemüller T
- Reineking B
- Skidmore AK
- Lautenbach S.
- Ferreira JA
- Guedes Soares C
- Fristrup KM
- Sequeira AMM
- Whoriskey F
- Simpfendorfer C
- Aarestrup K
- de Bruyn PJN
- du Dot T Jeanniard
- Ferreira LC
- Fernández-Gracia J
- Hückstädt LA
- Huveneers C
- Kittiwattanawong K
- Thorstad EB
- Treasure AM
- Williams GD
- Labrousse S
- McKinzie MK
- Muelbert MMC
- Whoriskey FG
- Woodward B.
- Sakamoto KQ
- Nicholls DG
- Petovello MG
- Lachapelle G
- Juarez-Martinez I
- Pennycuick C
- Pennycuick CJ
- Abourachid A
- Tobalske BW
- Crandell KE
- Richardson PL
- Takahashi A
- Sánchez-Román A
- Gómez-Navarro L
- Katsumata N
- Shimatani IK
- Anderson DJ
- Poloczanska E
- Thompson SA
- Bôas AB Villas
- Bourassa MA
- Cornuelle BD
- Fox-Kemper B
- Gommenginger C
- Merrifield ST
- Rodriguez E
- Subramanian AC
- Bonadonna F
- Guitteaud A
- de Grissac S
- Whitford DJ
- Hebblewhite M
- Yamagishi H
- Murakoshi M
Article and author information
For correspondence:.
© 2023, Uesaka et al.
This article is distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use and redistribution provided that the original author and source are credited.
Be the first to read new articles from eLife
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Sign up for alerts
- NEWS FEATURE
- 15 June 2023
Mice preying on adult albatross population in major global nesting site
- Leonie Joubert
You can also search for this author in PubMed Google Scholar

The grim result of a mouse attack. Credit: Anton Wolfaardt
Lire en français
Mice are gnawing on adult wandering albatrosses causing injuries that later lead to their deaths. The discovery in April on Marion Island, in the southern Indian Ocean, where 25% of the global albatross population nests, has raised fears for their future.
Marion Island and Prince Edward Island some 20km away, make up the Prince Edward Islands marine protected area, a South African territory about 2,200 km south-east of Cape Town in the southern Indian Ocean. The Prince Edward Islands are home to half the world’s breeding wandering albatrosses.
Mice arrived on Marion when it was a stop-off point for sealing vessels in the early 1800s. For two centuries, the rodents survived on insects and other invertebrates, mostly a local flightless moth, as well as weevils, mites, spiders, and worms.

Researchers on Marion Island examine an albatross carcass. Credit: Christopher Jones
The bodies of the two wandering albatross adults, fresh enough for evidence of the cause of their death, turned up on the northern coastline of Marion Island in the sub-Antarctic this April. They were among eight adult albatross bodies found in the immediate vicinity. All had died within weeks of each other. The carcasses had deep wounds at the elbows. The surrounding blood pattern indicated the injuries had been inflicted while the birds were still alive. The likely cause of death: secondary infection, or starvation as the crippled birds would have been unable to feed at sea.
“It’s very unusual to find albatross carcasses on land, because these birds spend most of their lives at sea,” says marine ecologist, Maëlle Connan, from the Institute for Coastal and Marine Research at Nelson Mandela University, South Africa.

Young grey headed albatrosses, with scalp injuries from mice attacks. Credit: Ben Dilley
Marion Island has become notably warmer and drier in the past three decades, giving mice a longer summer breeding season, allowing their numbers and food demands to grow exponentially. Researchers estimate that invertebrate numbers have fallen by almost 90% on Marion since the mid-1970s. This is starkly different to neighbouring Prince Edward, which remained mouse-free. Insects are still abundant on Prince Edward, and the plant life is noticeably undamaged by comparison. In 2015, doctoral researcher Ben Dilley with the Percy Fitzpatrick Institute of African Ornithology at the University of Cape Town captured footage of nocturnal mouse attacks on grey-headed albatross chicks on south Marion, confirming a trend that had escalated at an “unprecedented” rate over the previous two decades: When mice find young, down-covered chicks, they attack the birds’ wings or rumps. With older fledglings, whose feathers have toughened, the mice find easier pickings on the chicks’ heads, where the soft crown feathers still give easy access to the skin.
The scalped, weakened chicks are left vulnerable to secondary, often fatal infections, or are easy pickings for predators such as petrels. Even though these first adult albatross fatalities are small in number, this change in mouse behaviour is extremely concerning because of the implications for the bird population, according to Peter Ryan, emeritus professor at the PercyFitzpatrick Institute.
Wandering albatrosses only start breeding at eight to 10 years of age, they only have one chick every two years, and it takes almost 12 months for chicks to grow large enough to fly. Wandering albatrosses may be able to reach 60 but from the age of about 30 their breeding potential drops. “To have a sustainable albatross population, you need to have a high adult survival rate,” Ryan says.
In 2025, the South African Department of Forestry, Fisheries and the Environment plans a rodent eradication programme. From April until August, helicopters flying for the Mouse-Free Marion project will sweep the island, spreading blood-thinning rodent-targeted bait. This is the method used in other similar eradication programmes on sub-Antarctic islands such as South Georgia, Macquarie and Campbell, all of which were successful and allowed heavily impacted seabird colonies to recover. The Mouse-Free Marion initiative will be the biggest of its kind, according to Birdlife South Africa, which will run it on behalf of the South African government.
doi: https://doi.org/10.1038/d44148-023-00150-y
Reprints and permissions
Junior Group Leader Position at IMBA - Institute of Molecular Biotechnology
The Institute of Molecular Biotechnology (IMBA) is one of Europe’s leading institutes for basic research in the life sciences. IMBA is located on t...
Austria (AT)
IMBA - Institute of Molecular Biotechnology
Open Rank Faculty, Center for Public Health Genomics
Center for Public Health Genomics & UVA Comprehensive Cancer Center seek 2 tenure-track faculty members in Cancer Precision Medicine/Precision Health.
Charlottesville, Virginia
Center for Public Health Genomics at the University of Virginia
Husbandry Technician I
Memphis, Tennessee
St. Jude Children's Research Hospital (St. Jude)
Lead Researcher – Department of Bone Marrow Transplantation & Cellular Therapy
Researcher in the center for in vivo imaging and therapy.
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
- Reference Manager
- Simple TEXT file
People also looked at
Original research article, foraging behavior and energetics of albatrosses in contrasting breeding environments.

- 1 Department of Ecology and Evolutionary Biology, University of California, Santa Cruz, Santa Cruz, CA, United States
- 2 Centre d'Études Biologiques de Chizé, Centre National de la Récherche Scientifique, Villiers en Bois, France
Animals can maximize fitness by optimizing energy acquisition through the selection of favorable foraging habitats, but trade-offs exist between time spent in preferred feeding habitats, energetic costs of travel, and reproductive constraints. For pelagic seabirds, geographic distribution of suitable breeding islands can restrict access to marine prey resources and influence foraging strategies. Laysan ( Phoebastria immutabilis ) and black-footed albatrosses ( P. nigripes ) breeding in the Northwest Hawaiian Islands, and Indian yellow-nosed albatrosses ( Thalassarche carteri ) breeding in the Southern Indian Ocean, utilize productive subtropical-subpolar transition zones during their breeding and non-breeding periods, but this marine feature is at a comparatively greater distance for Hawaiian albatrosses during the breeding period due to location of nesting islands. We investigated the foraging behavior and energetics of these three species to evaluate how proximity to preferred marine habitats has influenced their overall foraging strategies. During incubation, all three species traveled to subtropical-subpolar transition zones, however, Hawaiian albatrosses ranged farther to reach this habitat. All species reduced time at sea during brooding, and Hawaiian albatrosses reduced their foraging ranges to distances similar to yellow-nosed albatrosses. As a consequence, Hawaiian albatrosses foraged in the warm, oligotrophic environment of the subtropical gyre during brooding while yellow-nosed albatrosses continued to forage in a subtropical-subpolar transition zone. Landing rates, an indicator of foraging effort, did not differ between reproductive stages and were highly variable within and among species. Hawaiian albatrosses generally spent more time in flight compared to yellow-nosed albatrosses, a strategy that may relate to searching for dispersed and unpredictable prey. Mean absolute field-metabolic rate (FMR) was greatest for black-footed albatrosses, and similar between Laysan and yellow-nosed albatrosses, but mass-specific FMR did not differ between species. Hawaiian albatrosses had lower total body water than yellow-nosed albatrosses (indicating greater lipid reserves), and had FMRs that fell below the allometric relationship for studied albatross species, attributes that likely reflect physiological adaptations for foraging in a low-productivity environment.
Introduction
Animals can maximize fitness by optimizing energy acquisition through the selection of preferred habitats ( Emlen, 1966 ; MacArthur and Pianka, 1966 ; Levins, 1968 ; Pyke, 1984 ), however, trade-offs exist when preferred foraging habitats are distant to breeding habitats ( Charnov, 1976 ; Orians and Pearson, 1979 ; Alerstam and Högstedt, 1982 ; Weimerskirch and Cherel, 1998 ). In the marine environment, animals that breed on land but forage at sea should adopt a strategy that optimizes energy gain while minimizing the cost of transporting energy resources (e.g., food, oil, milk) back to the breeding site ( Ricklefs, 1983 ; Pennycuick et al., 1984 ; Costa, 1991 ; Houston, 1993 ; Costa and Shaffer, 2012 ). Because marine prey are patchily distributed within a fluid, dynamic environment ( MacKas and Boyd, 1979 ; Russell et al., 1992 ; Fauchald et al., 2000 ; Weimerskirch, 2007 ), marine predators can optimize energy acquisition by exploiting physical oceanographic features that aggregate prey resources ( Schneider, 1990 ; Hunt, 1997 ; Hunt et al., 1998 ; Croll et al., 2005 ; Keiper et al., 2005 ). According to central place foraging theory, whether or not an individual selects a prey patch depends on its distance to the central place as well as its quality ( Orians and Pearson, 1979 ; Olsson and Bolin, 2014 ). Therefore, proximity of the breeding site to productive marine habitat is likely to play a role in shaping foraging strategies of marine predators ( Costa, 1993 ; Harding et al., 2013 ).
For pelagic seabirds, access to preferred marine habitats during the breeding season depends on location of the breeding colony, reproductive stage, and energetic costs of travel ( Orians and Pearson, 1979 ; Weimerskirch et al., 1993 ; Guinet et al., 1997 ; Shaffer et al., 2003 ). Albatrosses are well-adapted to long-distance travel due to their economical mode of flight ( Pennycuick, 1982 ; Costa and Shaffer, 2008 ; Sibly et al., 2012 ) and anatomical specialization for soaring and gliding ( Pennycuick, 1982 ), which enable low flight costs ( Costa and Prince, 1987 ; Shaffer et al., 2004 ). Albatross foraging range is variably constrained during the breeding period, however, due to changing energetic requirements at the nest. During incubation, the fasting capabilities of adults allow breeding pairs to alternate long shifts at the nest (~2–3 weeks) with far-ranging trips to sea. Foraging range contracts toward the end of incubation, and becomes most restricted during the brooding period, when young chicks require frequent meals and adults alternate short trips to sea (~3 days) with time spent at the nest provisioning young chicks. The chick-rearing period begins when the fasting and thermoregulatory capabilities of chicks have developed sufficiently for them to remain at the nest independently, enabling both adults to take longer trips to sea (~2–3 weeks). Albatrosses are therefore able to search for prey resources in productive habitats on basin-wide scales during the incubation and chick-rearing periods ( Jouventin and Weimerskirch, 1990 ; BirdLife International, 2004 ; Kappes et al., 2015 ), but are limited to short-distance trips during brooding, when energy deficits can occur in order to maximize food delivery to the chick ( Ricklefs, 1983 ; Shaffer et al., 2003 ). When rearing larger chicks, albatrosses allocate resources between themselves and their offspring, and may employ a dual foraging strategy, whereby adults maximize prey delivery to chicks by making short-distance trips, and restore their body condition when making long-distance trips ( Weimerskirch et al., 1997 ).
Laysan ( Phoebastria immutabilis ) and black-footed albatrosses ( P. nigripes ) breeding in the Hawaiian Islands, and Indian yellow-nosed albatrosses ( Thalassarche carteri ) breeding on Amsterdam Island in the southern Indian Ocean, utilize similar marine habitats (in two different ocean basins) when making long-range movements, but differ in terms of accessibility of preferred foraging habitats during the breeding period. On long foraging trips during incubation and chick-rearing, all three species utilize subtropical-subpolar transition zones ( Hyrenbach et al., 2002 ; Pinaud and Weimerskirch, 2005 ; Pinaud et al., 2005 ; Kappes et al., 2015 ) where warm, subtropical waters come into contact with cooler, subpolar waters ( Backus, 1986 ; Olson, 2001 ). These are highly productive pelagic habitats ( Lutjeharms and Valentine, 1984 ; Barange et al., 1998 ; Read et al., 2000 ; Olson, 2001 ; Polovina et al., 2001 ) that provide enhanced foraging opportunities for surface-feeding predators like albatrosses because surface convergence along frontal boundaries can aggregate neustonic or buoyant prey ( Olson and Backus, 1985 ; Franks, 1992 ; Govoni and Grimes, 1992 ; Olson et al., 1994 ). During the brooding period, however, when albatrosses take shorter foraging trips, Laysan and black-footed albatrosses are restricted to foraging in warm, oligotrophic waters ( Fernández et al., 2001 ; Kappes et al., 2010 , 2015 ) where prey abundance may be lower ( Ashmole, 1971 ; Ballance et al., 1997 ). Conversely, yellow-nosed albatrosses have access to cooler, more productive waters similar to habitats utilized during incubation and chick-rearing (Figure 1 ; Pinaud and Weimerskirch, 2005 ; Pinaud et al., 2005 ). The latter case is more characteristic of albatrosses in general; most albatrosses breed on islands in productive pelagic or coastal upwelling environments ( Tickell, 2000 ).
Figure 1 . Foraging trips of Laysan, black-footed, and Indian yellow-nosed albatrosses during late incubation (A–C) and brooding (D–F) . Laysan and black-footed albatrosses were tracked at Tern Island, Northwest Hawaiian Islands during 2002–2003, 2004–2005, and 2005–2006, and yellow-nosed albatrosses were tracked at Amsterdam Island, southern Indian Ocean, during 2006–2007 (study colonies indicated with a star). Tracks are superimposed on time-averaged Blended 5-day sea surface temperature (°C) for the respective study periods retrieved via the NOAA OceanWatch Live Access Server ( http://coastwatch.pfel.noaa.gov/erddap/ ).
We compared the foraging movements and activity patterns of Laysan, black-footed, and yellow-nosed albatrosses during the incubation and brooding periods, and measured energy expenditure during the brooding phase. By comparing closely related species with breeding locations that differ in terms of proximity to preferred marine habitats, we evaluated how these species respond behaviorally and physiologically to differing environmental conditions and reproductive demands. We hypothesized that during incubation, when all three species forage in productive subtropical-subpolar transition zones, activity patterns would be similar among species, but that differences would emerge during brooding when Hawaiian albatrosses are constrained to forage in an oligotrophic environment and competition for resources is likely high. Specifically, we hypothesized that Hawaiian albatrosses would spend more time in flight than yellow-nosed albatrosses during brooding, due to greater time spent in transit between more dispersed prey patches. We also hypothesized that Laysan and black-footed albatrosses would have lower landing rates and expend less energy at sea than yellow-nosed albatrosses during brooding, as a means of reducing energetic costs of foraging in a low-productivity environment.
Tracking Activities
We studied Laysan and black-footed albatrosses at Tern Island (23.87°N, 166.28°W), French Frigate Shoals, Northwest Hawaiian Islands during the 2002–2003, 2004–2005, and 2005–2006 breeding seasons, and Indian yellow-nosed albatrosses at Amsterdam Island (37.86°S, 77.52°E), Southern Indian Ocean, during the 2006-07 breeding season. We used satellite telemetry to determine at-sea locations of foraging albatrosses during late incubation and brooding to characterize differences in behavior between species and reproductive stages. During brooding, we also measured field metabolic rates of tracked albatrosses using the doubly labeled water technique ( Lifson and McClintock, 1966 ; Nagy, 1980 ; Speakman, 1997 ).
Seventy-five adult albatrosses were equipped with satellite platform terminal transmitters (30 g Pico-100, Microwave Telemetry, Columbia, MD; and 35 g SPOT4, Wildlife Computers, Redmond, WA) during late incubation (within 2 weeks of hatch date; 10 Laysan, 11 black-footed, 11 yellow-nosed albatrosses) and brooding (15 Laysan, 13 black-footed, 15 yellow-nosed albatrosses). Satellite tags were attached to dorsal feathers with adhesive tape (tesa ® , Hamburg, Germany), and satellite transmissions were downloaded via the Argos satellite system (Service Argos, Inc., Largo, MD). Individuals were also equipped with temperature recorders (10 g Lotek LTD 2400 and 1100, Lotek Wireless, St. John's, Newfoundland) attached to a plastic leg band so that temperature recordings (±0.05°C) could be used to characterize activity patterns while at sea ( Wilson et al., 1995 ). Foraging activity is only presented for the brooding period, when high-resolution (12 s) temperature records were available. In all cases, total mass of deployed devices was <2% of bird body mass, which is under the recommended limit for albatross tracking studies ( Phillips et al., 2003 ). Sex was determined from blood samples ( Fridolfsson and Ellegren, 1999 ) for all individuals tracked. All protocols employed in this study were approved by the Institutional Animal Care and Use Committees, University of California Santa Cruz.
Foraging Behavior
We delimited albatross foraging tracks based on visual observations of departure and arrival times from twice-daily nest checks during incubation, and hourly nest checks from dawn to dusk during brooding. To remove unlikely Argos locations, tracks were filtered using the Iknos Toolkit (Y. Tremblay, unpublished program) for Matlab (The MathWorks, Natick, MA), following Kappes et al. (2010) . First, a speed filter of 80 km h −1 was applied to transit rates between successive locations (following Hyrenbach et al., 2002 ; Suryan et al., 2006 ) to remove unrealistic flight speeds ( Spear and Ainley, 1997 ). Next, the maximum change in azimuth was set to 170° to remove track spikes between successive locations that are likely to be erroneous ( Keating, 1994 ; Freitas et al., 2008 ). Finally, to avoid errors in transit rate determination ( Hays et al., 2001 ), the minimum time between successive fixes was set to 10 min.
We calculated maximum distance traveled from the colony using great-circle distances to account for the earth's curvature. We divided great-circle distances between off-colony Argos locations by the time between successive locations to calculate average transit rate. To characterize albatross foraging activity patterns, we determined the proportion of time spent in flight and the frequency of landings on the sea surface. Landing rates are indicative of feeding effort ( Weimerskirch et al., 2000 ; Shaffer et al., 2001a ) because albatrosses must land on the sea surface in order to consume prey ( Conners et al., 2015 ). Previous research has demonstrated that take-offs and landings are the most energetically demanding activities albatrosses engage in at sea ( Weimerskirch et al., 2000 ), and landing rates of wandering albatrosses ( Diomedea exulans ) are correlated with field metabolic rates ( Shaffer et al., 2001a ). We used an algorithm (Iknos toolkit for Matlab; Y. Tremblay, unpublished program; Kappes et al., 2015 ) to identify landings based on rapid changes in temperature, and stable periods associated with sitting on the sea surface ( Wilson et al., 1995 ), for those individuals equipped with temperature records. We defined daylight hours based on civil twilight (sun no more than 6° below the horizon) using NOAA's solar calculator in the maptools package in R ( Lewin-Koh and Bivand, 2010 ) and temporally-matching to tracking locations.
Field Metabolic Rates
Doubly labeled water was used to determine field metabolic rates (FMR) of Laysan, black-footed, and yellow-nosed albatrosses at sea ( FMR at − sea ) and at the nest ( FMR on − nest ) during the brooding period ( Lifson and McClintock, 1966 ; Nagy, 1980 ; Speakman, 1997 ). Fifteen birds of each species were captured at the nest immediately following a mate switch, and an initial blood sample (0.5–3.5 ml) was collected from a vein on the tarsus. Albatrosses were given an intraperitoneal injection of 1.6–1.9 ml sterile water containing 0.9% NaCl, and either 34.5 atom percentage oxygen-18 and 35.9 atom percentage deuterium (Laysan and black-footed albatrosses), or 29.8 atom percentage oxygen-18 and 5.0 Mbq g −1 of tritiated water (yellow-nosed albatrosses). Mass of injectate (±0.01 g) was determined by weighing the syringe before and after injection using a portable field balance (Ohaus Corp., Pine Brook, NJ). Each bird was weighed to the nearest 50 g using either a spring-loaded Pesola (Pesola AG, Baar, Switzerland) or Salter scale (Salter Weightronix Ltd, West Bromwich, UK) and then placed in a box or holding pen; isotopes were allowed to equilibrate for ~90 min ( Shaffer et al., 2001b ) before a second blood sample was collected. Three Laysan, two black-footed, and three yellow-nosed albatrosses were held for 3 h to help ensure equilibration when there was evidence that injections may have been made into the gastrointestinal cavity or cutaneous fat. All individuals were equipped with satellite tags and/or temperature recorders and released at the nest. After completion of a foraging trip, each bird was recaptured and a third blood sample was collected, within 2–3 h of returning to the colony. Satellite tags and temperature recorders were then removed and final body mass was measured. To determine FMR on − nest , three Laysan, four black-footed, and three yellow-nosed albatrosses were subsequently captured after 2 days at the nest and a fourth blood sample was collected from these individuals. In one instance, a Laysan albatross did not depart to sea after release, but instead switched with its mate again and remained on the nest; for this individual only FMR on − nest was calculated. This double-switching behavior was also observed at Laysan and black-footed albatross nests checked daily to determine attendance patterns of control individuals.
Due to equipment limitations, one black-footed albatross was equipped with a temperature recorder only, two yellow-nosed albatrosses were equipped with satellite tags only, and one black-footed albatross was equipped with a GPS tag (TechnoSmart, Rome, Italy) rather than a satellite tag; in this case, the GPS record demonstrated that the individual stayed near Tern Island overnight and then returned to the nest (short departures were also observed in control pairs, with both members of the pair remaining at the nest for several days in some cases). As this was not representative of FMR at − sea or FMR on − nest , the metabolic rate measured for this individual is not included in the subsequent analyses.
All blood samples were collected with a syringe and 21–25 gauge needle, transferred to a vacutainer (B-D brand with spray-coated lithium heparin, Beckton-Dickinson, Franklin Lakes, NJ) and stored in a cooler with cold packs until centrifugation on the day of collection. Plasma was transferred to 2 ml cryogenic plastic screw cap vials (with silicon O-rings; Corning Inc., Corning, NY) and frozen until isotopic analyses were performed. Aliquots of water distilled from plasma samples (following Ortiz et al., 1978 ) were then used to determine specific activity of deuterium by laser-absorption spectroscopy (University of California Davis, Davis, CA) or tritium by scintillation spectrometry (LS 6500, Beckman Coulter Inc., Fullterton, CA) in triplicate; specific activity of oxygen-18 was determined by mass ratio spectrometry (Metabolic Solutions, Nashua, NH).
The initial dilution space of oxygen-18 was used to calculate the volume of initial total body water. To calculate final body water volume, body mass at recapture was multiplied by the initial fractional water content, which has been validated in Shaffer et al. (2006) . CO 2 production was calculated using equation 2 in Nagy (1980) ; this equation assumes that body mass of the animal changes linearly with time. For two yellow-nosed albatrosses and one Laysan albatross, FMR was determined using the single sample method described in Speakman (1997) , because initial total body water calculations suggested isotopes were not fully equilibrated when the post-equilibration blood sample was collected. FMR (mL g −1 h −1 ) was converted to kJ (and W) by applying a conversion factor of 24.7 kJ = 1 L CO 2 following ( Pettit et al., 1988 ), based on chemical composition of the diet of Laysan albatrosses ( Harrison et al., 1983 ); this conversion factor was assumed to approximate CO 2 yield from the diet of black-footed and yellow-nosed albatrosses. Mass-specific FMR (W kg −1 ) was determined by dividing FMR by mean body mass so that energy expenditure is more directly comparable among species. Because FMR calculations included time spent at the nest before departure and after arrival at the nest, measured FMR was corrected based on visual observations of departure and arrival times at the nest. Following methods of Costa and Prince (1987) , FMR at − sea was calculated as:
We compiled average FMR and body mass values for Hawaiian and yellow-nosed albatrosses with values from other studies (male and female wandering albatrosses ( Shaffer et al., 2001a ), shy albatrosses ( Thalassarche cauta ; Green and Brothers, 1995, Abstract from First International Albatross and Petrel Conference, Hobart, Australia), gray-headed albatrosses (T. chrysostoma ; Costa and Prince, 1987 ), black-browed albatrosses ( Thalassarche melanophrys ; Shaffer et al., 2004 ), and Laysan albatrosses during incubation ( Pettit et al., 1988 ) to provide a mechanism for comparing energy expenditure among species ( Shaffer, 2011 ). Given large differences in mass between wandering albatrosses and other studied albatross species, we tested for the effect of genus Diomedea in the relationship between log-transformed FMR and body mass values.
Statistical Analysis
Statistical analyses were implemented in the program R ( R Development Core Team, 2010 ). We used ANOVA and Tukey multiple comparison tests ( Hothorn et al., 2008 ) to investigate differences in foraging behavior between species and reproductive stages, and species differences in FMR, total body water, body mass, and foraging activity; Bonferroni-corrected P -values are presented for multiple comparisons. Trip characteristics, landing rates, time in flight, total body water, and FMR for each species did not differ significantly between the sexes, therefore males and females were grouped for all analyses. To meet normality assumptions, percent time in flight was arcsine transformed and landing rate was log transformed prior to analysis. Simple linear regression was used to investigate relationships between foraging behavior and FMR at − sea for each species, and to examine the allometric relationship between log-transformed FMR and body mass for albatrosses from this and other studies. P -values reported are two-tailed, and the significance level was set as P = 0.05. All averages are reported as Mean ± SD .
Hawaiian albatrosses traveled significantly farther during incubation compared to brooding (more than four times as far on average; Table 1 ; P < 0.001 for pair-wise tests), whereas maximum foraging range did not differ between incubation and brooding for yellow-nosed albatrosses ( P = 0.08). All three albatross species took foraging trips of longer duration during incubation compared to brooding ( P < 0.01 for all pair-wise tests): Hawaiian albatrosses took trips that were more than three times longer, whereas yellow-nosed albatrosses took trips that were just under two times longer on average (Table 1 ). During the incubation period, the majority of Hawaiian albatrosses traveled north of the Tern Island colony to cooler waters of the North Pacific Transition Zone; during brooding, their movements were restricted to warmer waters of the subtropical gyre (Figures 1 , 2 ). Yellow-nosed albatrosses foraged in similar thermal environments during the incubation and brooding periods (Figure 2 ). Black-footed albatrosses traveled more rapidly during incubation compared to brooding ( P = 0.02); transit rates did not differ between breeding stages for Laysan and yellow-nosed albatrosses (Table 1 ).
Table 1 . Summary characteristics (Mean ± SD ) of foraging trips of Laysan and black-footed albatrosses tracked at Tern Island, Northwest Hawaiian Islands, and Indian yellow-nosed albatrosses tracked at Amsterdam Island, southern Indian Ocean, during late incubation and brooding.
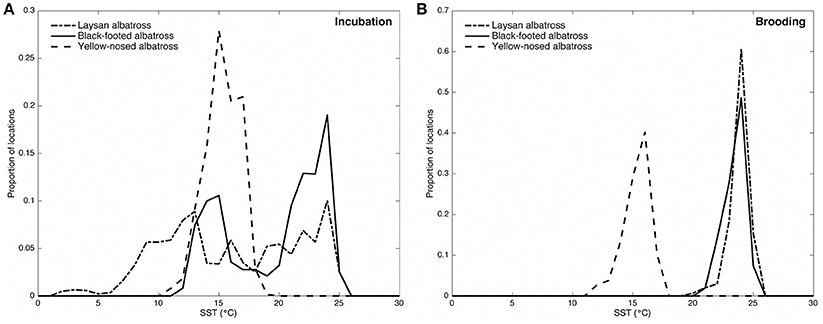
Figure 2 . Distribution of sea surface temperatures (°C) along Laysan, black-footed, and Indian yellow-nosed albatrosses foraging routes during incubation (A) and brooding (B) . Laysan and black-footed albatrosses were tracked at Tern Island, Northwest Hawaiian Islands during 2002–2003, 2004–2005, and 2005–2006, and yellow-nosed albatrosses were tracked at Amsterdam Island, southern Indian Ocean, during 2006–2007. Blended 5-day sea surface temperature retrieved via the NOAA OceanWatch Live Access Server ( http://coastwatch.pfel.noaa.gov/erddap/ ).
During the incubation period, Hawaiian albatrosses traveled farther (over 700 km on average) and more rapidly (9 km h −1 on average) than yellow-nosed albatrosses ( P < 0.02 for pair-wise tests), but spent a similar amount of time at sea (between 7 and 10 days on average). During brooding, all three species traveled similar distances (~400 km) and durations (3–4 days). Laysan albatrosses traveled more rapidly than yellow-nosed albatrosses during brooding (8 km h −1 on average; P = 0.004), but this was the only species difference in transit rate observed. Sea surface temperature along foraging tracks was most similar among species during incubation; Hawaiian albatrosses used warmer waters than yellow-nosed albatrosses during brooding (Figure 2 ).
Percent time in flight and landing rates did not differ between reproductive stages for any of the three species (Table 2 ). During incubation, Hawaiian albatrosses spent more time in flight at night (70–80%) compared to yellow-nosed albatrosses (~50%; Table 2 ; P < 0.01 for pair-wise tests). During brooding, Hawaiian albatrosses spent more time in flight both during the day (~90%) and at night (80–90%) compared to yellow-nosed albatrosses (~70% during day; ~40% at night; Table 2 ; P < 0.01 for pair-wise tests). Yellow-nosed albatrosses had higher overall and daytime landing rates than black-footed albatrosses during incubation ( P < 0.001), but there were no species differences in overall or daytime landing rates during brooding. All three species demonstrated diel patterns in foraging activity (Table 2 ).
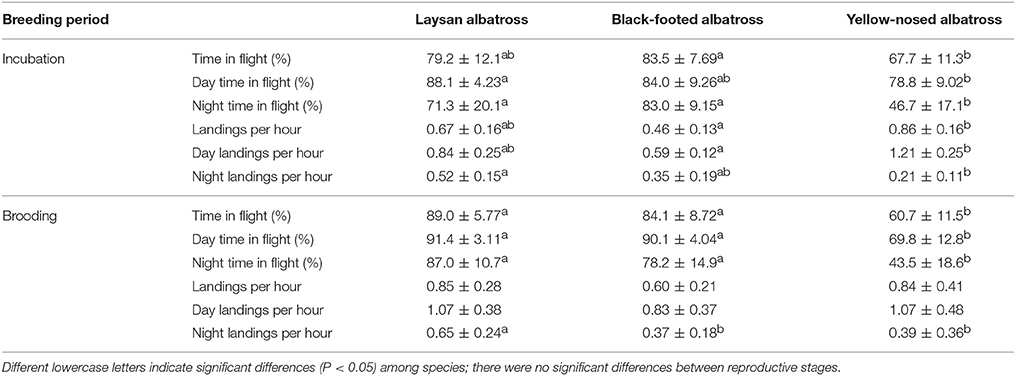
Table 2 . Summary characteristics (Mean ± SD ) of at-sea activity patterns of Laysan and black-footed albatrosses breeding at Tern Island, Northwest Hawaiian Islands, and Indian yellow-nosed albatrosses breeding at Amsterdam Island, southern Indian Ocean, during late incubation and brooding.
Body mass differed between the three species studied; black-footed albatrosses were significantly heavier than Laysan albatrosses ( P < 0.001), which were significantly heavier than yellow-nosed albatrosses ( P = 0.002; Table 3 ). Total body water (%) did not differ significantly between Laysan and black-footed albatrosses, but each species of Hawaiian albatross had a lower percentage of total body water compared to yellow-nosed albatrosses ( P < 0.001 for pair-wise tests). On average, albatrosses gained mass during the foraging trip but the change in mass did not differ between species and was highly variable among individuals (Table 3 ).
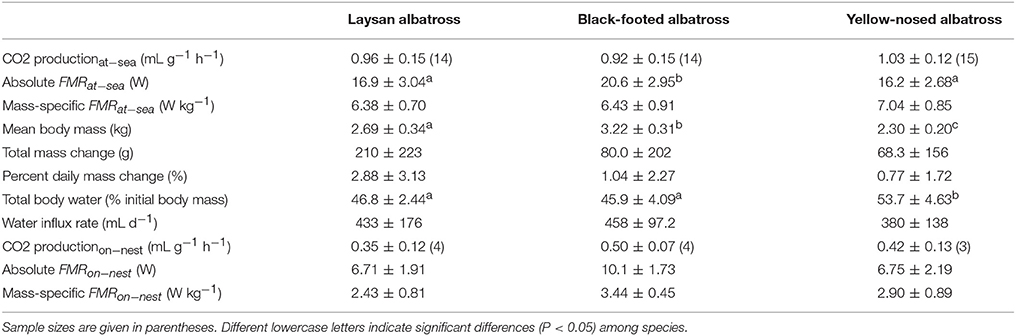
Table 3 . Energy expenditure (Mean ± SD ) of Laysan and black-footed albatrosses breeding at Tern Island, Northwest Hawaiian Islands, and Indian yellow-nosed albatrosses breeding at Amsterdam Island, southern Indian Ocean, during the brooding period.
Mean absolute FMR at − sea (W) was greatest for black-footed albatrosses ( P = 0.005, Laysan pair-wise test; P < 0.001, yellow-nosed pair-wise test), and similar for Laysan and yellow-nosed albatrosses (Table 3 ). Mean absolute FMR on − nest (W) did not differ between albatross species but sample sizes were low for this parameter (Table 3 ). Mass-specific FMR at − sea (W kg −1 ) and FMR on − nest (W kg −1 ) did not differ between species. The ratio of FMR at − sea to FMR on − nest was lowest for black-footed albatrosses (1.9), and similar for Laysan (2.6) and yellow-nosed albatrosses (2.4).
For each species, we investigated the relationship between at-sea behavior and mass-specific field metabolic rates, however, FMR at − sea (W kg −1 ) was not statistically related to foraging range, trip duration, transit rates, the percent time in flight, the number or frequency of landings, change in mass, or water influx rates.
We plotted the allometric relationship between FMR and body mass for albatrosses from this and other doubly labeled water studies to provide a mechanism for appropriately comparing energy expenditure among species (Figure 3 ; Shaffer, 2011 ). We found a significant effect of the genus Diomedea on the relationship between log-transformed FMR and body mass [β = 0.60, t (6) = 3.19, P = 0.02], therefore our discussion focuses on the allometric relationship of the smaller albatross species (solid line; Figure 3 ) rather than the line for all studied species (dashed line; Figure 3 ). We found that Hawaiian albatrosses during brooding fall below the regression line for smaller albatross species, whereas yellow-nosed albatrosses fall above this line (Figure 3 ).
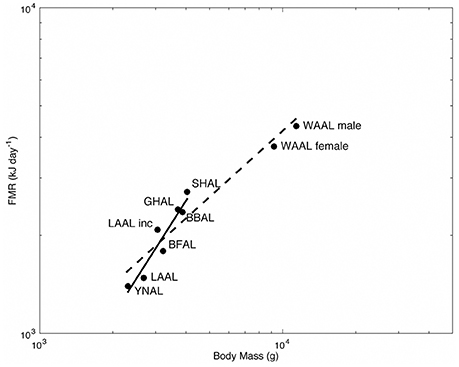
Figure 3 . Allometry of field metabolic rate (FMR; kJ d −1 ) for albatrosses measured using the doubly labeled water method. FMR and body mass (g) are plotted on a logarithmic scale for WAAL male and female (wandering albatross Diomedea exulans ; Shaffer et al., 2001a ), SHAL (shy albatross Thalassarche cauta ; Green and Brothers 1995, Abstract from First International Albatross and Petrel Conference, Hobart, Australia), GHAL (gray-headed albatross T. chrysostoma ; Costa and Prince, 1987 ), BBAL (black-browed albatross T. melanophrys ; Shaffer et al., 2004 ), LAAL Inc. (Laysan albatross Phoebastria immutabilis during incubation; Pettit et al., 1988 ), and BFAL (black-footed albatross P. nigripes ), LAAL (Laysan albatross), and YNAL (Indian yellow-nosed albatross T. carteri ) during brooding (this study).
Comparative Foraging Behavior
During the incubation period, all three albatross species foraged within convergence zones between warm subtropical waters and cool subpolar waters (Figure 1 ) where productivity is regionally enhanced ( Lutjeharms and Valentine, 1984 ; Barange et al., 1998 ; Read et al., 2000 ; Olson, 2001 ; Polovina et al., 2001 ) and albatross prey resources are aggregated ( Harrison et al., 1983 ; Gong et al., 1993 ; Yatsu et al., 1993 ; Pearcy et al., 1996 ; Pinaud et al., 2005 ; Conners, 2015 ). To reach these habitats, Laysan and black-footed albatrosses traveled significantly farther than yellow-nosed albatrosses and spent more time at sea. During brooding, all species reduced time at sea and Hawaiian albatrosses retracted their foraging ranges to the warm, oligotrophic environment close to the breeding colony (Figures 1 , 2 ), where prey abundance is likely lower ( Ashmole, 1971 ; Ballance et al., 1997 ). Conversely, yellow-nosed albatrosses were able to forage in a similar thermal environment during both reproductive stages, despite restricted movements during brooding (Figures 1 , 2 ).
Hawaiian albatrosses spent more time in flight than yellow-nosed albatrosses, especially at night and during the brooding period. This is in agreement with our hypothesis, as we expected that spending more time in flight would be suitable for foraging in a low-productivity environment with unpredictable prey resources ( Weimerskirch et al., 2005 ). Reliance on flight to travel between dispersed prey patches is seen in other tropical seabirds where greater flight proficiency is associated with lower productivity habitats ( Ballance et al., 1997 ). Compared to other albatross species that forage in more productive marine habitats such as subtropical and polar convergences, continental shelf-breaks and slopes, and coastal upwelling zones ( Tickell, 2000 ; BirdLife International, 2004 ), yellow-nosed albatrosses spent a similar proportion of time in flight (44–69%; Weimerskirch and Guionnet, 2002 ; Phalan et al., 2007 ). Contrary to our hypothesis, we did not detect a consistent difference in overall landing rates between Hawaiian albatrosses and yellow-nosed albatrosses during brooding, despite contrasting foraging environments. Landing rates during brooding were similar between species during the day, but higher for Laysan albatrosses at night. Higher landing rates at night for Laysan albatrosses may be related to nocturnal feeding in this species ( Harrison et al., 1983 ; Conners et al., 2015 ). The tendency of all species to forage during daylight hours is supported by previous research ( Fernández and Anderson, 2000 ; Weimerskirch and Guionnet, 2002 ; Kappes et al., 2015 ).
Foraging Energetics
Brooding Laysan and black-footed albatrosses did not differ in terms of body composition; however, both Hawaiian albatrosses demonstrated significantly lower total body water when compared to yellow-nosed albatrosses. Lower total body water suggests that Hawaiian albatrosses have comparatively greater lipid reserves ( Reilly and Fedak, 1990 ; Ellis and Jehl, 1991 ; Groscolas et al., 1991 ), which may be related to foraging in a low-productivity environment. If foraging conditions are poor during brooding, adults may rely on lipid body stores obtained during the incubation period for self-maintenance and then allocate food resources acquired at sea during the brooding period to rapidly-growing chicks ( Weimerskirch and Lys, 2000 ).
Mean absolute FMR at − sea was greatest for black-footed albatrosses, and similar for Laysan and yellow-nosed albatrosses. Greater absolute FMR at − sea in black-footed albatrosses can be explained by larger body size ( Nagy, 2005 ) and higher wing loading in this species ( Suryan et al., 2008 ). Contrary to our prediction, Laysan and black-footed albatrosses did not exhibit lower mass-specific FMR at − sea compared to yellow-nosed albatrosses. We expected that Hawaiian albatrosses would minimize foraging costs by employing a comparatively economical foraging strategy in response to sparse, unpredictable local prey resources ( Flint and Nagy, 1984 ; Weimerskirch et al., 2005 ). We therefore also combined our results with published research and examined residual variation in the allometric relationship between body mass and FMR at − sea to further evaluate the comparative energy expenditure of Hawaiian and yellow-nosed albatrosses (discussed below).
We did not find significant relationships between FMR at − sea and foraging range, trip duration, transit rates, the percent time in flight, the number or frequency of landings, change in mass, or water influx rates within each species. As predicted, Hawaiian albatrosses spent more time in flight than yellow-nosed albatrosses, however, we were not able to detect a relationship between time in flight and energetic costs within species. During brooding, overall landing rates did not differ between species and were highly variable. Previous research using the doubly labeled water method demonstrated a relationship between energetic costs and landing rates in wandering albatrosses ( Shaffer et al., 2001a ), therefore we expected that landing rates would be related to energy expenditure within species in this study. The lack of a relationship between landing rates and field metabolic rates may be explained by the relatively smaller size of Hawaiian and yellow-nosed albatrosses (2–4 kg) compared to the larger wandering albatross (8–10 kg; Tickell, 1968 ).
While field metabolic rates of black-footed and yellow-nosed albatrosses have not been studied during incubation, Pettit et al. (1988) measured FMR at − sea and FMR on − nest of Laysan albatrosses at Tern Island during the incubation period. Estimates of FMR on − nest during incubation were similar to our estimates of FMR on − nest during brooding, however, estimates of FMR at − sea during incubation were higher than our estimates of FMR at − sea during brooding for all three species ( Pettit et al., 1988 ; Figure 3 ). This contrasts with a study of wandering albatrosses, where FMR at − sea was higher during brooding, compared to the incubation period ( Shaffer et al., 2003 ). Although estimated FMR at − sea was greater during incubation for Laysan albatrosses, activity patterns during incubation and brooding were similar (this study; Kappes et al., 2015 ). This provides further evidence that activity patterns do not relate directly to energy expenditure in this species, contrary to findings for wandering albatrosses ( Weimerskirch et al., 2000 ; Shaffer et al., 2001a ). Higher FMR at − sea during the incubation period could reflect effort directed at assimilating lipid stores while foraging at distant, preferred habitats ( Welcker et al., 2009 ), so that adults are able to effectively provision young chicks while foraging in an oligotrophic marine habitat during brooding.
To further examine variation in energy expenditure among species, we evaluated the allometric relationship between FMR and body mass for smaller albatross species and examined where Hawaiian and yellow-nosed albatrosses fell in relation to the plotted regression line (Figure 3 ). We found that Hawaiian albatrosses during brooding fell below the regression line, whereas yellow-nosed albatrosses fell above this line (Figure 3 ). This indicates that Hawaiian albatrosses expend comparatively less energy at sea during brooding after accounting for species differences in mass, which may be related to foraging in an oligotrophic environment during this reproductive stage. It may also be related to the fact that Hawaiian albatrosses breed during boreal winter when winds are stronger, compared to conditions during the Indian Ocean austral summer when yellow-nosed albatrosses breed. Laysan albatrosses during incubation fall well above the regression line, indicating that this species expends comparatively more energy during this reproductive stage, which may be related to effort directed at assimilating lipid stores in distant, preferred habitats.
Conclusions
Among albatrosses, Hawaiian albatrosses are unique in that they are constrained to forage in a warm, oligotrophic marine environment during the energetically demanding brooding period. As hypothesized, Hawaiian albatrosses spent more time in flight than yellow-nosed albatrosses during brooding, a behavior suited for traveling between dispersed prey patches. Contrary to our predictions, we did not detect species differences in overall landing rates or mass-specific FMR at − sea during brooding, measures indicative of foraging energy expenditure. However, compared to yellow-nosed albatrosses, Hawaiian albatrosses had lower total body water (greater lipid reserves) and field metabolic rates that fell below the allometric relationship for studied albatross species, attributes which may reflect physiological adaptations of these species to foraging in a low-productivity environment. Given the relative lack of information on the physiological constraints of species movements ( Hays et al., 2016 ), our comparative approach provides a valuable case study as to how a group of related species responds physiologically and behaviorally to differing environmental conditions and reproductive demands.
Data Availability
Satellite tracking datasets generated from this research are available by request from the BirdLife Tracking Ocean Wanderers database ( http://seabirdtracking.org ).
Author Contributions
All authors participated in study design and interpretation of results. MA and YT: collected tracking data; MA, DC, and SS: implemented the doubly labeled water technique in the field; MA: performed laboratory work, conducted data analysis, and implemented statistical analyses; MA: wrote the first draft of the manuscript, and all authors contributed substantially to revisions.
This research was part of the Tagging of Pacific Pelagics (TOPP) program, funded in part by the National Ocean Partnership Program (N00014-02-1-1012), the Office of Naval Research (N00014-00-1-0880 & N00014-03-1-0651), the Gordon and Betty Moore, David and Lucille Packard, and Alfred P. Sloan Foundations. MA was supported by a Fulbright grant to France, a Chancellor's Dissertation-Year Fellowship and a UCSC Regents Fellowship, and grants from the ARCS Foundation, the Earl H. Myers and Ethel M. Myers Trust, Friends of Long Marine Lab, the Society for Integrative and Comparative Biology, the STEPS Institute, and the UCSC Ecology & Evolutionary Biology department. Cambridge Isotope Laboratories, Inc. provided deuterium and oxygen-18 isotopes for this study as a research award. S & A Machine and Tool Co. generously provided overseas shipping costs of research supplies for use on Amsterdam Island.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer GS and handling Editor declared their shared affiliation.
Acknowledgments
We thank Jill Awkerman, Sarah Chisholm, Peter Kappes, Nicolas Mignot, Scott Seganti, and Jean-Baptiste Thiebot for invaluable assistance in the field, and Lindsay Young for conducting genetic sexing. We are grateful to the Hawaiian Islands National Wildlife Refuge, U.S. Fish and Wildlife Service, Department of the Interior, for logistic support and permission to conduct research on Tern Island; to L'Institut Polaire Français and Terres Australes et Antarctiques Françaises for supporting field activities on Amsterdam Island; and to the Fulbright Commission for supporting research on Amsterdam Island, and while in residence at Centre d'Études Biologiques de Chizé (CNRS).
Alerstam, T., and Högstedt, G. (1982). Bird migration and reproduction in relation to habitats for survival and breeding. Ornis Scand. 13, 25–37. doi: 10.2307/3675970
CrossRef Full Text | Google Scholar
Ashmole, N. P. (1971). “Seabird ecology and the marine environment,” in Avian Biology , eds D. S. Farner and J. R. King (London: Academic Press), 223–286.
Google Scholar
Backus, R. H. (1986). “Biogeographic boundaries in the open ocean,” in Pelagic Biogeography , Vol. 49, eds A. C. Pierrot-Bults, S. van der Spoel, B. J. Zahuranec, and R. K. Johnson (UNESCO Technical Paper in Marine Science), 9–13.
Ballance, L. T., Pitman, R. L., and Reilly, S. B. (1997). Seabird community structure along a productivity gradient: importance of competition and energetic constraint. Ecology 78, 1502–1518. doi: 10.1890/0012-9658(1997)078[1502:SCSAAP]2.0.CO;2
Barange, M., Pakhomov, E. A., Perissinotto, R., Froneman, P. W., Verheye, H. M., Taunton-Clark, J., et al. (1998). Pelagic community structure of the subtropical convergence region south of Africa and in the mid-Atlantic Ocean. Deep Sea Res. I 45, 1663–1687. doi: 10.1016/S0967-0637(98)00037-5
BirdLife International (2004). “Tracking ocean wanderers: the global distribution of albatrosses and petrels,” in Results from the Global Procellariiform Tracking Workshop, (1-5 September, 2003) (Gordon's Bay; Cambridge, UK: BirdLife International).
Charnov, E. L. (1976). Optimal foraging, the marginal value theorem. Theor. Popul. Biol. 9, 129–136. doi: 10.1016/0040-5809(76)90040-X
PubMed Abstract | CrossRef Full Text | Google Scholar
Conners, M. G. (2015). Comparative Behavior, Diet, and Post-Breeding Strategies of Two Sympatric North Pacific albatross Species (Phoebastria sp.) . Ph.D. dissertation, University of California, Santa Cruz.
Conners, M. G., Hazen, E. L., Costa, D. P., and Shaffer, S. A. (2015). Shadowed by scale: subtle behavioral niche partitioning in two sympatric, tropical breeding albatross species. Mov. Ecol. 3, 28. doi: 10.1186/s40462-015-0060-7
Costa, D. P. (1991). Reproductive and foraging energetics of high latitude penguins, albatrosses and pinnipeds: implications for life history patterns. Am. Zool. 31, 111–130. doi: 10.1093/icb/31.1.111
Costa, D. P. (1993). The relationship between reproductive and foraging energetics and the evolution of the Pinnipedia. Symp. Zool. Soc. Lond. 66, 293–314.
Costa, D. P., and Prince, P. A. (1987). Foraging energetics of gray-headed albatrosses Diomedea Chrysostoma at Bird Island, South Georgia. Ibis 129, 149–158. doi: 10.1111/j.1474-919X.1987.tb03196.x
Costa, D. P., and Shaffer, S. A. (2008). “Physiological constraints on the foraging ecology and energetics of albatrosses and other large seabirds,” in 4th CPB Meeting in Africa: Mara 2008. Molecules to Migration: The Pressures of Life , eds S. Morris and A. Vosloo (Bologna: Medimond Publishing).
Costa, D. P., and Shaffer, S. A. (2012). “Seabirds and marine mammals,” in Metabolic Ecology: A Scaling Approach , eds R. M. Sibly, J. H. Brown, and A. Kodric-Brown (Oxford: John Wiley & Sons, Ltd.), 225–233.
Croll, D. A., Marinovic, B., Benson, S., Chavez, F. P., Black, N., Ternullo, R., et al. (2005). From wind to whales: trophic links in a coastal upwelling system. Mar. Ecol. Prog. Ser. 289, 117–130. doi: 10.3354/meps289117
Ellis, H. I., and Jehl, J. R. (1991). Total body water and body composition in phalaropes and other birds. Physiol. Zool. 64, 973–984. doi: 10.1086/physzool.64.4.30157952
Emlen, J. M. (1966). The role of time and energy in food preference. Am. Nat. 100, 611–617. doi: 10.1086/282455
Fauchald, P., Erikstad, K. E., and Skarsfjord, H. (2000). Scale-dependent predator-prey interactions: the hierarchical spatial distribution of seabirds and prey. Ecology 81, 773–783. doi: 10.3354/meps231279
Fernández, P., and Anderson, D. J. (2000). Nocturnal and diurnal foraging activity of Hawaiian albatrosses detected with a new immersion monitor. Condor 102, 577–584. doi: 10.1650/0010-5422(2000)102[0577:NADFAO]2.0.CO;2
Fernández, P., Anderson, D. J., Sievert, P. R., and Huyvaert, K. P. (2001). Foraging destinations of three low-latitude albatross ( Phoebastria ) species. J. Zool. 254, 391–404. doi: 10.1017/S0952836901000899
Flint, E. N., and Nagy, K. A. (1984). Flight energetics of free-living sooty terns. Auk 101, 288–294.
Franks, P. J. S. (1992). Sink or swim: accumulation of biomass at fronts. Mar. Ecol. Prog. Ser. 82, 1–12. doi: 10.3354/meps082001
Freitas, C., Lydersen, C., Fedak, M. A., and Kovacs, K. M. (2008). A simple new algorithm to filter marine mammal Argos locations. Mar. Mamm. Sci. 24, 315–325. doi: 10.1111/j.1748-7692.2007.00180.x
Fridolfsson, A.-K., and Ellegren, H. (1999). A simple and universal method for molecular sexing of non-ratite birds. J. Avian Biol. 30, 116–121. doi: 10.2307/3677252
Gong, Y., Kim, S., and An, D. H. (1993). Abundance of neon flying squid in relation to oceanographic conditions in the North Pacific. Int. N. Pac. Fish. Comm. Bull. 53, 191–204.
Govoni, J. J., and Grimes, C. B. (1992). The surface accumulation of larval fishes by hydrodynamic convergence within the Mississippi River plume front. Cont. Shelf Res. 12, 1265–1276. doi: 10.1016/0278-4343(92)90063-P
Groscolas, R., Schreiber, L., and Morin, F. (1991). The use of tritiated water to determine protein and lipid utilization in fasting birds: a validation study in incubating great-winged petrels, Pterodroma macroptera . Physiol. Zool. 64, 1217–1233. doi: 10.1086/physzool.64.5.30156241
Guinet, C., Koudil, M., Bost, C.-A., Durbec, J. P., Georges, J. Y., Mouchot, M. C., et al. (1997). Foraging behaviour of satellite-tracked king penguins in relation to sea-surface temperatures obtained by satellite telemetry at Crozet Archipelago, a study during three austral summers. Mar. Ecol. Prog. Ser. 150, 11–20. doi: 10.3354/meps150011
Harding, A., Paredes, R., Suryan, R., Roby, D., Irons, D., Orben, R., et al. (2013). Does location really matter? An inter-colony comparison of seabirds breeding at varying distances from productive oceanographic features in the Bering Sea. Deep-Sea Res. II 94, 178–191. doi: 10.1016/j.dsr2.2013.03.013
Harrison, C. S., Hida, T. S., and Seki, M. P. (1983). Hawaiian seabird feeding ecology. Wildlife Wildl. Monogr. 85, 1–71.
Hays, G. C., Åkesson, S., Godley, B. J., Luschi, P., and Santidrian, P. (2001). The implications of location accuracy for the interpretation of satellite-tracking data. Anim. Behav. 61, 1035–1040. doi: 10.1006/anbe.2001.1685
CrossRef Full Text
Hays, G. C., Ferreira, L. C., Sequeira, A. M. M., Meekan, M. G., Duarte, C. M., Bailey, H., et al. (2016). Key questions in marine megafauna movement ecology. Trends Ecol. Evol. 31, 463–475. doi: 10.1016/j.tree.2016.02.015
Hothorn, T., Bretz, F., and Westfall, P. (2008). Simultaneous inference in general parametric models. Biometr. J. 50, 346–363. doi: 10.1002/bimj.200810425
Houston, A. I. (1993). The efficiency of mass loss in breeding birds. Proc. R. Soc. Lond. B 254, 221–225. doi: 10.1098/rspb.1993.0149
Hunt, G. L. (1997). Physics, zooplankton, and the distribution of least auklets in the Bering Sea - a review. ICES J. Mar. Sci. 54, 600–607. doi: 10.1006/jmsc.1997.0267
Hunt, G. L., Russell, R. W., Coyle, K. O., and Weingartner, T. (1998). Comparative foraging ecology of planktivorous auklets in relation to ocean physics and prey availability. Mar. Ecol. Prog. Ser. 167, 241–259. doi: 10.3354/meps167241
Hyrenbach, K. D., Fernandez, P., and Anderson, D. J. (2002). Oceanographic habitats of two sympatric North Pacific albatrosses during the breeding season. Mar. Ecol. Prog. Ser. 233, 283–301. doi: 10.3354/meps233283
Jouventin, P., and Weimerskirch, H. (1990). Satellite tracking of wandering albatrosses. Nature 343, 746–748. doi: 10.1038/343746a0
Kappes, M. A., Shaffer, S. A., Tremblay, Y., Foley, D. G., Palacios, D. M., Bograd, S. J., et al. (2015). Reproductive constraints influence habitat accessibility, segregation, and preference of sympatric albatross species. Mov. Ecol. 3:34. doi: 10.1186/s40462-015-0063-4
Kappes, M. A., Shaffer, S. A., Tremblay, Y., Foley, D. G., Palacios, D. M., Robinson, P. W., et al. (2010). Hawaiian albatrosses track interannual variability of marine habitats in the North Pacific. Prog. Oceanogr. 86, 246–260. doi: 10.1016/j.pocean.2010.04.012
Keating, K. A. (1994). An alternative index of satellite telemetry location error. J. Wildl. Manage. 58, 414–421. doi: 10.2307/3809311
Keiper, C. A., Ainley, D. G., Allen, S. G., and Harvey, J. T. (2005). Marine mammal occurrence and ocean climate off central California, 1986 to 1994 and 1997 to 1999. Mar. Ecol. Prog. Ser. 289, 285–306. doi: 10.3354/meps289285
Levins, R. (1968). Evolution in Changing Environments: Some Theoretical Explorations . Princeton, NJ: Princeton University Press.
Lewin-Koh, N. J., and Bivand, R. (2010). Maptools: Tools for Reading and Handling Spatial Objects. R Package Version 0.7-34.
Lifson, N., and McClintock, R. (1966). Theory of use of the turnover rates of body water for measuring energy and material balance. J. Theor. Biol. 12, 46–74. doi: 10.1016/0022-5193(66)90185-8
Lutjeharms, J. R. E., and Valentine, H. R. (1984). Southern Ocean thermal fronts south of Africa. Deep Sea Res. 31, 1461–1475. doi: 10.1016/0198-0149(84)90082-7
MacArthur, R. H., and Pianka, E. R. (1966). On optimal use of a patchy environment. Am. Nat. 100, 603–609. doi: 10.1086/282454
MacKas, D. L., and Boyd, C. M. (1979). Spectral analysis of zooplankton spatial heterogeneity. Science 204, 62–64. doi: 10.1126/science.204.4388.62
Nagy, K. A. (1980). CO 2 production in animals: analysis of potential errors in the doubly labeled water method. Am. J. Physiol. 238, R466–R473.
PubMed Abstract | Google Scholar
Nagy, K. A. (2005). Field metabolic rate and body size. J. Exp. Biol. 208, 1621–1625. doi: 10.1242/jeb.01553
Olson, D. B. (2001). Biophysical dynamics of western transition zones: a preliminary synthesis. Fish. Oceanogr. 10, 133–150. doi: 10.1046/j.1365-2419.2001.00161.x
Olson, D. B., and Backus, R. H. (1985). The concentrating of organisms at fronts: a cold-water fish and a warm-core ring. J. Mar. Res. 43, 113–137. doi: 10.1357/002224085788437325
Olson, D. B., Hitchcock, G. L., Mariano, A. J., Ashjian, C. J., Peng, G., Nero, R. W., et al. (1994). Life on the edge: marine life and fronts. Oceanography 7, 52–59. doi: 10.5670/oceanog.1994.03
Olsson, O., and Bolin, A. (2014). A model for habitat selection and species distribution derived from central place foraging theory. Oecologia 175, 537–548. doi: 10.1007/s00442-014-2931-9
Orians, G. H., and Pearson, N. E. (1979). “On the theory of central place foraging,” in Analysis of Ecological Systems , eds D. J. Horn, G. R. Stairs, and R. D. Mitchell (Columbus, OH: Ohio State University Press), 155–177.
Ortiz, C. L., Costa, D. P., and Le Boeuf, B. J. (1978). Water and energy flux in elephant seal pups fasting under natural conditions. Physiol. Zool. 51, 166–178. doi: 10.1086/physzool.51.2.30157864
Pearcy, W. G., Fisher, J. P., Anma, G., and Meguro, T. (1996). Species associations of epipelagic nekton of the North Pacific Ocean, 1978-1993. Fish. Oceanogr. 5, 1–20. doi: 10.1111/j.1365-2419.1996.tb00013.x
Pennycuick, C. J. (1982). The flight of petrels and albatrosses ( Procellariiformes ), observed in South Georgia and its vicinity. Philos. Trans. R. Soc. Lond. B 300, 75–106. doi: 10.1098/rstb.1982.0158
Pennycuick, C. J., Croxall, J. P., and Prince, P. A. (1984). Scaling of foraging radius and growth rate in petrels and albatrosses ( Procellariiformes ). Ornis Scand. 15, 145–154. doi: 10.2307/3675955
Pettit, T. N., Nagy, K. A., Ellis, H. I., and Whittow, G. C. (1988). Incubation energetics of the Laysan albatross. Oecologia 74, 546–550. doi: 10.1007/BF00380052
Phalan, B., Phillips, R. A., Silk, J. R. D., Afanasyev, V., Fukuda, A., Fox, J., et al. (2007). Foraging behaviour of four albatross species by night and day. Mar. Ecol. Prog. Ser. 340, 271–286. doi: 10.3354/meps340271
Phillips, R. A., Xavier, J. C., and Croxall, J. P. (2003). Effects of satellite transmitters on albatrosses and petrels. Auk 120, 1082–1090. doi: 10.1642/0004-8038(2003)120[1082:EOSTOA]2.0.CO;2
Pinaud, D., Cherel, Y., and Weimerskirch, H. (2005). Effect of environmental variability on habitat selection, diet, provisioning behaviour and chick growth in yellow-nosed albatrosses. Mar. Ecol. Prog. Ser. 298, 295–304. doi: 10.3354/meps298295
Pinaud, D., and Weimerskirch, H. (2005). Scale-dependent habitat use in a long-ranging central place predator. J. Anim. Ecol. 74, 852–863. doi: 10.1111/j.1365-2656.2005.00984.x
Polovina, J. J., Howell, E., Kobayashi, D. R., and Seki, M. P. (2001). The transition zone chlorophyll front, a dynamic global feature defining migration and forage habitat for marine resources. Prog. Oceanogr. 49, 469–483. doi: 10.1016/S0079-6611(01)00036-2
Pyke, G. H. (1984). Optimal foraging theory: a critical review. Annu. Rev. Ecol. Syst. 15, 523–575. doi: 10.1146/annurev.es.15.110184.002515
R Development Core Team (2010). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Read, J. F., Lucas, M. I., Holley, S. E., and Pollard, R. T. (2000). Phytoplankton, nutrients and hydrography in the frontal zone between the Southwest Indian Subtropical gyre and the Southern Ocean. Deep Sea Res. I 47, 2341–2368. doi: 10.1016/S0967-0637(00)00021-2
Reilly, J. J., and Fedak, M. A. (1990). Measurement of the body composition of living gray seals by hydrogen isotope dilution. J. Appl. Physiol. 69, 885–891.
Ricklefs, R. E. (1983). Some considerations on the reproductive energetics of pelagic seabirds. Stud. Avian Biol. 8, 84–94.
Russell, R. W., Hunt, G. L., Coyle, K. O., and Cooney, R. T. (1992). Foraging in a fractal environment: spatial patterns in a marine predator-prey system. Landsc. Ecol. 7, 195–209. doi: 10.1007/BF00133310
Schneider, D. C. (1990). Seabirds and fronts: a brief overview. Polar Res. 8, 17–21. doi: 10.3402/polar.v8i1.6798
Shaffer, S. A. (2011). A review of seabird energetics using the doubly labeled water method. Comp. Biochem. Physiol. A 158, 315–322. doi: 10.1016/j.cbpa.2010.07.012
Shaffer, S. A., Costa, D. P., and Weimerskirch, H. (2001a). Behavioural factors affecting foraging effort of breeding wandering albatrosses. J. Anim. Ecol. 70, 864–874. doi: 10.1046/j.0021-8790.2001.00548.x
Shaffer, S. A., Costa, D. P., and Weimerskirch, H. (2001b). Comparison of methods for evaluating energy expenditure of incubating wandering albatrosses. Physiol. Biochem. Zool. 74, 823–831. doi: 10.1086/323650
Shaffer, S. A., Costa, D. P., and Weimerskirch, H. (2003). Foraging effort in relation to the constraints of reproduction in free-ranging albatrosses. Funct. Ecol. 17, 66–74. doi: 10.1046/j.1365-2435.2003.00705.x
Shaffer, S. A., Costa, D. P., and Weimerskirch, H. (2004). Field metabolic rates of black-browed albatrosses Thalassarche melanophrys during the incubation stage. J. Avian Biol. 35, 551–558. doi: 10.1111/j.0908-8857.2004.03264.x
Shaffer, S. A., Gabrielsen, G. W., Verreault, J., and Costa, D. P. (2006). Validation of water flux and body composition in glaucous gulls ( Larus hyperboreus ). Physiol. Biochem. Zool. 79, 836–845. doi: 10.1086/504611
Sibly, R. M., Brown, J. H., and Kodric-Brown, A. (2012). Metabolic Ecology: A Scaling Approach. Oxford: John Wiley & Sons, Ltd.
Speakman, J. R. (1997). Doubly Labelled Water: Theory and Practice. London: Chapman and Hall.
Spear, L. B., and Ainley, D. G. (1997). Flight speed of seabirds in relation to wind speed and direction. Ibis 139, 234–251. doi: 10.1111/j.1474-919X.1997.tb04621.x
Suryan, R. M., Anderson, D. J., Shaffer, S. A., Roby, D. D., Tremblay, Y., Costa, D. P., et al. (2008). Wind, waves, and wing loading: morphological specialization may limit range expansion of endangered albatrosses. PLoS ONE 3:e4016. doi: 10.1371/journal.pone.0004016
Suryan, R. M., Sato, F., Balogh, G. R., Hyrenbach, K. D., Sievert, P. R., and Ozaki, K. (2006). Foraging destinations and marine habitat use of short-tailed albatrosses: a multi-scale approach using first-passage time analysis. Deep-Sea Res. II 53, 370–386. doi: 10.1016/j.dsr2.2006.01.012
Tickell, W. L. N. (1968). The biology of the great albatrosses, Diomedea exulans and Diomedea epomophora . Antarct. Res. Ser. 12, 1–55.
Tickell, W. L. N. (2000). Albatrosses. New Haven, CT: Yale University Press.
Weimerskirch, H. (2007). Are seabirds foraging for unpredictable resources? Deep-Sea Res. II 54, 211–223. doi: 10.1016/j.dsr2.2006.11.013
Weimerskirch, H., and Cherel, Y. (1998). Feeding ecology of short-tailed shearwaters: breeding in Tasmania and foraging in the Antarctic? Mar. Ecol. Prog. Ser. 167, 261–274. doi: 10.3354/meps167261
Weimerskirch, H., Cherel, Y., Cuenot-Chaillet, F., and Ridoux, V. (1997). Alternative foraging strategies and resource allocation by male and female wandering albatrosses. Ecology 78, 2051–2063. doi: 10.1890/0012-9658(1997)078[2051:AFSARA]2.0.CO;2
Weimerskirch, H., Gault, A., and Cherel, Y. (2005). Prey distribution and patchiness: factors in foraging success and efficiency of wandering albatrosses. Ecology 86, 2611–2622. doi: 10.1890/04-1866
Weimerskirch, H., and Guionnet, T. (2002). Comparative activity pattern during foraging of four albatross species. Ibis 144, 40–50. doi: 10.1046/j.0019-1019.2001.00021.x
Weimerskirch, H., Guionnet, T., Martin, J., Shaffer, S. A., and Costa, D. P. (2000). Fast and fuel efficient? Optimal use of wind by flying albatrosses. Proc. R. Soc. Lond. B 267, 1869–1874. doi: 10.1098/rspb.2000.1223
Weimerskirch, H., and Lys, P. (2000). Seasonal changes in the provisioning behaviour and mass of male and female wandering albatrosses in relation to the growth of their chick. Polar Biol. 23, 733–744. doi: 10.1007/s003000000144
Weimerskirch, H., Salamolard, M., Sarrazin, F., and Jouventin, P. (1993). Foraging strategy of wandering albatrosses through the breeding season: a study using satellite telemetry. Auk 110, 325–342.
Welcker, J., Harding, A. M. A., Kitaysky, A. S., Speakman, J. R., and Gabrielsen, G. W. (2009). Daily energy expenditure increases in response to low nutritional stress in an Arctic-breeding seabird with no effect on mortality. Funct. Ecol. 23, 1081–1090. doi: 10.1111/j.1365-2435.2009.01585.x
Wilson, R. P., Weimerskirch, H., and Lys, P. (1995). A device for measuring seabird activity at sea. J. Avian Biol. 26, 172–175. doi: 10.2307/3677067
Yatsu, A., Shimada, H., and Murata, M. (1993). Distributions of epipelagic fishes, squids, marine mammals, seabirds and sea turtles in the Central North Pacific. Int. N. Pac. Fish Comm. Bull. 53, 111–146.
Keywords: Laysan albatross, black-footed albatross, Indian yellow-nosed albatross, foraging behavior, activity patterns, satellite tracking, doubly labeled water, energetics
Citation: Antolos M, Shaffer SA, Weimerskirch H, Tremblay Y and Costa DP (2017) Foraging Behavior and Energetics of Albatrosses in Contrasting Breeding Environments. Front. Mar. Sci . 4:414. doi: 10.3389/fmars.2017.00414
Received: 19 September 2017; Accepted: 01 December 2017; Published: 15 December 2017.
Reviewed by:
Copyright © 2017 Antolos, Shaffer, Weimerskirch, Tremblay and Costa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michelle Antolos, [email protected]
† Present Address: Michelle Antolos, Department of Fisheries and Wildlife, Oregon State University, Corvallis, OR, United States Scott A. Shaffer, Department of Biological Sciences, San Jose State University, San Jose, CA, United States Yann Tremblay, UMR MARBEC, IRD - Ifremer - Univ. Montpellier - CNRS, Sète, France
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article
Vultures of the Seas: Hyperacidic Stomachs in Wandering Albatrosses as an Adaptation to Dispersed Food Resources, including Fishery Wastes
* E-mail: [email protected]
Affiliations CEFE-CNRS, UMR5175, Montpellier, France, Percy FitzPatrick Institute and DST-NRF Centre of Excellence at the University of Cape Town, Rondebosch, South Africa
Affiliation CEBC-CNRS, UPR1934, Villiers en bois, France
Affiliation Institut Pluridisciplinaire Hubert Curien, Université de Strasbourg and CNRS UMR7178, Strasbourg, France
- David Grémillet,
- Aurélien Prudor,
- Yvon le Maho,
- Henri Weimerskirch

- Published: June 6, 2012
- https://doi.org/10.1371/journal.pone.0037834
- Reader Comments
Animals are primarily limited by their capacity to acquire food, yet digestive performance also conditions energy acquisition, and ultimately fitness. Optimal foraging theory predicts that organisms feeding on patchy resources should maximize their food loads within each patch, and should digest these loads quickly to minimize travelling costs between food patches. We tested the prediction of high digestive performance in wandering albatrosses, which can ingest prey of up to 3 kg, and feed on highly dispersed food resources across the southern ocean. GPS-tracking of 40 wandering albatrosses from the Crozet archipelago during the incubation phase confirmed foraging movements of between 475–4705 km, which give birds access to a variety of prey, including fishery wastes. Moreover, using miniaturized, autonomous data recorders placed in the stomach of three birds, we performed the first-ever measurements of gastric pH and temperature in procellariformes. These revealed surprisingly low pH levels (average 1.50±0.13), markedly lower than in other seabirds, and comparable to those of vultures feeding on carrion. Such low stomach pH gives wandering albatrosses a strategic advantage since it allows them a rapid chemical breakdown of ingested food and therefore a rapid digestion. This is useful for feeding on patchy, natural prey, but also on fishery wastes, which might be an important additional food resource for wandering albatrosses.
Citation: Grémillet D, Prudor A, le Maho Y, Weimerskirch H (2012) Vultures of the Seas: Hyperacidic Stomachs in Wandering Albatrosses as an Adaptation to Dispersed Food Resources, including Fishery Wastes. PLoS ONE 7(6): e37834. https://doi.org/10.1371/journal.pone.0037834
Editor: Hans-Ulrich Peter, Institute of Ecology, Germany
Received: March 12, 2012; Accepted: April 25, 2012; Published: June 6, 2012
Copyright: © 2012 Grémillet et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Funding: This study was funded by Centre National de la Recherche Scientifique and by the French Polar Institute Paul-Emile Victor (programme 109). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: The authors have declared that no competing interests exist.
Introduction
The capacity of animals to survive and reproduce in a given environment is often seen as primarily limited by energy acquisition (the metabolic theory of ecology [1] ). Yet two additional bottlenecks occur: (a) their ability to shed excess heat generated by muscle activity (heat dissipation limit theory [2] ), and (b) their capacity to digest food. This latter alternative has long been neglected, yet Karasov, Diamond and colleagues demonstrated the existence of digestive bottlenecks in a series of species, hummingbirds (e.g. Selasphorus rufus ) being the classic example [3] , [4] . Ecologically, digestion is a fundamental process since it does not only condition the fate of individual organisms, but also the flow of matter and energy across food webs [5] .
Biologically, digestion serves the purpose of breaking down and assimilating ingested food. In the digestive tract it is aided by mechanical churning, low pH, digestive enzymes, and the occasional symbiont [6] . The severity of this process largely depends upon the texture and hardiness of the food: when the aforementioned hummingbird feeds, nectar is easy to break down. At the other extreme, ostrich ( Struthio struthio ) food is proverbially tough.
In particular, generalists and/or scavengers need to be able to digest a broad diet, including hardy food [7] . Moreover, foraging theory predicts that animals feeding on patchy food should be capable of ingesting large amounts, and to digest them as quickly as possible [8] . This is particularly marked in birds which need to become airborne, even after the largest meals. A prime example of this strategy is found in vultures feeding on carrion. These species have large stomachs, and also very low stomach pH (1.5) which plays a crucial role in chemically dissolving hard parts, especially bones [9] . A pH of 1 to 2 is also optimal for proteolytic enzymes that play a crucial role in the breakdown of food [10] .
In the Southern Ocean, series of studies have addressed the capacity of marine predators to acquire food [11] , but little is known about their digestive physiology and potential digestive bottlenecks. In seabirds, pioneering work demonstrated that some prey, in particular squid, are more difficult to digest than others, that feeding on squid leads to delayed gastric emptying [12] , and that birds eating squid tend to have longer digestive tracts [13] .
Wandering albatrosses ( Diomedea exulans ), the largest extant seabird species, primarily feed on squid caught at the ocean’s surface [14] . However their diet is not restricted to squid, but shows a large variety of other prey such as fishes, carrion of seabirds and marine mammals, as well as fishery wastes, whose proportion vary according to sites or stages of the breeding season [15] – [18] . Wandering albatross food occurs in discrete and unpredictable patches; birds fly for extended periods before ingesting large squid or other prey at irregular intervals [19] . The most profitable predatory strategy is therefore to ingest as much food as possible whenever available and to move to another patch [20] . Albatross stomach morphology reflects this evolutionary constraint, with an estimated volume of 3–4 L [21] , which allows birds to ingest large single prey items of up to 3.2 kg [19] , i.e. over 30% of their own body mass. After such large meals, wandering albatrosses may have difficulties to take off if wind conditions are not favourable, which explains why they often remain at the ocean surface for several hours [22] . If they do manage to take off rapidly (in strong winds), such additional food load may increase their flight costs by increasing wing loading [23] . Wandering albatrosses therefore clearly should process large meals as quickly as possible, a strategy that they theoretically share with vultures that face similar foraging and flight constraints.
In this context, we tested the hypothesis that wandering albatrosses are vultures of the seas, designed to rapidly digest large volumes of hardy food such as squid, and are therefore pre-adapted to rapidly process fishery waste, a recently occurring resource that provides large quantities of food during a short period of time. To address this issue, we performed GPS-tracking of wandering albatrosses at sea, and recorded their stomach pH during, and in-between meals. These pH levels were then compared with those of other seabird species feeding on a variety of food types and with vulture stomach pH to test the prediction that wandering albatross stomach pH is as low as that of vultures.
Ethics Statement
All scientific procedures were validated by the ethics committee of the French Polar Institute (IPEV), were conducted according to its guidelines and under permits of the Réserve Naturelle des Terres Australes and of the Comité de l’Environnement Polaire.
The study was conducted in January – March 2011 on Possession Island (46°S, 51°E), Crozet Archipelago, Southern Ocean. Wandering albatrosses were studied while incubating, a period during which parents take shifts at the nest while a partner forages at sea for periods of a few days to a month [24] . Birds were caught at the nest within the framework of a long-term monitoring program of their foraging behaviour. Great care was taken to minimize stress while handling, which lasted <10 min in all cases. Birds were either fitted with a GPS data logger to record their movements at sea, or with a pH data logger to record stomach pH.
GPS Positioning
We used miniaturized GPS recorders (i-gotU, Mobile Action Technology Inc, New Taipei City, Taiwan; 44.5×28.5×13 mm, 20 g i.e. 0.2% bird body mass) attached with waterproof tape to feathers. Birds were captured and fitted with the GPS after they have been relieved by their partner and were about to leave for a foraging trip at sea. Device and tape were removed upon return to the colony after a single foraging trip. This technique has been successfully used on this species for nearly two decades [25] , with no measurable effects on behaviour, reproductive output or survival [26] . Devices were programmed to record a GPS position every 15 min across the foraging trip. Stored data were mapped on Google Earth® to illustrate wandering albatross at-sea home range.
Stomach pH and Temperature Recordings
We studied stomach pH and temperature using autonomous, miniaturized recorders enclosed in a titanium housing that was swallowed by the birds and remained in the stomach for the time of the measurement. The devices used (pH-meter, Earth & Ocean Technologies, Kiel, Germany, 11 cm long, 2 cm in diameter, mass 80 g i.e. 0.9% of bird body mass) are fully described in [27] , which also provide all necessary details about preparation, calibration procedures and data handling. Devices were set to record pH (accuracy 0.02 pH units) and temperature (accuracy <0.1°C) every ten seconds. Temperature data were analysed following [21] and [28] so as to estimate the mass of prey caught at sea using the amplitude and the duration of the temperature drop recorded in the stomach after prey ingestion.
The deployment procedure in the field closely followed previous investigations conducted in the same species [28] , using devices of the same mass and size, which nonetheless only recorded stomach temperature: Birds were induced to swallow the pH-meter at the beginning of the experiment, and it was recovered at the end of the measurement by stomach flushing, a technique which has been routinely used to gather stomach contents of seabirds for the purpose of dietary studies [29] .
GPS-tracking
We equipped a total of 43 birds with GPS recorders. One device did not collect data, a second was lost at sea, and a third only collected data for 12 hours. Therefore a total of 40 complete tracks were collected, for at-sea journeys of between 3.6 and 21.1 days (mean 9.3±4.9), during which birds travelled between 475 and 4507 km (mean 3511±2718). As demonstrated in previous work, the duration of trips was very variable, with trips occurring over oceanic waters, as well as over the shelf edge ( Fig. 1 ).
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
(A). Five birds performed long trips towards northwest, three performed long trips towards southeast, five birds performed intermediate trips, nine birds remained between the Crozet Archipelago and the westward Prince Edward Islands, and 18 birds remained on the Crozet plateau (B), extensively foraging along its edge; suggesting local interactions with fishing vessels.
https://doi.org/10.1371/journal.pone.0037834.g001
Stomach Temperature and pH Patterns
We equipped a total of 5 birds with pH-meters. Two individuals were equipped for a few hours at the nest during an initial test phase, while three were equipped before going out to sea. Within the latter group, only one bird came back to the nest with its pH-meter, the two others regurgitated the device at sea, something which had already occurred in previous studies using similar stomach loggers [28] , as it is the natural mechanisms by which wandering albatrosses and other seabirds evacuate indigestible food parts, such as squid beaks.
We therefore analyzed stomach pH and temperature recordings for three birds. In the bird that went out to sea (for a period of 7 days, Fig. 2 ), basal stomach pH was extremely low (pH 1.35±0.14), occasionally decreasing to pH 0.51. Parallel temperature recordings indicated ingestion of cold prey ( Fig. 2 ), who’s estimated mass was on average 110±280 g. Prey items were occasionally large, up to an estimated 1160 g. After the intake of such large items, stomach pH rose sharply (up to pH 4.88), and re-acidification to baseline levels only occurred within several hours to one day ( Fig. 2 ). The two birds that stayed on the nest and did not feed showed stable, very low stomach pH levels (average pH 1.50±0.13 and 1.65±0.10, respectively). These values are in line with the ground pH level recorded in the bird that went out to sea, and the average baseline pH was therefore pH 1.50±0.13 across all three birds.
https://doi.org/10.1371/journal.pone.0037834.g002
Using the first stomach pH recording ever conducted in a foraging petrel, we validate our prediction that the stomach pH of wandering albatrosses is extremely low ( Fig. 2 ). Such low pH is very close to the baseline stomach pH recorded in white-backed griffon vultures ( Fig. 3 , [30] ), and is significantly lower than pH levels recorded in a variety of other seabird species that mainly feed on fish and were previously studied using the same miniaturised, autonomous pH-meters ( Fig. 3 ).
https://doi.org/10.1371/journal.pone.0037834.g003
Our findings are based upon a very limited sample size, consisting of only one recording at sea and two for birds at the nest. They should be complemented by further recordings on a larger number of birds across different stages of the breeding cycle and also across different petrel species showing contrasting dietary preferences. However, our three recordings show consistent, extremely low baseline pH levels of 1.5 on average. Such physiological parameters are unlikely to show strong inter-individual variability, and indeed standard deviations for stomach pH measurements conducted in other bird species are within the same pH unit. We are therefore confident that our recordings demonstrate highly acidic (<2) stomach pH in wandering albatrosses.
Such low pH favours rapid chemical digestion of the food and is also optimal for proteolytic enzyme kinetic [10] . It is likely that this physiological characteristic evolved as a response to a diet largely composed of squid, and to a patchy distribution of this food resource resulting in large, infrequent meals. The strategy of wandering albatrosses is indeed to cover long distances rapidly and at low costs, to increase the probability of encountering dispersed prey patches whose distribution is unpredictable [22] , [31] . They catch on average one prey every 200 km, and some prey can be as heavy as 3.2 kg [22] , an additional load that increases wing loading and reduce optimality of flight [23] , [32] . As indicated above, they often remain at the sea surface for several hours after having swallowed large prey items [22] . This time spent on the sea surface without capturing additional prey probably corresponds to their digestion time, a period during which low stomach pH allows them to process food quickly, to become airborne again and fly at the lowest-possible energetic costs [31] . Being able to digest rapidly large meals represents an important advantage by reducing time spent on the water, or flight costs. This strategy is the marine equivalent to that of foraging vultures, which also remain on the ground after large meals.
However, low stomach pH represents also a strategic advantage for seabirds feeding upon fishery wastes: they can absorb large volumes of this patchy resource, and digest them rapidly. Direct observations around the Crozet-Kerguelen islands conducted from long-liners producing wastes (A. Prudor, unpubl data) show that wandering albatrosses are the dominant species within multi-species flocks attending fishing vessels because of their large body size and aggressive behaviour [31] . They also have sufficient stomach volume to ingest large volumes of these wastes, yet after a large meal they typically stay at the ocean’s surface for several hours.
Wandering albatrosses from the Crozet islands are thought to feed to some extent on wastes from long liners harvesting Patagonian toothfish ( Dissostichus eleginoides ), yet the amount of fishery waste that they actually consume remains to be determined, as well as the incidence of this artificial food resource upon seabird apparent fitness. Indeed, fishery wastes are generally beneficial to scavenging seabirds [33] , yet in certain cases they set ecological traps and diminish reproductive success [34] .
Acknowledgments
We are grateful to all participants of the 48th Crozet overwintering team, in particular Maxime Loubon, Anaëlle Atamaniuk, Simon-Pierre Babski and Jérémy Tornos for their dedicated help during fieldwork. Many thanks also to Emilie Tew Kai and Bénédicte Martin for computing and illustrative assistance.
Author Contributions
Conceived and designed the experiments: DG HW YLM. Performed the experiments: DG AP. Analyzed the data: DG AP. Contributed reagents/materials/analysis tools: YLM HW DG. Wrote the paper: DG.
- View Article
- Google Scholar
- 8. Stephens DW, Krebs JR (1986) Foraging theory. Chichester, West Sussex: Princeton University Press. 247 p.
- 23. Pennycuick CJ (1989) Bird flight performance: a practical calculation manual. Oxford: Oxford University Press. 153 p.
- 26. Barbraud C, Weimerskirch H (2012) Assessing the effect of satellite transmitters on the demography of the wandering albatross Diomedea exulans. Journal of Ornithology. DOI 10.1007/s10336-011-0752-8.
- 35. Peters G (1997) Die Regulation der Verdauungsprozesse bein Pinguinen ( Spheniscidae ). PhD Thesis, Kiel University, Kiel. 121 p.
Advertisement
Short- and long-term consistency in the foraging niche of wandering albatrosses
- Original Paper
- Published: 01 May 2012
- Volume 159 , pages 1581–1591, ( 2012 )
Cite this article
- Filipe R. Ceia 1 ,
- Richard A. Phillips 2 ,
- Jaime A. Ramos 1 ,
- Yves Cherel 3 ,
- Rui P. Vieira 1 ,
- Pierre Richard 4 &
- José C. Xavier 1 , 2
1023 Accesses
68 Citations
8 Altmetric
Explore all metrics
The wandering albatross ( Diomedea exulans ) is regarded as a generalist predator, but can it be consistent in its foraging niche at an individual level? This study tested short- and long-term consistency in the foraging niche in terms of habitat use, trophic level and, by inference, prey selection. Fieldwork was carried out at Bird Island, South Georgia, in May–October 2009, during the chick-rearing period. Blood (plasma and cells) and feathers for stable isotope analyses (δ 13 C and δ 15 N) were sampled from 35 adults on their return from a foraging trip during which they carried stomach temperature, activity and global positioning system loggers. Results suggest short-term consistency in foraging niche in relation to both oceanic water mass and trophic level, and long-term consistency in use of habitat. Consistent differences between individuals partly reflected sex-specific habitat preferences. The proportion of consistent individuals (i.e., with a narrow foraging niche) was estimated at c. 40 % for short-term habitat and trophic level (prey) preferences and 29 % for longer-term habitat preference, suggesting this is an important characteristic of this population and potentially of pelagic seabirds in general. Foraging consistency was not related to body condition or level of breeding experience; instead, it may reduce intraspecific competition.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others
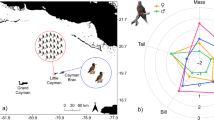
Interspecific and intraspecific foraging differentiation of neighbouring tropical seabirds
R. E. Austin, F. De Pascalis, … J. A. Green
Can variations in the spatial distribution at sea and isotopic niche width be associated with consistency in the isotopic niche of a pelagic seabird species?
Filipe R. Ceia, Vitor H. Paiva, … Jaime A. Ramos

Flexible foraging strategies in a highly pelagic seabird revealed by seasonal isotopic niche variation
Karen Bourgeois, Jemma R. Welch, … James C. Russell
Anderson ORJ, Phillips RA, Shore RF, McGill RAR, McDonald RA, Bearhop S (2009) Diet, individual specialisation and breeding of brown skuas ( Catharacta antarctica lonnbergi ): an investigation using stable isotopes. Polar Biol 32:27–33
Article Google Scholar
Araújo MS, Bolnick DI, Machado G, Giaretta AA, dos Reis SF (2007) Using d13C stable isotopes to quantify individual-level diet variation. Oecologia 152:643–654
Bearhop S, Waldron S, Votier SC, Furness RW (2002) Factors that influence assimilation rates and fractionation of nitrogen and carbon stable isotopes in avian blood and feathers. Physiol Biochem Zool 75:451–458
Article CAS Google Scholar
Bearhop S, Phillips RA, McGill R, Cherel Y, Dawson DA, Croxall JP (2006) Stable isotopes indicate sex-specific and long-term individual foraging specialisation in diving seabirds. Mar Eco Prog Ser 311:157–164
Bolnick DI, Svanbäck R, Fordyce JA, Yang LH, Davis JM, Hulsey CD, Forister ML (2003) The ecology of individuals: incidence and implications of individual specialization. Am Nat 161:1–28
Bolnick DI, Svanbäck R, Araújo MS, Persson L (2007) Comparative support for the niche variation hypothesis that more generalized populations also are more heterogeneous. Proc Natl Acad Sci USA 104:10075–10079
Bond AL, Jones IL (2009) A practical introduction to stable-isotope analysis for seabird biologists: approaches, cautions and caveats. Mar Ornithol 37:183–188
Google Scholar
Caut S, Angulo E, Courchamp F (2009) Variation in discrimination factors (Δ15N and Δ13C): the effect of diet isotopic values and applications for diet reconstruction. J Appl Ecol 46:443–453
Cherel Y, Klages N (1998) A review of the food of albatrosses. In: Robertson G, Gales R (eds) Albatross biology and conservation. Surrey Beatty & Sons, Chipping Norton, pp 113–136
Cherel Y, Hobson KA, Hassani S (2005a) Isotopic discrimination between food and blood and feathers of captive penguins: implications for dietary studies in the wild. Physiol Biochem Zool 78:106–115
Cherel Y, Hobson KA, Weimerskirch H (2005b) Using stable isotopes to study resource acquisition and allocation in procellariiform seabirds. Oecologia 145:533–540
Cherel Y, Hobson KA, Guinet C, Vanpe C (2007) Stable isotopes document seasonal changes in trophic niches and winter foraging individual specialization in diving predators from the Southern Ocean. J Anim Ecol 76:826–836
Cook TR, Cherel Y, Tremblay Y (2006) Foraging tactics of chick-rearing Crozet shags: individuals display repetitive activity and diving patterns over time. Polar Biol 29:562–569
Dias MP, Granadeiro JP, Phillips RA, Alonso H, Catry P (2010) Breaking the routine: individual Cory’s shearwaters shift winter destinations between hemispheres and across ocean basins. Proc R Soc B 2:1786–1793
Elliott KH, Woo KJ, Gaston AJ (2009) Specialization in murres: the story of eight specialists. Waterbirds 32:491–506
Fritz H, Said S, Weimerskirch H (2003) Scale-dependent hierarchical adjustments of movement patterns in a long-range foraging seabird. Proc R Soc Lond B 270:1143–1148
Hecht T (1987) A guide to the otoliths of Southern Ocean fishes. S Afr J Antarct Res 17:2–87
Hobson KA, Clark RG (1992) Assessing avian diets using stable isotopes II: factors influencing diet-tissue fractionation. Condor 94:189–197
Hobson KA, Clark RG (1993) Turnover of δ 13 C in cellular and plasma reactions of blood: implications for nondestructive sampling in avian dietary studies. Auk 110:638–641
Jaeger A, Blanchard P, Richard P, Cherel Y (2009) Using carbon and nitrogen isotopic values of body feathers to infer inter- and intra-individual variations of seabird feeding ecology during moult. Mar Biol 156:1233–1240
Jaeger A, Connan M, Richard P, Cherel Y (2010a) Use of stable isotopes to quantify seasonal changes of trophic niche and levels of population and individual specialisation in seabirds. Mar Ecol Prog Ser 401:269–277
Jaeger A, Lecomte VJ, Weimerskirch H, Richard P, Cherel Y (2010b) Seabird satellite tracking validates the use of latitudinal isoscapes to depict predators’ foraging areas in the Southern Ocean. Rapid Commun Mass Spectrom 24:3456–3460
Kelly JF (2000) Stable isotopes of carbon and nitrogen in the study of avian and mammalian trophic ecology. Can J Zool 78:1–27
Lecomte VJ, Sorci G, Cornet S, Jaeger A, Faivre B, Arnoux E, Gaillard M, Trouvé C, Besson D, Chastel O, Weimerskirch H (2010) Patterns of aging in the long-lived wandering albatross. Proc Natl Acad Sci USA 107:6370–6375
Louzao M, Pinaud D, Péron C, Delord K, Wiegand T, Weimerskirch H (2010) Conserving pelagic habitats: seascape modelling of an oceanic top predator. J Appl Ecol 48:121–132
MacArthur RH, Pianka ER (1966) On optimal use of a patchy environment. Am Nat 100:603–610
Matich P, Heithaus MR, Layman CA (2010) Contrasting patterns of individual specialization and trophic coupling in two marine apex predators. J Anim Ecol 80:294–305
Newsome SD, Rio CMD, Bearhop S, Phillips DL (2006) A niche for isotopic ecology. Front Ecol Environ 5:429–436
Parnell AC, Inger R, Bearhop S, Jackson AL (2010) Source partitioning using stable isotopes: coping with too much variation. PLoS ONE 5:e9672
Passos C, Navarro J, Giudici A, González-Solís J (2010) Effects of extra mass on the pelagic behavior of a seabird. Auk 127:100–107
Phillips RA, Xavier JC, Croxall JP (2003) Effects of satellite transmitters on albatrosses and petrels. Auk 120:1082–1090
Phillips RA, Bearhop S, McGill RAR, Dawson DA (2009) Stable isotopes reveal individual variation in migration strategies and habitat preferences in a suite of seabirds during the nonbreeding period. Oecologia 160:795–806
Phillips RA, McGill RAR, Dawson DA, Bearhop S (2011) Sexual segregation in distribution, diet and trophic level of seabirds: insights from stable isotope analysis. Mar Biol 158:2199–2208
Quillfeldt P, McGill RAR, Masello JF, Weiss F, Strange IJ, Brickle P, Furness RW (2008) Stable isotope analysis reveals sexual and environmental variability and individual consistency in foraging of thin-billed prions. Mar Ecol Prog Ser 373:137–148
Rubenstein DR, Hobson KA (2004) From birds to butterflies: animal movement patterns and stable isotopes. Trends Ecol Evol 19:256–263
Schoener TW (1971) Theory of feeding strategies. Annu Rev Ecol Syst 2:369–404
Smale MJ, Watson G, Hecht T (1995) Otolith atlas of Southern African marine fishes. Ichyological monographs, vol 1. JLB Smith Institute of Ichthyology, Grahamstown
Tickell WLN (1968) The biology of the great albatrosses Diomedea exulans and Diomedea epomophora . Antarct Res Ser 12:1–55
Votier SC, Bearhop S, Witt MJ, Inger R, Thompson D, Newton J (2010) Individual responses of seabirds to commercial fisheries revealed using GPS tracking, stable isotopes and vessel monitoring systems. J Appl Ecol 47:487–497
Warburton K, Retif S, Hume D (1998) Generalists as sequential specialists: diets and prey switching in juvenile silver perch. Environ Biol Fish 51:445–454
Weimerskirch H (2007) Are seabirds foraging for unpredictable resources? Deep Sea Res Part 2 Top Stud Oceanogr 54:211–223
Weimerskirch H, Wilson RP (2000) Oceanic respite for wandering albatrosses. Nature 406:955–956
Weimerskirch H, Cherel Y, Cuénot-Chaillet F, Ridoux V (1997) Alternative foraging strategies and resource allocation by male and female wandering albatrosses. Ecology 78:2051–2063
Weimerskirch H, Gault A, Cherel Y (2005) Prey distribution and patchiness: factors in foraging success and efficiency of wandering albatrosses. Ecology 86:2611–2622
Weimerskirch H, Pinaud D, Pawlowski F, Bost CA (2007) Does prey capture induce area-restricted search? A fine-scale study using GPS in a marine predator, the wandering albatross. Am Nat 170:734–743
Weimerskirch H, Shaffer SA, Tremblay Y, Costa DP, Gadenne H, Kato A, Ropert-Coudert Y, Sato K, Aurioles D (2009) Species- and sex-specific differences in foraging behaviour and foraging zones in blue-footed and brown boobies in the Gulf of California. Mar Ecol Prog Ser 391:267–278
Williams R, McEldowney A (1990) A guide to the fish otoliths from waters off the Australian Antarctic Territory, Heard and Macquarie Island. ANARE Res Notes 75:1–173
Wilson RP, Cooper J, Plötz J (1992) Can we determine when marine endotherms feed? A case study with seabirds. J Exp Biol 167:267–275
Wilson RP, Pütz K, Grémillet D, Culik BM, Kierspel M, Regel J, Bost CA, Lage J, Cooper J (1995) Reliability of stomach temperature changes in determining feeding characteristics of seabirds. J Exp Biol 198:1115–1135
Woo KJ, Elliott KH, Davidson M, Gaston AJ, Davoren GK (2008) Individual specialization in diet by a generalist marine predator reflects specialization in foraging behaviour. J Anim Ecol 77:1082–1091
Xavier JC, Cherel Y (2009) Cephalopod beak guide for the Southern Ocean. British Antarctic Survey, Cambridge
Xavier JC, Trathan PN, Croxall JP, Wood AG, Podestá G, Rodhouse PG (2004) Foraging ecology and interactions with fisheries of wandering albatrosses ( Diomedea exulans ) breeding at South Georgia. Fish Oceanogr 13:324–344
Zwarts L, Hulscher JB, Koopman K, Piersma T, Zegers PM (1996) Seasonal and annual variation in body weight, nutrient stores and mortality of oystercatchers Haematopus ostralegus . Ardea 84A:327–356
Download references
Acknowledgments
This research was co-sponsored by the Foundation for Science and Technology (Portugal) through several grants (PTDC/BIA-BDE/64539/2006; SFRH/BD/64558/2009), Centre d’Etudes Biologiques de Chizé (France) and the British Antarctic Survey (UK). We thank Derren Fox, Ewan Edwards and Stacey Adlard for help in the field and G. Guillou for running stable isotope samples.
Author information
Authors and affiliations.
Department of Life Sciences, Faculty of Sciences and Technology, IMAR-CMA-Institute of Marine Research, University of Coimbra, 3004-517, Coimbra, Portugal
Filipe R. Ceia, Jaime A. Ramos, Rui P. Vieira & José C. Xavier
British Antarctic Survey, Natural Environment Research Council, High Cross Madingley Road, Cambridge, CB3 0ET, UK
Richard A. Phillips & José C. Xavier
Centre d’Etudes Biologiques de Chizé, UPR 1934 du CNRS, BP 14, 79360, Villiers-en-Bois, France
Yves Cherel
Laboratoire Littoral, Environnement et Sociétés, UMR 6250 du CNRS-Université de La Rochelle, 2 Rue Olympe de Gouges, 17000, La Rochelle, France
Pierre Richard
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Filipe R. Ceia .
Additional information
Communicated by C. Harrod.
Rights and permissions
Reprints and permissions
About this article
Ceia, F.R., Phillips, R.A., Ramos, J.A. et al. Short- and long-term consistency in the foraging niche of wandering albatrosses. Mar Biol 159 , 1581–1591 (2012). https://doi.org/10.1007/s00227-012-1946-1
Download citation
Received : 27 January 2012
Accepted : 19 April 2012
Published : 01 May 2012
Issue Date : July 2012
DOI : https://doi.org/10.1007/s00227-012-1946-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Stable Isotope
- Trophic Level
- Stable Isotope Analysis
- Stable Isotope Ratio
- Find a journal
- Publish with us
- Track your research

IMAGES
VIDEO
COMMENTS
The wandering albatross (Diomedea exulans) is the world's largest flying bird, with a wingspan reaching an incredible 3.5 metres.These birds are oceanic nomads: they spend most of their 60 years ...
The Wandering Albatross and Global Warming. ... It is hard for a person to detect climate change at this scale, even though global temperature measurements clearly show that the planet has warmed.
Wandering albatross. (Elizabeth Crapo, NOAA) A wandering albatross has the largest wingspan of any bird, 3.5 meters (11.5 feet) tip to wing tip.
Length. 107-135. cm inch. Wingspan. 2.5-3.5. m ft. Described as "The bird which made the breeze to blow" the wingspan of a Wandering albatross ( Diomedea exulans) is the longest of any bird. It lives up to its name when it takes fishing trips that last 10-20 days and can cover 10,000 km while using hardly more energy than when sitting on its nest.
Wandering Albatross (Diomedea exulans) in the Drake's Passage. Credit: 3HEADEDDOG/Wikimedia Commons, CC BY-SA 1/6. ... By 2003, Marion Island's temperature had increased by 1.2°C compared
Wandering albatrosses are large seabirds with one of the most impressive wingspans found in the animal kingdom. While they spend most of their time efficiently gliding above the waves, they do have to regularly land on sea to snatch their prey. ... Doppler velocity (5 Hz), temperature (6 Hz), pressure (6 Hz), geomagnetism (6 Hz), and angular ...
There are 22 species of albatross that share the gift of efficient long-distance gliding flight. They are famously recognized by their lengthy wingspans with the Wandering Albatross holding the record at nearly 12 feet. These remarkable wingspans are vital for a lifetime at sea. With the help of air currents and temperature changes, these wings ...
The wandering albatross () is the largest extant bird. These birds spend most of their lives out at sea, using their large wings to ride the ocean's winds. They can glide for hours without flapping their wings. They are so efficient at flying that they use less energy in the air than they do sitting on a nest.
It is known that albatrosses are affected by climate change in more than one way: changed winds influence albatrosses' foraging flights 6; changes in sea temperature alter their prey ...
The wandering albatross (Diomedea exulans) is the world's largest flying bird, with a wingspan reaching an incredible 3.5 metres.These birds are oceanic nomads: they spend most of their 60 years of life at sea and only come to land to breed approximately every two years once they have reached sexual maturity.
wandering albatross facts - Basics. Average Weight: 5.9 to 12.7 kg, commonly 6.4 - 11.9 kg, males are typically around 20% heavier than females. Immature birds have been reported at up to 16.1 kg shortly after leaving the nest due to still having fat reserves that sustained while on the nest waiting for the adults to return.
Of the southern hemisphere albatrosses, data are available only for the Wandering Albatross ( Diomedea exulans) from Marion Island (Brown and Adams 1988 ), where thermistors in dummy eggs recorded an incubation temperature of 32.1 ± 1.2°C, well below the body temperature of 39.2°C of this species (Warham 1971 ).
The Wandering Albatross is a truly remarkable bird that captivates the imagination of wildlife enthusiasts and researchers alike. ... They must withstand strong winds, freezing temperatures, and potential threats from predators. Despite these difficulties, the dedicated parents remain vigilant, ensuring the survival of their offspring.
Woods Hole, Mass. — Wandering albatrosses, which are an iconic sight in the Southern Ocean, are highly adapted to long-distance soaring flight. Their wingspan of up to 11 feet is the largest known of any living bird, and yet wandering albatrosses fly while hardly flapping their wings.
These remarkably efficient gliders, named after the Greek hero Diomedes, have the largest wingspan of any bird on the planet. Name: Wandering Albatross, Snowy Albatross, White-winged Albatross ( Diomedea exulans) Length: Up to 135 cm. Weight: 6 to 12kg. Location: All oceans except in the North Atlantic.
Temperature data were analysed following and so as to estimate the mass of prey caught at sea using the amplitude and the duration of the temperature drop recorded in the stomach after prey ingestion. The ... Foraging paths of 40 incubating wandering albatrosses from Possession Island, Crozet archipelago (white square) in January - March 2011
The snowy albatross boasts a wingspan that can exceed 3.5 meters (11 feet), with an average span of around 3.1 meters (10 feet 2 inches). Body length ranges from 107 to 135 cm (3 feet 6 inches to 4 feet 5 inches), with females being slightly smaller than males. Adults typically weigh between 5.9 to 12.7 kg (13 to 28 lb).
Threats To Wandering Albatross Population. Climate change has caused a significant impact on the wandering albatross population. Changes in water temperature and ice cover affect the bird's food supply, which can result in lower breeding success rates. The increase in plastic waste has also led to many albatrosses suffering entanglement and ...
Our results demonstrate that wandering albatrosses can take-off in a variety of environmental conditions (wind speed: 0.7 ∼ 15.4 m/s, wave height: 1.6 ∼ 6.4 m). A previous study on wandering albatrosses identified the transition state from resting to flying tended to increase as the wind speed increased (Clay et al., 2020). Our results ...
Wandering albatrosses only start breeding at eight to 10 years of age, they only have one chick every two years, and it takes almost 12 months for chicks to grow large enough to fly. Wandering ...
Figure 2.Distribution of sea surface temperatures (°C) along Laysan, black-footed, and Indian yellow-nosed albatrosses foraging routes during incubation (A) and brooding (B).Laysan and black-footed albatrosses were tracked at Tern Island, Northwest Hawaiian Islands during 2002-2003, 2004-2005, and 2005-2006, and yellow-nosed albatrosses were tracked at Amsterdam Island, southern Indian ...
GPS-tracking of 40 wandering albatrosses from the Crozet archipelago during the incubation phase confirmed foraging movements of between 475-4705 km, which give birds access to a variety of prey, including fishery wastes. ... Devices were set to record pH (accuracy 0.02 pH units) and temperature (accuracy <0.1°C) every ten seconds ...
The wandering albatross (Diomedea exulans) is regarded as a generalist predator, but can it be consistent in its foraging niche at an individual level? This study tested short- and long-term consistency in the foraging niche in terms of habitat use, trophic level and, by inference, prey selection. Fieldwork was carried out at Bird Island, South Georgia, in May-October 2009, during the chick ...