

Temperature Excursion Management – A Novel Approach Of Quality System In Pharmaceutical Industry
Authors: nirmal kumar, ajey jha.
Quality of pharmaceutical product largely depends upon by the environment controls during its storage and handling. Each pharmaceutical product should be handled and stored under specified storage condition labelled on product information data sheet or product pack. Hence the temperature excursions during receipt of raw materials, manufacturing of pharmaceutical products and distribution should be managed during entire product life cycle with holistic approach. The research is based on primary data and exploratory study through literature review. The temperature excursion may be observed during transportation of raw materials manufacturing as well as distribution of pharmaceutical products, which have potential to deteriorate the product quality. Temperature excursion in pharmaceutical industry should be recorded and reported to the manufacturer for further investigation and risk analysis. The concept of temperature excursions, its reasons, consequences and handling mechanism should be well understood to ensure the concerted efforts under the aegis of Quality Management System. Based on the reasons and consequences of temperature excursions during pharmaceutical operations, a system based quality management has been envisaged through this study. The concept an d procedure to handle temperature excursion have evolved after this study which shall be useful to pharmaceutical industry as well as to medicine distributors and consumers.
GMP; Temperature excursions management; Formulation; Quality by Design; Degradation products
Citation: Kumar, N., Jha, A., Temperature excursion management – A novel approach of quality system in pharmaceutical industry, Saudi Pharmaceutical Journal(2016),doi: http://dx.doi.org/10.1016/j.jsps.2016.07.001
Received: 12 December 2015, Accepted: 1 July 2016, Available online: 9 July 2016
Copyright: © 2016 Production and hosting by Elsevier B.V. on behalf of King Saud University.Open Access funded by King Saud University
Temperature excursion is a general term that represents the environmental excursions. However, there is a need of holistic approach to handle the temperature excursions starting from raw material manufacturing site to medicine retailers shop to protect quality of product. The temperature excursion at any stage of pharmaceutical business operation must be reported as soon as possible and investigated appropriately. The consequences of deviation against temperature and humidity limits should be studied appropriately by quality assurance personnel. The risk of temperature excursions cannot be ruled out, but it can be minimized through effective system. Alternative is to use thermal resistant packaging and stringent control measures during transit and shipment, to avert the undesired quality impact on pharmaceutical product. The systematic approach to handle the issues related temperature excursions become inevitable for pharmaceutical manufacture.

Temperature Excursion Requirements for Refrigerated Medications

Related Content
A review of infused allergy treatments, fda grants accelerated approval for controversial novel alzheimer’s drug, new guidelines shore up smart infusion pump safety, analgesia and sedation strategies in covid-19 patients.

- Advertising Contacts
- Editorial Staff
- Professional Organizations
- Submitting a Manuscript
- Privacy Policy
- Classifieds
Explore solutions built for your industry
Our customer-proven solutions monitor medications and food inventories for some of the most recognizable names in the industries of healthcare, food service, and transportation, and logistics. See how our solutions adapt to your industry needs.
BY INDUSTRY
- Retail Grocery
- Food Service
- K-12 Education
- Conveniences Stores
- Supply Chain Monitoring
BY USE CASE
- COVID-19 Solutions
- Pharmacy Monitoring
- VFC Monitoring
- Food Safety Monitoring
- Asset Monitoring
- Advanced Analytics
SYSTEM COMPONENTS
- System Overview
- Cloud Dashboard
- Digital Checklists
- Sensors & Data Loggers
- Implementation Services
Festival Foods Protects its Profits & People with SmartSense
Share SmartSense Solutions with your team.
Resource Center
Work smarter. Explore our videos, webinars, and customer stories.
Learn how our Sensing-as-a-Service solutions can fit your business.
Review technical specifications for our solutions.
- Customer Videos
- Customer Stories
- Thought Leadership
- Media Coverage
- Wireless Sensors
- Installation
- See all categories
- Contact Support
Questions? Call +1 (866) 806-2653 to speak to our experts.
Questions contact us..
Call +1 (866) 806-2653 to speak with our experts or get started with a demo.
SmartSense was created to use the power of the Internet of Things (IoT) to help our customers protect the assets most critical to the success of their business.
Create the future of IoT by joining our team.
CONNECT. PROTECT. RESULTS.
- Leadership Team
- In the News
Select your login
- Help Center
- COVID-19 Monitoring
- SCHEDULE DEMO
- There are no suggestions because the search field is empty.
June 21, 2017
6 Steps for Handling Temperature Excursions
Written by SmartSense | Pharmacy Safety
What is a temperature excursion? In the pharmaceutical industry , it refers to any temperature reading outside recommended ranges from the manufacturer’s package insert. Why is it important? Because out-of-range storage temperatures or inappropriate conditions for any vaccine can negatively impact the efficacy, and will require immediate action.
Fortunately, the Centers for Disease Control (CDC) have developed best practices to handle this emergency situation. If there is any question about whether vaccines may have been exposed to a temperature excursion due to the unit becoming too cold or too hot, take the following steps.
Step 1: Notify Supervisors
Any staff member who hears an alarm, receives an alert message, or notices a temperature excursion should notify the vaccine coordinator immediately or report the problem to a supervisor.
Step 2: Quarantine Vaccines
If a vaccine has been compromised, quarantine it immediately. Label exposed vaccines, “DO NOT USE,” and place them separately from other vaccines in the storage unit. Do NOT discard the compromised vaccines.
Step 3: Document the Event
The vaccine coordinator, supervisor, or if necessary, the person reporting the problem, should document the event . Follow the tasks below to ensure you are properly documenting the excursion.
- Name of the person completing the report
- Date and time of the temperature excursion
- Inventory of affected vaccines
- Description of the event
- Minimum and maximum storage unit temperature and room temperature during the time of the event
- Length of time vaccine may have been affected
- Listing of items in the unit (including water bottles) other than vaccines
- Any problems with the storage unit and/or affected vaccines before the event
- Other relevant information

Step 4: Get Guidance
Contact your immunization program or vaccine manufacturer for additional guidance on whether to use affected vaccines and for information about whether patients will need to be recalled for re-vaccinations. Be prepared to provide documentation of the event (e.g., temperature log data).
Step 5: Implement SOPs
Implement your facility’s Standard Operating Procedures (SOPs) to adjust the unit temperature to the appropriate range. At a minimum, check the temperature monitoring device to make sure it is appropriately placed in the center of the fridge.
Step 6: Wrap Up
Complete your documentation of the event, including the following:
- What happened to affected vaccines
- What you did with the vaccine and when
- Whom you have contacted and instructions received
- What you have done to prevent a similar future event
You never know when a temperature excursion may happen. If you have not yet incorporated these best practices into your monitoring program, now is a good time to start!
Subscribe to Our Blog!
Subscribe to our blog to get weekly email updates about quality and safety issues important to the pharmaceutical industry.
Subscribe to the SmartSense Blog
Stay up-to-date on the evolution of IoT connectivity.
Learn how our complete critical environment monitoring solution will help you connect and transform your business.
Call +1 (866) 806-2653 to speak with our industry experts or get started by requesting a demo.
- Terms of Service
- Return Policy
- Privacy Policy
- Cookie Policy
- System Status

Guest Column | March 20, 2024
How to prevent and manage temperature excursions in clinical trials.
By Ana-Zeralda Canals Hamann, Debiopharm

In clinical trials, the quality and integrity of the drug products and the biological samples are crucial for ensuring the validity and reliability of the results. However, maintaining the optimal storage conditions for these products can be challenging, especially when they must be transported or handled across different locations and environments.
Temperature excursions, which are deviations from the specified temperature range for a product or sample, can compromise the product's quality, safety, and efficacy and may have serious consequences for the trial outcomes, regulatory compliance, and patient safety. Therefore, clinical trial professionals must effectively prevent and manage temperature excursions, especially for temperature-sensitive products such as vaccines, biologics, and cell and gene therapies.
This article will provide an overview of temperature excursions, why avoiding them is essential, and how they can be prevented and managed in clinical trials. It will also offer some practical tips and tricks for cold chain management, which ensures that the product is kept within the required temperature range throughout the supply chain. By following the best practices and the guidance in this article, clinical trial professionals can reduce the risk and the impact of temperature excursions and ensure the quality and integrity of the product.
What Are Temperature Excursions, And Why Are They Important?
Temperature excursions are defined as any deviation from the specified temperature range for a product during transport, storage, or handling. The manufacturer or the sponsor usually determines the specified temperature range based on the stability data and the regulatory requirements for the product or sample. For example, some products may need to be stored at 2 degrees to 8 degrees C (refrigerated), while others may need to be stored at minus 20 degrees C or below (frozen).
Temperature excursions can occur due to various factors, such as weather delays, customs clearance, equipment failure, human error, or inadequate packaging or monitoring. Temperature excursions can affect the quality, safety, and efficacy of the product and may cause the following problems:
- Loss of potency or activity of the product may reduce its effectiveness or accuracy.
- Alteration of the physical or chemical properties of the product may affect its appearance, solubility, viscosity, or pH.
- Formation of aggregates, precipitates, or crystals in the product may compromise its stability or sterility.
- Generation of toxic or immunogenic substances in the product may pose a risk to the patient.
- Non-compliance with the regulatory standards or the trial protocols may result in audit findings, quality issues, or legal actions.
- Waste of resources, time, and money, as the product may need to be replaced, retested, or discarded.
Therefore, preventing and managing temperature excursions in clinical trials is essential, as they can have serious implications for trial outcomes, regulatory compliance, and patient safety.
How Do We Prevent Temperature Excursions?
The best way to prevent temperature excursions is to plan ahead and follow the best practices for cold chain management. Cold chain management ensures that the product is kept within the required temperature range throughout the supply chain, from the manufacturer or the sponsor to the site. Cold chain management involves selecting and validating the packaging and shipping materials, monitoring and tracking the temperature history, labeling and documenting the shipment, training and communicating with the staff, and coordinating and collaborating among the partners. Some of the tips and tricks to prevent temperature excursions are:
- Select the product's appropriate packaging and shipping materials based on the required temperature range, transit time, and destination. For example, you may use insulated containers, gel packs, dry ice, or liquid nitrogen to maintain the desired temperature during transport. You may also use passive or active packaging systems, depending on the level of control and automation you need. Passive packaging systems rely on preconditioned materials to maintain the temperature, while active packaging systems use electrical or mechanical devices to regulate the temperature. You should validate the performance and the suitability of the packaging and shipping materials before using them and follow the manufacturer's or the sponsor's instructions for their preparation and use.
- Use validated temperature monitors or indicators to track the temperature history of the shipment and alert the recipient of any potential excursions. Temperature monitors are devices that record or display the shipment temperature during transport, storage, or handling. They can be either electronic or chemical, and they can provide either continuous or single-point measurements. You should choose the type and the number of temperature monitors or indicators based on the product characteristics, the shipment size and duration, and the regulatory requirements. You should also validate the accuracy and reliability of the temperature monitors before using them and follow the manufacturer's or the sponsor's instructions for their placement and activation.
- Label the shipment clearly and accurately with the product name, storage conditions, and contact information. The label should indicate the product's name and description, the required temperature range for its storage, and the contact details of the sender and recipient. The label should also include any special instructions or warnings for the transport, storage, or handling of the product, such as "Do not freeze", "Keep upright", or "Protect from light". The label should be visible and legible and should be attached securely to the outer surface of the shipment. You should also include a copy of the label inside the shipment in case the outer label is damaged or lost.
- Train the staff involved in transporting, storing, and handling the product on the proper procedures and protocols. The staff should be familiar with the product characteristics, the packaging and shipping materials, the temperature monitors, the labeling and documentation, and the contingency plans for preventing and managing temperature excursions. The staff should also be aware of the potential risks and consequences of temperature excursions, as well as the importance of following the best practices and guidance for cold chain management. You should provide regular and updated training to the staff and evaluate their knowledge and performance periodically.
- Communicate and coordinate with the shipping partners, sites, and sponsors to ensure timely and secure delivery and receipt of the product. You should establish and maintain clear and effective communication and coordination with the shipping partners, such as the courier companies, the airlines, or the customs agents, to ensure that they understand and comply with the requirements and expectations for the transport, storage, and handling of the product. You should also inform and confirm with the sites, such as the clinics, the hospitals, or the laboratories, about the arrival and acceptance of the shipment and verify that they have the appropriate facilities and equipment to store and handle the product or sample. You should also report and update the sponsors, such as the manufacturers, the investigators, or the regulators, about the product's status and condition and notify them of any issues or incidents that may affect the quality and integrity of the product or sample.
How Do You Manage Temperature Excursions?
Despite the best efforts, temperature excursions may occur due to unforeseen circumstances, such as weather delays, customs clearance, equipment failure, or human error. In such cases, it is important to follow the steps below to manage the temperature excursions.
Report the excursion
Report the temperature excursion to the relevant stakeholders, most importantly the Analytical Development and Quality Control (ADQC) staff, as soon as possible and document the event's details, such as the date, time, duration, location, and temperature range. You should inform the sender, the recipient, and the sponsor of the temperature excursion as soon as you detect it and provide them with the relevant information and data from the temperature monitors. It would be best if you also documented any major temperature excursions in a written report, which should include the following information:
- the product or sample name, description, and batch or lot number,
- the shipment details, such as the origin, the destination, the transit time, and the carrier.
- the temperature excursion details, such as the date, time, duration, location, and temperature range,
- the temperature monitor or indicator details, such as the type, the number, the placement, and the readings,
- the actions taken to address the temperature excursion, such as the quarantine, the assessment, the CAPA, or the disposal, and
- the impact and the outcome of the temperature excursion, such as the product or sample status, the quality and safety issues, or the regulatory implications.
Quarantine the affected product until the ADQC department has performed the impact assessment and if needed, the corrective and preventive actions (CAPA) are completed. You should isolate the affected product from the rest of the shipment and store it in a separate and secure area within the required temperature range. You should also label the affected product as "Quarantined" or "Under Investigation" and restrict its access and use until the impact assessment. You should not release, distribute, or administer the affected product until you receive confirmation and approval from the sponsor or the manufacturer.
Read the instructions
Depending on the product that has experienced the temperature excursion, consult the product label, the package insert, the stability data, or the manufacturer or sponsor for guidance on the acceptability of the product after the temperature excursion. It would be best if you referred to the product label, the package insert, the stability data, or the manufacturer or sponsor for the information and the criteria on the acceptable temperature range, the maximum allowable excursion time, and the potential impact of the temperature excursion on the product quality, safety, and efficacy.
You should also follow the instructions and recommendations from the manufacturer or sponsor on handling and using the affected product after the temperature excursion. It would be best if you did not make any assumptions or decisions about the acceptability of the product without consulting the manufacturer or sponsor, as they are the ultimate authority and are responsible for the product’s quality and integrity.
Develop and execute a CAPA plan
Implement the CAPA plan to address the root cause of the temperature excursion, if needed, and prevent its recurrence. You should investigate and identify the root cause of the temperature excursion and analyze the contributing factors and the corrective actions that were taken. You should also develop and execute a CAPA plan, which should include the following elements:
- the objectives and scope of the CAPA plan, as well as the roles and responsibilities of the staff involved,
- the corrective actions to resolve the immediate problem and restore the regular operation,
- the preventive actions to eliminate or reduce the risk of the problem from happening again,
- the verification and validation of the effectiveness and the suitability of the corrective and preventive actions,
- the monitoring and evaluation of the performance and the outcome of the CAPA plan, and
- the documentation and communication of the CAPA plan and its results.
What To Do If The Product Is Unusable
If the product is deemed unusable or unsafe, you should discard the affected product safely and appropriately, following the local regulations and the sponsor's instructions.
You should also document and record the disposal of the product and provide the reason for and evidence of disposal. You should not use, distribute, or administer the product if deemed unusable or unsafe, as it may pose a risk to the trial outcomes, regulatory compliance, and patient safety.
About The Author:

Like what you are reading?
Sign up for our free newsletter.


How to Troubleshoot Temperature Excursions?
Download our free temperature excursions poster below detailing everything from this blog including:
- Warning signs of a temperature excursion
- Actions items to take during an excursion event
- Preventative measures you can take
As a primer, we’ve detailed below everything you need to know about temperature excursions so you can be prepared if one happens inside your healthcare facility.
What Is a Temperature Excursion?
In the United States, the CDC defines a temperature excursion as "any break in the cold chain of a pharmaceutical product" . The "cold chain", is a series of refrigerated production , storage and distribution activities. These processes ensure that vaccines and other pharmaceuticals remain at the correct temperature. Maintaining proper temperatures and avoiding excursions ensures that materials remain safe and effective.
Meanwhile, according to the European Compliance Academy , a temperature excursion is defined as: " a deviation from the labeled storage condition of a product for any duration " .
This includes excursions during transportation or distribution.

As a result of this degradation, there will be a generation of impurities in the product. Degradation products like this are not wanted in the manufacturing chain. Additionally, they present a threat to patients' health.
As such, the raw materials needed to produce pharmaceuticals need to be maintained as well . Active pharmaceutical ingredients (API) need proper temperatures during the manufacturing process. If not properly handled, they can lose potency, effectiveness and safety before they're even distributed.
Deviating from optimal storage conditions can result in significant changes in the API. This includes degradation, decay, polymerization, and an increase in impurity levels.
Reasons Why Temperature Excursions Occur
Air handling units (AHU) maintain the required temperature in pharmaceutical factories. So, the design and capacity of these units reflect the API manufacturers temperature requirements during the production process. Regardless, temperature excursions are unavoidable. Even in the manufacturing area and during transportation.

- An insufficient number of air handling units to maintain the desired temperature conditions
- A leaked or ruptured air duct
- Mechanical failure in air handling units
- Unprecedented temperature fluctuations.
- Power outages interrupting the AHU's operation
- Poor adherence to good manufacturing practices (GMP) on the production floor
- Poor staff oversight of temperatures/ Poor Quality Control
- Extreme weather conditions and poor or absent SOPs for handling them

Effects Of Temperature Excursion
A temperature excursion affects product quality in two potential ways. But, the impacts of high temperatures are different than those of temperatures that are too low. Though the result is the same nonetheless - a compromised product.
Products sensitive to high temperatures experience degradation that can cause a decrease in the active ingredient content of the product .
This happens due transformations in the affected, degraded components within. The transformation could be oxidative, hydrolytic, or some other form. As a result, much more toxic variants of the compound begin to appear. The amount of degradation/toxicity is dependent on length and severity of the temperature excursion, which can result in:
- Components becoming discolored.
- Changes in dissolution rates
- Separation of emulsions
Products sensitive to a low temperature usually get damaged by phase changes caused by the freezing process . As a result, the physical atomic structures of the chemicals experience permanent changes.
Products with large quantities of water, like creams or biologicals, are especially prone. Often, they'll lose their properties after experiencing excessive freeze-thaw or temperature cycles. Because water can easily change temperature, the presence of ice quickly damages product.
How To Measure Temperature Excursions
One responsibility of pharmaceutical manufacturers is guaranteeing every batch is a safe and effective high quality product. One of the ways to make this happen is through continuous temperature monitoring. By monitoring the temperature range, they can catch any excursions that occur. After which, they can take the necessary steps to address them immediately.
The Center for Disease Control recommends using a continuous temperature monitoring device (TMD) to track excursions . The recommended TMD is a digital data logger (DDL) that has the following characteristics:
- Ability to record the logged temperature values to a computer system for review.
- The digital data logger's calibration status must be regularly verified, with up-to-date software. This ensures accurate information about storage conditions.
The following information is required when documenting temperature excursions:
- The date and time the temperature excursion occurred
- An inventory of affected products
- The storage unit air temperatures. Including the minimum and maximum temperatures observed during the temperature excursion, if available
- Ambient temperature (also referred to as “room temperature”).
- A general description of the event, such as the length of the temperature event. The digital data logger should provide this data.
- A list of any other items in the storage unit
- Documentation of any problems with the storage unit
All the data should be compiled, and a copy given to the distributor and recipient of the affected product(s).

How To Avoid and Combat Temperature Excursions
Simply put, any temperature excursion is a result of prolonged exposure to air that is room temperature or above. For end users of pharmaceuticals, the most likely scenario for them to experience a temperature excursion is because they’ve left the door open to a medical refrigerator/medical freezer or it has stopped running.
If Your refrigerator/freezer temperature has dropped below 35°F (2.0°C) for 15 or more minutes consecutively:
- Check the Placement of the Thermometer probe – Place in the middle and monitor temperature in 30-minute intervals
- Adjust Refrigerator/Freezer Temperature - Change temperature of appliance, if possible, to a warmer setting. Monitor and record = temperature every 30 minutes for next 2 hours.
- If temperatures remain out of range — implement facilities’ relocation plan. Immediately call vaccine manufacturer/distributor.
If your Refrigerator has risen above 46°F (7.7°C) or Freezer has risen above 5°F(-15°C) for 60 minutes or more consecutively:
- Check your power supply — if your area is experiencing a power outage estimated to last 2 or more hours and your facility does NOT have a dedicated backup power source for your cold storage appliances, implement emergency relocation plan
- Check the door to the storage unit — ensure nothing is preventing door from securely closing. If so, adjust accordingly and shut door completely.
- Adjust the refrigerator/freezer temperature — Change temperature of appliance to a cooler temperature is possible. Monitor and record temperature every 30 minutes for next 2 hours.
- If temperatures remain out of range — implement facilities’ relocation plan. Immediately call vaccine manufacturer/distributor. In short, unless total failure of the appliance has occurred, the most common solutions to these problems are:
- A door alarm to ensure staff always close it
- A backup power solution to ensure any medical and pharmaceutical refrigerators continue to operate as normal.
While door alarms are fairly easy to source tools, a reliable and powerful backup power system for refrigerators can be harder to find.
Luckily, battery backup systems offer instant and automatic power for medical appliances as soon as the power goes out. As a result, no staff needs to be on-site to keep track of or start the generator and vaccines will continue to remain safe—with no extra work required.
Additionally, their vertical, cabinet-like design and leak-proof batteries mean they can be installed in even the tightest spaces and oriented in anyway to make them fit. Plus, if your medication or vaccine room is truly tight on space, a hardwired backup power unit can instantly supply remote power to your appliance—directly via the outlet its already plugged into.
Regardless of what kind of system is the best fit, they ensure that your entire stock of vaccines are protected from a sudden loss of power (and the resulting temperature excursions) by guaranteeing a seamless transition from utility power to backup power.

- Over a weekend
- Or even for a whole week.
So, to protect your facility from tens of thousands of dollars in lost vaccine stock, speak to a Medi-Products battery backup expert.
They’ll help design you a system that both meets your power needs and will fit inside your facility—for a much lower cost than what your vaccines are worth. So a backup power system pays for itself the first time your power goes out.
Designing a system for you is as easy as taking a picture of your appliance’s nameplate, and a photo of the room where it’s in.
Then, you just email both photos to our Product experts, and we’ll provide you with multiple options for backup power protection.
For more information contact:
1.800.7653237
Battery Backup for Vaccine Refrigerators and Freezers.

Our powerful battery backup systems will instantly power multiple appliances during a power outage. These custom sized systems can provide power for up to 72 hours of runtime!
POPULAR QUESTIONS
Blog categories.
- Healthcare Compliance (30)
- Healthcare Management (26)
- Healthcare Storage (22)
- Healthcare Design (21)
- Vaccine Storage (20)
- Backup Generators (18)
- Power Outages (18)
- Energy-Efficient Solutions (1)
VISIT OUR LEARNING CENTER

We would like to hear from you
Our sales and technical support staff are available 8-5 EST, Mon-Fri

Vaccine Storage and Handling Resources
Recommendations and guidelines.
Proper vaccine storage and handling practices play a very important role in protecting individuals and communities from vaccine-preventable diseases. Vaccine quality is the shared responsibility of everyone, from the time vaccine is manufactured until it is administered.
Resources on Proper Vaccine Storage and Handling
- COVID-19 Vaccine Storage Temperature Logs:
- Refrigerator Storage Temperature Log (Celsius)
- Refrigerator Storage Temperature Log (Fahrenheit)
- Ultra-Cold Vaccine Storage Temperature Log (Celsius)
- Ultra-Cold Vaccine Storage Temperature Log (Fahrenheit)
- Safe and Proper Sharps Disposal During the COVID-19 Mass Vaccination Campaign This fact sheet reinforces how you can protect yourself from needlestick injuries while administering COVID-19 vaccines or while helping at vaccination sites.
- Temperature Monitoring Best Practices for Refrigerated Vaccines [2 pages] (Feb 2018) Fahrenheit (F) | Celsius (C)
- Temperature Monitoring Best Practices for Frozen Vaccines [2 pages] (Feb 2018) Fahrenheit (F) | Celsius (C)
- Storage Best Practices for Refrigerated Vaccines [2 pages] (Feb 2018) Fahrenheit (F) | Celsius (C)
- Storage Best Practices for Frozen Vaccines [2 pages] (Dec 2020) Fahrenheit (F) | Celsius (C)
- Vaccine Storage and Handling Toolkit (Mar 2021) A comprehensive resource for health care providers on vaccine storage and handling recommendations and best practice strategies. The Toolkit includes guidance on managing and storing vaccine inventory, using and maintaining storage unit and temperature monitoring equipment, preparing for emergency situations, and training staff.
- You Call the Shots: Vaccine Storage and Handling Module (Jan 2020) An interactive, web-based module which provides learning opportunities, self-test practice questions, reference and resource materials, and an extensive glossary. Continuing education credit is available.
- PDF [9 pages] (Oct 2023)
- PDF [9 pages] (Feb 2024)
- Provider’s Role: Importance of Vaccine Admin. & Storage Includes vaccine administration, timing and spacing of vaccine doses, observation of precautions and contraindications, management of vaccine side effects, etc.
- Vaccines for Children Program: Vulnerabilities in Vaccine Management (Jun 2012) Report and recommendations released by HHS Office of Inspector General following a routine assessment of the Vaccines for Children program.
- Handling a Temperature Excursion in Your Vaccine Storage Unit [1 page, 508] (Mar 2020) This document describes immediate corrective actions following a vaccine storage unit temperature excursion.
- Identifying, Disposing, and Reporting COVID-19 Vaccine Wastage (Feb 2022) Information to help providers and immunization managers in COVID-19 vaccination programs identify, handle, dispose of, and report COVID-19 vaccine waste.
- Packing Vaccines for Transport During Emergencies [2 pages] (Aug 2015) Do you know how to protect your vaccines during equipment failures, power outages, natural disasters? This document illustrates how to pack your refrigerated vaccines during emergency situations to prevent compromising vaccine storage conditions and damage to your vaccine supply.
Additional Resources for Vaccine Storage and Handling
Note: Storage and handling information in previously published CDC documents, including the 13th Edition Pink Book, may be superseded by the materials listed in above section on this page. If inconsistencies are seen, follow newer guidelines in toolkit or more recently dated materials.
- Vaccine Storage Temperatures [1 page]
- Vaccine Administration and Storage and Handling Resources Guide [1 page]
- Storage and Handling Best Practices: Hawaii (2018) Video highlighting Hawaii’s 2016 CDC Childhood Immunization Champion Award winner applying vaccine storage and handling best practices.
- Contact Information for State and Local Health Department Immunization Programs
- Storage and Handling Resources Refrigerator Temperature Logs (Fahrenheit)(Celsius), Freezer Temperature Logs, Checklist for Safe Storage and Handling, Don’t Be Guilty of These Errors in Storage & Handling, Do Not Unplug Sign…
- Manufacturer Product Information Package inserts
- Pink Book Chapter on Vaccine Storage and Handling See note above about referencing this document.
- National Institute of Standards and Technology: Vaccine Storage and Handling Research
Top of Page
- Vaccine Administration
- Recalled Vaccines
- Current Vaccine Shortages and Delays
- Vaccines & Immunizations
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.

Industry News
Temperature excursion management in pharmaceutical storage.
AKCP 06.2020 Articles , Blog

The quality of pharmaceuticals relies on environmental controls during their storage and handling. Every pharmaceutical item ought to be taken care of and stored under manufacturer-recommended storage conditions marked on the data information sheet or packaging.
Temperature Excursion Management in Pharmaceutical Storage is important during receipt of raw materials, manufacturing, and distribution of drugs.
System failures or human carelessness can cause circumstances leading to temperature excursions . The most significant environmental condition having the capacity to affect the nature of the pharmaceutical product is temperature. If the temperature excursions are not taken care of efficiently, there will be an adverse impact on product quality . There is a developing need to monitor for environmental excursions during pharmaceutical logistics to negate effects on the quality of the product. Quality Management System (QMS) should be implemented to avoid temperature deviations during the storage, transport, and distribution of pharmaceuticals.
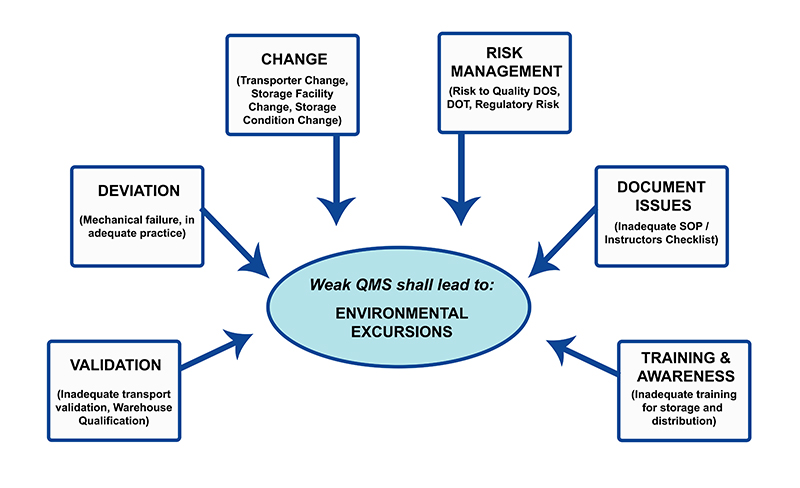
Pharmaceutical Temperature Excursion : An integrated approach for managing the quality system should include temperature excursion management. The overall temperature excursion management can be laid down in the following steps:
- Storage temperature and humidity limits: Specified directions are stated for pharmaceutical products with reference to the temperature and humidity at which articles shall be stored, transported, and distributed. Pharmaceutical supply chain products require cold storage or climate-controlled storage to meet the manufacturer’s recommendations. For products from the class of cold chain, the storage condition is maintained at 2°C–8 °C. Similarly, the relative humidity shall be maintained below 60% and above 40% depending upon the hygroscopic nature of the product. When environmental stability data indicates there has been temperature deviation with storage and distribution at a lower or higher temperature and humidity there are protocols in place that may require the disposal of the product, or at the least taken out of circulation until tested and verified.
- Measurement devices: Temperature and Humidity measuring devices (popularly referred to as data loggers) are available. They log the temperature at a preset interval which will be downloaded to a computer system or QMS for review, evaluation, and recording. Periodic verification of the calibration status of temperature data loggers and upgrading of software is a prerequisite for uninterrupted and accurate information about product storage conditions. The temperature and relative humidity sensor should be placed on the hottest spot, concluded after temperature mapping of the area.
- Reason for Temperature excursion: In pharmaceutical factories and cargo areas, the required temperature is maintained with help of air handling units (AHU). The design and capacity of AHU are selected on the temperature required to be maintained.
Temperature excursions in a manufacturing area are caused due to the following reasons (not limited to):
- An inadequate number of air handling units (AHU) were installed to maintain the desired temperature conditions inside the manufacturing shop floor.
- Leakage or rupture from the air duct, resulting in an insufficient cooling effect.
- Mechanical failure in air handling unit (AHU). unprecedented temperature fluctuations.
- Power failure makes the AHU operation defunct.
- Lack of quality system and weak discipline of Good Manufacturing Practices (GMP) on the production shop floor.
- General awareness about the consequences of not maintaining the temperature within limits.
- Extreme weather changes and obsolete contingency plans to handle
Temperature excursions during transport are caused due to the following reasons:
- An unexpected delay in transportation due to which the temperature control cannot be maintained effectively
- Product pallets are kept in hot zones of airports or shipping yards.
- Reefer containers or refrigerated control vans are not deployed for transportation.
- The transport agency fails to maintain the planned transport condition.
- Higher cost to maintain the temperature within limits.
- Power failure due to short longevity of power bank during longer travel time.
- Good Distribution Practices (GDP) understanding amongst supply chain personnel about the adverse impact on product quality.
Consequences Of Temperature Excursion
The storage condition for the product is assigned based on scientific studies into the deterioration of the product during its life cycle. If the temperature excursion isn’t addressed immediately, the subsequent negative impacts are common:
- Loss of product.
- Decreased efficacy.
- Separation of layers in liquid products.
- Change in dissolution pattern of solid dosage.
- Discoloration of products.
Control of Temperature Excursions
To handle the temperature excursion strategic planning , effective packaging, and well-documented procedures are recommended. The development of a QMS database of pharmaceutical products is beneficial to assign quality storage conditions.
The storage conditions suitable for the product are assigned through the following tests:
At the merchandise development stage, the semi-finished product and final pharmaceutical product dosage are subjected to challenging conditions to monitor the potential impact on quality attributes.
- Hold time studies are administered to determine the allowable period of time at a specified storage condition without impacting quality. the standard attributes of product intermediates include chemical, microbiological and pharmacological determinants at various time points of stability.
- Accelerated condition stability study data of the product form an assurance for the storage condition that shall be suitable to product safety. The time stability data is generated in the laboratory to assess the product’s change in quality and attributes throughout the expiration date. The accelerated stability data is generated to gauge the impact on the quality of the product under a stressed condition.
- A freeze/thaw study for multiple cycles should be conducted to specify the effect of freezing, if any, and therefore the subsequent thawing. Samples from different layers (top, middle)
Thermal Packing During Transportations:
Packaging of pharmaceutical products for transport should include the supply of thermal packing and display of appropriate storage conditions. Caution notes to avoid storage outside the specified conditions shall help supply chain personnel protect the merchandise quality. The storage condition should be effectively displayed on the packaging of the pharmaceutical product . The packaging configuration card must contain the small print of knowledge loggers.
Procedure To Minimize Temperature Excursions: The temperature excursion has regulatory implications as well as an impact on business operations. A standard procedure (SOP) should be established and adherence ensured through adequate training to concerned personnel. An operational checklist of the Integrated Quality Management System (QMS) approach should include the qualification status of a manufacturing facility with special attention to environmental controls during storage and transportation.
Control of Temperature Excursion By Using AKCP Wireless Temperature and Humidity Sensor:
With AKCP wireless environmental monitoring solution, you can monitor temperature excursions with real-time alerts, data logging , and reporting.
A monitoring system gives an accurate picture of the temperature and humidity conditions of drug storage . Have confidence in the quality and safety of pharmaceutical products. End-to-end monitoring prevents the loss of thousands of dollars of perished products
Wireless Tunnel radio technology penetrates even thick secure storage and refrigerated cabinets. Battery-powered sensors with a 10-year battery life guarantee easy installation.
Industry Articles and News
Datacenter blog 2.
Data Center Cooling Optimization
Use differential air pressure sensors to monitor and optimize your data center cooling, improving your PUE numbers and saving you money in Opex
Find out more
Homepage Blog 16

Optimizing Data Center Cooling
Proper cooling in a data center environment has always been a critical system component. Computer systems generate heat, and this heat must be removed.
Homepage Blog 7

Warehouse Flood Control and Monitoring
Water damage to warehouses from storms or leaks can cost huge sums in building and inventory damage.
Case Study: AKCP Monitors Simon Fraser University Data Center

AKCP, the world's oldest and largest supplier of networked wired and wireless sensor solutions, monitor Simon Fraser University data center.
The university recently updated their data center facilities, installing racks with rear door heat exchangers (RDHx) for high density computing. To guard against disasters caused by water leaks from the RDHx the data center administrators installed a leak detection system. AKCP rope and spot water sensors were deployed. Rope water sensors running down each aile under the rear doors will alert immediately should water leaks occur. This allows rapid response toguard against severe damage that water in the data center can cause.
- LEADING MINDS NETWORK
- GxP Services
- Book a Demo
- Jetzt Demo buchen
- Login / Register
Search our website
- Production & Cleanroom
- Lab & Equipment
- Cryogenic Storage & Shipment
- Dry Ice Transport
Cold Chain in Transport
- Warehouse & Cold room
- Direct-to-Patient Shipment
- Clinical Trials
- Pharmacy & Hospital
- WHO Vaccines
- Server Room
- Cold Chain Monitoring
- MONITORING OF ROOMS & EQUIPMENT
- Gxp Services
- All Solutions
- liberoMANAGER
- LIBERO Cx multi-use
- LIBERO Cx single-use
- ECOLOG-PRO xG
- ECOLOG Unlimited
- Temperature Data Loggers
- Produktion & Reinraum
- Labor & Geräte
- Kryolagerung & -Versand
- Trockeneis-Transport
- Kühlkette im Transport
- Lagerhaus & Kühlraum
- Direct-to-Patient Versand
- Klinische Studien
- Apotheke & Krankenhaus
- WHO-Impfstoffe
- Lebensmittel
- KÜHLKETTEN ÜBERWACHUNG
- ÜBERWACHUNG VON RÄUMEN UND GERÄTEN
- ALLE PRODUKTE
- Temperatur Datenlogger

Documenting Temperature Excursions
It is the responsibility of every pharmaceutical manufacturer to guarantee every medicine they deliver to a patient is of the highest quality. Maintaining the quality of a pharmaceutical product requires adhering to strict precautions, including storing or transporting the medication at specified temperatures.
If a shipment is complete, a decision needs to be made to release or quarantine the product. The following data must be available:
Complete measurement record from a calibrated sensor
Start time stamp (clear date and time)
Stop time stamp (clear date and time)
Stability budget (assessment criteria) with clear conditions of temperature zones/limits and allowed times
Sometimes additional criteria are defined, like number of allowed excursions or number of freeze-thaw cycles. As soon as all the data is available, the assessment can be performed a clear OK (= release) or ALARM (=quarantine) decision can be made. Information between stakeholders usually takes place via email or SMS.
A temperature excursion could result in patients ending up with unsafe products that may cause adverse reactions. Therefore, every pharmaceutical manufacturer must ensure all products are kept in the specified conditions until they reach the patient.
So, what are the right tools to use to guarantee that patients are getting the right quality and, most importantly, safe medicine?
Temperature Excursion Allowance: A GxP Compliant Solution
By programming stability information into data loggers, you can prevent products from being discarded prematurely.
What Are Temperature Excursions and Why Do They Even Matter?
Under the World Health Organization (WHO) Model Guidance, temperature excursion is “an excursion event in which a Time Temperature Sensitive Pharmaceutical Product (TTSPP) is exposed to temperatures outside the range(s) prescribed for storage and/or transport. Temperature ranges for storage and transport may be the same or different, as they are based on individual product stability data.”
Like many products, some medicinal products are sensitive to temperature and need to be stored and/or transported within a limited temperature range until expiration. However, unlike a flower that has lost the vibrancy and aromas, it isn't easy to tell when pharmaceutical products have lost their quality or efficacy.
Whenever temperature-sensitive pharmaceutical products are exposed to temperatures above or below the specified range, they experience temperature excursions. These excursions can affect their potency or result in an adverse reaction to the patient’s health. A temperature excursion may occur at the manufacturing site, in transit, or at the repository.
The Role of Regulators
Both the European and United States Pharmacopeia outline that pharmaceutical manufacturers are accountable for a product’s quality until it reaches the patient. While temperature conditions at manufacturing and packaging sites are usually under strict control (GMP regulation), this control is likely to decrease as soon as the product is dispatched for transportation.
As such, manufacturers should guarantee the safety, efficacy, and quality of the product until its final use. When temperature excursions occur, they have to take the required measures.
5 Examples of False Excursions and How to Correct Them
Temperature alarms may not be a final result. Sometimes there are false/positive excursions that can be corrected by a cold chain database. From the experience of millions of pharma shipments analyzed in our database, we know that less in than 10 percent of all cases, a true temperature alert (excursion outside defined shipping conditions) is found.
Typically, half of the temperature excursion cases are caused by a late stop or other mistakes that happen at destination – which could be considered false excursions.
The following are examples of common causes of false alarms and their interventions:
Early start, sensor measures too early (before product has been loaded or conditioned)
Intervention: Cold chain database can reassess the data using the correct time stamps.
No temperature values available due to sensor failure (sensor not started or no sensor added)
Intervention: If the shipment contains more than one device, it might be possible to use this data to release the entire shipment.
Minor temperature excursion during shipment
Intervention: Cold chain database can reassess the data using the stability data of the product.
Wrong sensor setting triggers a temperature alarm
Intervention: Cold chain database can reassess the data using the correct stability data.
Late stop at destination, sensor continues to measure (at room temperature)
In every case, it is important to have a two-level process in place whereby both logistics and quality review excursions before the product is released.
How to Document Temperature Excursions
Manufacturers that wish to follow GDP and GMP regulations are required to have procedures in place to document, investigate, and handle temperature excursions. The approach below outlines best practices for the documentation of temperature excursions at the manufacturing site, during transportation, or in warehouse storage.
Collect the following information:
- Details of the person completing the report
- Date and time of the temperature excursion
- Inventory of affected products
- Storage unit temperature (including minimum/maximum temperatures during the time of the event, if available)
- Room temperature (if available)
- General description of the event (i.e., what happened) • The length of the exposure if using a digital data logger (DDL)
- List of other items in the unit
- Any problems with the storage unit or affected products before the event
- Other relevant information
The distributor and recipient of the affected product(s) should receive a notification should a temperature excursion occur.
7 Components of a Cost-Saving CAPAs for Temperature Excursions
In case of a temperature excursion, GMP and GDP regulations require a Corrective Action & Preventive Action (CAPA). It is a structured process, which investigates and identifies root causes of problems and defines corrective action to prevent recurrences.
To create a useful CAPA, ask these questions:
Who found the temperature excursion, when, and where?
What is the scope of the case (shipment number, delivery, handling unit, pallet, product, batch)?
What is the severity of the excursion?
What was the label/transport condition?
What was the highest (or lowest) temperature measure?
What was the number of excursion hours (can the product still be released based on stability budget)?
What was the root cause of the excursion?
What are corrective actions to eliminate this specific problem?
Have similar cases happened before? Are there patterns in the data?
Can we define preventative actions to make sure similar root causes are eliminated?
For a reliable CAPA process in cold chain management, a database is necessary where all data is available in a structured and well-documented way.
Practical Guide: Cold Chain Logistics Choices
Learn how the choice of transportation mode & equipment affect products, & how to best handle temperature excursions & subsequent CAPAs.
Related Articles
Stability budget explained, making the right cold chain logistics and packaging choices ..., get everything under control with the right software, let's talk about temperature monitoring .
Schedule a call with our experts today. We're here to support your cold chain monitoring project and help ensure it is successful.

- Monitoring for Rooms & Equipment
- Product Catalog
- ECOLOG Connected Monitoring
- LIBERO Stability Monitoring
- INDEPENDENT Monitoring
- News & Events
- Compliance & whistleblower system
- Leading Minds Network
we prove it.

- Privacy Policy
- Legal Notice
- Terms of Use
- General terms and conditions
- General terms of conditions and purchase
Copyright © ELPRO-BUCHS AG 2024. All Rights Reserved.
The Federal Register
The daily journal of the united states government, request access.
Due to aggressive automated scraping of FederalRegister.gov and eCFR.gov, programmatic access to these sites is limited to access to our extensive developer APIs.
If you are human user receiving this message, we can add your IP address to a set of IPs that can access FederalRegister.gov & eCFR.gov; complete the CAPTCHA (bot test) below and click "Request Access". This process will be necessary for each IP address you wish to access the site from, requests are valid for approximately one quarter (three months) after which the process may need to be repeated.
An official website of the United States government.
If you want to request a wider IP range, first request access for your current IP, and then use the "Site Feedback" button found in the lower left-hand side to make the request.

IMAGES
VIDEO
COMMENTS
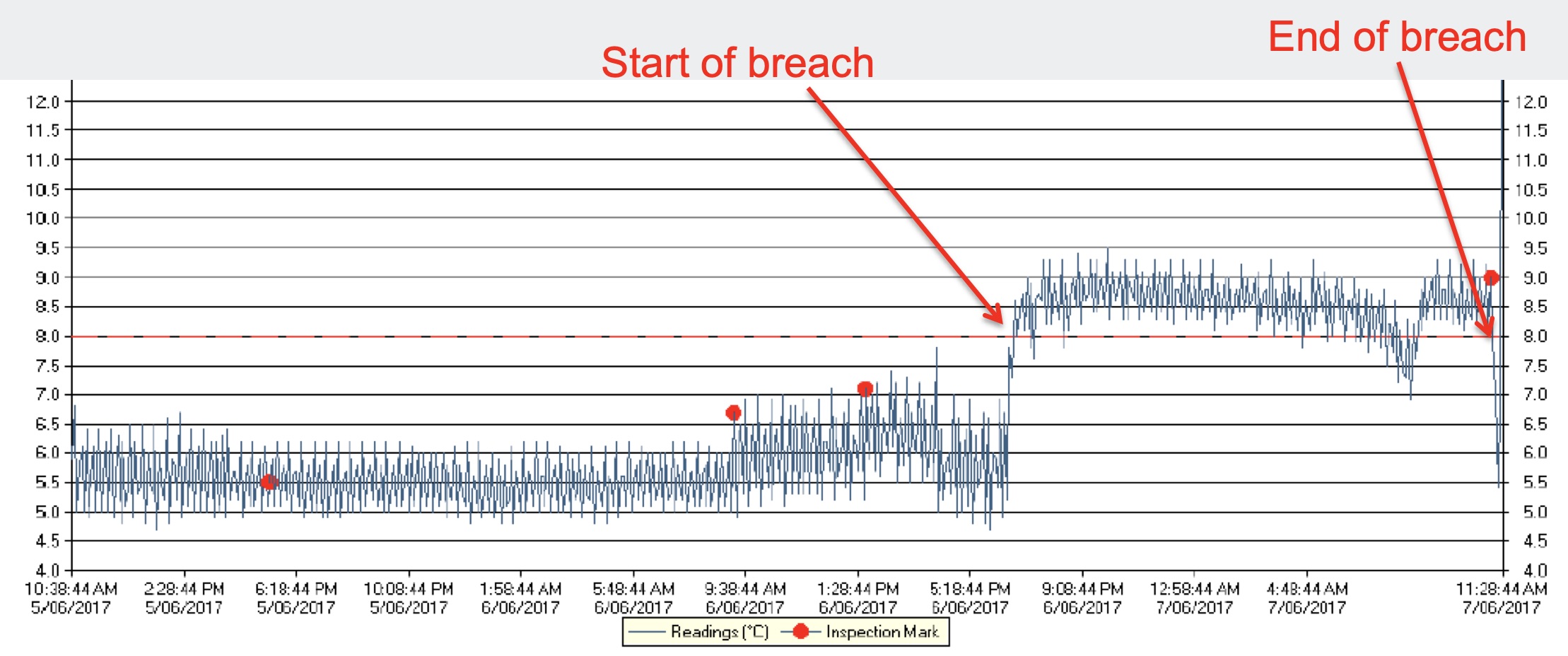
Temperature excursion procedure should clearly define the situations that are covered by studies and in which a batch can be released and those where it cannot ... Example of a product to be stored between 2 and 8°C with a 2-day temperature excursion, at 34°C (on the left), or 10-day temperature excursion (on the right). If supported by the ...
A temperature cycling study intended to identify those articles affected by multiple, short-term excursions beyond the storage temperature limits should be performed. These data provide wholesalers and distributors with clearer identification of those drug products that may require special handling during particular climate conditions.
If the excursion was the result of a temperature fluctuation, refer to the section, "Vaccine Storage and Temperature Monitoring Equipment," in CDC's Vaccine Storage and Handling Toolkit for detailed guidance on adjusting storage unit temperature to the appropriate range. If you believe the storage unit has failed, implement your emergency ...
Temperature excursion in pharmaceutical industry should be recorded and reported to the manufacturer for further investigation and risk analysis. The concept of temperature excursions, its reasons, consequences and handling mechanism should be well understood to ensure the concerted efforts under the aegis of Quality Management System. Based on ...
Patients use mail delivery as a convenient alternative to acquiring medications in person. Federal laws require nonspecialty oral medications to be stored at controlled room temperature during distribution; however, no laws or regulations govern temperature requirements for medication transport among patients, which may expose medications to harmful temperature excursions.
For Temperature Excursion. This same representation can be useful for decisions relating to temperature excursions. The information is reported directly on the product temperature profile. The decisions that have to be taken are supported by real data. In the example below, one batch of product has been stored at 34°C for 8 h (see Fig. 5a ...
Temperature is one of the most important parameters to control. Drugs must be stored, and transported according to predetermined conditions (for example, temperature, etc.) as supported by stability data. Temperature excursions outside of their respective labelled storage conditions,
Biopharmaceutical drug products may be exposed to temperatures outside of the intended storage temperature range (typically 2-8°C) during commercial distribution due to uncontrolled variables and unexpected events. Pharmaceutical companies are expected to ensure that product quality and stability are not negatively impacted by temperature excursions defined as being acceptable for the ...
The temperature excursion may be observed during transportation of raw materials manufacturing as well as distribution of pharmaceutical products, which have potential to deteriorate the product quality. Temperature excursion in pharmaceutical industry should be recorded and reported to the manufacturer for further investigation and risk analysis.
used to support temperature excursions), GDPs to minimize tem-perature excursions and use of theoretical methods/mathematical simulation models to assess temperature excursions. The infor-mation provided in this article may be useful for the pharmaceu-tical companies to design a temperature excursion management
RT was defined as 20 to 25 degrees Celsius (68-77 degrees Fahrenheit) based on the United States Pharmacopoeia's definition of "controlled RT." Refrigeration was defined as 2 to 8 degrees Celsius (36-46 degrees Fahrenheit). ... unaltered product from RT and for allowable temperature excursions for the drug during various stages of preparation ...
A comprehensive temperature excursion management program is expected to ensure product quality and help minimize, assess, and justify temperature excursions more efficiently, ensure regulatory compliance and avoid business impact caused by the loss of products or inadequate supply. ... Stability studies needed to define the handling and ...
All excursions must also be documented as a deviation in the corresponding chamber's log, regardless of the length of the excursion. However, the SOP should define the difference between routine activities such as opening the door, electrical surges, and an excursion to properly document these incidents (Table 2). An investigation should be ...
Step 3: Document the Event. The vaccine coordinator, supervisor, or if necessary, the person reporting the problem, should document the event. Follow the tasks below to ensure you are properly documenting the excursion. Name of the person completing the report. Date and time of the temperature excursion.
Some of the tips and tricks to prevent temperature excursions are: Select the product's appropriate packaging and shipping materials based on the required temperature range, transit time, and destination. For example, you may use insulated containers, gel packs, dry ice, or liquid nitrogen to maintain the desired temperature during transport.
The following information is required when documenting temperature excursions: The date and time the temperature excursion occurred; An inventory of affected products; The storage unit air temperatures. Including the minimum and maximum temperatures observed during the temperature excursion, if available; Ambient temperature (also referred to ...
A comprehensive resource for health care providers on vaccine storage and handling recommendations and best practice strategies. The Toolkit includes guidance on managing and storing vaccine inventory, using and maintaining storage unit and temperature monitoring equipment, preparing for emergency situations, and training staff.
Temperature Excursion: A documented event whereby a clinical product is shipped or stored at a temperature outside of its temperature range as defined in the protocol/MOP. A temperature excursion may occur in transit, at the repository, or at the site. Quarantine: Effective restriction of the availability product for use until released.
Temperature Excursion Management in Pharmaceutical Storage. The quality of pharmaceuticals relies on environmental controls during their storage and handling. Every pharmaceutical item ought to be taken care of and stored under manufacturer-recommended storage conditions marked on the data information sheet or packaging.. Temperature Excursion Management in Pharmaceutical Storage is important ...
Temperature excursion queries that may arise during this stage due to improper handling (as communicated by the drug product user) may be handled by the manufacture's Medical Information department, which may have data on file to provide information in response to specific questions on temperature excursions (e.g., information based on in-use ...
Minor temperature excursion during shipment. Intervention: Cold chain database can reassess the data using the stability data of the product. Wrong sensor setting triggers a temperature alarm. Intervention: Cold chain database can reassess the data using the correct stability data. Late stop at destination, sensor continues to measure (at room ...
BRIEFING. ( 1079.2} Mean Kinetic Temperature in the Evaluation of Temperature Excursions During Storage and Transportation of Drug Products. This proposal is based on the version of the chapter oficial as of December 1, 2020. MKT cannot be used to normalize storage conditions situations that are out of control but can be a valuable tool to ...
excursion? An excursion is any temperature outside the recommended temperature range for a vaccine. However, it is the total amount of time, or cumulative time, out of range that affects the viability of a vaccine. For example, if your temperature probe shows that the temperature of a refrigerated vaccine rose to 4 8. ⁰. F (9. ⁰
The blood is on your hands, Biden, we can see it all. And fuck no, I'm not votin' for you in the fall (Woo) Undecided. You can't twist the truth, the people out here united. Never be defeated when ...
To be considered a valid service dog, the dog must meet the definition of a "service animal" under 14 CFR 382.3 and accompany an "individual with a disability" as defined under 14 CFR 382.3. This exemption is limited to foreign-vaccinated service dogs entering the United States via seaports and is not available to foreign vaccinated ...