
Open Navigation
Close Navigation
Hemophilia is a genetic disease that prevents blood from clotting properly leading to prolonged internal and external bleeding. Learn how gene therapy works to slow or stop disease progression by instructing cells to produce the missing clotting factor, along with information on approved therapies and clinical trials.
HEMGENIX is an FDA-approved gene therapy for the treatment of adults with hemophilia B (congenital Factor IX deficiency).
ROCTAVIAN is an FDA-approved gene therapy for the treatment of adults with severe hemophilia A (congenital factor VIII deficiency).

About Hemophilia
Hemophilia is a genetic disease that prevents blood from clotting properly leading to prolonged internal and external bleeding. Clotting factors are proteins in blood that help the body stop bleeds. Without the ability to effectively stop bleeding, a simple cut or bruise can lead to external bleeds, and internal bleeding inside joints and muscles can damage organs including the brain. There are 12 different clotting factors, commonly referred to as factors I through XII. The most common types of the disease are hemophilia A and hemophilia B.
Hemophilia A is caused by a gene variant that leads to a deficiency (not enough) of clotting factor VIII.
Hemophilia B is caused by a gene variant that leads to a deficiency (not enough) of clotting factor IX.
Gene Therapy Approach
Gene therapy aims to be given one time with the goal of eliminating the need for recurring treatments. Gene therapy for hemophilia A or hemophilia B would deliver a working copy of the faulty gene into the liver cells with instructions to produce the missing clotting factor. The gene with its new instructions is delivered to the cells using a viral vector. Vectors are often derived from viruses because viruses have evolved to be good at getting into cells. But the viral genes are removed so only therapeutic (good) genes are delivered.
The liver has a variety of jobs in our body, and one of them is to create different elements in our blood—including clotting factors VIII and IX. By delivering the vector with the working gene into liver cells, the cells is given instructions to produce stable amounts of clotting factors to control bleeds. Gene therapy has been shown to reduce the rate of annual bleeds with hopes of improving the quality of life for people living with the disease.
Researchers are also exploring the use of gene editing to correct clotting factor production. Gene editing goes directly inside the cell to edit pieces of DNA using technology that is highly precise to make this change.
FDA-Approved Gene Therapies
HEMGENIX is an AAV-based gene therapy given one-time for the treatment of adults with Hemophilia B (congenital Factor IX deficiency) who:
- Currently use Factor IX prophylaxis therapy, or
- Have current or historical life-threatening hemorrhage, or
- Have repeated, serious spontaneous bleeding episodes.
ROCTAVIAN an AAV-based gene therapy given one-time for the treatment of adults with severe Hemophilia A (congenital factor VIII deficiency) who:
Have FVIII activity < 1 IU/dL
Don't have antibodies to adeno-associated virus serotype 5 (AAV5) detected by an FDA-approved test
It is important to inform your primary medical provider or hematologist to understand if a gene therapy is the best option for you and to understand health insurance coverage, along with short- and long-term risks. For instance, gene therapy can be an alteration for the lifetime, so people should be aware that there could be long term effects (both good and bad) that are not known at this time.
Other treatment options These include infusions, medications, physical therapy, or surgery to repair tissue damage. The most common option is the infusion to administer clotting factor replacements directly into the bloodstream. These treatments are required throughout a person’s entire lifetime to prevent or treat bleeding. Visit the hemophilia treatment centers page from CDC for more information.
Treatment Pipeline
Gene therapy for different types of hemophilia are being investigated in clinic trials and preclinical studies. Clinical trials are a required part of the research process that help scientists understand the way a drug or treatment will interact with the human body and whether it is safe and effective. Preclinical studies are an even earlier stage of research that test the safety and effectiveness of a treatment in animal or cell-based models before proceeding with a human clinical trial. Clinical trials may differ on various aspects of their design. Speak with a trusted provider or member of the clinical trial research team if you are considering participating in a clinical trial.
To stay up to date on open clinical trials in the U.S. or globally, visit the ASGCT Clinical Trials Finder and search using the "diagnosis" filter.
Participating in a Clinical Trial
It is important to be well informed when deciding to participate in a clinical trial. Below are some key points to consider. Visit the considering a clinical trial page for more information and resources to help guide you.
- Eligibility - Eligibility for a trial is based on strict inclusion and exclusion criteria. These are specific factors that determine whether a person can or cannot enroll in a clinical trial. This is an important way for researchers to understand if the gene therapy is working properly and to ensure participant safety. For hemophilia a key factor is age. Since the liver grows with us from childhood to adulthood, gene therapy trials are currently only being conducted in eligible adults with hemophilia. If a child was given the treatment, the therapy’s beneficial effects could become weaker as their liver grows with age. Other criteria may include factors such as how advanced the disease is, or previous use of treatments. Speak with a healthcare provider or a member of the clinical trial research team to help determine if you may be eligible for a clinical trial.
- Risks - As with any medical intervention, there are risks that need to be carefully considered. Before participating in a clinical trial, a member of the research team should review any potential risks and benefits with the patient or caregiver. Therapies being studied in clinical trials are not a guaranteed cure and cannot guarantee beneficial results. There is always a chance that the investigational treatment may not work. In the event a person is not satisfied with the outcome, the person may not receive another dose of the gene therapy. In addition, participating in a clinical trial may prevent future participation in other trials or from receiving other types of treatments. Gene therapy can be an alteration for the lifetime, so people should be aware that there could be long term effects (both good or bad) that are not known at this time.
- Benefits - Participating in a trial may offer many potential benefits compared to not receiving any form of intervention for a fatal disease. Gene therapy aims to be a one-time treatment with lasting positive effects that slow or stop disease progression for a lifetime. However, there is no guarantee. If gene therapy is received earlier in the course of disease, it has the potential to stop damage before it occurs.
- Long-term follow up - It is the patient’s responsibility to comply with the long-term follow-up of a trial. The Food and Drug Administration (FDA) guidelines require the clinical trial research team to monitor safety and potential long-term effects of a gene therapy. Follow up may require in-person appointments that vary in frequency and location, or completion of mailed packets with response forms. The need for long-term data collection for a gene therapy trial can last up to 15 years—another reason to consider all outcomes and responsibilities that come with committing to a clinical trial. There are a limited number of participants in trials so a lack of attendance at follow-up appointments leads to not enough study data. This could negatively affect FDA approval of a new therapy and thereby limit access to the therapy by patients who did not participate in the clinical trial.
Access
At this time, we do not know if or when more gene therapies will be approved by the FDA and commercially available for people living with different types of hemophilia. The overall process may take several more years, until it is deemed safe and effective by the FDA or regulatory agencies in other countries.
Stay Informed
A number of organizations provide individuals with blood disorders, and their families, with resources, research updates, and support:
- Hemophilia Federation of America (and check out their Learning Central )
- National Hemophilia Foundation
- The Coalition for Hemophilia B
- World Federation of Hemophilia
Related Pages
- FDA-Approved Cell & Gene Therapy Products
- Gaucher Disease
Last Updated: 07/06/2023
- Open access
- Published: 04 April 2022
The experiences of people with haemophilia and their families of gene therapy in a clinical trial setting: regaining control, the Exigency study
- Simon Fletcher ORCID: orcid.org/0000-0001-9018-6176 1 ,
- Kathryn Jenner ORCID: orcid.org/0000-0002-2704-0606 2 ,
- Luke Pembroke ORCID: orcid.org/0000-0002-2024-6898 2 ,
- Michael Holland ORCID: orcid.org/0000-0002-9173-4100 2 &
- Kate Khair ORCID: orcid.org/0000-0003-2001-5958 2
Orphanet Journal of Rare Diseases volume 17 , Article number: 155 ( 2022 ) Cite this article
3088 Accesses
20 Citations
8 Altmetric
Metrics details
An Editorial to this article was published on 04 April 2022
Gene therapy has the potential to change the life experience of people with haemophilia and family members. Few studies have sought to explore the impact of gene therapy on both individuals and families. The aim of this study was to capture real-life experiences of gene therapy in People with haemophilia and their families.
Sixteen participants with severe haemophilia (11 haemophilia A, five haemophilia B), mean age 41.4 years (range 23–75 years), took part in a single qualitative interview; ten were accompanied by a family member. Mean time since transfection was 3.56 years (range 1–10 years). Participants saw their involvement in gene therapy as a positive experience, freeing them from the personal burden of haemophilia and furthering treatment options for the wider haemophilia community. However, participants reported being unprepared for the side effects of immunosuppression. Some also reported feeling unsupported and having little control over what was happening as their factor levels became the focus of the process.
The results suggest that strategies need to be put into place to enable PwH fully to understand the process of gene therapy, and thereby make an informed choice as to whether it is a treatment they might wish for themselves. These include early and ongoing education, increased provision of psychosocial support and ongoing qualitative research.
Haemophilia affects 1:3333 men worldwide [ 1 ], resulting in recurrent joint and muscle bleeding leading to joint arthropathy, muscle contracture and significant disability [ 2 , 3 ]. The treatment of affected individuals involves the prophylactic replacement of the missing factor, which reduces the incidence of spontaneous bleeding events and resultant joint damage [ 4 , 5 ]. Replacement therapy has improved life expectancy and quality of life of people with haemophilia (PwH), though limitations such as high costs and the treatment burden of frequent intravenous infusions remain [ 6 , 7 ]. The latter has decreased with the development of extended half-life factor replacement products and factor VIII (FVIII) mimetics [ 8 , 9 , 10 ]. With the development of a number of gene therapy platforms for both haemophilia A and B, a potential cure also appears to be ever closer.
Gene therapies for haemophilia currently use an adeno-associated virus to insert the gene of interest (B domain deleted FVIII or factor IX [FIX] Padua) into hepatocytes, which then begin to produce the relevant clotting factor [ 11 , 12 , 13 , 14 ]. In the UK, 31 individuals (22 haemophilia A, nine haemophilia B) have so far undergone gene therapy in clinical trials examining the safety and efficacy of the technology [ 15 ]. Once biotechnology companies receive authorisation for their gene therapies [ 16 ] gene therapy may become a standard of care [ 17 ].
Qualitative studies have begun to explore the reasons why PwH might wish to consider gene therapy [ 18 , 19 , 20 ]. Some have sought to examine the impact gene therapy has had for those in clinical trials [ 21 , 22 ], but none has considered the nature and impact of gene therapy itself and the immediate follow-up care it requires. While follow-up processes and requirements may change as gene therapy moves from clinical trials to a standard of care for haemophilia, many are likely to remain similar, including the need to monitor liver enzymes and factor levels and the need for immunosuppression. Without a clear understanding of the experiences of PwH who have had gene therapy, those who opt to have it in future and the haemophilia treatment centres that provide it will not truly understand the potential implications and may be ill prepared to deal with them.
The Exigency programme was designed to explore the knowledge, expectations and experiences of gene therapy among a range of stakeholders in the UK haemophilia community (Fig. 1 ). This sub-study assesses the experiences of men with severe haemophilia who have undergone gene therapy in clinical trials. It is the first investigation by a team not involved with or affiliated to a gene therapy dosing centre.
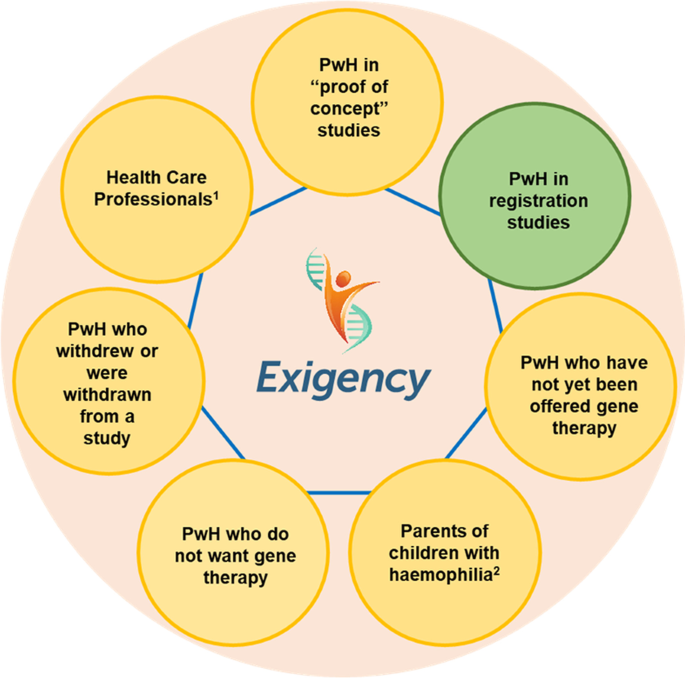
Exigency diagram
Sample characteristics
We invited 27 PwH (87.1% of those known to the UKHDCO) who had undergone gene therapy in the UK to participate. Sixteen PwH (51.6%) were interviewed along with 10 family members. Eleven participants had haemophilia A and five haemophilia B. The mean age of participants was 44.1 years (range 23–75 years). The mean time since gene therapy transfection was 3.56 years (range 1–10 years) and the mean self-reported factor level at the time of the interview was 0.33iu/ml (range < 0.01–1.37iu/ml). Three participants had been in phase 1 safety studies; the others had participated in subsequent phase 3 safety and efficacy studies. Recruitment was discontinued after 16 interviews as data saturation had been achieved. Three participants were known to SF and Six to KK prior to taking part in the study. None were known to both. For participant data see Table 1 .
Overview of findings
Four major themes emerged from the interviews: altruism, side effects of immunosuppression, control, and liberation.
All participants spoke of their reasons for wanting to take part in gene therapy. Nine spoke of their desire to help future generations of PwH.
“I’ve done it for the next generation. I don’t want anyone to have to go through what I went through.” [Exi06] “One of the big factors of moving it forward was, of course, our daughter being a carrier, because clearly, from our point of view, it was all about if by the time she gets to the point of having a family and fate rolls the dice and she has a haemophiliac then wouldn’t it be amazing if someone went, ‘That’s not a problem’.” [Exi11]
This was especially true for those who had participated in the phase 1 studies, who knew they would only see minimal increases in their factor levels.
“I don’t want to sound like I’m a saint because I’m not a saint – but I felt I ought to give something back.” [Exi09]
For others the primary reason for trial participation was more personal; they were seeking a cure for themselves.
“I think to be a cure for me, to be honest.” [Exi12]
“I did [it] for my own little kind of mental state and my ability to be able to do things.” [Exi06]
Side effects of immunosuppression
Thirteen participants required immunosuppression (eleven haemophilia A and two haemophilia B) either prophylactically, to prevent transaminitis, or to treat a transaminitis that occurred. The mean length of time on immunosuppressive therapy was 16 weeks (17.9 weeks haemophilia A (range 6–36 weeks) and 21 weeks haemophilia B (range 6–36 weeks)), with some requiring multiple courses of therapy. Ten participants and six family members stated immunosuppression and its side effects were the worst part of the gene therapy experience. One participant described the experience as “ absolutely horrendous ” [Exi06] . Another said he would only think about having gene therapy again (if the technology reaches a point where redosing is possible) if he was certain he would not have to have immunosuppressive therapy:
“[If they said], ‘You could have this gene therapy again, you don’t need to go on steroids, we’ve found another drug you can do that, will do the same, there’s no real side-effects,’ I would probably take it again.” [Exi03]
Both participants and family members described insomnia (n = 7), anger (n = 5) and feelings of depression (n = 2).
“I did not sleep. I didn’t need to.” [Exi06]
“I felt like it wouldn’t take much for me to flip out at someone, so I’d think, ‘If I just keep myself to myself, then I can’t upset anybody.’” [Exi02]
“That was a real dark, depressed… after a couple of weeks on them. I was angry, I was just… I broke down.” [Exi03]
Six participants said they had received immunosuppression for longer than they had expected and four had needed multiple courses.
“It was longer than I thought it was going to be for. I thought… I remember being told it would be six to eight weeks.” [Exi02]
“So, yes, in this next course of immune suppression – this is like chapter three of the immune suppression, the immune suppression diaries – that was the most intense time, for sure.” [Exi07]
While the overwhelming response to immunosuppression was negative, four participants reported some positive effects.
“Once I started taking the steroids and the tacro[limus] I felt quite good […] I had the sort of… the rush of blood to the head sort of energy of steroids.” [Exi07]
Others reported relief from their usual hay fever (n = 1) and relief from pain caused by arthropathy (n = 4):
“My inflammation that I keep getting in my joints or my muscles just did not happen at all for one month. So, I felt extremely healthy.” [Exi16]
For a full list of side effects experienced see Table 2 .
When asked to reflect on participation in gene therapy, all participants said it had been worthwhile, including those who now had no appreciable factor expression and were back on factor prophylaxis.
“I’d say yes, but just be prepared, really. Because it sounds really, really good – and it is good when it works – but you’ve got that period where – well, not for everyone – where it could be not very nice. Just be prepared for that, really.” [Exi02]
Half of the participants (n = 8), reported a need to control their haemophilia and its effect as important.
“It’s a bit difficult for somebody who’s not affected by the haemophilia to understand that you have to be able to control your life, and the home treatment was something that changed my life beyond all recognition. It allowed me to hold down a full-time job, which otherwise I would not have been able to do. It allowed me to go out of the house. It allowed me, or facilitated me rather, gradually overcoming my psychological fear of the world.” [Exi09]
For some, this search for control involved pushing boundaries of what was ‘allowable’ or ‘advisable’ to see what they were capable of. Four participants said this was important to their own sense of identity and wellbeing, although they admitted it had also led them to ignore their haemophilia and caused more harm than good.
“I think I’ve probably only just recently calmed down a little bit more. I was definitely the one that… I would… I put my body through probably more than I should have.” [Exi07]
“I’d had a really difficult probably three years of my life, with probably my physical and mental health, I suppose. And the haemophilia, I got really, really neglectful and I ended up… I ended up in hospital, very unwell.” [Exi05]
Half of the participants (n = 8) reported that rather than gaining control they had lost both control and individuality as they became subject to study-specific requirements.
“It was just everything for the results, and the blood tests and everything were more important than anything.” [Exi03]
“I suppose I’m saying that it’s the protocols that treat you as a number rather than the people.” [Exi04]
Some participants (n = 4) and family members (n = 2) felt this meant many of their concerns and issues were neither recognised nor adequately responded to.
“There was naturally stuff happening throughout the trial that I was noticing, and I was recognising and trying to have a conversation with them about – and it was like just falling on deaf ears.” [Exi05]
“Looking back, I’m starting to question a bit more why was I not just taken off that treatment the minute I expressed the level of discomfort that I was feeling.” [Exi07]
Two participants and their family members felt that mental health concerns were particularly poorly dealt with.
“Like, anything around mental health or psychological wellbeing was just like, nah… they did not want to know about that.” [ExiF03]
“I felt like at the time the trial was more important, the results of the trial were more important than [husband’s] mental health. I don’t think we really had the support for his mental health at the time.” [ExiF05]
Three participants thought some short-term loss of control was inevitable due to the constraining nature of study protocols. Four felt they had to wrest back some level of control, which took the form of refusing to attend appointments, weaning immunosuppression more quickly than advised, or refusing to have further courses of immunosuppression.
“They told me to prepare for it, because basically my liver enzymes kept rising and my factor’s been on a consistent downward slope. So, there was that time where… I think they said to me if I didn’t go on… Because they wanted me to go on immune suppression a fourth time and I said no. I said, ‘I can’t… for my own physical and mental health, and for my partner’s mental health, I don’t think we can go through that, so I’ll take my chances.’” [Exi07]
Despite the issues discussed above, the majority of those interviewed (participants, n = 12; family members, n = 3) described gene therapy as life changing.
“I can do most of the physical actions that I couldn’t do before. I can work in the garden, I can easily carry heavy bags from the grocery shop… And I don’t have to worry that my elbows or my shoulder joint or anything like that will just give me a bleed. So, it’s a peace of mind.” [Exi15]
“It’s unbelievably life-changing. Life-changing.” [ExiF08]
For others (n = 3) their improvement was down to ease of travel (not have to take large volumes of factor with them and navigate customs with needles and syringes) or the ability to participate in sports in ways not previously open to them.
“I play golf twice a weekend, I carried a bag five and a half miles, swung a golf club, and I never had a single problem. I’d get back and be completely fine. I wouldn’t even dream of doing that when I had haemophilia.” [Exi06]
Fourteen participants, including those in the early safety studies, had experienced a rise and then a decline in their factor levels. Four were on a prophylactic factor therapy regimen at the time of their interviews: two had returned to baseline levels of < 0.01iu/ml and two were experiencing bleeds despite having a factor level > 0.01iu/ml. The remaining 12 were not receiving factor replacement and 11 had not had any factor replacement therapy since transfection.
Of the 12 participants not currently on prophylaxis, all were aware there was a possibility of their levels dropping and that, at some point in the future, they may need to restart factor treatment, though there was hope this would not happen.
“I’m hoping that it comes down to such a level that I actually don’t need factor anymore at any time in the future.” [Exi01]
One participant thought gene therapy had “ not really made much difference ” [Exi03], as it was not able to fix the problems he had with his joints. He felt that if he had had it at age 18 “ it would have been probably a different story ”.
Further supporting quotes can be found in Additional file 1 .
A growing number of studies have sought to examine the impact of gene therapy on the lives of individuals who have undergone the procedure [ 21 , 22 ]. Most have focused on the positive results, many of which were also seen in this study, including ‘liberation’ from their condition and the worry of bleeds, the ability to participate in sports in ways previously not open to them, and to holiday without worrying about taking factor with them. The nature of the questions asked in a number of these studies have, however, been leading, guiding participants to talk about certain predefined negative aspects rather than those that were important to them.
Previous studies have also been undertaken by research teams involved in the dosing of the participants, which is a concern. There are well documented ethical concerns about unequal power relationships in clinician-led research, including coercion and bias, as participants can feel indebted to the interviewers and therefore inhibited talking about concerns they have [ 23 , 24 , 25 , 26 ]. A strength of our study is that neither of the interviewers worked at any of the dosing sites, and although several participants were known to one or other of the interviewers, none were known to both.
There are clearly many positives to gene therapy, but this study has highlighted a number of concerns that have not been described elsewhere, with the side effects of immunosuppressive therapy being the most difficult and troubling element. Although not seen in all cases, post vector infusion transaminitis is a recognised side effect of gene therapy [ 27 , 28 ]. The underlying pathophysiology of this inflammation, and why some individuals are affected and others not, has not yet been fully described [ 29 , 30 ]. However, even moderate rises in transaminase levels are associated with dramatic falls in factor expression [ 13 , 31 ]. Many gene therapy studies have therefore included the use of immunosuppression, either prophylactically or reactively, in an attempt to prevent this [ 29 ]. The duration of immunosuppression required is not fully understood and, as has been shown in this study, can vary between individuals.
Immunosuppression is associated with significant safety concerns due to the side effects profile of the medications, including weight gain, hypertension, hyperglycaemia, altered mood, muscle spasm, neuropathy and psychiatric reactions [ 32 ]. Many of these were reported by participants in this study. The use of immunosuppression and perceived pressure from research staff to continue immunosuppressive treatment, despite side effects, meant some participants felt they were losing control rather than gaining it. There was recognition and understanding that this pressure existed due to concerns that factor levels could drop, but a feeling that maintenance of expression became the primary focus for research staff and that other questions and concerns were ignored or downplayed. Four participants felt self-advocacy was the only way to regain control and took themselves off immunosuppression sooner and more quickly than study teams advised. The need for control (over individuals’ lives, conditions and the research process) has been described in other studies [ 33 ].
Lack of psychosocial support, including lack of recognition of the need for it, was perceived by a number of participants as a concern. Provision of psychosocial support has been an ongoing concern within the UK haemophilia treatment community, with two thirds of comprehensive care centres and most haemophilia treatment centres having little or no access to services [ 34 ]. While access to support services is a wider issue, the concerns raised by the interviewees suggests that there should be greater emphasis on psychosocial needs, and that this should be integral to the package of care if gene therapy is to become a standard therapy. Psychosocial needs should also be acknowledged by the biotechnology companies running gene therapy studies and supportive measures incorporated into trials.
Future recipients of gene therapy, either in clinical trials or through licensed treatment must fully understand the therapeutic goals, the processes involved, and potential side effects. Known and unknown complications should be discussed alongside mitigation strategies that might be necessary. Consent to treatment should therefore be a process rather than an event, particularly as it is not possible to discontinue treatment once the vector has been given. This information process should begin in childhood and continue throughout life [ 35 , 36 ]. In this way, when PwH decide that gene therapy is something they wish to receive, they will have a greater understanding and expectation of the process and potential outcomes.
Limitations
This study involved a self-selecting, UK-based sample of participants with ready access to prophylactic haemophilia treatment prior to their gene therapy. There could therefore be an inherent, unintended selection bias in this group. This bias has however been mitigated to a degree by the size of the sample (> 50% of the UK gene therapy cohort).
Data saturation usually requires 20–25 individual interviews [ 37 , 38 ] but there is a degree of consistency in this study due to the homogeneity inherent in the gene therapy participant group. As no new codes or themes emerged in interviews 15 and 16, the research team felt that data saturation had been achieved. There may be a greater diversity of opinion and experience as gene therapy becomes more widely available, and it will be necessary to continue to interview future recipients and family members to continue to understand what affect it has.
The Exigency programme [ 19 , 35 ] has been carried out in a high-income country where PwH have good access to intensive treatment. The concerns and issues raised may differ from those of low- and middle-income countries, or the emphasis placed on them may be different. Further research needs to be undertaken to delineate a greater understanding of these concerns. We believe it is important that such studies are undertaken by groups not linked to any single dosing centre to avoid researcher bias, thereby enabling participants to voice their concerns without fear that their comments could upset the teams looking after them.
When it becomes more widely available, gene therapy for haemophilia may become a standard of care, potentially changing the face of future haemophilia care. If this is to happen and is to be seen as a safe and attractive treatment, PwH need a greater understanding of the processes and implications of the therapy, some of which have been highlighted in this study. Strategies including early and ongoing education, and the adequate provision of psychosocial support throughout the process should be established. Ongoing longitudinal qualitative research will be needed to understand what impact gene therapy for haemophilia has throughout all life stages.
Study design
A qualitative interview study was conducted with men with severe haemophilia who had undergone gene therapy in the UK. Interviews were undertaken between 1 August 2020 and 31 August 2021.
The interviews followed an interview guide based on a review of the literature and the experience of the study team (see Additional file 2 ). Questions addressed the individual’s haemophilia and treatment history, the decision-making process of opting for gene therapy, and their experience of gene therapy.
Recruitment and data collection
Participants were recruited through haemophilia centre referral, social media, and word of mouth referral. All participants took part in a single 1 h interview conducted by two researchers (SF and KK) via the video conferencing platform, Zoom®. Participants were given the option to be interviewed with a family member. The initial recruitment target was 25 individual interviews though recruitment could be discontinued at the discretion of the researchers if data saturation was achieved, or further recruitment was unlikely. The latter condition was added as UK data show that just 31 PwH have received gene therapy [ 16 ].
Each interviewee was randomly assigned a study number (PwH, Exi01-Exi16; family members, ExiF01-ExiF10). All interviews, which were recorded and transcribed verbatim, were facilitated by SF and KK who each have more than 30 years’ experience in nursing. Transcripts were thematically analysed by both researchers after each interview using inductive coding (SF: NVivo® for Mac; KK: manual coding). Prior to each scheduled interview the researchers met to discuss, review and refine emergent codes, enabling their exploration in subsequent interviews. On completion and analysis of the final interview, the researchers met to discuss all transcripts, further refine codes and identify final themes.
Data availability
The datasets generated and/or analysed during the current study are not publicly available as it contains un-anonymised participant information. Data sets are available from the corresponding author on reasonable request.
Stonebraker JS, Bolton-Maggs PHB, Brooker M, Evatt B, Iorio A, Makris M, O’Mahony B, Skinner MW, Coffin D, Pierce GF, Tootoonchian E. The world federation of Hemophilia Annual Global Survey 1999–2018. Haemophilia. 2020;27:591–600. https://doi.org/10.1111/hae.14012 .
Article Google Scholar
Curtis R, Baker J, Riske B, et al. Young adults with hemophilia in the U.S.: demographics, comorbidities, and health status. Am J Hematol. 2015;90:S11–6. https://doi.org/10.1002/ajh.24218 .
Article PubMed Google Scholar
Manco-Johnson M, Abshire TC, Shapiro AD, Riske B, Hacker MR, Kilcoyne R, Ingram JD, Manco-Johnson ML, Funk S, Jacobson L, Valentino LA, Hoots WK, Buchanan GR, DiMichele D, Recht M, Brown D, Cindy L, Bleak S, Cohen A, Mathew P, Matsunaga A, Medeiros D, Nugent D, Thomas GA, Thompson AA, McRedmond K, Soucie JM, Austin H, Evatt BL. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe Hemophilia. N Engl J Med. 2007;357(6):535–44. https://doi.org/10.1056/nejme078098 .
Article CAS PubMed Google Scholar
Richards M, Williams M, Chalmers E, Liesner R, Collins P, Vidler V, Hanley J, on behalf of the Paediatric Working Party of the United Kingdom Haemophilia Doctors’ Organisation. A United Kingdom Haemophilia Centre Doctors’ Organization guideline approved by the British Committee for Standards in Haematology: guideline on the use of prophylactic factor VIII concentrate in children and adults with severe haemophilia A. Br J Haematol. 2010;149:498–507. https://doi.org/10.1111/j.1365-2141.2010.08139.x .
Srivastava A, Santagostino E, Dougall A, Kitchen SS, Sutherland M, Pipe SW, Carcao M, Mahlangu J, Ragni MV, Windyga J, Linás A, Goddard NJ, Mohan R, Poonose PM, Feldman BM, Lewis SZ, van den Berg H, Pierce GF, on behalf of the WFH Guidelines for the Management of Hemophilia panellists and co-authors. WFH guidelines for the management of Hemophilia, 3rd edition. Haemophilia. 2020;26:S1-158. https://doi.org/10.1111/hae.14046 .
Farrugia A, Cassar J, Kimber MC, Bansal M, Fischer K, Auserswald G, O’Mahony B, Tolley K, Noone D, Balboniet S. Treatment for life for severe hemophilia A-A cost-utility model for prophylaxis vs. on-demand treatment. Haemophilia. 2013;19:e228–38. https://doi.org/10.1111/hae.12121 .
Li N, Sawyer EK, Maruszczyk K, Guzauskas G, Slomka MT, Burke T, Martin AP, O’Hara J, Stevenson M, Recht M. Adult lifetime cost of hemophilia B management in the US: Payer and societal perspectives from a decision analytic model. J Med Econ. 2021;24:363–72. https://doi.org/10.1080/13696998.2021.1891088 .
Oldenburg J, Mahlangu JN, Kim B, Schmitt C, Callaghan MU, Young G, Santagostino E, Kruse-Jarres R, Negrier C, Kessler C, Valente N, Asikanius E, Levy GG, Windyga J, Shima M. Emicizumab prophylaxis in Hemophilia A with inhibitors. NEJM. 2017;377:809–18. https://doi.org/10.1056/NEJMoa1703068 .
Young G, Liesner R, Chang T, Sidonio R Jr, Oldenburg J, Jiménez-Yuste V, Mahlangu J, Kruse-Jarres R, Wang M, Uguen M, Doral MY, Wright LY, Schmitt C, Levy GG, Shima M, Mancuso ME. A multicenter, open-label phase 3 study of emicizumab prophylaxis in children with hemophilia A with inhibitors. Blood. 2019;134:2127–38. https://doi.org/10.1182/blood.2019001869 .
Article CAS PubMed PubMed Central Google Scholar
Pipe S, Shima M, Lehle M, Shapiro A, Chebon S, Fukutake K, Key N, Portron A, Schmitt C, Podolak-Dawidziak M, Bienz N, Hermans C, Campinha-Bacote A, Kiialainen A, Peerlinck K, Levy G, Jimenez-Yuste V. Efficacy, safety, and pharmacokinetics of emicizumab prophylaxis given every 4 weeks in people with haemophilia A (HAVEN 4): a multicentre, open-label, non-randomised phase 3 study. Lancet Haematol. 2019;6(6):e295–305. https://doi.org/10.1016/S2352-3026(19)30054-7 .
Nathwani AC, Tuddenham EGD, Rangarajan S, Rosales C, McIntosh J, Linch DC. Adenovirus-associated virus vector-mediated gene transfer in haemophilia B. N Engl J Med. 2011;365:2357–65. https://doi.org/10.1056/NEJMoa1108046 .
Nathwani AC, Reiss UM, Tuddenham EGD, Rosales C, Chowdary P, McIntosh J, Peruta MD, Lheriteau E, Patel N, Raj D, Riddell A, Pie J, Rangarajan S, Bevan D, Recht M, Shen YM, Halka KG, Basner-Tschakarjan E, Mingozzi F, High KA, Allay J, Kay MA, Ng CYC, Zhou J, Cancio M, Morton CL, Gray JT, Srivastava D, Nienhuis AW, Davidoff AM. Long-term safety and efficacy of factor IX gene therapy in haemophilia B. N Engl J Med. 2014;371:1994–2004. https://doi.org/10.1056/NEJMoa1407309 .
Perin GQ, Herzog RW, Markusic DM. Update of clinical gene therapy for haemophilia. Blood. 2018;33:407–14. https://doi.org/10.1182/blood-2018-07-820720 .
Article CAS Google Scholar
Rangarajan S, Walsh L, Lester W, Perry D, Madan B, Laffan M, Yu H, Vettermann C, Pierce GF, Wong WY, Pasi KJ. AAV5–factor VIII gene transfer in severe haemophilia A. N Engl J Med. 2017;377:2519–30. https://doi.org/10.1056/NEJMoa1708483 .
United Kingdom Haemophilia Doctors’ Organisation. The UKHCDO annual report and bleeding disorder statistics for the financial year 2019–2020. Manchester: United Kingdom Haemophilia Doctors’ Organisation; 2021. http://www.ukhcdo.org/wp-content/uploads/2021/03/UKHCDO-Annual-Report-2020-2019-20-Data_FINAL.pdf . Accessed 12 Mar 2021.
Brown K, Green G. The haemophilia drug market. Nat Rev Drug Discov. 2018;17:541–2. https://doi.org/10.1182/blood-2018-07-820720 .
Pierce G, Coffin D, Members of the WFH Gene Therapy Round Table Program Committee and Organizing Committee. The 1st WFH Gene Therapy Round Table: understanding the landscape and challenges of gene therapy for haemophilia around the world. Haemophilia. 2019;25:189–94. https://doi.org/10.1111/hae.13673 .
van Overbeeke E, Michelsen S, Hauber B, et al. Patient perspectives regarding gene therapy in haemophilia: interviews from the PAVING study. Haemophilia. 2020;27:129–36. https://doi.org/10.1111/hae.14190 .
Article PubMed PubMed Central Google Scholar
Fletcher S, Jenner K, Holland M, Chaplin S, Khair K. An exploration of why men with severe haemophilia might not want gene therapy: the exigency study. Haemophilia. 2021;27:760–8. https://doi.org/10.1111/hae.14378 .
van Balen EC, Wesselo ML, Baker BL, Westerman MJ, Coppens M, Smit C, Driessens MHE, Leebeek FWG, van der Bom JG, Gouw SC. Patient perspectives on novel treatments in haemophilia: a qualitative study. Patient. 2020;13:201–10. https://doi.org/10.1007/s40271-019-00395-6 .
Meisbach W, Klamroth R. The patient experience of gene therapy for haemophilia: qualitative interviews with trial patients. Patient Preference Adherence. 2020;14:767–70. https://doi.org/10.2147/PPA.S239810 .
Aradom E, Gomez K. The patient gene therapy journey: findings from qualitative interviews with trial participants at one UK Haemophilia centre. J Haemophilia Pract. 2021. https://doi.org/10.17225/jhp00174 .
Miller FG. Recruiting research participants. In: Emanuel EJ, Grady C, Crouch RA, Lie RK, Miller FG, Wender D, editors. The Oxford textbook of clinical research ethics. Oxford: Oxford University Press; 2008. p. 397–403.
Google Scholar
Whitmore E. To tell the truth: Working with oppressed groups in participatory approaches to inquiry. In: Reason P, editor. Participation in human inquiry. London: Sage; 1994. p. 82–98.
Berg DN, Smith KK. The clinical demands of research methods. In: Berg DN, Smith KK, editors. Exploring clinical methods for social research. Beverly Hills: Sage; 1985. p. 21–34.
Simmons M. Insider ethnography: tinker, tailor, researcher or spy? Nurse Res. 2007;14(4):7–17. https://doi.org/10.7748/nr2007.07.14.4.7.c6039 .
Rangarajan S, Kim B, Lester W, Symington E, Madan B, Laffan M, Tavakkoli F, Pierce G, Wong WY, Pasi J. Achievement of normal factor VIII activity following gene transfer with valoctocogene roxaparvovec (BMN 270): long-term efficacy and safety results in patients with severe hemophilia A. Haemophilia. 2018;24:S65.
High KA, George LA, Eyster E, Sullivan SK, Ragni MV, Croteau SE, Samelson-Jones BJ, Evans M, Joseney-Antoine M, Macdougall A, et al. A phase 1/2 trial of investigational Spk-8011 in hemophilia a demonstrates durable expression and prevention of bleeds. Blood. 2018;132:92–5856. https://doi.org/10.1182/BLOOD-2018-99-115495 .
Batty P, Lillicrap D. Advances and challenges for hemophilia gene therapy. Hum Mol Genet. 2019;28:R95–101. https://doi.org/10.1093/hmg/ddz157 .
Nathwani AC, Rosales C, McIntosh J, Rastegarlari G, Nathwani D, Raj D, Nawathe S, Waddington SN, Bronson R, Jackson S, Donahue RE, High KA, Mingozzi F, Ng CYC, Zhou J, Spence Y, McCarville MB, Valentine M, Allay J, Coleman J, Sleep S, Gray JT, Nienhuis AW, Davidoff AM. Long-term safety and efficacy following systemic administration of a self-complementary AAV vector encoding human FIX pseudotypedwith serotype 5 and 8 capsid proteins. Mol Ther. 2011;19:876–85.
Pipe S, Stine K, Rajasekhar A, Everington T, Poma A, Crombez E, Hay CRM. 101HEMB01 is a phase 1/2 open-label, single ascending dose-finding trial of DTX101 AAVrh10FIX in patients with moderate/severe hemophilia B that demonstrated meaningful but transient expression of human factor IX hFIX. Blood. 2017;130:S3331. https://doi.org/10.1182/blood.V130.Suppl_1.3331.3331 .
National Institute of Health and Care Excellence. British National Formulary. 2021. https://bnf.nice.org.uk/treatment-summary/corticosteroids-general-use.html . Accessed 12 Sept 2021.
Dresser R. Silent partners: human subjects and research ethics. Oxford: Oxford University Press; 2016.
Book Google Scholar
Quality Review Services. Inherited and acquired haemophilia and other bleeding disorders peer review programme: overview report . 2020. https://images.qualityreviewservicewm.nhs.uk/wp-content/uploads/2020/05/28154818/IABD-Overview-Report-2020-V1-20200527-1.pdf . Accessed 14 Sept 2021.
Khair K, Steadman L, Chaplin S, Holland M, Jenner K, Fletcher S. Parental perspectives on gene therapy for children with haemophilia: the Exigency study. Haemophilia. 2021;27:120–8. https://doi.org/10.1111/hae.14188 .
Woollard L, Gorman R, Rosenfelt DJ. Improving patient informed consent for haemophilia gene therapy: the case for change. Ther Adv Rare Dis. 2021;2:1–16. https://doi.org/10.1177/26330040211047244 .
Fusch PI, Ness LR. Are we there yet? Data saturation in qualitative research. Qual Rep. 2015;20:1408–16. https://doi.org/10.46743/2160-3715/2015.2281 .
Bernard RH. Social research methods: qualitative and quantitative approaches. 2nd ed. Thousand Oaks: Sage; 2012.
Download references
Acknowledgements
We would like to thank the centres who identified potential participants as well as the participants of this study for sharing their views and time.
Authors information
S Fletcher. This paper and others from the Exigency study programme will be offered in evidence for a phD by published works.
The Exigency study programme is funded by an unrestricted education grant from uniQure Biopharma.
Author information
Authors and affiliations.
Oxford Haemophilia and Thrombosis Centre, Oxford University Hospitals NHS Foundation Trust, Oxford, OX3 7LE, UK
Simon Fletcher
Haemnet, London, N15 3JR, UK
Kathryn Jenner, Luke Pembroke, Michael Holland & Kate Khair
You can also search for this author in PubMed Google Scholar
Contributions
SF Study and interview guide design, interview facilitation, analysis of interviews primary authorship of manuscript. KJ Transcription of interviews. LP Study and interview guide design. MH, Study design. KK. Study and interview guide design, interview facilitation, and analysis of interviews. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Simon Fletcher .
Ethics declarations
Ethics approval and consent to participate.
All participants were sent detailed information sheets informing them of the nature and purposes of the research. Written informed consent was obtained. All participants received a gift voucher as a ‘thank you’ for the time they gave attending interviews. Ethical approval for all elements of the study was granted by the UK Healthcare Research Authority and the South East Scotland Research Ethics Committee (20/SS/0061).
Consent for publication
No applicable.
Competing interests
S Fletcher. Unrestricted educational grant from Roche Products Limited and Chugai Pharma UK Ltd, speaker honoraria from Roche Products Limited, Bayer and Novo Nordisk, meeting support from Novo Nordisk and Bayer. K Jenner. Unrestricted educational grant from Roche Products Limited and Chugai Pharma UK Ltd. L. Pembroke. Unrestricted educational grant from Roche Products Limited and Chugai Pharma UK Ltd, Consultancy fees from Roche Products Limited, Bayer, Biomarin and Sobi, Speaker Honoraria from Biomarin, Sobi, Novo Nordisk and Chugai. M Holland. Unrestricted educational grant from Roche Products Limited and Chugai Pharma UK Ltd. K Khair. Unrestricted educational grant from Roche Products Limited and Chugai Pharma UK Ltd, Consultancy fees from Bayer, Novo Nordisk, Sobi and Takeda, Speaker honoraria from Bayer, Biomarin, Novo Nordisk, Sobi, Pfizer and Takeda.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1..
Supporting Quotes.
Additional file 2.
Interview Guide.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Fletcher, S., Jenner, K., Pembroke, L. et al. The experiences of people with haemophilia and their families of gene therapy in a clinical trial setting: regaining control, the Exigency study. Orphanet J Rare Dis 17 , 155 (2022). https://doi.org/10.1186/s13023-022-02256-2
Download citation
Received : 12 November 2021
Accepted : 13 February 2022
Published : 04 April 2022
DOI : https://doi.org/10.1186/s13023-022-02256-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Haemophilia A
- Haemophilia B
- Genetic therapy
- Decision making
- Informed consent
- Clinical trial
Orphanet Journal of Rare Diseases
ISSN: 1750-1172
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
- Type 2 Diabetes
- Heart Disease
- Digestive Health
- Multiple Sclerosis
- COVID-19 Vaccines
- Occupational Therapy
- Healthy Aging
- Health Insurance
- Public Health
- Patient Rights
- Caregivers & Loved Ones
- End of Life Concerns
- Health News
- Thyroid Test Analyzer
- Doctor Discussion Guides
- Hemoglobin A1c Test Analyzer
- Lipid Test Analyzer
- Complete Blood Count (CBC) Analyzer
- What to Buy
- Editorial Process
- Meet Our Medical Expert Board
- Symptoms and Causes
Coping With Hemophilia A
Giving Yourself the Self-Care You Deserve
- Next in Hemophilia A Guide What Is Hemophilia A?
Hemophilia A can have a big impact on your life and your family. It requires long-term treatment, and sometimes the treatment can be complicated, especially if you develop inhibitors (your immune system works against the treatment).
In addition to getting medical treatment, you also can get help managing your feelings about your condition, staying safe, and with family planning. This article will discuss how to cope and live well with hemophilia A.
Verywell / Jessica Olah
Living with hemophilia A means that you must limit some of your activities to avoid injuries that could lead to bleeding. This can make you feel sad, anxious, or angry. It is completely normal to have these feelings.
You might have times in your life when you feel more emotional distress or less emotional distress about your condition, and your range of feelings is normal, too. There are different ways to manage your feelings, and some might be right for you at various times in your life.
Strategies include:
- Meeting with a qualified therapist
- Joining a support group so you can talk to other people who have bleeding disorders
- Talking to a friend or family member
- Working on projects that promote hemophilia A support, awareness, or advocacy
- Learning about your condition
- Meditation
You might feel that one or more of these suits your personality and needs. If you try a strategy and it isn’t working for you, consider trying a different one.
It’s important that you take care of your health so you can minimize the risks and effects of hemophilia A. Medical treatment is an important aspect of managing the physical effects of your condition, and there are other things you need to do to take care of your body.
Physical considerations include:
- Safety : Avoiding injuries that could cause internal bleeding or open wounds is vital. This means not participating in contact sports, high-impact sports, or activities with a high risk of injury. Activities, sports, and exercises you can do include swimming, running, jogging, Pilates, yoga , golf, tennis, dance, and more.
- Safety gear : Sometimes protective equipment can help prevent injuries during certain activities. These types of shields can include helmets, knee pads, elbow pads, or other protection.
- Exercise : Staying active is an important way to maintain physical strength, balance, and flexibility. Strength and balance can reduce your risk of injuries, and joint flexibility is especially important because hemophilia A can cause joint damage due to bleeding .
- Healthy diet : A well-balanced diet can help optimize your body’s functions, preventing other causes of bleeding, such as vitamin deficiencies.
- A plan for managing bleeding at home : It is likely that you could have occasional minor injuries. Discuss a plan with your doctor so you will know how to prevent excess bleeding. Strategies like using an ice pack if you become injured can help. Also, be sure to cover wounds to prevent infections.
- Recognizing emergencies : While most injuries are likely to be mild, you can experience serious injuries . Discuss a plan with your doctor and with your family members so you will know when to get help and whom to call. Severe bleeding can cause organ damage and can be life-threatening.
With concerns about injuries and having frequent medical visits, you might feel alone and unable to participate in social events. This can be a feature of your life at any age, but it can be especially difficult for children who are living with hemophilia A.
There are things you can do to help build and maintain social connections with others so you will have the healthy and enjoyable interactions you need and crave.
Things to consider:
Decide how much you want to share about your condition : Sometimes you might want to explain what you are going through to certain friends, classmates, or colleagues. And sometimes you might want to keep your health issues to yourself. It’s OK to share sometimes but not other times. It all depends on what feels right for you.
Find activities you can enjoy : You might feel somewhat left out of certain groups, such as an athletic team, when you can’t participate in their activity. Consider developing group hobbies that are safe for hemophilia A and that don’t have an inherent risk of bleeding.
For children, this might include swimming, dance, choir, theater, art, debate, music, and many more options. For adults, this can include things like golf, bowling, yoga, and more.
Independence for children : If your child has hemophilia A, it’s normal for you to be protective. Your child also needs to learn how to be confident and to become independent as they learn to manage their health and their life.
Empower them to learn about their treatment. Remember to be supportive if they face issues such as feeling left out. You can help them build the self-assurance they need to thrive throughout life.
There are several important issues you need to pay attention to if you have hemophilia A.
Family Planning
The implications of hemophilia A for your potential offspring are important to consider. If you have the hereditary form of hemophilia A, you could have a child with the condition. There are varying levels of severity, and you or your child might have a mild, moderate, or severe form.
It’s important to discuss the risks with your partner so you can decide about genetic testing and family planning together. There may also be risks associated with pregnancy and childbirth, so discuss these with your physician.
Time Off From School or Work
You might have many medical appointments for your treatment or for physical therapy. It can take a good deal of planning to work out the timing of your medical care along with your other obligations and your social life and hobbies. Be patient with yourself as you balance all these different things in your life.
If you need a flexible schedule, consider enrolling in some online courses instead of taking all your classes in person. And think about finding a career that gives you flexibility so you can achieve everything you want and take good care of your health.
Living with hemophilia A involves reducing your injury risk, having a plan for managing injuries, acknowledging your emotions, and maintaining social connections despite the limitations you may have on your activities.
Additionally, genetic testing might be important for you and your family. You might need accommodations as you balance your illness with everything else you want to do in your life.
A Word From Verywell
Living with hemophilia A is about more than getting medical care. You and your family may have many concerns about how your condition affects your life. Reach out for help and take advantage of the resources available to you so you can live your best life with hemophilia A.
Parviniannasab AM, Rakhshan M, Momennasab M, Soltanian M, Rambod M, Akbarzadeh M. Haemophiliac adolescents' perspectives of resilience: A qualitative study based on the resilience in illness model . Clin Child Psychol Psychiatry. 2020 Apr;25(2):346-358. doi:10.1177/1359104519890905
Torres-Ortuño A, Cuesta-Barriuso R, Nieto-Munuera J, Galindo-Piñana P, López-Pina JA. Coping strategies in young and adult haemophilia patients: A tool for the adaptation to the disease . Haemophilia. 2019 May;25(3):392-397. doi:10.1111/hae.13743
Reinicke K, Søgaard IS, Mentzler S. Masculinity challenges for men with severe hemophilia . Am J Mens Health. 2019 Jul-Aug;13(4):1557988319872626. doi:10.1177/1557988319872626
By Heidi Moawad, MD Dr. Moawad is a neurologist and expert in brain health. She regularly writes and edits health content for medical books and publications.
Educational needs of patients, families, and healthcare professionals to support the patient journey in haemophilia gene therapy in the UK
Affiliations.
- 1 University Hospital Southampton, Southampton, UK. [email protected].
- 2 Oxford University Hospitals NHS Foundation Trust, Oxford, UK.
- 3 The Royal Victoria Infirmary, Newcastle upon Tyne, UK.
- 4 CSL Behring, West Sussex, UK.
- 5 Haemophilia, Haemostasis and Thrombosis Centre, Hampshire Hospitals NHS Foundation Trust, Basingstoke, UK.
- 6 Sheffield Children's NHS Foundation Trust, Sheffield, UK.
- 7 Haemophilia and Thrombosis Centre, Royal Free Hospital, London, UK.
- 8 East Kent Hospitals University NHS Foundation Trust, Kent, UK.
- 9 University Hospital Southampton, Southampton, UK.
- PMID: 38007560
- PMCID: PMC10676600
- DOI: 10.1186/s13023-023-02977-y
With the first gene therapies for haemophilia approved by the European Commission, the US Food and Drug Administration, and the Medicines and Healthcare products Regulatory Agency, it is important to consider the remaining unmet needs and challenges that may arise throughout patients' treatment journeys. We discuss existing unmet needs and important considerations prior to, during, and following haemophilia gene therapy treatment in the UK, and propose potential next steps. Key areas for attention are education, psychological support, and guidance on implementation. Strategies are urgently required to fulfil these needs. An immediate priority for information providers should be comprehensive education for people with haemophilia (PWH) and healthcare professionals (HCPs). Greater access to resources and training in psychological services will be required throughout the treatment pathway. More specific guidance is required to define the implementation model, criteria for accreditation, and responsibilities of care centres. Furthermore, PWH may revisit discussions with HCPs multiple times pre-infusion, thus the patient journey is unlikely to be linear. Consideration of these challenges, and of potential strategies to address them, will be integral to optimising the future success of this promising therapy.
Keywords: Gene therapy; Haemophilia A; Haemophilia B; Health services needs and demand; Patient navigation.
© 2023. The Author(s).
Publication types
- Delivery of Health Care
- Health Personnel
- Hemophilia A* / therapy
- United Kingdom

Realizing I Was a Runner, Despite My Hemophilia
Thomas Carine’s story of living an active life with the rare bleeding disorder.

An Early Diagnosis
Carine was diagnosed with hemophilia A soon after birth, when his parents noticed substantial bruising from crawling and delayed healing time after his circumcision. Like many medical conditions, hemophilia is not experienced the same way by everybody. Carine’s condition is severe and can cause life-threatening internal bleeding even in the absence of an inciting injury. At the time of his diagnosis, there weren’t many hematology departments around Carine’s hometown of San Jose, California, and finding adequate care (and clinical understanding) was difficult.
“The early years were very hard,” he says. “Luckily my dad was a doctor, so he was there to handle my factor VIII replacement infusions at home.” Bleeding episodes in joints—such as the ankles, knees, and elbows—sometimes occurred with little warning. These prevented him from participating in many childhood activities.
Outside Looking In
Carine recalls gearing up for a sleepover with a laundry list of to-dos. “My parents and I prepared so many questions for the doctors, had calls with the parents and the other kids, talked through everything I’d need to bring with me,” he recalls. “Finally, we get to the sleepover. That night there was blood in my urine. The next morning, we had to go to the hospital where I found out I was bleeding into my kidneys. That was a linchpin moment: I realized that I couldn’t wish this away.” At age 10, Carine’s doctor recommended treating with factor VIII prophylactically, aiming to prevent bleeding episodes rather than treating them after they happen in order to help improve his symptoms.
Even then, sports were still daunting. Carine remembers trying out for the baseball team: “By the second week of tryouts, my joints were painful and swollen.” Doubting his ability to lead a physically active life, he began to explore the theater program in high school, building sets and doing audio-visual work—a serendipitous pivot that would ultimately lead Carine to a job in the A/V department for a professional hockey team.
Embracing His Reality
As Carine transitioned into adulthood, his experience managing the physical and mental effects of hemophilia A evolved. “It just becomes your life. It becomes another day,” he says. “The next day becomes a little easier, and then you get through the one after that. I remember the first factor VIII infusion I did for myself was a huge milestone: How do I give myself my own infusion so I don’t have to wait for my parents, so that I don’t have to go to a hospital?” With more confidence in learning to manage his condition, Thomas started participating in more physical activities too.
Still, independence was not without challenges. While he had a career he loved, managing his condition and treatment schedule still posed a burden. His factor VIII infusions required careful preparation and administration several times a week. Carine has always looked for new strategies to streamline his routine and at one point he optimistically pursued a gene-therapy trial, but was denied eligibility due to certain existing antibodies to the virus used in that therapy.
Still, he continued searching for new treatment options.
Finding the Right Treatment
Four years ago, upon his doctor’s advice, he began using Hemlibra® (emicizumab-kxwh)*, a prescription medicine used to prevent or reduce the frequency of bleeding episodes in adults and children living with hemophilia A. It is the first medicine for hemophilia A that can be subcutaneously injected, like a shot under the skin, similar to the way someone with diabetes injects insulin. Because Hemlibra has a unique four-week half-life (how long it takes for half the amount of a medicine to leave the body), the medicine sustains longer in the body between doses, enabling patients to self-administer once a week, every two weeks, or every four weeks after loading doses.
*What is the most important information I should know about HEMLIBRA? HEMLIBRA increases the potential for your blood to clot. Discontinue prophylactic use of bypassing agents the day before starting HEMLIBRA prophylaxis. Carefully follow your healthcare provider’s instructions regarding when to use an on-demand bypassing agent, and the dose and schedule you should use. HEMLIBRA may cause serious side effects when used with aPCC (FEIBA ® ), including thrombotic microangiopathy (TMA) and blood clots (thrombotic events). If aPCC (FEIBA ® ) is needed, talk to your healthcare provider in case you feel you need more than 100 U/kg of aPCC (FEIBA ® ) total. See more safety information below.
Running Forward
The administration of Hemlibra, taking less than a minute to inject after preparation, allowed Carine to integrate Hemlibra into his busy schedule, and he turned his main focus back to his life. “I made an effort to figure out my workout schedule and run with other people to encourage me,” he says. Carine talked to his doctor to make sure it was okay for him to participate in these activities and to learn how to take the necessary precautions.
While he still struggles with joint damage and issues from childhood, Carine continues to regularly participate in physical activities and recreational sports. With more flexibility in his schedule, he runs every day, plays on an intramural softball team with friends, and practices calisthenics with weights regularly throughout the week. His experience makes him hopeful for the future of people with hemophilia. “I just want people with this condition to lead more normal, active lives,” he says. “It’s all about the next generation.”
Every person's hemophilia A journey is different, and Thomas' physical practices may not be appropriate for everyone.
*In the Phase III HAVEN 3 study in people aged 12 years and older without factor VIII inhibitors, the average number of treated bleeds per year (ABR, annualized bleed rate) for people receiving HEMLIBRA prophylaxis every week (N=36) or every two weeks (N=35) was 1.5 (95% CI: 0.9, 2.5) and 1.3 (95% CI: 0.8, 2.3), respectively, compared to 38.2 (95% CI: 22.9, 63.8) for people receiving no prophylaxis (N=18). The median time on HEMLIBRA for people in HAVEN 3 study was 30 weeks (once every week), 31 weeks (once every 2 weeks), and 24 weeks (no prophylaxis). In the Phase III HAVEN 4 study, the ABR for people aged 12 years or older with hemophilia A with or without factor VIII inhibitors receiving HEMLIBRA prophylaxis every four weeks (N=41) was 2.4 (95% CI: 1.4, 4.3). The median time on Hemlibra for people in HAVEN 4 study was 26 weeks.
Important Safety Information
What is the most important information to know about Hemlibra?
Hemlibra increases the potential for blood to clot. People who use activated prothrombin complex concentrate (aPCC; Feiba®) to treat breakthrough bleeds while taking Hemlibra may be at risk of serious side effects related to blood clots.
These serious side effects include:
• Thrombotic microangiopathy (TMA), a condition involving blood clots and injury to small blood vessels that may cause harm to one’s kidneys, brain, and other organs
• Blood clots (thrombotic events), which may form in blood vessels in the arm, leg, lung, or head
Patients should talk to their doctor about the signs and symptoms of these serious side effects, which can include:
• Confusion
• Stomach, chest, or back pain
• Nausea or vomiting
• Swelling, pain, or redness
• Feeling sick or faint
• Decreased urination
• Swelling of arms and legs
• Yellowing of skin and eyes
• Eye pain, swelling, or trouble seeing
• Fast heart rate
• Numbness in your face
• Shortness of breath
• Coughing up blood
If patients experience any of these symptoms during or after treatment with Hemlibra, they should get medical help right away.
Patients should carefully follow their healthcare provider’s instructions regarding when to use an on demand bypassing agent or factor VIII, and the dose and schedule to use for breakthrough bleed treatment. If aPCC (Feiba®) is needed, patients should talk to their healthcare provider in case they feel they need more than 100 U/kg of aPCC (Feiba®) total.
Patients’ bodies may make antibodies against Hemlibra, which may stop Hemlibra from working properly. Patients should contact their healthcare provider immediately if they notice that Hemlibra has stopped working for them (e.g., an increase in bleeds).
The most common side effects of Hemlibra include: injection site reactions (redness, tenderness, warmth, or itching at the site of injection), headache, and joint pain. These are not all of the possible side effects of Hemlibra. Patients can speak with their healthcare provider for more information.
What else should patients know about Hemlibra?
Patients should see the detailed “Instructions for Use” that comes with Hemlibra for information on how to prepare and inject a dose of Hemlibra, and how to properly throw away (dispose of) used needles and syringes.
• Patients should stop taking their prophylactic bypassing therapy the day before they start Hemlibra.
• Patients may continue taking their prophylactic factor VIII for the first week of Hemlibra.
Hemlibra may interfere with laboratory tests that measure how well blood is clotting and create an inaccurate result. Patients should speak with their healthcare provider about how this may affect their care.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide.
Patients should only use Hemlibra for the condition it was prescribed. Patients should not give Hemlibra to other people, even if they have the same symptoms that they have. It may harm them.
Patients should tell their healthcare provider about all the medicines they take, including prescription medicines, over-the-counter medicines, vitamins, or herbal supplements. Patients should keep a list of them to show their healthcare provider and pharmacist.
Before using Hemlibra, patients should tell their healthcare provider about all of their medical conditions, including if they are pregnant, plan to become pregnant, are breastfeeding, or plan to breastfeed.
Since Hemlibra was tested in males, there is no information on whether Hemlibra may impact an unborn baby or breast milk. Females who are able to become pregnant should use birth control during treatment.
Side effects may be reported to the FDA at (800) FDA-1088 or www.fda.gov/medwatch . Side effects may also be reported to Genentech at (888) 835-2555.
Please see Important Safety Information, including Serious Side Effects, as well as the Hemlibra full Prescribing Information and Medication Guide .
M-US-00022169(v1.0)
© 2024 Genentech USA, Inc. All rights reserved.
This is a paid partnership between Genentech and Men’s Health.
San Bernardino 5-year-old living active life thanks to innovative hemophilia treatment

Kevin Sanchez Baca presented as a challenge to doctors trying to treat him after he fell while playing with his siblings. The one-year-old had injured the connecting tissue from his lip to his gums and began bleeding. And Kevin kept bleeding every time he ate. It wasn’t until the third trip to the emergency department that his parents found out why.
Kevin had hemophilia, a blood disorder in which the person’s coagulation system lacks the ability to produce blood clotting factors that are critical in arresting a bleed every time the body undergoes a trauma. The only way these patients can be kept alive without bleeding out, is by intravenous clotting factor infusion two or three times a week. A simple paper cut could be fatal.
Akshat Jain, MD, MPH, director of Inherited Bleeding Disorders at Loma Linda University Children's Health diagnosed a rare genetic aberration in Kevin. Genome sequencing conducted on Kevin’s blood led to the identification of a rare mutation known to afflict families suffering from hemophilia. Unfortunately, Kevin was the first member of his family to develop this mutation, known as inhibitor development—which renders standard lifesaving treatments ineffective.
Hemophilia treatments for Hispanics, African Americans, and Indigenous people are often complicated by the development of this rare complication. This makes the disease fatal for people in these groups at double the rates of Caucasian hemophilia patients, Jain says.
With demographics in the Inland Empire, Jain says many patients coming to Loma Linda University Health for hemophilia treatment have higher rates of inhibitor development given their ethnicity. Previously, all such patients used to be referred outside of LLUH, forcing them to endure long drives and fractured care due to language barriers. Kevin would have been one such baby, who developed an aggressive inhibitor in his infancy.
Children receiving hemophilia treatment usually have a PORT device surgically implanted in their chest (similar to devices used for cancer patients). This allows their parents to infuse the lifesaving factor three times a week for the rest of their lives. But Kevin’s inhibitors developed so early in life, which meant he couldn’t be a candidate for PORT surgery. Doctors weren’t able to give him the necessary clotting factor to stop him from bleeding out.
Instead, Jain decided to offer a new treatment that had been attempted with only two other patients internationally at that time. Kevin received a unique hybrid treatment model with a newly approved FDA immunotherapy called Emicizumab, an injection given once every week along with blood clotting factor therapy into his veins at LLUH’s comprehensive hemophilia center, Jain said.
“Our novel experimental aggressive hybrid therapy for Kevin worked amazingly well,” Jain recalled. “His life-threatening continuous bleeding stopped, and within four months our team eradicated the antibody that was killing the factor in this bloodstream successfully forever.”
Now five years old, Kevin is now living a healthy, active life of riding his bicycle and playing soccer. “Those things could have been a deadly risk for him a decade ago,” Jain said.
World Hemophilia Day is commemorated annually each April 17 to honor of the birthday of Canadian Businessman Frank Schnabel, who founded the Montreal-based World Federation of Hemophilia in 1963. Today the federation works with national member organizations in 147 countries.
Jain said this year’s theme is particularly meaningful for those in the Inland Empire Region: “Equitable access for all: recognizing all bleeding disorders.” The theme, he says, speaks directly to people like Kevin, who is Hispanic and lives in a home where Spanish is spoken.
Jain said Loma Linda University Health is the only facility to offer hemophilia care to service four Southeastern California counties, and it’s the only such center from Central Arizona to downtown Los Angeles.
Loma Linda University Health’s care for hemophilia patients started in 2017 and now serves more than 500 families with bleeding disorders.
More information is available on the Loma Linda University Pediatric Hemophilia & Bleeding Disorders webpage .
—William Castillo, MSW, contributed to this story.

Hemophilia treatment model
Dr. Akshat Jain shows Kevin and his mother Lucia how untreated hemophilia can affect joints throughout the body.
- NBDF’s Established Initiatives & Impact >

- Mission & History
- What Do We Value?
- Health Equity
- Accomplishments
- Financial Statements
- Media Newsroom
- The National Hemophilia Foundation Has a New Name
- The Red Thread: 75th Anniversary Celebration
- Board of Directors
- Meet the NYLI
- Nursing Working Group
- Physical Therapy Working Group
- Social Work Working Group
- Partners In Progress
- The ACT Initiative
- Red Tie Society
- CDC Coop Agreement
- Pathway to Cures
- Career Opportunities
- Volunteer Opportunities
- What is a Bleeding Disorder?
- Types of Bleeds
- Women and Bleeding Disorders
- Hemophilia A
- Hemophilia B
- Von Willebrand Disease
- Other Factor Deficiencies
- Inherited Platelet Disorders
- Comprehensive Medical Care
- MASAC For You
- Treatment Guidelines (MASAC)
- Current Treatments
- Future Therapies
- Clinical Trials
- Shared Decision-Making
- Choosing an Insurance Plan
- Private Insurance
- Public Insurance
- Health Insurance Toolkit
- Bleeding Disorders Conference
- Inhibitor Education
- Online Education
- Rare Bleeding Disorders
- Mental Health
- Gene and Innovative Therapies
- Educational Programming
- Own Your Path
- Clotting Cascade
- Youth Leadership (NYLI)
- Undiagnosed
- Guías Culturales
- Access to Care
- Federal Programs
- Blood & Blood Product Safety
- Make All Copays Count
- Utilization Management
- Patient Out-of-Pocket Expenses
- Register to Vote
- Washington Days
- Advocacy Do’s and Don’ts
- 6 Steps for Grassroots Advocacy
- Tell Your Story
- Collaborating on Coverage
- Quality of Care Guidelines
- CME/CE Webcast Series
- Educational Web Portal
- Quality Improvement & Cost Management
- Foundation Research
- Funded by Foundation
- Presented at Our Conference
- Research Journal Club
- Clinical Trial Essentials
- coreHem Mental Health Tool
- Judith Graham Pool Postdoctoral Research Fellowship
- Career Development Award
- Nursing Excellence Fellowship
- Physical Therapy Excellence Fellowship
- Social Work Excellence Fellowship
- What is CVR?
- How & Why Should I Participate?
- Impact on Research
- Frequently Asked Questions
- What We’ve Learned So Far
- What is the National Research Blueprint?
- Involvement from Across the Community
- Lived experience experts (LEEs) leading the way
- Building the Blueprint
- Research Priorities
- Comprehensive Care
- MASAC Documents
- Products Licensed (US)
- Emergency Management
- Snapshots of VWD Guidelines
- NBDF-Takeda Clinical Fellowship Program
- NBDF’s Collaborative Learning Exchange
- Online Education for Providers
- Live & Online Learning (Partners)
- Rare Coagulation Resource Room
- Peer-reviewed Journals
- NBDF Publications
- Other Associations
- Physical Therapy
- Social Work
- HANDI - NBDF's Information Resource Center
- Read Our Publications
- Subscribe for Email Updates
- Get HemAware Magazine
- Newsletter Archive
- Wednesday Webinar Series
- Hemophilia Treatment Centers
- Bleeding Disorders Camps
- Scholarships
- Patient Assistance Programs
- Make a Donation
- Give Monthly
- Honor a Loved One
- Support Research
- Kevin Child Scholarship Donation Form
- Physician Partners
- Be a Corporate Partner
- Donate Your Car
- Make a Planned Gift
- Create a Will
- Donor Advised Funds
- Run in a Marathon
- 2024 United Airlines New York City Half Marathon
- Create Your Own Fundraiser
- Fundraise on Facebook
- Red Tie Soiree
- Bleeding Disorders Awareness Month
- Find a Walk
- For Young Professionals
MASAC Document 278 - MASAC Recommendations on Administration of Vaccines to Individuals with Bleeding Disorders
Replaces: 221
There has been considerable discussion in the hemophilia community regarding the optimal protocol for the administration of vaccines to individuals with bleeding disorders. Speculation that vaccines may induce the development of inhibitors to factor concentrates are not substantiated 1 . The MASAC Vaccine Working Group has reviewed the available literature, online and in print, and has developed the following recommendations.
1. Centers for Disease Control and Prevention (CDC) Guidelines
It is highly recommended (See MASAC Recommendation #218, page 5, Section G.1 and G.2) 1 that patients with bleeding disorders continue to follow the American Academy of Pediatrics’ and CDC’s vaccine recommendation route and schedule for their age. These recommendations can be found on the CDC website as follows:
- Infant Schedule Age (0-6yrs):
http://www.cdc.gov/vaccines/parents/downloads/parent-ver-sch-0-6yrs.pdf
- Child Schedule Age (7-18 yrs):
http://www.cdc.gov/vaccines/who/teens/downloads/parent-version-schedule-7-18yrs.pdf
- Adult Schedule Age (19 yrs and older):
http://www.cdc.gov/vaccines/schedules/downloads/adult/adult-schedule-easy-read.pdf
http://www.cdc.gov/vaccines/schedules/downloads/adult/adult-schedule-easy-read - bw.pdf
- Travel Recommendations:
http://wwwnc.cdc.gov/travel/page/vaccinations.htm
- Special Groups:
http://www.cdc.gov/vaccines/spec-grps/default.htm
2. Protocol for Administration of Vaccines
MASAC recommends that when giving immunizations, the following procedures may be considered:
- A fine-gauge needle (23 gauge or smaller caliber) be used. 2
- Firm pressure should be applied to the site for at least 2 minutes without rubbing. 2
- The patient and/or caregiver should be informed that there is risk of hematoma development at the injection site. 2 Patients with hemophilia depending on their factor level, may consider use of therapy to prevent hematoma formation, in consultation with their hemophilia treatment center.
- Anticipatory guidance should be given regarding when to call the physician or HTC regarding any adverse reactions such as hematoma, fever, warmth, redness. 2
- For pain/fever relief 2 , avoid aspirin and NSAIDS (such as ibuprofen, naproxen sodium) because of the potential risk of bleeding. Acetaminophen is a safe alternative, but should be used with caution, especially in individuals at risk for liver disease.
- Patients on Emicizumab prophylaxis may not require additional treatment prior to vaccinations. 3-5
- Patients with Type 1 or 2 Willebrand disease (VWD), depending on their baseline von Willebrand factor (VWF) activity levels, may consider use of therapy to prevent hematoma formation, in consultation with their hemophilia treatment center. Patients with Type 3 VWD should consider a VWF-containing infusion prior to vaccination. 5
- All rare bleeding disorder patients (including those with thrombocytopenia and/or platelet function disorders) should be vaccinated with the above general precautions.
- Patients on Vitamin K antagonists should have prothrombin time testing performed within 72 hours prior to injection to determine international normalized ratio (INR); if results are stable and within the therapeutic range, they can be vaccinated intramuscularly. No data are available in patients on DOACs/NOACs. 6
3. Vaccines that can be given subcutaneously
There is considerable variation regarding vaccine route of administration (IM vs SQ) among HTC providers (reference CDC data). Many vaccines have not undergone rigorous investigation to demonstrate that SQ administration is as effective as IM administration. Whether or not the potential reduction in intramuscular hematomas from SQ administration outweighs any potential reduction in vaccine efficacy is not known. The vaccines (single vaccines, not in combination with other vaccines) that have been tested and demonstrated to be effective when administered either IM or SQ include:
A. Pneumococcal polysaccharide (PPSV) 7
B. Polio, inactivated (IPV) 7
C. Hepatitis A 8
D. Hepatitis B 9-11
4. The safety, efficacy, and optimal protocols for administration of other existing, and existing and emerging vaccines will be evaluated by MASAC on an ongoing basis.
5. Live vaccines should not be administered in patients receiving high dose steroid or immune-modulating drugs as a part of immune tolerance therapies or gene therapy protocols. 12
- Platokouki H, Fischer K, Gouw SC, Rafowicz A, Carcao M, Kenet G, Liesner R, Kurnik K, Rivard GE, van den Berg HM. Vaccinations are not associated with inhibitor development in boys with severe haemophilia A. Haemophilia. 2018 Mar;24(2):283-290.
- MASAC recommendations concerning products licensed for the treatment of hemophilia and other bleeding disorders Revised October 2013. MASAC Document #218.
- Tiede A, Leise H, Horneff S, Oldenburg J, Halimeh S, Heller C, Königs C, Holstein K, Pfrepper C. Safety of intramuscular COVID-19 vaccination in patients with haemophilia. Haemophilia. 2022 Sep;28(5):687-693. doi: 10.1111/hae.14586. Epub 2022 May 13. PMID: 35561276; PMCID: PMC9348084.
- Pfrepper C, Holstein K, Königs C, Heller C, Krause M, Olivieri M, Bidlingmaier C, Sigl-Kraetzig M, Wendisch J, Halimeh S, Horneff S, Richter H, Wieland I, Klamroth R, Oldenburg J, Tiede A; Hemophilia Board of the German, Austrian, Swiss Society on Thrombosis Hemostasis Research (GTH). Consensus Recommendations for Intramuscular COVID-19 Vaccination in Patients with Hemophilia. Hamostaseologie. 2021 Jun;41(3):190-196. doi: 10.1055/a-1401-2691. Epub 2021 Apr 15. PMID: 33860513.
- Guidance from the World Federation of Hemophilia (WFH), European Association for Haemophilia and Allied Disorders (EAHAD), European Haemophilia Consortium (EHC), and U.S. National Hemophilia Foundation (NHF).
- Caldeira D, Rodrigues BS, Alves M, Pinto FJ, Ferreira JJ. Low risk of haematomas with intramuscular vaccines in anticoagulated patients: a systematic review with meta-analysis. Thromb J. 2022 Feb 16;20(1):9.
- Immunization Action Coalition, www.immunize.org , www.vaccineinformation.org , http://www.immunize.org/catg.d/p3085.pdf .
- Ragni MV, Lusher JM, Koerper MA, Manco-Johnson M, Krouse DS. Safety and immunogenicity of subcutaneous hepatitis A vaccine in children with haemophilia. Haemophilia 2000; 6(2): 98-103.
- Gazengel C, Courouce AM, Torchet MF, Kremp O, Brangier J, Brechot C, Degos F. Use of HBV vaccine in hemophiliacs. Scand J Haematol 1984; 40: 323-8.
- Janco RL. Immunogenicity of subcutaneous hepatitis B vaccine in hemophiliacs. J Pediatr 1985; 107: 316.
- Zanetti AR, AR, Mannucci PM, Tanzi E, Moroni GA, De Paschale M, Morfini M, Carnelli V, Tirindelli MC, De Biasi R, Ciavarella N, et al. Hepatitis B vaccination of 113 hemophiliacs: lower antibody response in anti-LAV/HTLV-III-positive patients. Am J Hematol, 1986; 23: 339- 45.
- Golli T, Kastrin A, Pokorn M, Rener-Primec Z. Immunosuppression and immunization: Vaccination in pediatric patients with neuromuscular diseases treated with steroids or immune-modulating drugs. Eur J Paediatr Neurol. 2021 Nov;35:158-164.

World Hemophilia Day 2024: Theme, History, Symptoms, Causes and Types
W orld Haemophilia Day, observed annually on April 17 by the World Federation of Hemophilia (WHF), aims to raise awareness about the rare genetic bleeding disorder. This global event provides a platform to educate people about haemophilia and other bleeding disorders while also encouraging improved accessibility for those affected. Its primary goals include raising funds to support patients.
Haemophilia can cause continuous bleeding both internally and externally, due to the absence of blood clotting proteins. As of now, while there is no cure for this condition, various treatments are available to manage the condition. As we observe World Haemophilia Day, let’s explore its theme, history, symptoms, causes and types of this disorder.
World Hemophilia Day 2024: Theme
The theme for this year’s World Haemophilia Day is, ‘Equitable access for all: recognising all bleeding disorders.’ The main idea of this theme is to make sure everyone gets treated, no matter what type of bleeding problem they have, how old they are, where they live or their gender.
World Hemophilia Day: History
The World Federation of Hemophilia (WFH) was founded in 1963 by Frank Schnabel, a Canadian businessman who had hemophilia A since birth. The initial WFH Congress took place in Copenhagen, Denmark, on June 25, 1963 and it was attended by representatives from 12 nations. The day was first celebrated on April 17, 1989, as a tribute to Schnabel’s birthday. During the 10th century, people began noticing that more males were dying from minor injuries compared to females.
This condition was known as abulcasis at that time. However, due to limited technology and knowledge, treatment was not available. In 1803, Dr John Conrad Otto from Philadelphia, USA started studying individuals with bleeding disorders and discovered that the disease was passed from mothers to sons.
Hemophilia Symptoms
The symptoms depend on how severe the deficiency is in an individual. Among them is the appearance of large and unexplained bruises. Bleeding in the gums, nosebleeds, pain and swelling in the joints are also common. Additionally, individuals may experience tightness in the joints, notice blood in their urine and stool. Unusual bleeding, even after vaccinations, can be a symptom of hemophilia.
Hemophilia Causes
In our bodies, blood clotting is a complex process involving different proteins known as coagulation factors. These factors play vital roles in clot formation.
When there’s damage to the skin or in the blood vessels, platelets rush to the injured area to form an initial plug, starting the coagulation cascade. This process helps to clot the blood and stop bleeding.
However, in haemophilia, clotting doesn’t happen naturally due to changes or mutations in genes responsible for producing essential proteins.
As a result, individuals with haemophilia may experience bleeding even from minor injuries or cuts.
Hemophilia Types
Hemophilia has four main forms: Hemophilia A, Hemophilia B, Hemophilia C, and Acquired Hemophilia. Each variant has its own abnormalities and causes.

Europe PMC requires Javascript to function effectively.
Either your web browser doesn't support Javascript or it is currently turned off. In the latter case, please turn on Javascript support in your web browser and reload this page.
Search life-sciences literature (43,947,359 articles, preprints and more)
- Free full text
- Citations & impact
- Similar Articles
The Hemophilia Gene Therapy Patient Journey: Questions and Answers for Shared Decision-Making.
Author information, affiliations.
- Negrier C 2
- Driessler F 3
- Goodman C 4
- Skinner MW 5
ORCIDs linked to this article
- Wang M | 0000-0001-9289-4862
- Skinner MW | 0000-0002-0934-0680
Patient Preference and Adherence , 09 Jun 2022 , 16: 1439-1447 https://doi.org/10.2147/ppa.s355627 PMID: 35707346 PMCID: PMC9191577
Abstract
Free full text , the hemophilia gene therapy patient journey: questions and answers for shared decision-making, michael wang.
1 University of Colorado Anschutz Medical Campus, Aurora, CO, USA
Claude Negrier
2 National Reference Center for Haemophilia, Louis Pradel Cardiology Hospital, University of Lyon, Lyon, France
Frank Driessler
3 Bayer, Basel, Switzerland

Clifford Goodman
4 The Lewin Group, Falls Church, VA, USA
Mark W Skinner
5 Institute for Policy Advancement Ltd, Washington, DC, USA
6 McMaster University, Hamilton, ON, Canada
The anticipated emergence of hemophilia gene therapy will present people with hemophilia (PWH) and treating clinicians with increasingly complex treatment options. It will be critical that PWH and their families be empowered to participate fully in decision-making through transparent communication and the development of targeted educational resources.
The Council of Hemophilia Community (CHC) convened across a series of roundtable meetings to define the patient journey for hemophilia gene therapy, and to develop a question-and-answer style resource to guide discussion between healthcare professionals (HCPs) and their patients. Patient groups were also consulted during the development of this tool.
The CHC defined 5 key stages in the hemophilia gene therapy patient journey: pre-gene therapy (information-seeking and decision-making), treatment initiation, short- and long-term post-gene therapy follow-up. PWH will have different questions and concerns at each stage of their journey, which should be discussed with their HCP to aid decision-making. The resulting patient journey infographic and Q&A resource (see Supplementary Materials ) has been developed for HCPs and PWH to provide a novel and practical roadmap of key issues and considerations throughout all stages.
These resources support a collaborative, patient-centric, shared decision-making approach to inform treatment decision discussions between HCPs and PWH. The value of such discussions will be influenced by the language adopted; health literacy is a particularly important consideration, and these discussions should be accessible and tailored to PWH. HCPs and PWH can benefit from awareness of the common questions and uncertainties as they progress together along the patient journey. While the contents of this article are specific to hemophilia gene therapy, the concepts developed here could be adapted to aid patients in other disease states.
- Introduction
Limited literature is available that combines the concepts of the patient journey in hemophilia gene therapy with corresponding life stages. As clinical developments to address the unmet need in hemophilia advance there is also a great need to address the educational and clinical decision-making challenges that will follow market authorization. Herein, we provide a new, interactive, question and answer (Q&A) PDF resource (see Supplementary Materials ) for shared decision-making along the patient journey.
The Unmet Need in Hemophilia
Hemophilia is a rare X-linked recessive genetic disease, with an estimated prevalence of 17.1 and 3.8 cases per 100,000 males worldwide for hemophilia A and B, respectively. 1 The current standard of care for severe hemophilia, in countries where it is available, is prophylaxis with factor or non-factor replacement therapy, which aims to prevent bleeds and greatly improves patient outcomes and quality of life. 2 However, people with hemophilia (PWH) remain at increased risk of bleeds (including joint and post-surgical) as well as hematomas (and ensuing synovitis and joint disease) 3 and life-threatening intracerebral hemorrhage. 4 , 5
As single-gene disorders, hemophilia A and B are promising candidates for gene therapy. Introduction of the missing F8 (hemophilia A) or F9 (hemophilia B) gene aims to deliver a potentially long-term, functionally curative treatment by restoring coagulation factor production to clinically effective levels. Simultaneous goals include ensuring no vector- or transgene-related toxicity and broad patient eligibility across age groups and in patients with inhibitors. 6–8 Gene therapy can therefore offer a new therapeutic option within this treatment landscape, to fit the needs of each individual.
Different gene therapy products are likely to meet varied unmet needs of PWH. While several hemophilia gene therapy clinical trials are in progress, no product had been approved at the time of publication. 8 Among the approaches in development, adeno-associated viral (AAV) gene transfer solutions are the most advanced in clinical trials, though these products have different constructs, serotypes, promoter/enhancer regions, and manufacturing methods that will affect patient eligibility and choice. 8 , 9
Defining the Patient Journey
The gene therapy patient journey extends throughout all life stages for PWH–from birth/diagnosis through to later adulthood–with different checkpoints corresponding to patient life stages. The constantly evolving treatment landscape involves numerous decisions that require guidance and support from healthcare professionals (HCPs). The patient journey for PWH undergoing gene therapy in particular can raise common concerns and experiences, eg, understanding benefits, risks, adverse events, impact on quality of life, and eligibility. 10 Therefore, full transparency and personalized information about the existing knowledge, therapeutic options, and unmet needs are essential to empower PWH to make informed decisions before, during, and after treatment. 7 , 10–12
- Patient Centricity and Shared Decision-Making in Clinical Practice
Current Gaps and Limitations
Patient engagement and personalized care are gaining attention in medication development, validation, and adoption, beyond the more traditional emphases on safety, efficacy, regulatory aspects, and communication with HCPs. PWH are typically instructed about their current and future treatments, rather than engaged in conversations and decision-making on treatment options. 13 Often, healthcare materials are written at a technical level in what may be the patient’s second language. 14 This has made it difficult for them to access, interpret, and apply health information to their decisions, contributing to inadequate health literacy. PWH and families are also exposed to a multitude of information sources via social media and traditional news channels, which often present incomplete, inaccurate, and contradictory content. 15 , 16 Furthermore, in some countries, direct-to-patient marketing may present an additional confounder to patient comprehension, particularly in vulnerable groups. 17 In addition to inadequate patient educational materials, a discordance often exists between the physician’s perception of their patients’ understanding and the patient’s actual comprehension. 14 , 18 This communication failure leads to postponement of treatment decision-making, non-adherence, and poorer health outcomes. 14
Appropriate, individualized communication is necessary to facilitate targeted treatment approaches and disease prevention. 19 This issue extends beyond patient understanding. Particularly, with emerging therapies and novel mechanisms of action, it is inappropriate to assume that HCPs have adequate understanding of gene therapy.
Proposed Solutions
A complementary approach includes patient centricity, defined as engaging with patients to achieve the best experience and outcomes for the individual and their family. 13 The process of shared decision-making can reduce the information and knowledge imbalance, and lead to more personalized treatment decisions, better medical outcomes, treatment adherence, improved quality of life, and decreased costs of treatment and absenteeism. 20–22 The introduction of a new option, such as gene therapy, provides opportunities for shared decision-making to ensure patients understand their best, individualized option. 22 This type of patient-centric decision-making is relevant to clinical practice and is particularly important for AAV gene therapy due to current limitations on re-treatment via that modality. Importantly, shared decision-making is distinct from informed consent, with a primary difference being the timing and extent of patient involvement. 23 Shared decision-making is more than a one-size-fits-all description of the risks and benefits associated with each treatment option in order to meet a legal requirement; patients, and family members where appropriate, should be actively involved in an individually tailored, two-way discussion with their care provider, with patient preferences and concerns at the forefront. 23
- Appropriate Language in Patient Communication
A key element in a patient-centric approach is dialogue, which must be accessible to PWH and their families according to widely diverse levels of health and medical comprehension, as well as personal circumstances. 13 , 18
PWH who are diagnosed and treated, and families, tend to be well informed about their condition and are committed to its management, including keeping abreast of treatment options. 24 However, the evolving treatment landscape poses an array of questions, so it is imperative that PWH have access to health information that they can easily find, understand, and use to inform their decisions.
General recommendations to improve physician–patient communication include: avoiding assumptions of low or high patient health literacy, avoiding jargon and unnecessary detail, implementing the “teach-back” method (reiterating in your own words what was learned) to ensure full patient understanding, and preparing written materials, with only key points and graphics or other visuals where relevant. 18 A recent review identified additional variables relevant to shared decision-making in hemophilia treatment, including bleeding phenotype, musculoskeletal status, treatment adherence, venous access, and lifestyle. 3
When considering health literacy, it is also important to understand the issues facing PWH enrolling in clinical trials, and the clinical and technical terminologies involved. Physicians and the surrounding care team can play an important role in ensuring that patients achieve clinical trial literacy and are fully informed when making decisions about participation. 25 This is also necessary when communicating with patients who have already started their gene therapy journey in clinical practice, 11 who may still have outstanding questions or concerns.
The Q&A resource presented here will help to tailor communications and support a patient-centric shared decision-making process.
The Council of the Hemophilia Community (CHC) convened with the aim of filling an information gap through the development of a resource to support discussions between HCPs and PWH about gene therapy. Here, we summarize the results of focus group discussions conducted by the CHC, which aimed to define the patient journey in hemophilia gene therapy within the different life stages of PWH and create an accompanying Q&A resource for HCPs and PWH. The intention is to support shared decision-making in real-world practice by giving HCPs insight into likely patient questions regarding gene therapy and clinical trial participation.
Resource Development Strategy
The resources described here are based on expert discussions involving HCPs and patients. The CHC was established in 2019 as a multidisciplinary expert group, formed of independent advisors, HCPs, industry leads, and patient representatives with experience in medicine, research, advocacy, patient support, health economics, data, and healthcare access. The CHC collaborate on behalf of PWH to provide guidance for: 1) overcoming clinical and access challenges in the hemophilia environment; 2) perceived gaps in programs and initiatives; and 3) developing initiatives based on emerging insights.
Over the course of 3 roundtable meetings held between November 2020 and May 2021, the CHC first conducted a process of insight identification to understand the elements of the specific patient journey that could best guide the hemophilia community and facilitate treatment decision-making. The life cycle of PWH was aligned to patient journey stages, based on exchanges of opinion within the interdisciplinary working group. The CHC then identified and refined key questions based on those defined in an early patient questionnaire and by the National Hemophilia Foundation (NHF). 26 The selection of these source materials was based on a consensus of expert opinion; no formal literature search was conducted.
Patient questions were then selected and assigned to the patient journey stages and refined by the CHC members during iterative CHC expert workstream discussions using a focus group format. Answers to the questions were refined separately by the authors following multiple rounds of review. The target audience for the developed questions and answers were hematologists and their patients.
Collaboration with Patients
This collaborative development and feedback process engaged all members of the CHC, patient groups, patient representatives, and nurses. In addition, both resources were reviewed by groups of European and US-based patients, caregivers, and parents of PWH (Bayer Global and European Patient Councils for Hemophilia) and the Bayer Hemophilia Employee Council, to seek their insight on the relevance and accessibility of the text, as well as their opinions on the need for any additional questions and answers. All members of the Bayer Global and European Patient Councils groups are PWH or a caregiver of a person with hemophilia. Members of the Bayer Hemophilia Council include Bayer employees with hemophilia. The resultant patient journey roadmap and Q&A resource were shared with the entire CHC for feedback. The authors met regularly to discuss this feedback, and to decide on the content and placement of key questions along the patient journey.
The Patient Journey: Key Stages and Aims
As an initial step in developing the Q&A resource, the CHC undertook defining the patient journey through gene therapy for hemophilia. The CHC has categorized 5 pillars of the patient decision-making journey in the current treatment landscape:
Pre-gene therapy information seeking
Pre-gene therapy decision-making
Treatment initiation
Short-term post-gene therapy follow-up (≤1 year)
Long-term post-gene therapy follow-up (>1 year)
The patient journey as mapped onto these 5 pillars is shown in Figure 1 .

The patient decision-making journey.
Key Themes of the Q&A Resource
The Q&A development process revealed several areas contributing to patient decision-making in hemophilia gene therapy. First, expectations of PWH need to be managed, as the potential benefits and risks of gene therapy are likely to vary among PWH. In addition, the need for a commitment to participating in a long-term registry 21 , 27 should be communicated before gene therapy is initiated. Further explanations of eligibility and effects should be clarified, notably that any gene expression occurs only in somatic cells (not germline cells), hence any effect is not conveyed to future generations. 21 The questions should also cover the current unknowns of treatment, including long-term durability, whether the achieved level of FVIII/FIX is sufficient to effectively prevent bleeds, and treatment options in the event that gene therapy does not achieve its therapeutic goals.
Summary of the Q&A Resource
The CHC agreed that questions from PWH concerning gene therapy tend to fall under the following broad categories: treatment regimen/adherence requirements, treatment predictability and variability, treatment durability, and the risk:benefit profile. The full, collated list of questions and answers is presented in the Supplementary Materials . The resource is organized from initial information-seeking to decision-making, treatment initiation and trial information, and short- and long-term follow-up. The reading level of the document is a grade 10–11 (high school) level, based on a Flesch-Kincaid readability test. It is fully referenced, as appropriate, for further context and can serve as a practical tool for the treating physician in shared decision-making. Information to facilitate discussion regarding participation in clinical trials is also included, from eligibility through follow-up. Some of the questions deal with complex queries, such as explaining what gene therapy is and how it works in the body, as well as efficacy and safety.
Additional online resources to support patients considering gene therapy are summarized at the end of the Supplementary Materials .
Hemophilia Gene Therapy Awareness
The CHC-defined patient journey and Q&A resource reported here are designed to help to inform HCPs regarding the questions and considerations likely to be encountered by PWH at different life stages, and when discussing gene therapy options. Each patient’s journey will encounter the emergence of new therapeutic options, growing evidence and evolving individual preferences, goals, aspirations, and risk tolerance.
By identifying and providing key information to aid these discussions on gene therapy, this tool will help to address knowledge gaps of HCPs and PWH. A recent global survey sent to patient organizations reported that although most (68%) PWH express that they have a basic understanding of gene therapy, only a small proportion (6%) consider themselves to have an advanced understanding. 28 A similar pattern was observed for HCPs, with 44% recording a basic or intermediate understanding, and only 12% reporting an advanced understanding. 28 A different international survey of physicians and scientists echoed these results, with 40% of physicians directly in hemophilia care claiming limited ability to discuss gene therapy with their patients. 29 As gene therapy emerges, it will be critical to meet the knowledge gaps of HCPs and PWH. Educating physicians, nurses, and the surrounding care teams who care for PWH will better enable them to meet the needs of their patients through constructive and informative discussions. 29 Although the majority of PWH report positive opinions of gene therapy, including with respect to such considerations as annual bleeding rates, factor levels, long-term impacts, use of factor replacement, and treatment costs, 30–32 many believe that they will not benefit from new products (eg, gene therapy and new factor/non-factor replacement therapies). 31 , 32 Therefore, HCPs will need to educate PWH about all available treatment options as they engage them in shared decision-making. 31
HCP-Patient Communication on Gene Therapy Advances
The resources will be shareable during in-person or virtual consultations and available for reference afterwards. In order to maximize the impact of this tool, HCPs should become familiar with appropriate language and educational materials to answer these questions. Results from a qualitative study across a variety of stakeholders suggest that consistency in the language and terminology used when discussing hemophilia can reduce miscommunication and facilitate conversations, promoting informed decision-making. In particular, the need for appropriate terminology to describe gene therapy has been previously highlighted and that it is preferable to discuss “what gene therapy is,” rather than what current treatments “are not.” 24 As HCPs will likely become increasingly engaged in the use of gene therapy, their adoption and comfort with the preferred terminology will enhance their communications. The usefulness of the resource provided here should be evaluated by an assessment of publication metrics as well as focus group discussions to understand the uptake of the tool by the hemophilia community.
Lessons on communicating with patients may be learned from past and ongoing miscommunications between the scientific community and the public, 33 eg, the harmful misconceptions around antimicrobial resistance or those that arose from the flawed and ultimately retracted report of an association of the measles, mumps and rubella vaccine with autism development. 34 , 35 Poorly delivered or incorrect information, including in clinical trials, can result in a lack of confidence in treatment, unnecessary costs, and discontinuity of care. Transparency is an essential criterion for gaining trust. 36 In particular, the need for adequate education of HCPs will be vital for facilitating these conversations, alongside the need to adjust the information and method of delivery to particular care contexts.
Strengths and Limitations
The Q&A resource provided here is a novel, readily available educational tool for HCPs and their patients. Its strengths include an easy-to-follow format, ready for use as a comprehensive shared decision-making resource in the clinic. Moreover, the development of the resource was heavily guided by its intended audience, therefore we hope it proves immediately useful.
The key limitation of the Q&A resource is its reading level (grade 10–11; high school), which is higher than the grade 5–8 level (10–14 years old) at which the average patient comfortably comprehends patient educational materials. 37 , 38 However, this resource is intended for use by HCPs to tailor their delivery of the material appropriately to different patients (for example, by omitting or describing content based on patient knowledge and/or age) and thus the reading level is targeted higher to include relevant details. In addition, while the Q&A resource is intended to guide conversations with PWH, the patient journey starts in infancy and hence will also involve discussions with parents or other caregivers of pediatric patients, who may have additional specific questions. While gene therapy is not currently available to children, advice to parents of pediatric PWH should be provided to ensure full awareness of future considerations. 39 The Q&A resource therefore functions as a starting point from which HCPs can individualize discussions. Other concerns, including those of reimbursement and financing, while important, will vary by region and are beyond the scope of this article and Q&A resource. Clinical evaluations to date have been conducted predominantly in high-income countries, 40 and therefore, this communication tool is currently limited to patients in those regions. Finally, despite good levels of engagement across key stakeholder groups, the relatively small size and composition of the CHC group could limit the generalizability of these conclusions to the global hemophilia community.
Future Directions
Ideally, the resource would be updated and published as needed to provide the latest relevant information and address any feedback from the community. This work would benefit from even wider stakeholder engagement including surveying a larger, more diverse group of PWH. It would incorporate findings of clinical trials and other research (such as from registries) that would be of potential relevance to decision-making (eg, probability of expressing FVIII, levels of FVIII expression, duration of expression, probability of side effects) and shared and reviewed with patients as it becomes available. 41–43
Furthermore, while this resource provides an educational and decision support tool to aid discussions between HCPs and patients, barriers to providing requisite biomedical and clinical education about gene therapy for HCPs (eg, time and travel costs) should also be identified and addressed. 29
- Conclusions and Key Takeaways
Gene therapy offers a potential alternative to lifelong infusions, with the promise of transformative changes in the lives of PWH 7 , 44 and a major shift within the treatment paradigm. 8 The emergence of gene therapy will also add to the complexity of treatment decisions faced by PWH and their families. HCPs will have a crucial role in providing the appropriate, personalized guidance and support to ensure that PWH fully understand the options available to them.
The educational and decision support resources described herein recognize that each patient’s decision journey will evolve throughout their lifetime with their individual preferences at different life stages, and with the emergence of new therapies and a growing evidence base.
The Q&A resource provides HCPs and PWH with timely, relevant information, facilitates discussions, and empowers PWH to engage in shared decision-making. As gene therapy products enter the market, the themes and questions mapped here should stimulate discussion and aid interactions among HCPs, PWH, and family members, to ensure that they are fully informed and realize the clinical potential of this treatment. While the issues discussed here pertain to hemophilia, they could also be applied to other hereditary diseases with multiple treatment options.
- Acknowledgments
The authors would like to acknowledge all other members of the Council of the Hemophilia Community for their contributions to this manuscript, particularly in the development of the Q&A resource: Erik Berntorp, Alfonso Iorio, Christoph Königs, Keiji Nogami, Johannes Oldenburg, Brian O’Mahony, Mark Reding, and Dawn Rotellini. This independent group was funded by Bayer. They would also like to thank members of the Bayer Global and European Patient Councils for Hemophilia, as well as Sophie Babicz and Alex Mehuys, and the Bayer Hemophilia Employee Council (particularly Gregory LeCleir) for their insight into the Q&A resource. The authors would like to thank Alexandra Wissle for her support in facilitating the survey. Editorial support (in the form of writing assistance, including development of the initial draft based on author direction, assembling tables and figures, collating authors’ comments, grammatical editing, and referencing) was provided by Ewa Kilinska, of Fishawack Communications Limited, part of Fishawack Health, UK, and was funded by Bayer. The authors would also like to acknowledge Sharon Eastwood, of Bayer, for editorial support during the development of the manuscript.
MW received honoraria for advisory boards from Bayer, BioMarin, Bioverativ, Catalyst Biosciences, CSL Behring, Genentech, Novo Nordisk, and Takeda; CN has received grants/research support/honoraria or consultation fees from Bayer, BioMarin, Catalyst Biosciences, CSL Behring, Freeline, Novo Nordisk, Pfizer, Roche, Sanofi, Sobi, Spark Therapeutics, Takeda, Uniqure; FD is an employee of Bayer; CG is a full-time employee of The Lewin Group, and has been assigned to projects with various life sciences companies, including AbbVie, Alkermes, Amgen, Bayer, BioMarin, bluebird bio, Genentech, GlaxoSmithKline, Janssen, Medtronic, Merck, Mitsubishi Tanabe, and Roche, as well as not-for-profit organizations including Hemophilia Alliance and National Hemophilia Foundation; MS reports grants and personal fees from Bayer, BioMarin, Roche/Genentech, Takeda, Novo Nordisk, and Sanofi; grants from Freeline, UniQure, and Sobi; other from Spark and Pfizer, outside the submitted work; and is a Member of the Institute for Clinical and Economic Review (ICER) Governing Board, Blue Cross Blue Shield Medical Advisory Panel, Consultant for National Hemophilia Foundation and the National Hemophilia Foundation’s Medical and Scientific Advisory Council (MASAC) Member. The authors report no other conflicts of interest in this work.
Full text links
Read article at publisher's site: https://doi.org/10.2147/ppa.s355627
Citations & impact
Impact metrics, citations of article over time, alternative metrics.

Article citations
Barriers to gene therapy, understanding the concerns people with haemophilia have: an exigency sub-study..
Fletcher S , Jenner K , Holland M , Khair K
Orphanet J Rare Dis , 19(1):59, 10 Feb 2024
Cited by: 0 articles | PMID: 38341591 | PMCID: PMC10859013
Infrastructural considerations of implementing gene therapy for hemophilia in the Nordic context.
Astermark J , Baghaei F , Strandberg K , Toplican PG , Birkedal MF , Grahn EE , Hansson C , Kampmann P , Lehtinen AE , Täckström K , Holme PA , Magnusson M
Ther Adv Hematol , 14:20406207231202306, 17 Oct 2023
Cited by: 0 articles | PMID: 37859645 | PMCID: PMC10583513
Gene Therapy in Hemophilia: A Transformational Patient Experience.
Rasul E , Hallock R , Hellmann M , Konduros J , Pembroke L , LeCleir G , Malacan J , von Mackensen S
J Patient Exp , 10:23743735231193573, 31 Aug 2023
Cited by: 1 article | PMID: 37663068 | PMCID: PMC10472832
Gene Therapy for Hemophilia A: A Mixed Methods Study of Patient Preferences and Shared Decision-Making.
Limjoco J , Thornburg CD
Patient Prefer Adherence , 17:1093-1105, 19 Apr 2023
Cited by: 0 articles | PMID: 37102127 | PMCID: PMC10123005
How to translate and implement the current science of gene therapy into haemophilia care?
Hermans C , Gruel Y , Frenzel L , Krumb E
Ther Adv Hematol , 14:20406207221145627, 12 Jan 2023
Cited by: 0 articles | PMID: 36654740 | PMCID: PMC9841832
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Considerations for shared decision management in previously untreated patients with hemophilia A or B.
Astermark J , Blatný J , Königs C , Hermans C , Jiménez-Yuste V , Hart DP
Ther Adv Hematol , 14:20406207231165857, 17 Apr 2023
Cited by: 1 article | PMID: 37113810 | PMCID: PMC10126613
Educational needs of patients, families, and healthcare professionals to support the patient journey in haemophilia gene therapy in the UK.
Boyce S , Fletcher S , Jones A , Kohli R , Mangles S , Ong M , Pollard D , Sivasubramaniyam S , Stephensen D , Stoner N , Kazmi R
Orphanet J Rare Dis , 18(1):366, 25 Nov 2023
Cited by: 0 articles | PMID: 38007560 | PMCID: PMC10676600
The GOAL-Hēm journey: Shared decision making and patient-centred outcomes.
Roberts JC , Richardson S , Miles ME , Stanley J , Chapman CAT , Denne M , Caicedo J , Rockwood K , Recht M
Haemophilia , 28(5):784-795, 21 Jun 2022
Cited by: 3 articles | PMID: 35728103 | PMCID: PMC9546188
A qualitative systematic review of internal and external influences on shared decision-making in all health care settings.
Truglio-Londrigan M , Slyer JT , Singleton JK , Worral P
JBI Libr Syst Rev , 10(58):4633-4646, 01 Jan 2012
Cited by: 38 articles | PMID: 27820528
Overcoming the challenges of treating hemophilia in resource-limited nations: a focus on medication access and adherence.
Ghosh K , Ghosh K
Expert Rev Hematol , 14(8):721-730, 29 Jul 2021
Cited by: 2 articles | PMID: 34278926
Europe PMC is part of the ELIXIR infrastructure
Fresh Call for Public Awareness and Action Against Hemophilia
- East African Congress of Accountants (EACOA) Concludes in Kigali with a Vision for Transformative Accounting Practices
- New Report Reveals Nestle’s Addition of Sugar to Infant Milk Sold in Africa and Elsewhere
- Mosquito Nets Prove Vital in Protecting Boarding Students from Malaria
- How Karongi’s Community Health Workers Tackle Malaria at the Grassroots Level

By Elias Hakizimana.
Members of the Rwanda Fraternity Against Hemophilia have issued a fresh call, urging everyone, especially parents, to become more aware of the deadly hemophilia disease and to seek early medical diagnosis and treatment.
Hemophilia is a disorder characterized by abnormal blood clotting, leading to excessive bleeding, both externally and internally, following any injury or damage.
Amos Nsengiyumva, 28, a hemophilia survivor since birth, shared his journey of coping with the disease.
“I have lived with hemophilia since I was 5 years old. It started with a small tumor on my leg, and my parents were unable to determine the cause. Playing with other children often resulted in immediate bleeding episodes. After a visit to King Faisal Hospital in 2007, I was finally diagnosed with hemophilia through blood tests conducted in France,” Nsengiyumva recounted.
The costs of coagulation factors, medicines that enhance blood clotting, coupled with prolonged absences from school, have been significant challenges for Nsengiyumva.
“A single bottle of medicine cost Rwf1.8 million, making this disease extremely burdensome. I often missed school for weeks due to bleeding episodes,” he stated, emphasizing the potential long-term impact of hemophilia.
In response to the challenges faced by hemophilia survivors, the Rwanda Fraternity Against Hemophilia was established in 2014. This association focuses on raising awareness about the disease, ensuring the availability of medications through external funding, and providing counseling to survivors who often experience traumatic events.
Clémentine Mujawimana, a mother of five, shared her struggle in obtaining medication for her hemophilia-surviving sons.
“It was challenging to diagnose the disease in my firstborn, who experienced frequent bleeding episodes since birth. The lack of awareness and support made the situation even more difficult,” Mujawimana expressed.
Sylvestre Murindabyuma, Acting President of Rwanda Fraternity Against Hemophilia and a father of a hemophilia survivor, highlighted the association’s efforts to increase awareness and advocate for better access to diagnosis and treatment.
“We faced challenges in diagnosing the disease and accessing affordable treatment, but with increased awareness and advocacy, progress has been made,” Murindabyuma stated.
Dr. Evariste Ntaganda from Rwanda Biomedical Center (RBC) emphasized the importance of early diagnosis and treatment of hemophilia. “Thanks to the efforts of organizations like Rwanda Fraternity Against Hemophilia, awareness about hemophilia has increased, leading to better outcomes for patients,” Dr. Ntaganda mentioned.
Currently, Rwanda has 90 to 100 registered hemophilia survivors, with ongoing efforts to identify and support others affected by the disease. RBC continues to train healthcare professionals at various levels to improve hemophilia diagnosis and treatment across the country.
RFH has made remarkable progress in advocating for the hemophilia cause with the support of WFH and Novo Nordisk, particularly on occasions like World Hemophilia Day, alongside other initiatives aimed at enhancing hemophilia care overall.

Elias Hakizimana
Elias Hakizimana, CEO&Founder of The Inspirer Ltd,(www.rwandainspirer.com) is a professional Rwandan Journalist with Bachelor’s Degree in Journalism and Communication, received from University of Rwanda’s College of Arts and Social Sciences (CASS) in 2014. He served various media houses in Rwanda including Rwanda Broadcasting Agency (RBA) in 2013 and became passionate with English Online and Print Media Publications where he exercised his talent as a Freelance News Reporter for The New Times, The Independent, The Rwanda Focus, Panorama and more before he became a Self-Entrepreneur as the CEO and Founder of The Inspirer Limited in early 2017.
You May Also Like

Meet Sibomana Emmanuel: Actor, Translator, Interpreter and Social Media Influencer

“Say No to Stoning of the LGBTQI+ Community in Burundi”: A Call for International Solidarity

GASABO: New Eco-Tourism Training Center launched to boost industry
Leave a reply cancel reply.
Your email address will not be published. Required fields are marked *
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- v.8(2); 2024 Apr
- PMC11023713

Management of Urgent Bleeding in Patients with Hemophilia A: Focus on the Use of Emicizumab
Víctor jiménez-yuste.
1 Hematology Department, La Paz University Hospital-IdiPaz, Universidad Autónoma, Madrid, Spain
María T. Álvarez-Román
Rubén berrueco.
2 Pediatric Hematology Unit, Hospital Sant Joan de Deu, Barcelona, Spain
Santiago Bonanad
3 Hematology Department, Hospital Universitari i Politècnic La Fe, Valencia, Spain
José M. Calvo-Villas
4 Hematology Department, Hospital Universitario Miguel Servet, Zaragoza, Spain
Rebeca González-González
5 Emergency Department, Hospital Universitario Severo Ochoa, Leganés, Madrid, Spain
José R. González Porras
6 Hematology Department, Hospital Clínico Universitario, Salamanca, Spain
Ramiro J. Núñez-Vázquez
7 Hematology Department, Hospital Universitario Virgen del Rocío, Instituto deBiomedicina de Sevilla (IBIS), Sevilla, Spain
Manuel Rodríguez-López
8 Hematology Department, Hospital Álvaro Cunqueiro, Vigo, Spain
Management of patients with hemophilia A (HA) requires the knowledge and experience of specialized health care professionals. However, these patients may need to be attended in emergencies, outside the referral hospital, where health care professionals do not know about hemophilia and/or new innovative treatments.
This study aimed to develop a simple and practical algorithm that could be used in emergency situations by nonspecialized treaters in HA and bleeding with or without factor VIII (FVIII) inhibitors under emicizumab prophylaxis.
A group of experts agreed on a simple algorithm, easy to operate, adapted from previous international guidelines, and based on their clinical experience.
The proposed algorithm starts with identifying the patient, confirming the diagnosis of HA, prophylaxis with emicizumab, and/or use of other treatments. After stabilizing the patient and stratifying the bleeding risk, the patient is managed according to the presence/absence of FVIII inhibitors. Patients without FVIII inhibitors should receive FVIII concentrate. Dose and follow-up depend on bleeding localization and severity. Patients with FVIII inhibitors should preferably receive recombinant activated factor VII as bypass agent. A basic coagulation assay, FVIII assessment, and FVIII inhibitors detection assays are necessary in an emergency. However, these tests should be interpreted with caution and appropriately chosen, as emicizumab may alter the results.
The management of patients with HA is challenging in emergency situations, especially if they are treated with new agents. Nonspecialized in coagulopathies health care professionals have limited understanding of the disease, highlighting the need for an algorithm to assist them in making informed decisions.
Introduction
Hemophilia A (HA), which is caused by congenital factor VIII (FVIII) deficiency, is a well-known coagulation disorder despite its rarity. It affects 1 in 10,000 cases in the population. 1 Due to its low prevalence, expertise is scarce outside the hemophilia care centers. Since their first edition, the World Federation of Hemophilia guidelines have promoted the creation of hemophilia treatment centers that, through multidisciplinary teams, would be able to bring knowledge, experience, and a comprehensive approach to patients with hemophilia. 2
The classic treatment of hemophilia has been based on the use of clotting factor concentrates, first from donor plasma and subsequently produced by genetic engineering to give rise to recombinant factors with a longer half-life, which in the case of HA is FVIII. 2 3 4 However, the development of inhibitory antibodies against FVIII is relatively common. 5 6 Up to 20 to 40% of patients with HA develop inhibitors against FVIII. 2 These inhibitors decrease the efficacy of conventional treatments with factor concentrates, increasing the rate of bleeding episodes and negatively impacting patients' quality of life and life expectancy. To address this situation, different agents are utilized, including bypass agents like activated prothrombin complex concentrates (aPCCs) and recombinant activated factor VII (rFVIIa) or more recently FVIII-mimetic bispecific antibodies (emicizumab). 2 3 4 7
Emicizumab is a chimeric bispecific monoclonal antibody that binds to FIXa and FX, mimicking the cofactor function of FVIII in patients with HA. This drug allows the activation of FX without the need for FVIII intervention, thus initiating the final common pathway that leads to thrombin generation. 8 9 The main benefits of emicizumab are its subcutaneous route of administration; prolonged half-life, which allows maintenance dosing up to every 4 weeks; high efficacy in the prevention of bleeding in HA patients with or without FVIII inhibitors. Data from studies have shown that long-term use of emicizumab prophylaxis resulted in statistically significant and clinically meaningful reductions in bleeding rates and target joint resolution in patients with HA with a favorable safety profile, regardless of age, FVIII inhibitor status, or dosing regimen. 8 10 11 12 13 14 These findings show that emicizumab is recommended for patients of all ages with HA who have FVIII inhibitors for regular prophylaxis of bleeding episodes. It is also recommended for those with severe (FVIII <1%) and moderate (FVIII ≥1 and ≤5%) HA without FVIII inhibitors. 8 Nevertheless, as emicizumab does not normalize completely the coagulation process, full protection from all bleeding episodes is not expected. 15
Under normal conditions, patients with HA go to their referral center for treatment. However, there may be situations in which they come at a time when a specialized hematologist is not available, does not have a referral center nearby, or must be treated at an emergency center with health care personnel without experience with hemophilia treatment. This is even more complicated due to the arrival of new innovative drugs such as emicizumab. This treatment has a different mechanism of action, its management differs from the other treatments used in hemophilia, and modifies the results of basic laboratory tests. Although there are international protocols and guidelines widely used by specialized health care professionals, 16 17 18 19 20 they do not solve the problem of managing these patients in emergency situations in which specialized personnel are not always available.
Therefore, with the aim of adapting these guidelines and recommendations to the reality of patients with HA with or without FVIII inhibitors under prophylaxis with emicizumab in emergency situations, after a review of the most recent literature on the topic and several discussion meetings, a group of experts in hemophilia agreed on a simple and practical algorithm adapted from previous international guidelines and based on their clinical experience that can be used in emergency situations by nonspecialized personnel.
Basic Recommendations for the Treatment of a Patient with Hemophilia A in the Event of a Bleeding Emergency
Patients with hemophilia, apart from being trained in self-management, are instructed to recognize bleeding and its management at home, especially joint and muscle bleeding. In addition, it is also important that family members and caregivers recognize the subtle signs of bleeding in young children with hemophilia.
In general, the main treatment for bleeding is with concentrates of FVIII as prescribed by health care professionals. Local measures should be considered in the case of mild bleeding, that is, compression, ice, and topical hemostatic agents, including tranexamic acid; moreover, systemic administration of tranexamic acid should be added. In people with HA and FVIII inhibitors, it would be necessary to treat bleeding with a bypass agent according to the hematologist's indications. Despite these basic recommendations, patients often need to be treated in an emergency department.
General Recommendations for Management of a Patient with Hemophilia in Emergency Situations
If someone with hemophilia seeks medical attention in an emergency situation, it is important that they are evaluated promptly. Even seemingly minor complications can worsen if they are made to wait for too long. 2 These patients require stabilization or treatment similar to other subjects in an emergency situation. 21 However, there are some precautions to be taken into account. For instance, it is recommended that intravenous access be performed by experienced personnel, as different attempts may result in hematoma formation. Unless it is necessary to infuse large volumes of fluid or blood, it is preferable to opt for small caliber catheters. 21
Emicizumab is a drug indicated for the prophylactic treatment of HA that is not useful for treating acute bleeds. Patients receiving emicizumab who experience breakthrough bleeding should be treated based on whether they have FVIII inhibitors or not. In the absence of inhibitor, they are treated with FVIII concentrates at doses considered necessary to achieve hemostasis. In the presence of inhibitor, a bypass agent should preferably be used (see details in the next section of the algorithm). 16 17 18 19 21
Algorithm for the Management of Patients with Hemophilia A in Emergency Situations
The algorithm starts when a patient arrives to the emergency department with a bleeding, a potential bleeding problem, or requiring an invasive procedure. According to the recommendations of the emergency departments, the first step is to identify and stabilize the patient in case of acute bleeding. It is recommended, in the first instance, to request the laboratory tests that will guide future decisions: a complete blood count, a basic coagulation study, and the measurements of FVIII levels and its inhibitor from each center, based on their respective capabilities.
Phase I: Patient Identification
The identification of the patient is the first step of the proposed algorithm and a key step because it allows guiding the health professional(s) in one direction or another, saving time in urgent situations. If patients are outside their referral hospital and electronic medical records are not available, patients are advised to carry, for example, an identification card or a small report. The experts' recommendation about the minimum data that the report should contain are the diagnosis of HA, severity (mild, moderate, or severe), the presence or absence of FVIII inhibitors, whether the patient is on treatment with emicizumab, what treatment has been prescribed in case of acute bleeding (FVIII or rFVIIa), and the contact details of the referral center. In those with FVIII inhibitors, the type of anamnestic response (high/low responder) and the last available inhibitor titer should be cited, although the implications rely on the treatment prescribed for acute bleeding. This information would be very useful in emergency situations so that the patient can be attended as quickly as possible.
Nevertheless, for the correct identification of the patient, it is recommended to contact the patient's referral center, but in case this is not possible, or there is not enough time, it is suggested to follow the algorithm shown in Fig. 1 . The present document only addresses the situation in which the patient has congenital HA.

Identification of patients with hemophilia A. aPCC, activated prothrombin complex concentrate; aPTT, activated partial thromboplastin time; FVIII, coagulation factor VIII; HA, hemophilia A; rFVIIa, recombinant activated factor VII.
After verifying that the patient has HA, it is necessary to know if the patient is being treated with emicizumab. If yes, check whether the patient has FVIII inhibitors. If treatment for hemophilia is unknown, while obtaining a response from the referral center, it is recommended to rely on laboratory tests, such as activated partial thromboplastin time (aPTT). It is important to note that false normalization of aPTT may be obtained in patients treated with emicizumab.
Depending on whether the patient has FVIII inhibitors or not, different recommendations will be followed according to the treatment algorithm for bleeding in HA (see “Phase III: Treatment for Bleeding” section for details). If the presence of FVIII inhibitors is unknown, either because the patient is unconscious or in an urgent and severe situation, the patient is treated as if the inhibitors are present by administering rFVIIa (with a half-life of 2 h) while identifying the presence of the inhibitors in order to continue treatment.
Phase II: Stabilization of the Patient
Patients with hemophilia may present to the emergency room for any cause unrelated to their disease. Therefore, it is always recommended to perform all appropriate instrumental (i.e., ultrasonography) and laboratory evaluations to confirm or reasonably exclude the presence of bleeding and to assess its severity. In addition to the location or type of bleeding, it is important to consider the hemodynamic status of the bleeding patient. Recommendations for different kind of bleedings, polytrauma, or potential bleeding are described below.
If the bleeding is mild and the patient is stable, clinical follow-up should be assessed, waiting for evaluation by the hematologist before administering a concomitant dose of treatment in the emergency room. If clinically indicated, local hemostatic approaches (e.g., compression, ice) or antifibrinolytic agents may be used in milder situations ( Fig. 2 ). Treatment of bleeding is described in section “Phase III: Treatment for Bleeding.”

Stabilization and treatment of the patient. *Hemodynamic instability defined by shock index (heart rate/systolic blood pressure) >1. **Locations requiring rapid action: head and neck bleeding, compartment syndrome, central nervous system, gastrointestinal, major surgery, and major trauma, among others. aPCC, activated prothrombin complex concentrate; FVIII, coagulation factor VIII; rFVIIa, recombinant activated factor VII.
If the bleeding is severe or critical, with unstable bleeding (defined by shock index [heart rate/systolic blood pressure] >1) or in a location that requires immediate treatment (head and neck, compartment syndrome, central nervous system, gastrointestinal, major surgery and major trauma, among others), intensive treatment will be performed without waiting for instructions from the referral center or Hematology department ( Fig. 2 ; see section “Phase III: Treatment for Bleeding” for details).
It is important to keep in mind the golden rule: when in doubt whether it is a hemorrhage, for patients with HA without FVIII inhibitors it is recommended to treat with 50 IU/kg of FVIII concentrate. For patients with HA with FVIII inhibitors, an rFVIIa bypass agent should be preferred (given the concomitant use of emicizumab in large proportion of cases) or aPCC. This rule should be applied, above all, in situations that do not admit of delay (severe and/or life-threatening bleeding, before waiting for possible complementary tests).
Phase III: Treatment for Bleeding
Once the patient has been stabilized (has stopped bleeding), treatment is performed according to the bleeding risk (mild, moderate, or severe) and the presence/absence of FVIII inhibitors ( Fig. 3 ). The following recommendations are valid both for patients taking emicizumab and those not taking emicizumab.

Bleeding treatment of patients with hemophilia A. aPCC, activated prothrombin complex concentrate; FVIII, coagulation factor VIII; rFVIIa, recombinant activated factor VII; TMA, thrombotic microangiopathy.
Patients without Factor VIII Inhibitors
Although prophylactic treatment with emicizumab provides stable hemostasis, in the event of bleeding, and/or some procedures such as major surgeries, FVIII replacement therapy should be used at a dose appropriate to the bleeding episode. In any case, in a bleeding event, the emicizumab prophylaxis regimen should be maintained.
In the case of mild muscle or joint bleeding or gross hematuria, the use of 30 IU/kg of FVIII concentrate is recommended, with a reevaluation at 12/24 hours. 17 To avoid a renal colic related to blood clots in the ureters in patients with gross hematuria, before the administration of FVIII concentrate it is recommended to give intravenous fluids until the color of the urine changes to a pinker shade. 2 22
If there has been moderate trauma, severe muscle bleeding, minor surgery, or an endoscopic procedure has been performed, 50 IU/kg of FVIII concentrate is recommended. Dose reduction of FVIII concentrate is conceivable for minor surgical procedures but not in children, who often show less favorable pharmacokinetics. Dental extractions and skin or other biopsies where adjuvant local hemostasis is possible would be excluded.
Finally, in the case of severe hemorrhages, such as those caused by bleeding in the head and neck, compartment syndrome, central nervous system, gastrointestinal, major surgery and major trauma, it is recommended to administer 50 IU/kg of FVIII concentrate with continuous monitoring of the patient. 17
If there is no risk to the patient and the bleeding has stopped, the patient may be discharged, but should be reevaluated by the Hematology Service in 12 to 24 hours.
Patient with Factor VIII Inhibitors
Prophylactic treatment with emicizumab provides stable hemostasis, but in case of bleeding, a bypass agent (rFVIIa) should be used. If rFVIIa is not available, aPCC could be used. If severe bleeding is not controlled with concentrates of clotting factors, it is recommended to rule out the presence of FVIII inhibitors. The FVIII inhibitors test should be performed with the chromogenic assay and the use of bovine reagents (to determine endogenous FVIII levels) or with human reagents (to measure the activity of endogenous FVIII and emicizumab; see section “Factor VIII Assessment”).
The use of rFVIIa is the preferred option. Depending on the bleeding episode, it would be used intensively at a dose of 90 μg/kg, monitoring the patient according to the severity of bleeding: every 6/8 hours for mild/moderate bleeding or continuously for severe bleeding. According to expert opinion, lower doses of bypass agent may be sufficient to control the bleeding episode. If necessary, rFVIIa administration can be repeated after 2 to 4 hours.
If rFVIIa is not available or an efficient response to this bypass agent is not achieved, aPCC would be used at a dose <50 IU/kg (not exceeding 100 IU/kg in the first 24 h), monitoring the risk of thrombotic microangiopathy (TMA) and thromboembolism until resolution of bleeding. It is important to note that serious and potentially life-threatening adverse effects have been observed using concomitant aPCC and emicizumab, such as TMA and thromboembolism, so both events should be closely monitored. 11
In patients with FVIII inhibitor at low titer, in the case of severe bleeding or failure of rFVIIa, the possible use of FVIII concentrates should be considered, although such indication and laboratory monitoring are up to specialized centers.
In both situations, if there is no risk to the patient and the bleeding has stopped (mild or moderate), the patient can be discharged, with reevaluation at 12 to 24 hours by the Hematology Service.
Laboratory Tests and Precautions to be Considered if Emicizumab is Used
The main laboratory tests that can be performed in patients with HA and acute hemorrhage are the basic coagulation test, a complete blood count including platelet count, FVIII assessment, FVIII inhibitors detection, and other global hemostasis techniques (thrombin generation test and viscoelastometric techniques such as thromboelastography and rotational thromboelastometry). The first two tests are necessary in an emergency. 16 17 18 19 In practice, baseline FVIII determination is not performed always in a well-identified patient. On the contrary, the subsequent follow-up if the patient has to receive treatment should include FVIII measurement. On the other hand, quantifying an inhibitor in an emergency situation may be ambitious, although this should be aimed at, especially, in patients with unknown inhibitor “status.” Fig. 4 shows laboratory tests and precautions to be taken into account if emicizumab is used.

Laboratory tests and precautions to be taken into account if emicizumab is used. FVIII, coagulation factor VIII; HA, hemophilia A; PTT, partial thromboplastin time.
Basic Coagulation Assay
The basic coagulation test would include prothrombin time test, partial thromboplastin time, and fibrinogen test (preferably fibrinogen Clauss assay if derived fibrinogen <150 mg/dL). 8 23 In patients with HA treated with emicizumab it is important to consider several aspects. On one hand, patients with HA have prolonged clotting times. On the other hand, emicizumab alters intrinsic pathway clotting tests. This is because it restores the missing tenase cofactor activity of activated FVIII, resulting in markedly reduced clotting times. This is the case for aPTT, which falsely normalizes in the presence of emicizumab. 24 25 26
Factor VIII Assessment
For FVIII assessment the recommendation is to use, if available, both the one-stage coagulometric method and the chromogenic method with bovine reagents. 27 However, it is important to differentiate the assessment of FVIII in patients with or without FVIII inhibitors.
If FVIII inhibitors are not present, the assessment of FVIII might be useful to diagnose the disease. In the absence of prophylactic treatment it reveals the patient's baseline FVIII level. If the patient is receiving replacement therapy with FVIII concentrate, FVIII assessment allows monitoring of FVIII levels. However, in the presence of emicizumab, two considerations must be taken into account. On one hand, to determine endogenous FVIII levels it is necessary to use chromogenic methods with bovine reagents. On the other hand, to measure the activity of endogenous FVIII and emicizumab, chromogenic methods with human reagents must be used. In the case of patients with FVIII inhibitors, FVIII activity is undetectable or close to zero. 24 25 26
Detection of Factor VIII Inhibitors
After exposure to FVIII concentrate, HA patients could develop alloantibody-type inhibitors. These inhibitors are usually uncorrected or partially corrected in the immediate mixing test; they are time- and temperature-dependent, and require incubation at 37°C for 2 hours. To confirm their presence and quantify them, the Bethesda test with the Nijmegen modification should be performed. 28 In the presence of emicizumab, it is necessary to use the Bethesda test (chromogenic method with bovine reagents) to know if there is an inhibitor and its titer. 24 25 26
The management of patients with HA is a challenge in emergency situations. The lack of knowledge about the management of the disease by nonspecialized health care personnel makes it necessary to develop an algorithm to help them make the most appropriate decisions, considering factors such as the presence or absence of FVIII inhibitors, or treatment with emicizumab, which can affect the results of many of the coagulation tests routinely performed on these patients.
In addition to the algorithm, and to facilitate patient identification in situations requiring immediate assistance, it would be very useful for patients with HA to have an identification card, either physical or digital, on which all the patient's diagnostic and therapeutic information is recorded. In order to disseminate or implement the present algorithm in clinical practice, it would be very useful to present it at congresses and in emergency medicine journals, to obtain the support of the related scientific societies, to hold local meetings with the experts involved, and to create material in the form of posters or infographics.
Moreover, in order to optimize the whole process, coordination between the different levels of care and, within the hospital, between the different specialists involved in the management of these patients would be necessary.
Acknowledgments
We wish to thank Content Ed Net (Madrid, Spain) and Fernando Sánchez Barbero, PhD, for their support on the preparation of this manuscript. In addition, we would like to thank the Spanish Society of Emergency Medicine, the Spanish Society of Thrombosis and Hemostasis, and the Spanish Society of Hematology for their endorsement of this document.
Funding Statement
Funding This work has been sponsored by ROCHE.
Conflict of Interest V.J-Y. has received reimbursement for attending symposia/congresses and/or honoraria for speaking and/or honoraria for consulting, and/or funds for research from Takeda, Bayer, BioMarin, CSL-Behring, Grifols, Novo Nordisk, Sobi, Roche, Octapharma, and Pfizer.
M.T.Á-R. has received reimbursement for attending symposia/congresses and/or honoraria for speaking and/or honoraria for consulting, and/or funds for research from Takeda, Bayer, CSL-Behring, Novo Nordisk, Sobi, Roche, Grifols, Novartis, Amgen, and Pfizer.
R.B.M. has received reimbursement for attending symposia/congresses and/or honoraria for speaking and/or honoraria for consulting, and/or funds for research from Takeda, Bayer, CSL-Behring, Novo Nordisk, Sobi, Roche, Boehringer Ingelheim, and Pfizer.
S.B. has received reimbursement for attending symposia/congresses and/or honoraria for speaking and/or honoraria for consulting, and/or funds for research from Takeda, Bayer, CSL-Behring, Novo Nordisk, SOBI, Roche, Biomarin, and Pfizer.
J.M.C.V. has received reimbursement for attending symposia/congresses and/or honoraria for speaking and/or honoraria for consulting, and/or funds for research from Takeda, Bayer, CSL-Behring, Novo Nordisk, Sobi, Roche, and Pfizer.
R.G.G. has received personal fees from Novo Nordisk.
J.R.G.P. has received reimbursement for attending symposia/congresses from Roche, Amgem, Novo Nordisk, and Pfizer, and funds for consulting from Roche.
R.J.N.V. has received reimbursement for attending symposia/congresses and/or honoraria for speaking and/or honoraria for consulting, and/or funds for research from Takeda, Octapharma, Bayer, CSL-Behring, Novo Nordisk, Grifols, Sobi, Roche, and Pfizer.
M.R.L. has received reimbursement for attending symposia/congresses and/or honoraria for speaking and/or honoraria for consulting, and/or funds for research from Takeda, Octapharma, CSL-Behring, Novo Nordisk, Sobi, Roche, and Pfizer.
Patient Consent
Not applicable.

IMAGES
VIDEO
COMMENTS
The CHC defined 5 key stages in the hemophilia gene therapy patient journey: pre-gene therapy (information-seeking and decision-making), treatment initiation, short- and long-term post-gene therapy follow-up. PWH will have different questions and concerns at each stage of their journey, which should be discussed with their HCP to aid decision ...
The Patient Journey: Key Stages and Aims. As an initial step in developing the Q&A resource, the CHC undertook defining the patient journey through gene therapy for hemophilia. The CHC has categorized 5 pillars of the patient decision-making journey in the current treatment landscape: Pre-gene therapy information seeking
Unmet needs throughout the patient journey, potential next steps, and educational requirements for PWH. A Current unmet needs throughout the gene therapy for haemophilia journey and potential starting points regarding education, psychological support, and guidance. Time periods are indicated by a light grey background; unmet needs are applicable to all time periods across which they span.
Patient groups were also consulted during the development of this tool. Results: The CHC defined 5 key stages in the hemophilia gene therapy patient journey: pre-gene therapy (information-seeking and decision-making), treatment initiation, short- and long-term post-gene therapy follow-up. PWH will have different questions and concerns at each ...
Currently, only one hemophilia A GT product (valoctocogene roxaparvovec) is available, marketed since 2022 in the European Union. 11 The GT patient journey has recently been described based on roundtable meetings between patients and healthcare professionals (HCPs) and can be broadly split into 5 stages: (1) pre-GT information seeking, (2 ...
Hemophilia. Hemophilia is a genetic disease that prevents blood from clotting properly leading to prolonged internal and external bleeding. Learn how gene therapy works to slow or stop disease progression by instructing cells to produce the missing clotting factor, along with information on approved therapies and clinical trials. HEMGENIX is an ...
The resulting patient journey infographic and Q&A resource has been developed for HCPs and PWH to provide a novel and practical roadmap of key issues and considerations throughout all stages of the hemophilia gene therapy patient journey. Purpose The anticipated emergence of hemophilia gene therapy will present people with hemophilia (PWH) and treating clinicians with increasingly complex ...
Patient groups were also consulted during the development of this tool.Results: The CHC defined 5 key stages in the hemophilia gene therapy patient journey: pre-gene therapy (information-seeking and decision-making), treatment initiation, short- and long-term post-gene therapy follow-up. PWH will have different questions and concerns at each ...
The patient journey in haemophilia gene therapy is complex and multifaceted, presenting challenges to the hub and spoke model of care. Understanding these from the patient viewpoint will help ensure people with haemophilia receive the information, guidance and support they need throughout their gene therapy journey.
Hemophilia A. Hemophilia A is one of three types of hemophilia, a rare, inherited blood disorder. It happens when your blood doesn't clot as it should, which is when bleeding stops or slows down. Hemophilia A usually affects men and people assigned male at birth (AMAB), but it may also affect women and people assigned female at birth (AFAB).
Hemophilia A, also called factor VIII (8) deficiency or classic hemophilia, is a genetic disorder caused by missing or defective factor VIII (FVIII), a clotting protein. Although it is passed down from parents to children, about 1/3 of cases found have no previous family history. According to the US Centers for Disease Control and Prevention ...
Each of the questions were subsequently assigned to the five stages of the patient "decision making journey.". These included 1) Pre-gene therapy information seeking 2) Pre-gene therapy decision making 3) Treat initiation 4) Short-term post-gene therapy follow up (less than one year since receiving gene therapy) 5) Long-term gene therapy ...
Aradom E, Gomez K. The patient gene therapy journey: findings from qualitative interviews with trial participants at one UK Haemophilia centre. ... Crombez E, Hay CRM. 101HEMB01 is a phase 1/2 open-label, single ascending dose-finding trial of DTX101 AAVrh10FIX in patients with moderate/severe hemophilia B that demonstrated meaningful but ...
Hemophilia, which means love (philia) of blood (hemo), manifests with prolonged and excessive bleeding either spontaneously or after insignificant trauma. Hemophilia encompasses a group of inherited ailments that alter the body's normal blood coagulation. A hereditary hemorrhagic disorder resulting from a congenital deficit or scarcity of factor VIII, hemophilia A, which is known as classical ...
Living with hemophilia A involves adapting your life to reduce injury risks, making time for treatment, and finding safe activities you can enjoy. Menu. Health A-Z COVID-19; ... Coping strategies in young and adult haemophilia patients: A tool for the adaptation to the disease. Haemophilia. 2019 May;25(3):392-397. doi:10.1111/hae.13743 ...
Herein, we provide a new, interactive, question and answer (Q&A) PDF resource (see Supplementary Materials) for. shared decision-making along the patient journey. The Unmet Need in Hemophilia ...
Bhavya S Doshi. In acquired hemophilia A (AHA), autoantibodies to coagulation factor VIII (FVIII) neutralize FVIII activity leading to a potentially severe bleeding diathesis that carries a high rate of morbidity and mortality. This disorder is rare and occurs mainly in adults over 60 years of age or in the postpartum period.
Patient journey and unmet needs. A total of 26 studies (19 papers and 7 thesis) addressed the concept "Patient journey and unmet needs" (Table 3 and Appendix IV). Six studies investigated the adherence to the available therapeutic alternatives, for which a range of 25.4 % to 72.5 % of the PwH were considered adherent to the treatment.
Educational needs of patients, families, and healthcare professionals to support the patient journey in haemophilia gene therapy in the UK Orphanet J Rare Dis . 2023 Nov 25;18(1):366. doi: 10.1186/s13023-023-02977-y.
From the start of this journey, it was apparent that the development of successful gene therapy strategies for the 2 forms of hemophilia would face different challenges. ... This treatment-related complication occurring in ~30% of severe hemophilia A and ~3% of severe hemophilia B patients is a major challenge to the effective hemostatic ...
The most common side effects of Hemlibra include: injection site reactions (redness, tenderness, warmth, or itching at the site of injection), headache, and joint pain. These are not all of the ...
Now five years old, Kevin is now living a healthy, active life of riding his bicycle and playing soccer. "Those things could have been a deadly risk for him a decade ago," Jain said. World Hemophilia Day is commemorated annually each April 17 to honor of the birthday of Canadian Businessman Frank Schnabel, who founded the Montreal-based ...
If the patient is receiving prophylaxis treatment for hemophilia, vaccination may be administered within 24 hours of Standard or Extended half-life FVIII or Standard half-life FIX concentrate and within 48 hours of administration of Extended half-life FIX concentrate, to decrease the risk of developing a hematoma. 3-4 For patients with a basal ...
World Haemophilia Day, observed annually on April 17 by the World Federation of Hemophilia (WHF), aims to raise awareness about the rare genetic bleeding disorder. This global event provides a ...
All members of the Bayer Global and European Patient Councils groups are PWH or a caregiver of a person with hemophilia. Members of the Bayer Hemophilia Council include Bayer employees with hemophilia. The resultant patient journey roadmap and Q&A resource were shared with the entire CHC for feedback.
Introduction. Hemophilia A and B are congenital bleeding disorders characterized by abnormalities in coagulation factors (F) VIII and IX, resulting in impaired coagulation factor production and a heightened propensity for bleeding. 1, 2 Patients with severe phenotypes produce less than 1% (1 IU/dL) of FVIII or FIX activity, experiencing recurrent musculoskeletal, soft tissue, and intracranial ...
Sunyani, April 16, GNA - The Komfo Anokye Teaching Hospital (KATH) has diagnosed more than 500 patients with Hemophilia, Dr Yaa Gyamfua Oppong Mensah, Specialist Pediatrician, Department of Child Health, KATH has said. Hemophilia is a bleeding disorder where an individual's blood does not clot properly due to lack of sufficient blood ...
RESULTS: A total of 40 publications, including 17 full-text papers, 21 abstracts, and 2 Institute for Clinical and Economic Review reports, met eligibility criteria. Total annual health care costs per patient ranged from $213,874 to $869,940 and are mainly driven by the cost and intensity of prophylaxis with FVIII replacement concentrates ...
Hemophilia is a disorder characterized by abnormal blood clotting, leading to excessive bleeding, both externally and internally, following any injury or damage. Amos Nsengiyumva, 28, a hemophilia survivor since birth, shared his journey of coping with the disease. "I have lived with hemophilia since I was 5 years old.
Algorithm for the Management of Patients with Hemophilia A in Emergency Situations. The algorithm starts when a patient arrives to the emergency department with a bleeding, a potential bleeding problem, or requiring an invasive procedure. According to the recommendations of the emergency departments, the first step is to identify and stabilize ...