Sperm Whale Migration
Female sperm whales ( Physeter macrocephalus ) travel in groups with their young, circling the oceans to find food, they may travel a million miles in a lifetime. Inhabiting warmer waters than the males who thrive in the Arctic, they must meet up in the middle near the Azores.
Biology, Geography

Media Credits
The audio, illustrations, photos, and videos are credited beneath the media asset, except for promotional images, which generally link to another page that contains the media credit. The Rights Holder for media is the person or group credited.
Last Updated
January 11, 2024
User Permissions
For information on user permissions, please read our Terms of Service. If you have questions about how to cite anything on our website in your project or classroom presentation, please contact your teacher. They will best know the preferred format. When you reach out to them, you will need the page title, URL, and the date you accessed the resource.
If a media asset is downloadable, a download button appears in the corner of the media viewer. If no button appears, you cannot download or save the media.
Text on this page is printable and can be used according to our Terms of Service .
Interactives
Any interactives on this page can only be played while you are visiting our website. You cannot download interactives.
Related Resources
Your Impact Will Go Twice as Far for a Pup
Do you notice all those folds of skin? They tell a heartbreaking story of a pup far too thin.
Right now, there’s a $5,000 gift match waiting for the pups , and your support is needed to unlock it.
Yes, your $5 = $10 today, meaning you can provide double the fish meals for a pup like Horseshoe.
- Learn About Marine Mammals /
- Cetaceans /
Sperm Whale
Physeter macroephalus
Learn More About Sperm Whales
Sperm whales are named after the waxy substance, spermaceti, found in their heads. These whales are the largest of the toothed whales. Males can reach 60 feet in length and smaller females reach 37 feet.
Sperm whales are dark gray in color, have a hump rather than a dorsal fin and have triangular shaped flukes, or tails. Just behind their large head (which is about one-third of their total body length!) their skin is wrinkled to increase surface area, leading to greater heat loss. This gives them a shriveled look.
These whales have a single, S-shaped blowhole located on the left side of the top of their head. This unique blowhole produces a distinctive angled spout, or blow. Males have about 40-50 teeth, located only in their narrow lower jaw and females have even less.
What do they sound like?
Your browser doesn't support HTML5 audio. Download the audio file.
The greatest threats to marine mammals are caused by people, but we can also be their greatest champions.
Sign up for email from the marine mammal center to stay updated on how you can be an advocate and champion for marine mammals like sperm whales..
Yes! I want to be a champion for marine mammals!
Habitat & Population Status
Sperm whales can be found around the world but typically stay away from the extremely cold waters near the polar ice in the north and the south. Females usually remain in temperate and tropical waters within 45-55° latitude, whereas males travel in temperate waters. In California, sperm whales can be seen in waters off the continental slope from November to April.
Sperm whales prefer deep water around ocean trenches, where strong currents flow in opposite directions that bring concentrated nutrients to the area thus attracting a large number of creatures that the sperm whales can eat.
Sperm whales were heavily hunted from 1800 to 1987 for spermaceti, the substance found only in a sperm whale’s head and used in echolocation. They were also hunted for ambergris, a waxy substance used in making perfume, that forms around squid beaks in the whale’s digestive system.
Today sperm whales are classified as endangered. The species is slowly recovering from decades of hunting. It’s estimated that the population is about 850,000 individuals worldwide.
Breeding & Behavior
Mating occurs in spring and summer, and females carry their young for 14 to 16 months, giving birth every three to five years. Newborn calves are 13 feet long and weigh about a ton. These calves nurse for two years but may continue nursing with their mother intermittently for up to eight years.
Female and young male sperm whales are social with each other and are sometimes are seen in pods, or groups, of up to 50 whales. The females form matriarchal, or female-led, pods. The young males will often leave these groups to form bachelor herds until they are able to compete in mating at about 20 years old. Aside from the breeding season, adult males lead a solitary life.
Sperm whales are champion divers and are thought to dive to depths greater than 3,000 feet and can stay underwater for up to two hours. They impressively get to these depths in a matter of minutes.
Squid is a sperm whale’s favorite food and it’s suspected that they spend a lot of their dive time hunting for prey using echolocation. Sperm whales also produce a series of clicks called codas. Each whale has a distinctive coda and scientists think that sperm whales recognize each other by these clicks. There is also evidence that sperm whales produce intense bursts of sound to stun their prey.
The sperm whale is a species known for stranding in large groups. It is not known why they strand in this manner, but some theories include mass illness, parasitic infection, following a sick leader and echolocation malfunctions due to gently sloping beaches and underwater magnetic anomalies leading to disorientation.
Learn About Another Animal
Pacific harbor seal, northern elephant seal, california sea lion, steller sea lion, northern fur seal, guadalupe fur seal, hawaiian monk seal, common bottlenose dolphin, humpback whale, pacific white-sided dolphin.

Sperm Whale
Physeter macrocephalus [=catodon].
- Species Status Native Imperiled
- View All Species
Listing Status
- Federal Status: Endangered
- FL Status: Federally-designated Endangered
- FNAI Ranks: Not ranked
- IUCN Status: VU (Vulnerable)

Sperm whales are the largest member of the toothed whale Family, Odontoceti. This species is sexually dimorphic by size and weight. Females can reach a length of 36 feet (11 meters) and a weight of 15 tons (13,607 kilograms), while males grow up to 52 feet (15.9 meters) and 45 tons (40,823 kilograms). Sperm whales can be distinguished from other whale species by their enormous head, which can take up to 35% of their body. Their brain is the largest of any animal. Sperm whales have 20-26 cone-shaped teeth on each side of the lower jaw; however, their teeth are not needed for feeding. Their blowhole is located on the left side of the head. Sperm whales uniquely shoot water forward from their blowholes, which is unlike other whales that shoot water straight up. Sperm whales are mainly dark grey with a white-colored interior mouth, triangle-shaped fluke (tail), and thick rounded pectoral fins (National Oceanic & Atmospheric Administration, n.d.).
The diet of the sperm whale primarily consists of large squids and fish including sharks (National Oceanic & Atmospheric Administration, n.d.).
Sperm whales are polygamous breeder – they breed with more than one partner. Breeding season peaks in the spring in both the Southern and Northern Hemisphere, and calves are born during the fall. During the breeding season, males join groups of females temporarily. The males become very aggressive towards each other when looking for a female to mate with. Sperm whales reach sexual maturity at a slow rate. Females reach sexual maturity at eight to eleven years old. Males do not begin mating until around 25-27 years old because they are not experienced enough. The gestation period for sperm whales is 14-16 months with the female giving birth to one calf every two to five years (NMFS 2010, Ballenger 2003). Adult female sperm whales and subadults form cohesive ‘social units’ (pods) that can remain together over a number of years. Adult male sperm whales typically travel in bachelor groups or alone.

Sperm whales can be found in all major oceans on Earth in waters 600 feet (182.9 meters) over the continental slope. (National Oceanic & Atmospheric Administration, n.d.). Females and subadults inhabit tropical and temperate waters. Adult males live in high-latitude regions and travel to lower latitudes in search of females for mating.
Historically, sperm whales have faced catastrophic population declines due to harvesting. From 1800 to 1987, humans captured and harvested around one million sperm whales (National Oceanic & Atmospheric Administration, n.d.). In 1988, the International Whaling Commission put a halt on all whaling; however, poaching still continues today. Poaching caused an extreme variation in the ratio of females to males, which affected reproduction of the species (American Cetacean Society, n.d.). Some countries (i.e. Japan) still harvest whales for research purposes. Sperm whales are also threatened by boat and ship hits. Human made noises, such as from oil drilling, can disturb a population’s ability to communicate due to the noise interfering with their vocalizations. Other threats include pollution from PCBs (polycholorobiphenyls), and PAHs (polycyclic aromatic hydrocarbons) (National Oceanic & Atmospheric Administration, n.d.).
Conservation and Management
The sperm whale is protected as an Endangered species by the Federal Endangered Species Act and as a Federally-designated Endangered species by Florida’s Endangered and Threatened Species Rule . It is also protected Federally protected as a Depleted species by the Marine Mammal Protection Act.
Federal Recovery Plan
Other Informative Links
Animal Diversity Web Aquarium of the Pacific International Union for Conservation of Nature National Geographic National Oceanic & Atmospheric Administration Printable version of this page
American Cetacean Society. (n.d.). Sperm Whale . Retrieved June 6, 2011, from American Cetacean Society Fact Sheet: https://www.acsonline.org/sperm-whale?
Ballenger, L. 2003. "Physeter catodon" (On-line), Animal Diversity Web. Accessed August 12, 2011 http://animaldiversity.ummz.umich.edu/site/accounts/information/Physeter_catodon.html
National Marine Fisheries Service. 2010. Recovery plan for the sperm whale ( Physeter macrocephalus ). National Marine Fisheries Service, Silver Spring, MD. 165pp.
National Oceanic and Atmospheric Association. (n.d.). Sperm Whale . Retrieved June 6, 2011, from NOAA National Marine Fisheries Service: http://www.nmfs.noaa.gov/pr/species/mammals/cetaceans/spermwhale.htm

Deep in the arctic winter off Norway, a boat searches the ocean for sperm whales
The world's largest toothed predator is well studied in the tropics, but close encounters in the high latitudes remain rare. These hardy researchers don‘t just want to find them—they want to dive with them.
I'm standing on the stern of the 37ft expedition yacht Barba . Ahead, the serrated cliffs of Andøya, a Norwegian island 300km north of the Arctic Circle, mask the partial winter sun. Moderate offshore winds whip off the surface of Andfjorden and bring the temperature to 20 below.
“Whale blow, 200 meters to port,” calls helmsman Emil Gundersen. The yacht pitches hard. Photographers Tord Karlsen and Sophie Bolseworth steady themselves. Crewman Aksel E. Ørstavik and I hand fins and cameras to Norwegian captain and marine biologist, Andreas B. Heide, and French acoustic engineer and marine researcher, Fabrice Schnöller. Three male sperm whales pass 50 meters to port and the men dive in. It's the first documented moment that people have free-dived with sperm whales in this region of Arctic Norway during winter.
Sperm whales near Andøya
The Bleik Canyon begins 8 nautical miles offshore from Andenes on the northern tip of Andøya. The 50km submarine trench reaches like a tendril from land out towards the continental shelf, where water depths plummet from 200m to over 2000m. Here, upwelling forces nutrient rich water to the surface and brings with it a diverse array of marine life. Of particular importance are the deep-water cephalopods, which are believed to form an integral part of the sperm whale diet. Subsequently, the region boasts one of the largest known aggregations of sperm whales near land. However, only adult males have been documented in the high latitudes.

Jonathan Gordon, who has spent the past 30 years studying sperm whales and is a Research Fellow at the University of St. Andrews’ Sea Mammal Research Unit, understands this is due to feeding opportunities. “Males leave as they mature and are found closer to the poles. The feeling is that the feeding must be better as they tend to grow a lot larger than females,” he says. “But if you ask for hard evidence that the feeding conditions are better, well… there really isn’t much.”
Tiu Similä, a Finnish scientist who pioneered long-term orca research in Norway, works closely with Gordon and has studied sperm whales in the Andenes region since 2016. “There is so much we do not know about the male sperm whales,” she says. “What is their diet? What is their habitat use pattern? How solitary or social are they? Do they compete, cooperate or maybe both?”
Since the late 1980s, tourist whale watching companies have operated in the area and often host scientists and researchers. But little time is spent at sea outside of the tourist season, which runs from May to September. During this period, the primary form of documentation is photo-ID. (Read: A guide to ethical whale tourism in the 21st century)

Based on data collected in this manner and collated over a 22-year period, it is estimated that the mean number of individuals in the area is 101. But this documentation accounts solely for the spring and summer months, and unlike the tropics, engaging with and recording these animals underwater is incredibly challenging; in winter, it has never been attempted.
Two adventurers align
Heide has spent the past decade guiding experts, free-diving and documenting whales in Arctic Norway aboard Barba, which he uses as a research and storytelling platform. In 2018, during his third season tracking orcas in the region, he first spotted sperm whales in the waters off Andøya. While his experience of documenting close encounters with orcas and humpbacks was extensive, he was yet to encounter sperm whales.
It was therefore fortuitous that he had been introduced to Schnöller earlier that season. “For me, they are one of the more mysterious whales due to their deep diving capabilities and because they spend a limited time on the surface, unlike orcas,” he tells me. “I wanted to go back with Schnöller to get a better idea of what I was seeing in the area.”
For me, they are one of the more mysterious whales due to their deep diving capabilities and because they spend a limited time on the surface, unlike orcas. Andreas Heide
Schnöller’s experience began with a remarkable encounter with a pod of 20 sperm whales in the warm waters off the coast of his home on the Réunion Islands some 15 years ago. He then went on to found the Darewin Project in 2012. The project’s objective is to collate data and present it in an open-source format to encourage the wider scientific community to invest additional resources into whale communication research—studying the vocal ‘clicks’ by which the mammals engage with each other. And while he has over 200 underwater encounters in the tropics, he had never documented sperm whales in the arctic and was initially sceptical. “I thought it was almost impossible, that perhaps there was only one male, and that the conditions would be too hard,” he says. “But I wanted to try.” (Related: Ground-breaking effort launched to decode whale language.)

And so, a symbiosis rapidly formed between the two and a question was raised: Can we find, document—and interact—with sperm whales underwater during the Arctic winter?
The north calls
Within weeks, the team is assembled in Tromsø. We sit in the intimate saloon aboard Barbaand gaze over the chart plotter. Heide’s route will take us past the island of Senja, across Andfjorden and around the tip of Andøya to Andenes and Bleik before a southward sail through Lofoten to Bodø. During the 10-day expedition, we will cover 300 nautical miles through some of Norway’s most marine-life-rich waters.
Research into whale communication is a vibrant field —but documentation of sperm whales in an environment such as this is challenging. Passive acoustic recordings help establish whale locations and aid with questions surrounding conspecific communication; photo-ID allows for the documentation of individuals; biopsy and faecal samples (whale poo is ejected in loosely aggregated plumes, which float until they break up) help understand genetics and diet; and tagging proffers information on orientations and movements. But in the Andenes region, these are limited, speculative and seasonal.

However, some observations have been made that will help guide our mission. Marine biologist and bioacoustics specialist Giulia Ercoletti has spent the past three years in Andenes observing sperm whales. She notes that while they remain far offshore and solitary during the whale watching season, they have been observed close to land and in groups during winter; it is widely believed that this is due to the aggregation of food.
(Related: this baby sperm whale was tangled in ocean trash for 3 years.)
“In winter you can see them in Andfjorden itself, and often in small groups,” she tells me. “They feed using echolocation, so it’s possible that when they’re close, their echolocation interacts with one another. But during winter, when they don’t have to dive so deep, perhaps the echolocation is less of a problem so they can hunt together.”
In evidence of Ercoletti’s observations, Heide’s original sighting places the whales at the opening of Andfjorden, so with the coordinates as our goal, we cast off. The conditions are treacherous, and we reach Andfjorden on our second day at sea under the onslaught of 40-knot gusts.
In the limited daylight, we’re proffered just 4-5 hours of searching per day, with darkness once again descending by 14:00. But it is as we approach Andfjorden that Heide spots the blow of a whale. We change course and tack in its direction. The whale dives, and from the fluke we confirm that it is a sperm whale. And then another surfaces, followed by two more close together. Karlsen and Bolesworth begin photo-identification as Heide consults the chart plotter. “We’re in the exact same area as when I saw them 2 years ago,” he comments. “This must be a hotspot.”

Gale-force gusts bellow from the mouth of Andfjorden and with the light quickly fading, we’re forced to sail onward to the safe harbour of Bleik. Excitement spreads through the team; the whales are here and with right conditions, free-diving will be possible. (Related: can today's whale species survive the age of humans?)
Arctic fieldwork
It is the danger of executing fieldwork in these remote and volatile conditions that Heide notes as the most challenging factor. The line between safety and alarm is a fine one when both sailing and freediving offshore in the arctic, particularly during winter—and explains why in-water encounters here have never been recorded before. In addition, searching for, finding and documenting cetaceans has always been a balance between instinct and experience. Heide says that when searching for orcas, it is best to encounter them when they are feeding, or just after they have fed, in which they will likely be socialising. But with sperm whales, it is far harder to establish such parameters. “It’s very difficult to find an area where it’s both easy to access the water and where the whales are interested in you,” says Schnöller. “Perhaps these whales have never seen a human in the water before so this very first interest could lead to a big encounter.”
We cast off before first light with the intention of sailing 12 nautical miles to the location we spotted the whales the day before. However, as we round the headland, fierce winds slam against the bow and cast the yacht into a ceaseless pendulum. Spindrift from cresting waves fills the air and makes searching for whale blows impossible. We travel back to port. A storm arrives the next day and we are forced to remain in harbour. It is the unpredictability of the conditions that makes fieldwork in the arctic so slow. But it also offers necessary time to prepare.

Documentation and tech
The primary tool used to determine sperm whale location is the hydrophone. Either towed or mounted into the hull, it allows researchers to listen for a variety of vocalisations (clicks) most commonly associated with feeding and communication. This, in partnership with vision, is the best means by which to find sperm whales. Once found, photo-ID is the most common way to document individuals. Heide explains that a whale’s fluke is unique, much like our own fingerprints, so by photographing the fluke when a whale dives, researchers are able to determine the individuals in a given area. “We saw three travelling together, so it will be interesting to see if the same three travel together again,” he says. “When the weather clears, we will travel to the last spot we saw them.
But the technology used to document sperm whales is evolving. “Some new approaches, such as suction cup tags (DTAGS) with cameras attached, are a good way of understanding what they’re doing underwater,” notes Gordon. “They’ve also been used to record orientation and movement in conjunction with passive acoustics.” These methods help to understand what the whales are doing at depth. However, effective deep ocean technology is still in its infancy.

For Schnöller, the importance comes from getting in the water itself to personally collect data to allow for a deeper understanding of these animals. Sperm whales possess the largest brain in the animal kingdom, and the neocortex, which controls higher-level brain functions such as cognition, perception and language, is not only larger, but far denser than our own. In addition, they also possess spindle cell neurones, which are directly linked with empathy.
This evidence suggests that sperm whales are capable of feeling, among other things, emotion and intuition, and goes some way to explain just how diverse their communications are. Utilising customised 360-degree camera systems, Schnöller hopes to capture a total underwater picture which, when paired with passive acoustic recordings and VR, will give scientists a broader picture and understanding of behavior and communication. He has even developed a gun-like lens which he hopes will be able to rebound whale click communications to start the process of deciphering not just how they communicate, but what information they are communicating.
Frigid free-diving
The weather doesn’t calm enough until our last day. We rise early and are once again at sea before first light. An onshore wind builds as we pass the headland, but as the dawn light splays across the turbid water, we spot our first whale blows.
In quick succession we spot a number of individuals traveling away from shore. Heide spots three individuals travelling in unison and by comparing them to the images captured on our first day, he is able confirm that they are the same three. Schnöller notes that sperm whales tend to travel in the same direction when feeding. On the surface, they take 5-10 breathes before diving, which they signal by the bobbing of their heads before their flukes rise. “They normally dive for 20-40 minutes and travel in the same direction, so we have to get ahead,” he says. “They are curious animals, so we have to make ourselves attractive.”
(Learn about the new National Geographic documentary Secrets of the Whales.)

As we sail, Schnöller listens to and records the click communications through the hydrophone mounted into the hull of Barba and is later able to determine the size of the whales through their acoustic signature. He explains that while diving, the sperm whale emits a steady click in order to explore the environment and to communicate. Each click has a multi-pulse phase within the whales’ head, known as the Stable Inter Pulse Interval. This interval is determined by using a set of algorithms, and the specific length of these intervals can help establish the size of an individual. “The acoustics tell me that they are 10-meter-long sperm whales,” he confirms. “That’s small, even for young males.”
Heide and Schnöller change into their wetsuits and ready themselves in the dinghy towed behind Barba. As anticipated, the whales surface nearby and the men enter the water. With powerful kicks they close the gap and are alone at sea with the world’s largest toothed predator. It is a moment we had all hope for; a moment that lasts just a handful of seconds before the whales dive once again. But the men have captured the first videos and photographs of sperm whales underwater in this region; vital to the further understanding of these animals. Back onboard, the elation is profound.
“The feeling of being underwater with these mysterious creatures is quite overwhelming,” says Heide. “We know so little about them but encounters like this help us to understand them better.” As the men go below deck, a snowstorm blows across the water and forms an opaque veneer over the already-fading light; our time with the sperm whales has come to an end and we begin our southward sail through the frozen fjords of Lofoten to Bodø.
Later, in the warmth of the saloon, Heide and Schnöller discuss their observations. “I really thought it would be impossible to have an encounter here. But what I observe is exactly what I have observed in other places; it is quite possible to interact with them,” says Schnöller. “They were curious, but also uncertain. The best thing is to find an individual that is curious,” he continues. “I’m sure that if you come to this area day after day, they will get closer, and the curiosity will overcome the uncertainty.”

For Heide, an expedition like this allows him to get specialists into areas so remote and volatile that they would otherwise never be able to. “My primary objective is to bring people like Schnöller into the field, to provide a platform so that we can learn more. From what we’ve seen, there’s a lot to study here,” he says. “So little is known about [sperm whale] biology, their behavior and what they’re eating. How big is the area they inhabit? Is it only around Andenes, or is it along a larger area of continental shelf?”
To the future
One year on, Heide hopes to be able to answer some of these questions as part of the Arctic Sense expedition, which aims to explore and assess the polar Atlantic ecosystem. Throughout the 3,000 nautical-mile, 4-month journey, the team will conduct world-first oceanographic research into cetaceans, climate change and pollution.
Utilising a bespoke towed hydrophone array designed and built by Gordon in conjunction with Marine Ecological Research, Barba will once again sail to Andenes to gather further data to help expand knowledge of sperm whale distribution, behavior and communication along the Norwegian continental shelf. The team will then sail north to Svalbard, across the Greenland and Norwegian Seas to explore the remote island and waters of Jan Mayen, before a southward journey to London via the Faroe Islands, the Shetland Islands and Edinburgh.
“With Barba , we are able to bring experts into the field to gather critical data in a sustainable way, non-invasive way,” says Heide. “What I witnessed outside Andenes is a beacon of hope for marine conservation. It is our obligation to ensure we understand and protect our oceans and their inhabitants.”
Related Topics
- SPERM WHALE
- POLAR REGIONS
- ANIMAL BEHAVIOR
- ANIMAL COMMUNICATION
You May Also Like

This video captures a rarely seen sperm whale birth. It’s beautiful.

These birds help humans find honey. But it’s rare—and getting rarer.
Free bonus issue.

Whales can sing underwater without drowning—now we know how

First-ever photos show humpback whales mating—and they’re males

Love them or hate them, hyenas are getting the last laugh

Polar bears are trying to adapt to a warming Arctic. It’s not working.

We might lose these whales for good if we don’t slow down
- Environment
- Perpetual Planet
- History & Culture
History & Culture
- History Magazine
- Mind, Body, Wonder
- Paid Content
- Terms of Use
- Privacy Policy
- Your US State Privacy Rights
- Children's Online Privacy Policy
- Interest-Based Ads
- About Nielsen Measurement
- Do Not Sell or Share My Personal Information
- Nat Geo Home
- Attend a Live Event
- Book a Trip
- Inspire Your Kids
- Shop Nat Geo
- Visit the D.C. Museum
- Learn About Our Impact
- Support Our Mission
- Advertise With Us
- Customer Service
- Renew Subscription
- Manage Your Subscription
- Work at Nat Geo
- Sign Up for Our Newsletters
- Contribute to Protect the Planet
Copyright © 1996-2015 National Geographic Society Copyright © 2015-2024 National Geographic Partners, LLC. All rights reserved

Cephalopods, Crustaceans & Other Shellfish
Corals & Other Invertebrates
Marine Mammals
Marine Science & Ecosystems
Ocean Fishes
Sea Turtles & Reptiles
Sharks & Rays
Marine Life Encyclopedia
Sperm Whale
Physeter macrocephalus
Distribution
Worldwide in tropical to polar latitudes
eCOSYSTEM/HABITAT
Open ocean (pelagic); deep diver
FEEDING HABITS
Active predator
Suborder Odontoceti (toothed whales), Family Physeteridae (sperm whales)
Sperm whales have several specialized physical characteristics that aid in this predatory behavior. They have large conical teeth for ensnaring their preferred prey. Like most active predators, they have large brains and in fact, the sperm whale has the largest brain of any animal on the planet. They also have the most powerful sonar of any animal, which they use to find their prey in the dark deep sea. Finally, they have an ability to dive to incredible depths (up to 1000 meters) and stay down for incredible lengths of time (up to two hours), both abilities increasing their likelihood of finding prey. As a result of their deep-sea behaviors, sperm whales typically live in waters of several thousand meters deep and are rarely seen along the coast except in areas where deep trenches or underwater canyons approach the shore.
The sperm whale’s very large brain and specialized sonar organ (called a melon) contribute to its characteristic block-shaped head. It is the only whale that has that shaped head and is typically quite easy to identify. The body is generally uniformly grey. The sperm whale’s lifecycle is very similar to that of humans. Individuals reach sexual maturity in their teenage years, and females reproduce until they reach their forties and go on to live into their seventies. Sperm whales give birth to only one calf at a time, and at birth, baby Sperm Whales are enormous – over 13 feet (4 m) long. Because calves cannot undertake the deep, long dives that their mothers do, groups of mothers form tight bonds and share the responsibility of protecting calves at the surface. While one or more mothers dive, others stay with at the surface with the young.
150 years of commercial whaling for sperm whales cut their numbers at least in half, and some scientists estimate that whaling reduced the population by 75% or more. During a time when whale oil was a primary energy/lighting source in the U.S. and Europe, sperm whale oil was some of the highest quality and highest volume per whale of any species. Though whaling has all but ceased since 1988, sperm whales have not yet fully recovered from this cruel practice and are still considered vulnerable to extinction by expert scientists. They have, however, recovered more significantly than the other large whales and are the most common large whale in the ocean today. It is difficult to obtain accurate numbers of sperm whales in the wild, so it is equally difficult to determine if populations are increasing or decreasing, but today’s primary threats include accidental entanglement in fishing gear, chemical pollution, and noise pollution. Several countries around the world have offered sperm whales some or extensive legal protection.
Fun Facts About Sperm Whales
1. Sperm whales are the largest of all toothed whales and can grow to a maximum length of 52 feet (15.8 m) and weight of 90,000 pounds (40 metric tons), with males growing much larger than females.
2. Sperm whales live for up to 60 years.
3. Sperm whales have one of the widest distributions of all marine mammals, living everywhere from the Arctic to the Antarctic.
4. Sperm whales are named after the spermaceti – a waxy substance that was used in oil lamps and candles – found on their heads.
5. Sperm whales are known for their large heads that account for one-third of their body length.
6. Sperm whales can stay underwater for up to 60 minutes at a time.
7. Sperm whales can dive more than 10,000 feet (3,048 m) in search of their preferred prey, which includes squid, sharks and fish.
8. Sperm whales eat up to 3.5 percent of their body weight in food every day.
9. Female sperm whales form lasting relationships with other females in their family and create social groups around these bonds. Males, on the other hand, leave their matriarchal groups between 4 and 21 years old to join “bachelor schools” before eventually leading solitary lives in their later years. 1
Engage Youth with Sailors for the Sea
Oceana joined forces with Sailors for the Sea, an ocean conservation organization dedicated to educating and engaging the world’s boating community. Sailors for the Sea developed the KELP (Kids Environmental Lesson Plans) program to create the next generation of ocean stewards. Click here or below to download hands-on marine science activities for kids.

References:
1 NOAA Fisheries
IUCN Red List
Get Involved

Donate Today
Support our work to protect the oceans by giving today.
With the support of more than 1 million activists like you, we have already protected nearly 4 million square miles of ocean.

TAKE ACTION NOW
Support policy change for the oceans.
Decision-makers need to hear from ocean lovers like you. Make your voice heard!

VISIT OUR ADOPTION CENTER
Symbolically adopt an animal today.
Visit our online store to see all the ocean animals you can symbolically adopt, either for yourself or as a gift for someone else.

DOWNLOAD OCEAN ACTIVITIES
Help kids discover our blue planet.
Our free KELP (Kids Environmental Lesson Plans) empower children to learn about and protect our oceans!

FEATURED CAMPAIGN
Save the oceans, feed the world.
We are restoring the world’s wild fish populations to serve as a sustainable source of protein for people.
More CAMPAIGNs
Protect Habitat
Oceana International Headquarters 1025 Connecticut Avenue, Suite 200 Washington, DC 20036 USA
General Inquiries +1(202)-833-3900 [email protected]
Donation Inquiries +1(202)-996-7174 [email protected]
Press Inquiries +1(202)-833-3900 [email protected]
OCEANA'S EFFICIENCY
BECOME A WAVEMAKER
Sign up today to get weekly updates and action alerts from Oceana.
SHOW YOUR SUPPORT WITH A DONATION
We have already protected nearly 4 million square miles of ocean and innumerable sea life - but there is still more to be done.
QUICK LINKS:
Press Oceana Store Marine Life Blog Careers Financials Privacy Policy Revisit Consent Terms of Use Contact
April 15, 2024
Do Sperm Whales Have Culture?
As hard as it is to study these denizens of the deep, researchers have found some intriguing evidence to support the idea that “sperm whale culture” exists.
By Joseph Polidoro
Scubazoo Images/Getty Images

Joseph Polidoro: Hal Whitehead can tell you exactly where he was when he discovered that sperm whales don’t all speak the same dialect.
Hal Whitehead: Luke Rendell and I made our big discovery off the Galápagos Islands.
Polidoro: The sperm whales they were studying seemed to live in two adjacent but distinct groups, each with its own dialect.
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing . By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
Whitehead: One clan whose social vocalizations, which were codas, went kind of, “Click click click click,” and the other went, “Click click click—click,” with a pause before the last one.
I’m Hal Whitehead . I’m a professor of biology at Dalhousie University in Canada.
That was a fundamental discovery. And then we began to show that there were a number of other behavioral characteristics which differed between the, between the clans ...
Polidoro: How they travel, how they feed and reproduce, how they babysit their calves.
Whitehead: And then clans started being found in other parts of the world.
Polidoro: These marine mammals are very hard to observe, but in the past two decades the roughly 20 or so people in the world who study sperm whales have found some compelling evidence of culture among them.
Hal summarized these findings in a review paper published in Royal Society Open Science in January. And he asked a natural follow-up question: What can we learn by comparing human and sperm whale cultures?
For Science, Quickly, I’m Joseph Polidoro.
Sperm whales use short strings of distinct clicks, known as codas, to signal their membership in a clan.
[Clip: Sperm Whale vocalization]
Scientists also use these click sequences to differentiate between sperm whale clans, naming each group after an attribute of their respective coda.
Whitehead: So a member of a sperm whale clan could listen to the codas of another whale and know immediately whether that whale is from its own clan or from a different clan.
Polidoro: Some clans are so large and widespread that many of their members may never meet. But if they did, they’d recognize each other as belonging to the same group, just like a New Yorker and a Texan connecting over their shared American English while traveling abroad.
Sperm whales’ basic social unit, the pod, comprises about 10 females and their offspring. Clans are much larger. On average ...
Whitehead: It’s about 20,000 females, although it almost certainly varies enormously between the different clans.
Polidoro: Clans’ geographic ranges can also vary from large to supersized. The Plus-one clan occupies a more than 600-mile range between the Galápagos and mainland Ecuador, while the Short clan stretches all the way from Japan to Chile.
Whitehead: These are huge scales for a social structure …
Polidoro : But not so different from the scale of human ethnolinguistic groups.
Andy Whiten : I think what’s really new and exciting here is this whole story of symbolic marking.
I’m Andy Whiten , professor of evolutionary and developmental psychology at the University of St Andrews in Scotland.
Polidoro: Andy’s referring to what bioacoustician Taylor Hersh, now at Oregon State University, and her colleagues have found: that differences in sperm whale codas are greatest when two separate clans live close to each other.
Whiten: It’s almost as if they’re turning up the volume on the communication that’s saying, “Hey, I’m from clan A. I’m different from you guys in clan B.”
Polidoro : But can we call this “culture”? Well, yes.
Cristina Moya: A more minimal definition would be just information that’s socially transmitted that affects behavior.
I’m Cristina Moya . I’m an assistant professor of evolutionary anthropology at [University of California], Davis. I’m interested in how humans as a species fit into the biodiversity that we know about from other species.
Polidoro: Cristina believes these researchers are onto something with sperm whale clans.
Moya: I think they’re right to point out that maybe they’re more similar to ethnic groups.
Whiten: It’s less than a century ago that it was thought that, well, only humans have culture and, you know, that’s not really a phenomenon amongst animals. But now we’ve learned it seems to pervade many aspects of many animals’ lives: culture.
Polidoro: It has led both to informal collaborations between anthropologists and animal biologists, as well as to interdisciplinary groups such as the Cultural Evolution Society, Cristina says.
Whiten: The discovery of animal culture—some of it done incidentally by people whose
background is in biology, others whose background is in anthropology—I think that really transforms our sort of understanding of evolution at large or biology at large. That's quite a fundamental cross-fertilization.
Polidoro: Animal culture is a “second inheritance system,” as Andy calls it—meaning it partners with natural selection in shaping evolution.
Whiten: You’ve got these two forms of evolution, and they can entwine in this phenomenon we call gene-culture co-evolution. And we’ve certainly got examples of that in this human sphere, the most famous one being lactose tolerance.
Polidoro: The persistence of lactose tolerance in the past 10,000 years is tied to agriculture, and this adaptation may have helped humans survive during famines.
The vocal culture of sperm whales appears to help them survive, too.
Whitehead: These animals depend heavily on each other. Without each other, they’re probably not going to live long, and their offspring aren’t going to survive. And so this bonding is vital. And the codas are an important way they do it. That sets up these patterns of cooperation and collaboration in groups, which are so important to the whales.
Polidoro: For Hal, the importance of language to sperm whale and human cultures invites comparison.
Whitehead: The fact that a pretty similar kind of social structure is found in a completely different creature in a completely different environment, for an evolutionary biologist, suggests looking [at], well, what do these two species have in common?
Polidoro: Cristina and other anthropologists agree there’s a strong case for comparing human and sperm whale cultures.
Moya: It’s not because of our shared common ancestry that we share these traits but because maybe we’ve experienced some of the same selective pressures.
Polidoro: Andy adds that there are a couple of different things we can learn from studying animal culture. Studying the cultural traits we share with our closest living relatives, other primates, sends us down one path of discovery.
Whiten: We can make inferences about what the cultures of our joint ancestors were like.
When we move to more distant species, I think the lessons are rather different.
Polidoro: For instance, humans teach, and other animals seem to teach—but other primates don’t, Andy says. We don’t share a close relative with meerkats, which teach their young how to remove stingers from scorpions before eating them, but both humans and meerkats are predators.
Whiten: Our evolutionary history went through an important stage of hunting, eventually big game hunting.
Polidoro: The comparison provides a clue as to why we, uniquely among primates, may have evolved to teach.
At the same time we need to be careful about comparisons. Do unacquainted whales who belong to a clan with tens of thousands of members really act like they belong to the same community?
Moya: I’d be surprised—but again, I’m willing to be surprised—if whales cooperated at a very large scale.
Polidoro: Still, Cristina believes animals with simpler cultures such as sperm whales may offer models for understanding the vastly more complex cultures of humans.
Moya: It’s nice to have, perhaps, simpler models, simpler cases from other species to help inspire us, in terms of some of the mechanisms that might be important.
Polidoro: She points to consensus decision-making and how ethnic groups form and splinter, two cultural phenomena mentioned in Hal’s paper.
And if his paper raises at least as many questions as it answers, well, that may be kind of the point. It’s a call to go deeper, to resist generalizations, to think harder about the diversity of all cultures.
Whitehead: We should be prepared for almost anything and not expect to be able to put hard-and-fast rules, models on what clans are and how they evolve.
Polidoro: For Science, Quickly, I’m Joseph Polidoro.
Science, Quickly is produced by Rachel Feltman, Kelso Harper, Carin Leong, Madison Goldberg and Jeff DelViscio. Our music is composed by Dominic Smith.
Subscribe to Science, Quickly wherever you get your podcasts. If you like the show, give us a rating or review. For more in-depth science news and features, go to ScientificAmerican.com .
Sperm Whale
The inspiration for the white whale of Moby Dick, sperm whales have the largest heads, biggest brains, and make the loudest sound of any animal on Earth

Region: Arctic, Antarctica
Destinations: Lofoten, Cape Verde, South Orkney Islands, Antarctic Peninsula, South Shetland Islands, Greenland, Svalbard, Falkland Islands, South Georgia, Ascension Island, St. Helena, Tristan da Cunha, Iceland
Name : Sperm whale (Physeter macrocephalus)
Length : 16-20 metres (53-66 feet)
Weight : 40,000-55,000 kg (88,000- 12,000 pounds)
Location : Sub-Arctic, sub-Antarctic, and Atlantic waters
Conservation status : Vulnerable
Diet : Mainly squid, but also fish, octopi, rays, and megamouth sharks
Appearance : Long, block-shaped head comprising up to 1/3 of the whale’s body, with grey or black skin, which tends to be wrinkled behind the head and on the sides. Short, wide flippers, broad, triangular fluke, and a single blowhole.

Picture by Thomas Laumeyer
How do sperm whales hunt?
Sperm whales usually eat a little over 900 kg (almost 2,000 pounds) of food per day. To find their prey (preferably giant squid), they dive somewhere between 300 and 1,200 metres (990 and 4,000 feet), though they can go as deep as 2 km (1.2 miles) while on the hunt. An average dive lasts about an hour. Using echolocation to focus on their prey, sperm whales generate a series of clicks that are the loudest animal-caused noises in the world. A sperm whale’s teeth along its bottom jaw are about 18 to 20 cm long (7.1 to 7.9 inches), fitting into sockets along the underside of the palate. The upper teeth of a sperm whale never grow out of its upper jaw. Scientists believe that sperm whales and giant squid are natural enemies. While no actual battles have ever been observed, sperm whales sometimes carry round scars believed to have come from the suckers of giant squid. When hunting smaller fish, sperm whale pods can work together to force feeder fish into ball-like clumps that are more substantial to eat than individuals.
Are sperm whales social?
When they are not breeding, adult male sperm whales live on their own. Female sperm whales and offspring, however, gather into pods of up to 20 members. The male sperm whale generally leave around 4 years old, sometimes forming a pod of its own with other young adult males. This pod will also eventually split up as the males age. Adult male sperm whales are the only members of the species that venture into the colder waters approaching the poles, while the pods of female and young sperm whales remain in tropical and temperate zones. Sperm whales spend most of their time on the hunt, but sometimes they break off in the afternoon to engage in more social behaviour. This includes calling to each other and rubbing against each other. When attacked, sperm whales form a “marguerite formation,” surrounding a vulnerable pod member with their tails thrust outward to ward off harassers.

How fast can sperm whales swim?
A sperm whale’s normal cruising speed ranges somewhere around 5 to 15 kph (3 to 9 mph). When they speed up, sperm whales can swim approximately 35 to 45 kph (22 to 28 mph), and they can maintain these speeds for about an hour.
What are sperm whale mating rituals like?
Sperm whale males reach sexual maturity around 18 years old and females at 9 years old. Males battle for mating rights, then breed with multiple females. Male sperm whales do not create harems of females like other animals. The sperm whale pregnancy term lasts about 15 months, resulting in a single calf. The birth is a social event, with the rest of the sperm whale pod forming a protective barrier around the birthing mother and her calf. Female sperm whales mate once every 4 to 20 years until they are about 40 years old.
How long do sperm whales live?
Sperm whales have a lifespan similar to humans, living about 70 years. Males do not reach full size until they are about 50.
How many sperm whales are there today?
Global abundance is not known but is broadly estimated to be about 360,000, making sperm whales one of the most abundant of all the great whales .
Do sperm whales have any predators?
Orcas are the largest natural threat to sperm whales, though pilot whales and false killer whales are also known to hunt them. Orcas go after entire sperm whale pods and will try to take a calf or even a female, but the male sperm whales are generally too big and aggressive to be hunted. Aside from the usual commodities (food, blubber, oil), sperm whales have other materials that were valuable during the high whaling eras:
- Spermaceti – A waxy substance used in a variety of pharmaceuticals as well as candles, ointments, cosmetics, and weather proofing
- Ambergris – Waxy and flammable, this material forms in the sperm whale’s digestive track by irritation from squid beaks. Sperm whales produce it over the course of years to help with the passing of objects that do not otherwise break down in their digestive track. Ambergris was heavily used by the perfume industry, but its rarity eventually led to the search for other substances.

Do sperm whales attack people?
While sperm whales generally retreat from ships, on very rare occasions they have been known to ram small boats. Some scientists believe sperm whales remember past human aggression and have become hostile, but others think their collisions are purely accidental.
Seven suitable sperm whale facts
- Sperm whales have the biggest heads and brains on Earth. Their brains are five times heavier than a human’s.
- The white whale in Moby Dick was based on two real-life sperm whales: a whale that rammed and sank the ship Essex and an albino adult male named Mocha Dick .
- Sperm whales are named after the spermaceti pulled from their bodies.
- Sperm whales fertilize the oceans with their feces, which floats upward and is consumed by phytoplankton.
- Male adult sperm whales very occasionally have been known to attack orcas to compete for food.
- A sperm whale’s heart weighs about the same as two average adult male humans (125 kg or 275 pounds).
- The highest sound pressure level ever recorded from an animal was from a sperm whale off the coast of northern Norway. The single click reached 235 (dB re 1 μ Pa), which is equal to the sound pressure of the Saturn V rocket heard at about one meter distance (3 feet). This recording proved the “the Big Bang” hypothesis, which stated that sperm whales could stun or even kill prey with sound.

Australian long-finned pilot whales (Globicephala melas) emit stereotypical, variable, biphonic, multi-component, and sequenced vocalisations, similar to those recorded in the northern hemisphere
Rachael Courts, Christine Erbe, … Micheline-N. Jenner
Foraging activity of sperm whales (Physeter macrocephalus) off the east coast of New Zealand
Giacomo Giorli & Kimberly T. Goetz
High Arctic “hotspots” for sperm whales (Physeter macrocephalus) off western and northern Svalbard, Norway, revealed by multi-year Passive Acoustic Monitoring (PAM)
Viivi Pöyhönen, Karolin Thomisch, … Heidi Ahonen
Introduction
Marine mammals rely heavily on sounds as their primary means of communication and sensing their word; where acoustic cues serve a fundamental role in all exchanges between individuals, from social interactions to the coordination of group activities 1 , 2 , 3 . Some of these sounds have been investigated quite extensively in several species such as sperm whale ( Physeter macrocephalus ), and their significance and diversity are relatively well-established. Sperm whales mostly produce a number of sharp onset, broadband, evenly spaced pulses of decaying amplitude known as ‘clicks’, with different properties and repetition rates, and a bandwidth of 100 Hz–30 kHz 4 , 5 , 6 , 7 , 8 . Clicks—generated by the massive sperm whale nasal complex—may be temporally arranged in different patterns, having both echolocation and communication functionality 4 , 7 , 8 . Usual clicks and creaks 9 , 10 are produced at depth and appear to be used primarily in searching for food and targeting the prey, respectively 8 . Codas, generally emitted at the surface, are stereotyped patterns of clicks thought to serve in social communication in both sexes 8 , 11 . Slow clicks, which are heard in the presence of mature or maturing males 5 , 7 , 12 at depth and at the surface, seem to be related with the sperm whale mating system, as long-range communication for attracting females or in male-male competition 8 . Long-range communication between males in foraging grounds has been also reported, suggesting that slow clicks functionality may vary depending on the behavioural context 12 . Some additional defined click patterns of surface creaks 8 (i.e. coda-creaks 10 ), rapid/fast clicks, and chirrups 9 , 13 ) have also been described in the acoustic repertoire of the species, and are possibly used for scanning their social partners 8 .
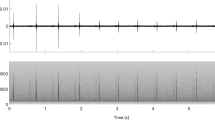
Sperm whales are also able to produce non-click sounds 8 . These include “squeals”, with a possible communicative social function 13 , 14 , ‘pips’ 13 , “short trumpets” 13 and “trumpets” 9 .
Little information is available in the literature regarding trumpets (Table 1 ). Gordon 9 wrote the earliest reference of these narrow-band sounds with harmonics and described these calls as similar to trumpets sounds produced by elephants: “This sound, like a muffled trumpeting call of an elephant, was recorded very clearly on three occasions after the fluking-up of one particular whale and before it started clicking”. Then, several research groups have recorded and identified occasional trumpet sounds 10 , 13 , 15 , 16 , 17 , 18 , 19 . These studies showed that sperm whale trumpets appear as tonal sounds relative to human hearing and in their spectrographic representation, consisting of units lasting about 0.2 s each and arranged in short sequences, with energy up to 20 kHz. It has been reported that the number of units ranges from 2 to 18 (Table 1 ), and the entire sequence in a trumpet takes between 0.6 and 4.3 s 18 , 19 . Even trumpets seem like tonal sounds, their structure can be seen as a fast sequence of evenly spaced pulses, but with varying inter-pulse intervals. The waveform and the harmonic structure support the hypothesis of the pulsed nature of trumpets 20 and suggest their possible source in the sperm whale monkey lips (i.e., specific valves for sound generation located in the nasal complex and associated with small fat bodies, the dorsal bursae, which can vibrate in the air current and produce sound waves in adjacent tissues 21 , 22 , 23 ).
Results by Teloni and colleagues 18 also showed that trumpets are produced by the same individual at the start of the descendant phase of a dive (at shallow depth) before the onset of a usual click sequence (confirming the observation reported by Gordon), and that the time interval from the trumpet to the first usual click averaged 28 s. Teloni 17 reported that in some instances the trumpet is preceded by codas, explaining this as a sort of preparation of the phonation organ for the following click emissions with echolocation function.
Trumpets are actually supposed to be by-products of the click generation mechanism when the sperm whale nasal complex is adjusted to switch from a configuration appropriate to respiration to one suitable for echolocation clicks 9 , 18 . Another suggestion is that the trumpet could be produced by a threatened whale as an alarm call due to the presence of the vessels 9 , 13 , but the variability, the stability and the functional significance of these sounds remains uncertain. The modest source level and the apparent lack of directionality 18 seem to exclude echolocation, and the possible trumpets’ communicative role as a signal (i.e., selected for conveying information to recipients to elicit responses that result in fitness consequences 24 , 25 ) or a cue (i.e., not shaped by natural selection for the purpose of transmitting information, but able to provide information to others as a by-product of an activity 24 , 25 ) is not clearly inferred from existing data.
Here, we present the features of sperm whale trumpets recorded in the Pelagos Sanctuary area (Mediterranean Sea), with the aim of expanding the knowledge on these less studied sounds and offer new insights on the emission context.
Materials and methods
The study site is located in the north-western portion of the “Pelagos Sanctuary for Mediterranean Marine Mammals” 26 (Fig. 1 ). The area is characterized by a complex geomorphology with a narrow continental shelf, deeply incised by several submarine canyons, followed by offshore waters deeper than 2500 m. The presence of a permanent frontal system and the interaction between geomorphologic and oceanographic factors makes the region one of the most productive of the Mediterranean 27 , 28 .
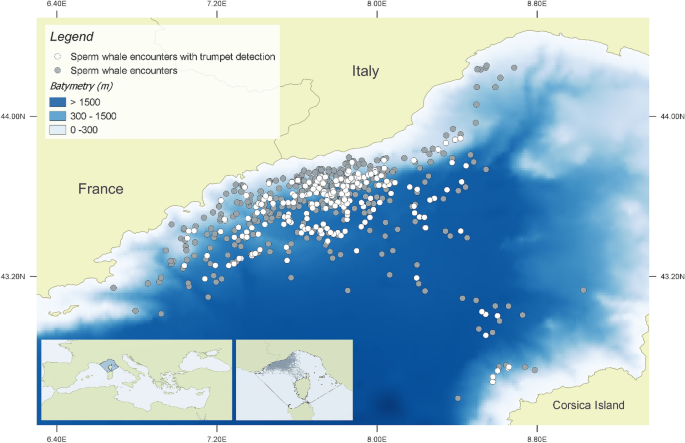
Tethys Research Institute/CSR project study area. All sperm whale encounters between 2007 and 2018 are shown. White dots indicate location of trumpets recordings. TRI/CSR cruise track lines, and the Pelagos Sanctuary borders are shown in the panel. Map created using the Free and Open Source QGIS.
Sperm whales have been reported in the area during the summer period since 1990 29 , 30 , with predominant foraging activities 31 , 32 . The estimated length of the encountered individuals suggests the area is primarily used by males 33 , 34 , 35 , 36 generally swimming or diving alone, or seen alone at the surface 37 , while females and calves in social units (sensu Whitehead 8 ) are infrequently sighted 38 or stranded 39 . Sperm whale habitat preference is related to regions with well-defined depth and slope gradients, as in other Mediterranean locations 30 , 31 , 40 , 41 , 42 , 43 , 44 . Sperm whale occurrence in this study area has been reported over a 25-year period 30 , providing key information on the population status of a species in suspected decline in the Mediterranean Sea 45 and listed as Endangered in the IUCN Red List.
Data collection and field procedures
We analysed two different sperm whale acoustic datasets. The first one derives from the Cetaceans Sanctuary Research long-term research program (1990-ongoing) run by Tethys Research Institute (TRI), Italy, and includes sperm whale recordings collected between 2007 and 2018. The second one originates from an acoustic campaign conducted by CIBRA-University of Pavia, Italy, in 1996. We used this CIBRA historical dataset as it contains the first trumpet recordings in the Mediterranean Sea and accounts for the permanence of these sounds in the basin.
TRI recordings were collected during visual and acoustic surveys conducted in spring/summer (May–September) using sailing vessels of 15–21 m. Two observers, positioned one at each side of the vessel at a height of approximately 3 m above the sea surface, visually scanned for cetaceans by using 7 × 50 binoculars during daylight. Visual effort was performed under ‘favourable conditions’ only (i.e. the vessel speed averaged 5–11 km h −1 in sea state conditions corresponding to a Beaufort scale lower than 3). Acoustic surveys were also conducted in higher sea state conditions.
A dedicated laptop, connected to a GPS receiver, automatically acquired and logged the GPS track every minute. The International Fund for Animal Welfare (IFAW) software Logger 2000 and Logger 2010, and the software PAMGuard (version 1.15) implemented by the University of St. Andrews were used for data logging. Acoustic detections were performed using a stereo hydrophone array incorporating two hydrophones (BENTHOS AQ4—frequency range 10 Hz to 15 kHz − 3 dB) with 2 pre-amps (Magrec HP02 with high pass filters set to − 3 dB at 100 Hz) towed on a 200 m cable. The system was connected to the laptop through an audio interface (Sample rates: 44.1 and 96 kHz, 16-bit resolution). Rainbow Click IFAW software ( http://www.marineconservationresearch.co.uk/downloads/logger-2000-rainbowclick-software-downloads/ ) or the “Click Detector” PAMGuard module ( https://www.pamguard.org/devDocs/clickDetector/package-summary.html ) were used to detect and track the sperm whale clicks. Once the sperm whales were detected, the vessel was maneuverer to determine the bearing of the vocalizing focal animal relative to it. In case of more than one clicking sperm whale, the focal animal was labelled as the one producing the more intense sound. To track the focal animal, the stereo signal was analysed using time of arrival differences between the same clicks on the two channels to estimate the bearing of each click source 46 , 47 . This approach allowed to track the sperm whale until the end of its dive (i.e., the time the whale was first sighted at the surface 48 ) having as final goal the identification of the animal through photo-identification. When the tracked whale stopped clicking, the acoustic operator informed the visual observers, since cessation of clicking was usually an indication of the end of the dive. When the sperm whale was sighted at the surface, surfacing time, geographic position and respiration pattern, were also collected.
During the surface period, the focal whale was approached to collect photo-identification data by using a Canon digital camera equipped with image stabilized telephoto zoom lens (70–200 mm F2.8). At the beginning of a new dive after the surface period (i.e., when the whale fluked-up 48 ), continuous acoustic recordings were initiated by using Sound Emission Analyzer Pro (SeaPro, developed by CIBRA). Patches, nicks, notches, scars, and other marks on the sperm whale flukes were used to identify individuals 49 , 50 , 51 , 52 . Photo-identification pictures were then coupled with recordings from the data logging system, in order to associate in real-time, the photo-identified focal whale to the relative acoustic files. The focal whale started its sounds production just after the fluke-up, when it is still in the first tens or few hundred meters below the surface. Accordingly, the sounds produced by the diving sperm whale have a much higher intensity than any other animal eventually present in the nearby. After 20 min, (while recordings were continuously collected) the boat started again to maneuverer to determine the bearing of the vocalizing focal whale. This “second cycle” had the final goal to confirm the identification of the animal and the correct association between the photo-identification and the recording. The entire process of finding, tracking, visually detecting, photo-identifying and recording the focal whale is summarized in the sequence of the activities shown in Fig. 2 . A total of 352 sperm whale encounters were completed by Tethys during the study period (2007–2018), where 149 different individuals were photo-identified.
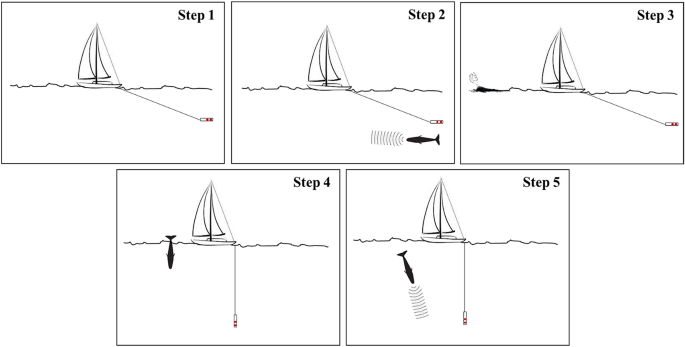
Sequence of activities adopted during the field work to univocally identify each focal whale. Step 1: Whale acoustic detection during surveys; Step 2: Whale acoustic tracking; Step 3: Surface visual detection; Step 4: Photo-identification; Step 5: Recordings.
As for the CIBRA dataset, in September 1996, a 12-day research cruise was conducted in the Pelagos Sanctuary. Due to bad weather conditions, the acoustic survey effort was limited to 7 days. A hydrophone dipole array 53 was towed with 150 m long cable by a 16 m schooner at a speed of 6 knots. The hydrophones in the array, spaced 8 m to give directional cues, recorded on a DAT recorder (Casio DA-2, 48 kHz sampling rate, 16-bit resolution). Towed array operations totalled 73 h, during which the array was monitored for at least 5 min every 30 min, on a 24-h basis. When sperm whales were detected, continuous monitoring and recording were activated. Sperm whales were then tracked acoustically and eventually approached at surface to obtain photo-identification images (a 35 mm film camera equipped with zoom lens 80–200 mm F2.8 was used) and close-range sound recording. A total of 32 h of DAT recordings were taken in two areas, on the Ligurian coast off Imperia and NW of Corsica off Calvi, where most of the encounters occurred. A group of 3 sperm whales was acoustically detected, tracked and approached off Calvi. Among series of usual clicks and codas, trumpets were also recorded from the same direction of the whales, but individual attribution was not possible.
Data analysis
A total of 765 h of recordings in 1091 wav files were investigated for trumpets. A total of 230 trumpets (226 TRI; 4 CIBRA) were detected in 227 wav files, through 122 h of recordings. Trumpet data presented here is related to these recordings only, coupled with photo-identification of the corresponding fluking-up (focal) whale whenever possible.
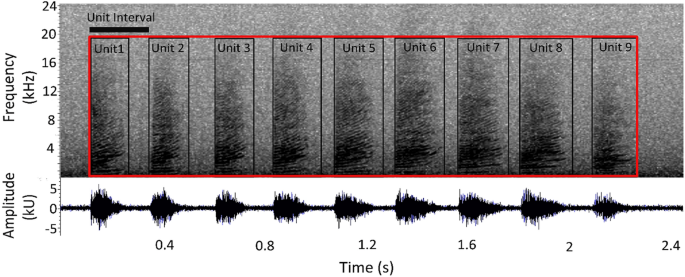
Trumpets resulted in sound elements composed by a rapid series of up-sweep units with extended harmonic structure with no apparent formants (Fig. 3 ). Different acoustic parameters (Table 2 ) were measured for each Trumpet and for each Unit in a Trumpet using Raven Pro Sound Analysis Software 54 . Depending on the acoustic parameters successfully measured, a quality score of 1 (High), 2 (Medium) or 3 (Low) was assigned to each trumpet (Table 2 and Fig. 4 ).
Spectrogram of a sperm whale trumpet using Raven 2.0 (FFT and Hanning window size 2048, 50% overlap).
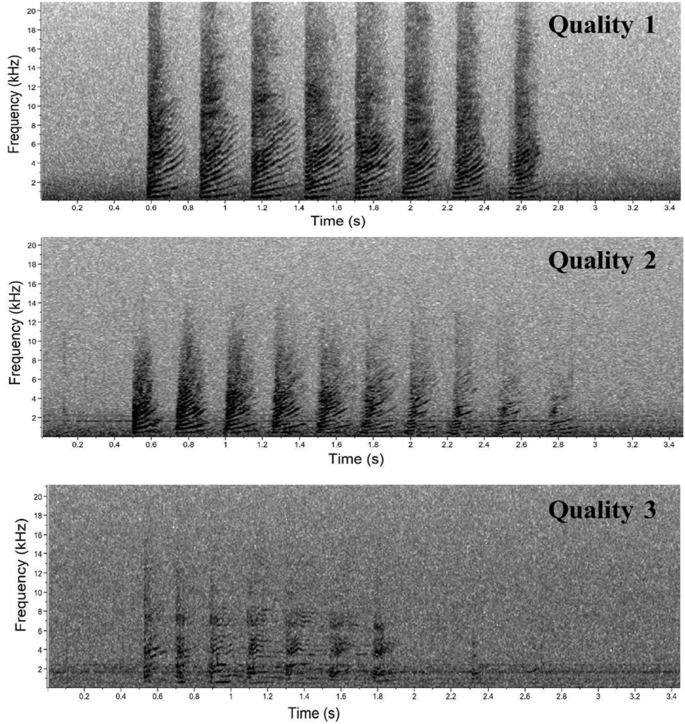
Spectrogram of sperm whale trumpets scored as quality 1 (upper panel), 2 (middle panel) and 3 (lower panel) using Raven 2.0. Upper and middle panels (FFT and Hanning window size 1024, 50% overlap), lower panel (FFT and Hanning window size 512, 50% overlap).
Spectrograms were generated with different settings depending on the sample rate of the recordings and the analysis. For all recordings collected at 44.1 kHz, FFT and Hanning window size of 512 was used to measure the Trumpet parameters, and FFT and Hanning window size of 1024 to measure the Unit parameters. For all recordings collected at 96 kHz, FFT and Hanning window size of 2048 used to measure the Trumpet parameters, and FFT and Hanning window size of 4096 for measuring the Unit parameters. Overlap of 50% was chosen for all different settings. A measurement rectangle was manually traced around each Trumpet, and for each Unit in the Trumpet, to assess acoustic parameters (Fig. 2 ). Duration-90% was introduced in the Unit analysis. This parameter was automatically computed as the time interval containing 90% of the signal energy (i.e. the difference between time points marking 95% and 5% of spectrogram power spectral density) in the rectangle selection drawn in Raven and was introduced to limit the variability and the potential errors that could be generated by manually identify the points on the spectrogram 55 , 56 .
The unit interval was calculated as the interval between the onset of two consecutive units 57 . The Unit Repetition Rate in a trumpet, in unit per second, was computed as 1/unit interval. Duration parameters were automatically extracted by the software while frequency parameters were manually measured moving the cursor on the spectrogram and selecting the most reliable measure points. The initial fundamental frequency and the final fundamental frequency were measured on the fundamental frequency when visible as the starting and the ending point of the Units composing the Trumpet, otherwise these parameters were estimated by measuring the frequency interval among visible harmonics.
Details on the context during trumpet recordings were also collected and reported as: DT interval (the time in seconds from the focal whale fluke-up to the onset of the trumpet), TFC interval (the distance in seconds from the end of the trumpet to the first usual click emitted by the focal whale), the estimated Group Size scored as 1 (the focal whale only) or > 1 (other whale(s) than the focal one visually/acoustically detected during each recording containing a trumpet), and the Acoustic Events (the of presence of other sounds around the trumpet after the focal whale fluke-up and before the start of the usual click sequence). The Acoustic Events were defined as “Regular” (a trumpet followed by an acoustic pause and then a series of usual click) and “Multi-Pattern” (a trumpet preceded/followed by different kind of click patterns, such as short sequences of 1–8 slow clicks with an inter-click-interval ≥ 2 s, codas, and/or rapid clicks; Fig. 5 ). For a subset of 214 trumpets (hereafter referred to as “Subset”), it was possible to assess both the Acoustic Events and the identity of the focal whales emitting trumpets (“Trumpet Whales”; n = 68) through photo-identification. The Acoustic Events in the 68 Trumpet Whales were assessed in both recordings with and without trumpets.
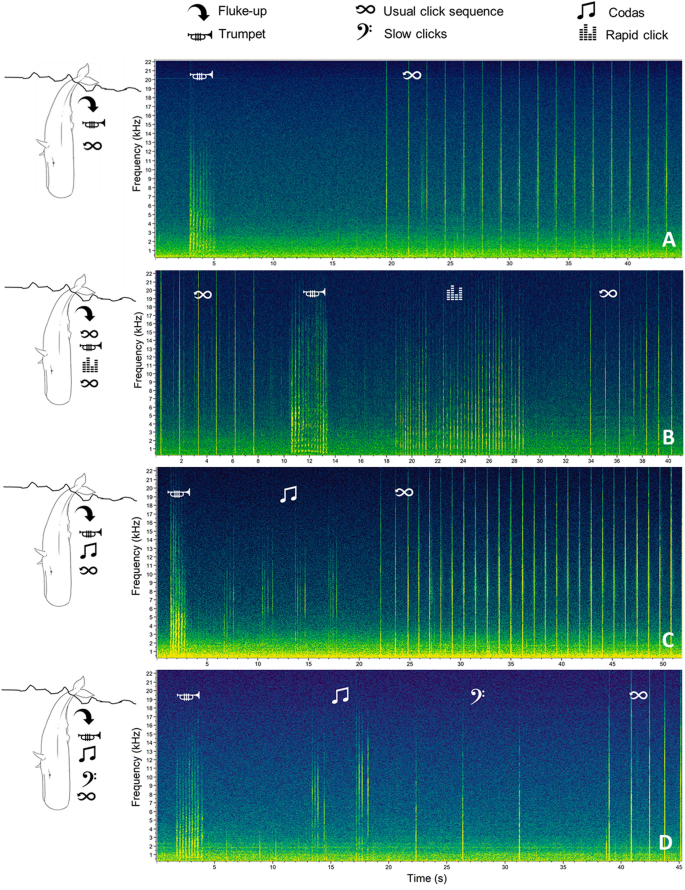
Spectrograms showing the Acoustic Events associated with trumpet emissions using Raven 2.0 (FFT and Hanning window size 1024 ( A ) and 512 ( B , C , D ), 50% overlap). A Regular; B , C , D multi-pattern arrangements.
Maps were generated using the software QGIS (Version 2.18.16). Slope values were calculated using the ESRI Arcview Spatial Analyst tool 58 and depth data was derived through the GEBCO One-minute Digital Atlas ( https://www.gebco.net/data_and_products/gridded_bathymetry_data/gebco_one_minute_grid/ ).
Statistical procedures
Given the multilevel structure of the data (Units composing a Trumpet, and Trumpets nested in Individuals), a Linear Mixed Model (LMM) approach 59 was applied to test the variation of the acoustic parameters (Table 3 ) within the same individuals and or between different individuals. Only high-quality Trumpets from 14 Trumpet Whales were used, choosing as the random effect the Trumpets nested in the Individuals (Model 1), Individuals only (Model 2), and Trumpets only (Model 3); the Initial Fundamental Frequency of each Unit was selected as the fixed effect (independent variable) and the Duration of each Unit was used as the dependent variable.
Overall, variations in the Trumpet acoustic parameters were examined in relation to Group Size (2 classes: 1 or > 1 whale) and Acoustic Events (2 classes: Regular or Multi-Pattern) using two-tailed Welch’s t-tests. The influence of Environmental Variables (2 classes: Depth and Slope) on Acoustic Events was examined through paired t-test. A binomial logistic regression approach 60 , 61 was employed to model the presence/absence of the Trumpet by using the Group Size and the Acoustic Events as predictors. A Pearson's chi-squared test was then applied to analyse the relationship between the Trumpet occurrence and the different click patterns (codas, rapid clicks, and slow clicks) in the Acoustic Events.
Analysis was performed in R (version 3.3.3, The R Foundation for Statistical Computing, Vienna, Austria, http://www.r-project.org ) using CRAN packages Seewave 2.1.5 62 , ggplot2 63 , dplyr 0.8.5 64 , lme4 65 , lmerTest 66 , and SPSS Statistics (version 26, IBM, New York, USA, https://www.ibm.com/it-it/products/spss-statistics ).
Trumpet recording locations are reported in Fig. 1 . All trumpets (n = 230) were recorded at the beginning of a new dive after the focal whale fluked-up following a period at the surface. The time interval from the beginning of a dive (after the fluke-up) and the trumpet (DT interval) had an average of 35.5 s, ranging from 1.8 to 131.5 s. The time interval from the end of the trumpet to the onset of the first usual click (TFC interval) averaged 18.22 s, ranging from 2.6 to 77.7 s.
Over a total of 230 analysed trumpets, 44 were scored as high-, 112 as medium- and 74 as low- acoustic quality. The initial frequency of each trumpet, measured on the first unit, ranged from 245 to 649 Hz, whereas the final frequency, measured on the last unit, ranged between 301 and 964 Hz (Table 3 ). As expected, the total duration of the trumpet was strongly related to the number of units, lasting between 1.1 and 6.6 s, per number of units ranging from 2 to 24 (Table 3 ). The Unit Repetition Rate in a trumpet showed an average of 3.9 s −1 , ranging from 1.4 to 5.9 s −1 . Trumpet durations were significantly longer in the Multi-Pattern (mean = 2.63 s) than the Regular Acoustic Events (mean = 2.36 s) (Welch’s t-test: t (213) = 2.8838; p < 0.01; Supplementary Figure S1 ). About 89% of the trumpets (n = 204) were documented when whale(s) other than the focal one was visually/acoustically detected during each recording with a trumpet (Group Size > 1). This proportion is comparable with recordings without trumpets. The trumpet initial frequency was significantly higher when Group Size was > 1 (mean = 439 Hz) than when there was just one whale in the area (mean = 412 Hz) (Welch’s t-tests: t (124) = − 2.1704), p < 0.05; Supplementary Figure S2 ).
The Units composing the trumpet were characterized by increasing frequency (sweep-up) throughout their duration. Their initial frequency ranged from 212 up to 672 Hz, whereas the final one between 332 and 1774 Hz (Table 3 ), regularly increased throughout each unit. Units lasted an average of 0.21 s, ranging between 0.11 and 0.52 s. The average Unit Interval was 0.26 s, ranging between 0.15 up to 0.61 s.
Unit duration 90% resulted higher in the Multi-Pattern than in the Regular Acoustic Events (Pair t-test: t (213) = − 16.339; p < 0.001). The Acoustic Events were mapped (Fig. 6 ) and related to Environmental Variables (i.e. depth and slope descriptive statistics). Multi-Pattern series turned out to be recorded in areas with higher slope values (Pair t-test: t (213) = − 13.521; p < 0.001) and lower slope variability (Pair t-test: t (213) = 14.083; p < 0.001), i.e. submarine canyons (Supplementary Figure S3 ).
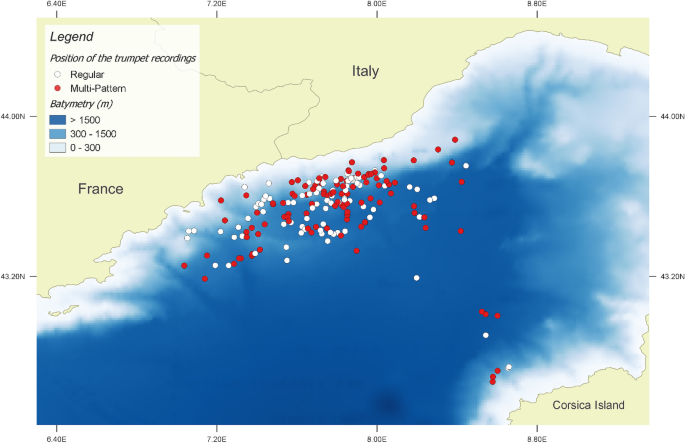
Position of the sperm whale TRI trumpet recordings showing Regular (white dots) or Multi-Pattern (red dots) Acoustic Events. Map created using the Free and Open Source QGIS.
As previously mentioned, for a subset of 214 trumpets, 68 Trumpet Whales were identified over a total of 149 that were catalogued in the study period. The proportion of the Trumpet Whales represented more than 50%, and up to 75% of the total number of photo-identified whales each year (Supplementary Table S1 ). The individual emission rate (Table 4 )—calculated as the number of encounters with trumpets over the total number of encounters per focal Trumpet Whale—has an average of 0.58. For almost 62% (n = 42) of the Trumpet Whales, more than one trumpet in the same survey season or in different ones was recorded, with 29 individuals producing trumpets over different years (maximum range: 10 years).
Three different LMMs were run to test the differences of the acoustic parameters within and between individuals (Table 5 ). Comparison of the AIC values showed that Model 1 (the one using the Trumpet nested in the Individual as random effect) better explained the correlation between the variables (AIC = 565.70), followed by the Model 2 (ΔAIC = 27.78). The Duration of each Unit significantly correlated with the Initial Fundamental Frequency (t-value = − 3.772; p < 0.001) and the variability of the Trumpet nested in the Individuals (SD = 0.67) was higher than between different Individuals (SD = 0.20). The model diagnostics considered the variable independence assessment and the residuals normal distribution (Shapiro–Wilk normality test: W = 0.988; p > 0.05).
The stepwise binary logistic analysis selected the Acoustic Events as the strongest predictors of Trumpet occurrence (Table 6 ). As shown in the confusion matrix, the model had a higher accuracy for predicting Trumpet presence (95%) and a lower accuracy for Trumpet absence (52%). However, the overall accuracy of the model was higher than 75% (Table 6 b). Regular Acoustic Event inversely correlated with the Trumpet presence (Table 6 a), suggesting that the Multi-Pattern Acoustic Events could be more associated with the trumpet emission than the Regular. Finally, Pearson chi-squared test highlighted a significant association between trumpet occurrence and sequences of slow clicks in the Multi-Pattern Acoustic Events (χ 2 (1, 505) = 148.9, p < 0.0001).
Here we explored a topic that was scarcely reported until now. Specifically, we investigated the sperm whale trumpets in the Mediterranean Sea, their acoustic characteristics, the context of emission, and the individual variability of these sounds.
Acoustic emissions generated by specialized anatomical structures are often presumed to be signals, even if their functional purpose is unclear or undetermined 25 . A signal evolved to deliver information that, on average, enhances long-term fitness of both the signaller and receiver(s) 67 , 68 , 69 . The aim of a signal (or of a signaling system) is communication 70 , and its goal is to change the receiver’s behavioural, physiological, or developmental responses 71 . If any information is obtained from traits that are not signals (i.e. not evolved for the purpose of conveying information), these traits are reported as cues 25 , 72 .
It is not easy to demonstrate if a sound is a putative signal or a cue, as different acoustic cues can be a source of information beneficial to both sender and receiver as well, and some vocal signals may have evolved from altered breathing patterns that once were cues 24 , 68 , 69 , 73 . It is still unclear if sperm whale trumpets are sound signals conveying informative aspects of the signaller (e.g., individual/species/population identity, age, physiological condition or motivation 73 ) or the context (referential signaling 73 ), or if they are cues, by-products of the click generation mechanism 9 , 18 . While the precise trumpet producing mechanism is still unknown, the by-product hypotheses may have been inferred from the extended harmonic series in the trumpets, which suggest an underlying pulsed structure possibly related to the click production. The suggestion that the trumpet could be produced by a threatened whale as an alarm call due to the presence of the vessels 9 , 16 introduce the signal hypotheses. This may derive from the well recognizable and stereotyped acoustic structure of the trumpet. Stereotypy is defined as one of the necessary components of signal evolution, to increase consistency of signal perception for effective communication and to allow receivers to reliably associate a particular signal with a conspecific 66 , or with a particular individual (i.e. , the vocalization that contains sufficient unique information to be individually distinctive and with a specific situation 74 ). Consequently, stereotyped sounds are commonly used as a way of individual recognition in a wide range of taxa, including mammals (e.g., Australian sea lion , Neophoca cinerea ) 75 ; African elephant, Loxodonta africana 76 ). From the existing data, however, it is difficult to understand which, if any, of the signal or cue hypotheses reflects the function of trumpets.
Hint #1: Trumpet stability
The results of this study indicated that trumpets in the Mediterranean are conserved in the sperm whale acoustic repertoire at the decadal timescale, demonstrating the persistence of these sounds over 22 years (1996–2018). Presumably, if a sound has a relevant function in a given context, it would be disadvantageous to change it over time. Even so, trumpets were not recorded for all individuals in all encounters during each dive, nor were they recorded in all dives by the same whale. The absence of trumpets, however, did not necessarily imply that they were absent from the individual repertoire, since it is unlikely that the recordings collected in this study comprise the whole acoustic repertoire of all whales of the entire population at any given time. Our results showed that at least half of the known whales frequenting the study area emitted trumpets each year, indicating the persistence of this sound type in space and in the same individuals across a wide time period. This stability was reported by Pace as well 19 in the Tyrrhenian Sea (Italy), advancing the hypothesis that trumpets may be a long-lasting component of the individual acoustic repertoire.
Hint #2: Trumpet variability in social context and different individuals
This study reported the persistence of the trumpets in individuals over time, but a variability in their fine-scale structural parameters with the context. The Initial Fundamental Frequency of the units and of the entire trumpet was significantly higher when more than one whale was visually/acoustically recorded (Group Size > 1) during the trumpet emissions. Higher fundamental frequencies seem to be mainly related to intense social interactions 77 , 78 . The African elephant, for example, produce sounds at a higher fundamental frequency during positive social situations and dominance circumstances 79 . The increase of the initial fundamental frequency here reported in social context might reflect a situation of “excitement” experienced by the whales when other individuals were detected in the area, a hypothesis that is further supported by the correlation between the initial fundamental frequency and the duration of the units in 14 Trumpet Whales. In intense social contexts, other species like baboons ( Papio sp.) 80 and chimpanzees ( Pan troglodytes ) 81 are reported to produce specific sounds both longer and at higher fundamental frequencies than the ones emitted in more relaxed situations. The higher initial fundamental frequency and the duration variability within each Trumpet Whales further suggested an individual plasticity in composing and arranging units in a trumpet.
Hint #3: Trumpet association with social click patterns
The results of this study showed that the trumpet emission was related to different sequences of click patterns. Trumpets were reported to be produced at the beginning of a dive, prior to the usual click series onset 12 , 15 , 16 , 17 , 18 . This was true for most of the trumpets analysed in this study although, on eleven occasions, the sequence of usual clicks began before the trumpet’s emission. In our knowledge to date, this is the first time that an observation of this kind has been reported. Teloni 17 illustrated that in some instances the trumpet was preceded by codas, as also observed in this study in one case, and that a few trumpets were followed by a short sequence of clicks (usually 2) with an inter click interval of about 5 s. No mention in the literature was found of codas or other social vocalizations, such as rapid clicks, following the emission of the trumpet, as here reported. It was postulated that the sperm whale can change the acoustic characteristics of the sound generated in the nasal complex when switching between codas and echolocation clicks, two highly different click patterns in terms of directionality and acoustic output 9 . Based on the description of their acoustic structure and considering that they are emitted at the beginning of a deep dive, Gordon 9 hypothesized that trumpets may be physiological sounds generated by the complex respiratory system of the whale in preparation for immersion. Teloni and colleagues 18 further proposed that trumpets might be a by-product of airflow in the vocal tract of the sperm whale as it modifies the sound production apparatus from a configuration appropriate for respiration and surface codas, to one appropriate for diving and sonar clicks. Observations from this study, which identified Regular and Multi-Pattern Acoustic Events (i.e. sound sequences around trumpet including social sounds such as codas and slow clicks), provided evidence of a more complex scheme than previously described, with trumpets being emitted both before and after the onset of either codas (and other social sounds) or usual echolocation clicks. In the majority of the Multi-Pattern Acoustic Events here reported, the trumpet was followed by some codas and, more frequently, by short sequences of 1–8 slow clicks 4 , 11 , 12 , a click type—only recorded in males—that has a possible long-range communicative function (e.g., presence, location, identity and perhaps size of the signaller 8 ) due to both the long inter-click interval and the waveform 11 . In addition, the trumpet duration (and so the number of units) and Units Repetition Rate were longer in the Multi-Pattern Acoustic Events. More stereotyped repeated units mean greater redundancy, and greater redundancy may improve sound detectability 82 .
Hint 4#: Trumpet association with steep slope habitat and foraging dives
Trumpets may also persist in the sperm whale repertoire in the North-western Mediterranean because they may be functionally specific to foraging activities in this region. It is widely recognised that sperm whales travel through the whole western Mediterranean Sea, with movements and exchanges of males within the area 50 , 83 . Male sperm whales use the North-Western Mediterranean Sea especially in the summer months, while social units of females with calves and juveniles tend to remain in southern areas 84 , 85 , 86 . Foraging appears to be the predominant activity performed by sub-adult/adult males while they are at the higher latitudes 32 , 33 , 87 , 88 , with a strong relation with the continental slope area 30 , 40 , 41 , 89 . In particular, sperm whale habitat preference seems associated with submarine canyons 32 , 44 , 87 , known to be extremely productive, comprising complex topographic features which serve as hotspots of biodiversity and key habitats for top predators 90 . The trumpet datasets analysed here were collected in a high-latitude habitat that is used predominantly as foraging ground for sperm whales, with higher foraging rates occurring in steep slope habitat 87 (i.e. a proxy of canyon systems). The Multi-Pattern Acoustic Events, with codas and slow clicks associated with the trumpet, appear to be produced in areas where the slope is steepest, suggesting a potential occurrence of communication dynamics among individuals at the beginning of a foraging dive. Coupling the linear distance between two different sperm whales emitting trumpets during the same survey (6.6 km; Supplementary Table S2 ), and the frequent association of the trumpet with slow clicks that potentially relays information regarding individual identity or behavioural states 12 , may be indicative of a long-range communication between males in a Mediterranean high latitude feeding ground.
This study provides the first evidence that sperm whale trumpets may persist across years and individuals in the same area. The higher fundamental frequency when multiple whales were visually/acoustically detected, the stereotyped characteristics of the trumpet acoustic structures, the frequent association with a male only communication signals (i.e., slow clicks), and the emission in feeding grounds, suggest the trumpet functions as a sound of maturing/mature males, perhaps having a role in male-male interaction context during foraging. Even with these totally new findings, it is still not possible to assess which one contributed most to the hypotheses of trumpet as a signal (intentional signal either to label an individual or a situation) or a cue (unintentional conveyers of information). In the absence of a more consistent dataset this remains an open question. Further investigation is needed and is strongly encouraged to better understand the role of temporally stable trumpets as well as individual variation, and trumpet usage across sex, age and size, social context, noise conditions, and sperm whale populations.
Fichtel, C. & Manser, M. B. Vocal communication in social groups. In Animal Behaviour: Evolution and Mechanisms (ed. Kappeler, P.) 29–54 (Springer, Berlin, 2010).
Chapter Google Scholar
Frankel, A. S. Sound production. In Encyclopedia of Marine Mammals (eds Perrin, W. F. et al. ) 1056–1071 (Academic Press, London, 2009).
Janik, V. Acoustic communication networks in marine mammals. In Animal Communication Networks (ed. McGregor, P. K.) 390–415 (Cambridge University Press, Cambridge, 2005).
Madsen, P. T. et al. Sperm whale sound production studied with ultrasound time/depth-recording tags. J. Exp. Biol. 205 (13), 1899–1906 (2002).
Article CAS PubMed Google Scholar
Weilgart, L. & Whitehead, H. Distinctive vocalisations from mature sperm whales ( Physeter macrocephalus ). Can. J. Zool. 66 , 1931–1937 (1988).
Article Google Scholar
Richardson, W. J., Greene, C. R., Malm, C. I. Jr. & Thomson, D. H. Marine Mammals and Noise (Academic Press, London, 1995).
Google Scholar
Madsen, P. T., Wahlberg, M. & Møhl, B. Male sperm whale ( Physeter macrocephalus ) acoustics in a high-latitude habitat: implications for echolocation and communication. Behav. Ecol. Sociobiol. 53 , 31–41 (2002).
Whitehead, H. Sperm Whale: Social Evolution in the Ocean (The University of Chicago Press, Chicago, 2003).
Gordon, J. C. D. The behaviour and ecology of sperm whales off Sri Lanka (Doctoral dissertation, Darwin College, Cambridge, 1987).
Weilgart, L. Vocalisations of the sperm whale ( Physeter macrocephalus ) off the Galapagos Islands as related to behavioural and circumstantial variables (Doctoral dissertation, Dalhousie University, Halifax, Canada, 1990).
Watkins, W. A. & Schevill, W. E. Sperm whale codas. J. Acoust. Soc. Am. 62 , 1485–1490 (1977).
Article ADS Google Scholar
Oliveira, C., Wahlberg, M., Johnson, M., Miller, P. J. O. & Madsen, P. T. The function of male sperm whale slow clicks in high latitude habitat: communication, echolocation, or prey debilitation?. J. Acoust. Soc. Am. 133 (5), 3135–3144 (2013).
Article ADS PubMed Google Scholar
Goold, J. C. Behavioural and acoustic observations of sperm whales in Scapa Flow, Orkney Islands. J. Mar. Biol. Assoc. U. K. 79 , 541–550 (1999).
Weir, C. R., Frantzis, A., Alexiadou, O. & Goold, J. C. The burst-pulse nature of ‘squeal’ sounds emitted by sperm whales ( Physeter macrocephalus ). J. Mar. Biol. Assoc. U. K. 87 , 39–46 (2007).
Priano, M., Pavan, G., Manghi, M. & Fossati, C. Rilieviacusticisulcapodoglio ( Physeter macrocephalus ) nel Mar MediterraneoCentrale. Natura 90 , 181–188 (2001).
Drouot, V. Ecology of sperm whale ( Physeter macrocephalus ) in the Mediterranean Sea (Doctoral dissertation, University of Wales, Bangor, 2003).
Teloni, V. Patterns of sound production in diving sperm whales in the Northwestern Mediterranean. Mar. Mamm. Sci. 21 (3), 446–457 (2005).
Teloni, V., Zimmer, W. M. X. & Tyack, P. L. Sperm whale trumpet sounds. Bioacoustics 15 (2), 163–174 (2005).
Pace, D. S. On the sperm whale ( Physeter macrocephalus ) ecology, sociality and behaviour off Ischia Island (Italy): patterns of sound production and acoustically measured growth (Doctoral dissertation, Sapienza University of Rome, Italy, 2016). https://iris.uniroma1.it/retrieve/handle/11573/1044620/549333/Tesi%20dottorato%20Pace .
Watkins, W. A. The harmonic interval: fact or artifact in spectral analysis of pulse trains. In Proceedings of the Second Symposium on Marine Bio-Acoustics , Vol. 2, No. 68, 15–43 (1968).
Cranford, T. W., Amundin, M. & Norris, K. S. Functional morphology and homology in the odontocete nasal complex: implications for sound generation. J. Morphol. 228 , 223–285 (1996).
Huggenberger, S., Andrè, M. & Oelschlager, H. H. A. An acoustic valve within the nose of sperm whales Physeter macrocephalus . Mamm. Rev. 44 , 81–87 (2014).
Huggenberger, S., Andrè, M. & Oelschlager, H. H. A. The nose of the sperm whale: overviews of functional design, structural homologies and evolution. J. Mar. Biol. Assoc. U. K. 96 , 783–806 (2016).
Laidre, M. E. & Johnstone, R. A. Animal signals. Curr. Biol. 23 (18), R829–R833 (2013).
Clark, C. J. Signal or cue? Locomotion-induced sounds and the evolution of communication. Anim. Behav. 143 , 83–91 (2018).
Notarbartolo di Sciara, G., Agardy, T., Hyrenbach, D., Scorazzi, T. & Van Klaveren, P. The Pelagos sanctuary for Mediterranean marine mammals. Aquat. Conserv. Mar. Freshw. Ecosyst. 18 , 367–391 (2008).
Barth, A., Alvera-Azcárate, A., Rixen, M. & Beckers, J. M. Two-way nested model of mesoscale circulation features in the Ligurian Sea. Prog. Oceanogr. 66 (2–4), 171–189 (2005).
Goffart, A., Hecq, J. H. & Prieur, L. Contrôle du phytoplancton du bassinLigure par le front liguroprovençal (secteur Corse). Oceanol. Acta 18 , 329–342 (1995).
Azzellino, A. et al. Predictive habitat models for managing marine areas: spatial and temporal distribution of marine mammals within the Pelagos Sanctuary (Northwestern Mediterranean Sea). Ocean Coast. Manag. 67 , 63–74 (2012).
Azzellino, A., Airoldi, S., Lanfredi, C., Podestà, M. & Zanardelli, M. Cetacean response to environmental and anthropogenic drivers of change: results of a 25-year distribution study in the north-western Mediterranean Sea. Deep Sea Res. Part II 146 , 104–117 (2017).
Watwood, S. L., Miller, P. J. O., Johnson, M., Madsen, P. T. & Tyack, P. L. Deep-diving foraging behaviour of sperm whales ( Physeter macrocephalus ). J. Anim. Ecol. 75 , 814–825 (2006).
Article PubMed Google Scholar
Giorli, G., Neuheimer, A. & Au, W. Spatial variation of deep diving odontocetes’ occurrence around a canyon region in the Ligurian Sea as measured with acoustic techniques. Deep-Sea Res. Part I 116 , 88–93 (2016).
Drouot-Dulau, V. & Gannier, A. Movements of sperm whale in the western Mediterranean Sea: preliminary photo-identification results. J. Mar. Biol. Assoc. U. K. 87 , 195–200 (2007).
Teloni, V., Zimmer, W. M. X., Wahlberg, M. & Madsen, P. T. Consistent acoustic size estimation of sperm whales using clicks recorded from unknown aspects. J. Cetacean Res. Manag. 9 (2), 127–136 (2007).
Pierantonio, N., Airoldi, S. Acoustically derived growth-rates of male sperm whales ( Physeter macrocephalus ) in the NW Mediterranean Sea. In Proceedings of the 28th Annual Conference of the European Cetacean Society. https://www.europeancetaceansociety.eu/sites/default/files/28th%20conference%20Liege%20abstract_book.pdf (2014).
Pierantonio, N., Soldano, G., Airoldi, S. 2016. A new equation to calculate the allometric inter-pulse interval to body length relationship in Mediterranean male sperm whales. In Proceedings of the 30th Annual Conference of the European Cetacean Society . https://www.europeancetaceansociety.eu/sites/default/files/30th%20ECS2016_MADEIRA_version_3.0.pdf (2016).
Lettevall, E. et al. Social structure and residency in aggregations of male sperm whales. Can. J. Zool. 80 , 1189–1196 (2002).
Moulins, A. & Wurtz, M. Occurrence of a herd of female sperm whales and their calves ( Physeter macrocephalus ) off Monaco in the Ligurian Sea. J. Mar. Biol. Assoc. U. K. 85 (1), 213–214 (2005).
Podestà, M. & Bortolotto, A. Il ProgettoSpiaggiamenti del Centro StudiCetacei: analisideirisultati di 11 anni di attività. Natura 90 , 145–158 (2001).
Azzellino, A., Airoldi, S., Gaspari, S. & Nani, B. Habitat use of cetaceans along the continental slope and adjacent waters in the western Ligurian Sea. Deep Sea Res. Part I 55 , 296–323 (2008).
Tepsich, P., Rosso, M., Halpin, P. N. & Moulins, A. Habitat preference of two deep diving cetacean species in the northern Ligurian Sea. Mar. Ecol. Prog. Ser. 508 , 247–260 (2014).
Pace, D. S. et al. Habitat suitability modelling in sperm whale ( Physeter macrocephalus ) social groups. J. Wildl. Manag. 82 (5), 1062–1073 (2018).
Pace, D. S. et al. An integrated approach for cetacean knowledge and conservation in the central Mediterranean Sea using research and social media data sources. Aquat. Conserv. Mar. Freshw. Ecosyst. 29 (8), 1302–1323 (2019).
Mussi, B., Miragliuolo, A., Zucchini, A. & Pace, D. S. Occurrence and spatiotemporal distribution of sperm whale ( Physeter macrocephalus ) in the submarine canyon of Cuma (Tyrrhenian Sea, Italy). Aquat. Conserv. Mar. Freshw. Ecosyst. 24 (S1), 59–70 (2014).
Pace, D. S., Mussi, B., Wurtz, M. & Gordon, J. D. C. Foreword. Aquat. Conserv. Mar. Freshw. Ecosyst. 24 (S1), 1–3 (2014).
Clark, R. A. et al. Cetacean sightings and acoustic detections in the offshore waters of the Maldives during the northeast monsoon seasons of 2003 and 2004. J. Cetacean Res. Manag. 12 (2), 227–234 (2012).
De Vos, A. et al. Cetacean sightings and acoustic detections in the offshore waters of Sri Lanka: March–June 2003. J. Cetacean Res. Manag. 12 (2), 185–193 (2012).
MathSciNet Google Scholar
Gannier, A., Petiau, E., Dulau, V. & Rendell, L. Foraging dives of sperm whales in the north-western Mediterranean Sea. J. Mar. Biol. Assoc. UK 92 , 1799–1808 (2012).
Würsig, B. & Jefferson, T. A. Methods of photo-identification for small cetaceans. Rep. Int. Whaling Comm. Spec. Issue 12 , 43–52 (1990).
Carpinelli, E. et al. Assessing sperm whale ( Physeter macrocephalus ) movements within the western Mediterranean Sea through photo-identification. Aquat. Conserv. Mar. Freshw. Ecosyst. 24 (S1), 23–30 (2014).
Mariani, M. et al. Analysis of the natural markings of Risso’s dolphin ( Grampus griseus ) in the central Mediterranean Sea. J. Mamm. 97 (6), 1512–1524 (2016).
Arnbom, T. Individual identification of sperm whales. Rep. Int. Whaling Comm. 37 , 201–204 (1987).
Pavan, G. & Borsani, J. F. Bioacoustic research on cetaceans in the Mediterranean Sea. Mar. Freshw. Behav. Phys. 30 , 99–123 (1997).
Center for Conservation Bioacoustics. Raven Pro: Interactive Sound Analysis Software (Version 2.0) [Computer Software]. Ithaca. Cornell Laboratory of Ornithology. Available from https://ravensoundsoftware.com/ (2019).
Charif, R. A., Waack, A. M. & Strickman, L. M. Raven Pro 1.4 User’s Manual (The Cornell Lab of Ornithology, Ithaca, 2010).
Rice, A. N., Palmer, K. J., Tielens, J. T., Muirhead, C. A. & Clark, C. W. Potential Bryde’s whale ( Balaenoptera edeni ) calls recorded in the northern Gulf of Mexico. J. Acoust. Soc. Am. 135 (5), 3066–3076 (2014).
Clink, D. J., Grote, M. N., Crofoot, M. C. & Marshall, A. J. Understanding sources of variance and correlation among features of Bornean gibbon ( Hylobates muelleri ) female calls. J. Acoust. Soc. Am. 144 (2), 698–708 (2018).
Burrough, P. A. Principles of Geographical Information Systems for Land Resources Assessment (Oxford University Press, Oxford, 1986).
Book Google Scholar
Zuur, A. et al. Mixed Effects Models and Extensions in Ecology with R (Springer, Berlin, 2009).
Book MATH Google Scholar
Afifi, A. & Clark, V. Computer-Aided Multivariate Analysis. Texts in Statistical Science (Chapman and Hall/CRC, Boca Raton, 1996).
Guisan, A. & Zimmermann, N. E. Predictive habitat distribution models in ecology. Ecol. Model. 135 , 147–186 (2000).
Sueur, J., Aubin, T. & Simonis, C. Seewave: a free modular tool for sound analysis and synthesis. Bioacoustics 18 , 213–226 (2008).
Wickham, H. ggplot2: Elegant Graphics for Data Analysis (Springer, Berlin, 2016). https://ggplot2.tidyverse.org .
Wickham, H., François, R., Henry, L., Müller, K. dplyr: A Grammar of Data Manipulation . R package version 0.8.5. https://CRAN.R-project.org/package=dplyr (2020).
Bates, D., Mächler, M., Bolker, B. & Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67 , 1–48 (2015).
Kuznetsova, A., Brockhoff, P. B. & Christensen, R. H. B. lmerTest package: tests in linear mixed effects models. J. Stat. Softw. 82 , 1–26 (2017).
Bradbury, J. W. & Vehrencamp, S. L. Principles of Animal Communication (Sinauer Associates, Inc., Sunderland, 2011).
Maynard Smith, J. & Harper, D. Animal Signals (Oxford University Press, Oxford, 2003).
Scott-Phillips, T. C. Defining biological communication. J. Evol. Biol. 21 , 387–395 (2008).
Darwin, C. The Descent of Man, and Selection in Relation to Sex (Princeton University Press, Princeton, 1871).
Donath, J. Signals, Cue and Meaning (Massachusetts Institute of Technology, Cambridge, 2011).
Naguib, M. Animal Communication: Overview. In Encyclopedia of Language and Linguistics (ed. Brown, K.) 276–284 (Elsevier, Amsterdam, 2006).
Murray, T. G., Zeil, J. & Magrath, R. D. Sounds of modified flight feathers reliably signal danger in a pigeon. Curr. Biol. 27 , 1–6 (2017).
Article CAS Google Scholar
Falls, J. B. Individual recognition by sound in birds. In Acoustic Communication in Birds (eds Kroodsma, D. E. & Miller, E. H.) 237–278 (Academic Press, London, 1982).
Gwilliam, J., Charrier, I. & Harcourt, R. G. Vocal identity and species recognition in male Australian sea lions Neophoca cinerea . J. Exp. Biol. 211 , 2288–2295 (2008).
McComb, K., Reby, D., Baker, L., Moss, C. & Sayialel, S. Long-distance communication of acoustic cues to social identity in African elephants. Anim. Behav. 65 , 317–329 (2003).
Briefer, E. F. Vocal expression of emotions in mammals: mechanisms of production and evidence. J. Zool. 288 (1), 1–20 (2012).
Morton, E. S. On the occurrence and significance of motivation-structural rules in some bird and mammal sounds. Am. Nat. 111 , 855–869 (1977).
Soltis, J. Emotional communication in African elephants ( Loxodonta africana ). In Evolution of Emotional Communication: From Sounds in Nonhuman Mammals to Speech and Music in Man (eds Altenmüller, E. et al. ) 105–115 (Oxford University Press, Oxford, 2013).
Rendall, D., Seyfarth, R. M., Cheney, D. L. & Owren, M. J. The meaning and function of grunt variants in baboons. Anim. Behav. 57 , 583–592 (1999).
Slocombe, K. E. & Zuberbühler, K. Chimpanzees modify recruitment screams as a function of audience composition. Proc. Natl. Acad. Sci. 104 , 17228–17233 (2007).
Article ADS CAS PubMed PubMed Central Google Scholar
Luo, J., Goerlitz, H., Brumm, H. & Wiegrebe, L. Linking the sender to the receiver: vocal adjustments by bats to maintain signal detection in noise. Sci. Rep. 5 , 18556 (2016).
Article ADS CAS Google Scholar
Rendell, L. et al. Abundance and movements of sperm whales in the western Mediterranean basin. Aquat. Conserv. Mar. Freshw. Ecosyst. 24 (S1), 31–40 (2014).
Drouot, V., Gannier, A. & Goold, J. C. Summer social distribution of sperm whales ( Physeter macrocephalus ) in the Mediterranean Sea. J. Mar. Biol. Assoc. U. K. 84 (3), 675–680 (2004).
Pace, D. S. et al. Sociality of sperm whales ( Physeter macrocephalus ) off Ischia Island (Tyrrhenian Sea, Italy). Aquat. Conserv. Mar. Freshw. Ecosyst. 24 (S1), 71–82 (2014).
Pace, D. S., Miragliuolo, A. & Mussi, B. Behaviour of a social unit of sperm whales ( Physeter macrocephalus ) entangled in a driftnet off Capo Palinuro (Southern Tyrrhenian Sea, Italy). J. Cetacean Res. Manag. 10 (2), 131–135 (2008).
Lanfredi, C. et al . Preliminary assessment of sperm whale foraging habitat in the Ligurian Sea (North-Western Mediterranean Sea) by using underwater vocalizations. In Proceedings of the 30th Annual Conference of the European Cetacean Society . https://www.europeancetaceansociety.eu/sites/default/files/30th%20ECS2016_MADEIRA_version_3.0.pdf (2016).
IUCN-MMPATF. North West Mediterranean Sea, Slope and Canyon system IMMA Factsheet. IUCN Joint SSC/WCPA Marine Mammal Protected Areas Task Force. https://www.marinemammalhabitat.org/portfolio-item/north-western-mediterranean-sea-slope-canyon-system/ (2017).
Claro, B., Perez-Jorge, S. & Frey, S. Seafloor geomorphic features as an alternative approach into modelling the distribution of cetaceans. Ecol. Inform. 58 , 101092 (2020).
Fernandez-Arcaya, U. et al. Ecological role of submarine canyons and need for canyon conservation: a review. Front. Mar. Sci. 4 , 5 (2017).
Download references
Acknowledgements
The authors would like to thank all the people (researchers, research assistants, collaborators, volunteers, under- and post-graduate students) who participated in the surveys of the Tethys Research Institute/CSR Project. Many thanks to the skippers, Roberto Raineri and Paolo Pinto, who have provided invaluable support during fieldwork. Recordings by CIBRA have been made possible by the collaboration of Marco Priano, Michele Manghi, Claudio Fossati and Gionata Montesi. Thanks to the International Fund of Animal Welfare (IFAW) for the use of the software LOGGER, St. Andrews University for PAMGuard Software and to Portosole Sanremo for the logistic support. We are especially grateful to Giovanna Jona Lasinio for her statistical advice; to Bobbi Estabrook for the English review; to Maria Cristina Gambi, Raffaella Tizzi and Elena Papale for their encouragements. Many thanks to the three anonymous reviewers whose comments significantly improved the manuscript.
Author information
Authors and affiliations.
Department of Environmental Biology, Sapienza University of Rome, Rome, Italy
D. S. Pace, G. Giacomini, M. Silvestri & D. Ardizzone
Tethys Research Institute, Milan, Italy
C. Lanfredi & S. Airoldi
Department of Earth and Environmental Sciences, CIBRA, University of Pavia, Pavia, Italy
You can also search for this author in PubMed Google Scholar
Contributions
D.S.P., D.A. and C.L. conceived the study; S.A., C.L. and G.P. conducted the fieldwork; D.S.P., M.S., G.P. and G.G. analysed the acoustic data; C.L., M.S. and D.S.P. performed statistical analysis. All authors reviewed and approved the manuscript.
Corresponding authors
Correspondence to D. S. Pace or C. Lanfredi .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary informations., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Pace, D.S., Lanfredi, C., Airoldi, S. et al. Trumpet sounds emitted by male sperm whales in the Mediterranean Sea. Sci Rep 11 , 5867 (2021). https://doi.org/10.1038/s41598-021-84126-8
Download citation
Received : 29 May 2020
Accepted : 11 February 2021
Published : 12 March 2021
DOI : https://doi.org/10.1038/s41598-021-84126-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Passive acoustic monitoring of sperm whales and anthropogenic noise using stereophonic recordings in the mediterranean sea, north west pelagos sanctuary.
- Marion Poupard
- Maxence Ferrari
- Hervé Glotin
Scientific Reports (2022)
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Where Do Sperm Whales Live?
Sperm whales (particularly adult males) can be found in all of the world’s major oceans, from the warm tropical climates in and around the equator to the northern and southern polar hemispheres. Young sperm whales generally stay close to their mothers until they reach maturity, and then they will go and venture out on their own.
Unlike adult male sperm whales, the female sperm whales and their calves prefer staying warm climates around the equator throughout the year. The adult male sperm whales travel to the colder climates during the off-season and return to the warmer climates during the mating season , which occurs during the colder winter months.
In fact, some male sperm whales can travel throughout all of the world’s major oceans over the course of their 70-year lifespan , allowing them to travel the world before they die.
Male sperm whales are also known to be solitary animals, often traveling alone or in small groups where they may form a loose bond with another male. In addition to traveling between warm and cold climates, sperm whales are also among the deepest diving species of all cetaceans.
During deep dives, sperm whales can dive to depths more than 3,000 ft. to look for giant squid and octopus to eat. Some sperm whales can even be found with marks and scars around their head from fights with large squid that tried to avoid being eaten by latching onto the whale’s head.
Being equipped with echolocation allows the sperm whale to use sound to navigate the ocean and search for prey, which is extremely important since these marine mammals often hunt in complete darkness. This makes echolocation essential for their survival and ability to obtain adequate food for their diet.
In fact, these marine mammals dive so deep that it has been difficult for researchers to observe or obtain footage of the exact hunting methods used by sperm whales.
In addition to traveling the world and diving to depths over 3,000 ft. these marine mammals are the largest animals within the toothed whale family and have the biggest brain of all living animals. At full maturity, the male sperm whale can reach lengths over 65 ft. long and weigh 45 tons when fully matured. Female sperm whales, on the other hand, measure 1/2 to 1/3 the size of their male counterparts.
Note : To use a comparison to show you just how big the sperm whale is, as stated early reaching lengths of up to 67 ft. the sperm whale is the largest of the toothed whale family and is significantly larger than the second-largest toothed whale, Baird’s beaked whale , which can reach lengths of 40+ ft. when fully matured. That’s a difference of over 20 ft. long!
Solitary lifestyle
While not always the case, it seems as though larger marine mammals such as male sperm whales tend to lead a more solitary lifestyle when compared to smaller cetacea like dolphins and porpoises . As stated earlier, they often travel alone when mating season is not in session and can wander off to various locations of their choosing.
In some cases, a male sperm whale may choose to form a bond with another male whale and form a small pod; however, the length of time that the relationship/pod lasts can vary from one male to the next.
Although the reason for their solitary lifestyle is unknown, it is possible that they do not need to rely on large groups to survive or protect themselves since they are fairly large and robust in size. After all, an adult male can reach lengths over 67 ft. and a weight of 45 tons (not common).
Once fully grown, a male sperm whale is largely protected by its size alone, which is likely to deter most would-be predators. In fact, the only known predators of the sperm whale are a pack of hungry killer whales and potentially large sharks , which may try to prey on young whales since they are easier to catch.
Motherly protection
While male sperm whales are fairly solitary, the female sperm whales can often be seen grouped with other females, and they’re young. As the males go off to wander the oceans, the females stay together to protect their young from potential predators (such as sharks) and nurture them to grow up to be strong in big.
During the first few years of birth, the baby whales are nurtured and cared for by being fed milk which can last for 1 1/2 – 4 years or more depending on the emotional bond they have with their mother.
When the young male whales reach the ages 4 – 21, they choose to leave their pod and go off independently. The young female whales continue to maintain relationships with their local pods and, upon sexual maturity, may begin mating and reproducing offspring of their own.
To protect their young from potential threats, the adult females may form a circle around the child with their heads facing the child and their flukes facing the outside circle. This formation is used as a form of defense as the flukes are powerful enough to immobilize or even kill a predator if they are struck hard enough.
In some cases, the female whales may also form an overwatch position where their flukes are pointed in towards the child, and their heads are facing the outside of the circle.
As far as pods/groups go, female sperm whales may form 6 – 10 members. However, larger variations may occur in some pods containing 20 or more members. Unlike other cetacea, however, female sperm whales do not appear to show a preference for maintaining relationships with pod members within their families.
Once a female sperm whale becomes part of a pod and spends several years together with its pod members, they rarely leave to join a new pod.
Related posts:
- Marine Mammal Conservation
- What Is A Group Of Whales Called?
- Whales For Kids – Questions and Answers
- Arnoux’s Beaked Whale Facts | Diet, Migration and Reproduction

Sex in Cetaceans pp 443–467 Cite as
Sperm Whale Reproductive Strategies: Current Knowledge and Future Directions
- Ana Eguiguren 3 ,
- Christine M. Konrad Clarke 4 &
- Mauricio Cantor 5 , 6
- Open Access
- First Online: 26 September 2023
4759 Accesses
1 Altmetric
Sperm whales’ reproductive strategies are centered around their extreme sexual dimorphism, both in morphology and behavior. Females are much smaller than males and are highly social. Females live in stable, matrilineally based social units with communal care of calves, including cooperative defense and allonursing. In contrast, male sperm whales are large nearly solitary nomads. Males disperse from their natal social unit and move toward the poles, where they eat and grow almost three times larger than females. Males’ great ranges span across and between ocean basins, allowing global genetic connectivity. As they rove the warm waters where females concentrate, mature males avoid each other; physical aggression on the breeding grounds is rarely observed. Instead, males may rely on powerful acoustic displays to establish dominance over potential competitors and provide females with an honest quality signal. Associations between sexually mature males and groups of females tend to be transitory. Disproportionate mating success of some males is suggested by evidence of paternal relatedness within female social units. Sperm whale mothers provide a substantial investment of time and energy to calves, resulting in the slowest reproductive rate among cetaceans. The peculiar characteristics of sperm whale mating systems reflect the evolutionary interplay between habitat structure, predation risk, sociality, and reproduction. A convergence of reproductive biology between sperm whales and African elephants likely results from similarities in these ecological pressures. Despite sperm whales being one of the most studied cetaceans, much remains unknown about their reproductive strategies. Most of what we know comes from whaling data and long-term observational and modeling studies. The rapid advances in technology for behavioral and physiological studies at sea can refine our understanding of these elusive deep-diving animals’ social, mating, and caring systems and the extent to which these vary across oceans.
You have full access to this open access chapter, Download chapter PDF
19.1 Introduction
The sperm whale ( Physeter macrocephalus ) is a unique creature shaped by the deep ocean—a dark desert where these whales live highly social lives (Whitehead 2003 ). Their distinctiveness is showcased in their morphology; sperm whales are the largest toothed predators, their massive heads contain the world’s biggest brains in absolute size, and they possess the most powerful biological sonar. Sperm whales use these extreme characteristics for hunting deep-sea creatures, communicating with conspecifics, and sustaining a rich social and cultural life (reviewed by Cantor et al. 2019 ). Sperm whales are the most sexually dimorphic of all cetaceans (Fig. 19.1 )—mature males can be 40% larger than females and three times as heavy (Rice 1989 ). The magnitude of such morphological differences between sperm whale sexes parallels sex-based distinctions in social behaviors, distribution, movements, and ecology, all of which have consequences for their reproductive strategies (Whitehead 2003 ).

Female and immature sperm whales gathered around a large mature male off Dominica, West Indies, showing their distinct body size dimorphism (credit: Marina Milligan, Dominica Sperm Whale Project)
Female sperm whales are highly social, whereas males are solitary nomads. The complex sociality of females is reflected in their multilevel societies (Whitehead et al. 2012 ; Cantor et al. 2015 ). At the lowest level, individual female sperm whales spend their lives in close contact with few other individuals (Christal et al. 1998 ; Gero et al. 2014 ) to whom they are often matrilineally related (Lyrholm and Gyllensten 1998 ; Mesnick 2001 ; Gero et al. 2008 ; Konrad et al. 2018b ). These long-term associations are called social units (Whitehead 2003 ). Members of different social units interact with other social units over a few hours to a few days, forming groups (Christal and Whitehead 2001 ). These temporary groups are exclusively formed among members of social units that share a considerable portion of their vocal repertoire of “codas” (acoustic communication signals used in social contexts). These social preferences give rise to the uppermost social level, the clan , comprising 100s–1000s of individuals with shared vocal repertoires (Rendell and Whitehead 2003 ). In contrast, male sperm whales leave their maternal social unit at a young age (6–16 years; Best, 1979 ), heading toward high-latitude waters where they eat, grow, and sexually mature. These bachelor males can form occasional associations with other males (Christal and Whitehead 1997 ; Kobayashi et al. 2020 ) but ultimately spend most of their adult lives solitarily (Whitehead 2003 ).
Females prefer warm waters, while males are distributed circumglobally. The multilevel societies of females inhabit warm tropical and subtropical waters within a 0–40°N/S latitudinal range; males can occupy waters from the equator to the polar regions (Best 1979 ; Whitehead 2003 ; Fig. 19.2 ). Females can travel hundreds of kilometers a year but are philopatric at the ocean basin scale, as demonstrated by the significant differences in the maternally inherited mitochondrial DNA between oceans (Lyrholm and Gyllensten 1998 ; Engelhaupt et al. 2009 ). Males can travel much further than females; mature male sperm whales travel 5000 km a year on average (Mizroch and Rice 2013 ). Males leave polar waters to visit tropical waters and interact with females in search of mating opportunities (Rice 1989 ; Mizroch and Rice 2013 ). Homogeneity in nuclear DNA around the globe suggests that males reproduce with females across ocean basins (Lyrholm et al. 1999 ; Engelhaupt et al. 2009 ). These spatial differences between the sexes and morphological and behavioral ones are associated with distinct trophic niches between females and males.
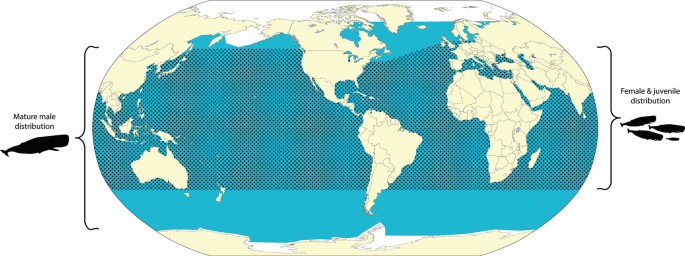
Approximate representation of the global sperm whale distribution. The range of mature males is in blue, and the range of females is in the dotted pattern (modified from Whitehead 2009 )
The sperm whale diet mainly comprises deep-sea squids (Kawakami 1980 ). Female sperm whales are effective hunters of their chosen prey, whereas males tend to have a more generalist diet which may include fish and crustaceans (Clarke et al. 1988 ; Best 1999 ; Mendes et al. 2007 ). These trophic differences result partly from the large-scale differences in the geographical distribution of males and females, yet small-scale habitat preferences also matter. For instance, in the Mediterranean Sea population, where male and female sperm whales are confined to the same ocean basin, sex-based trophic niche distinctions remain (Pirotta et al. 2020b ) and mirror the distinct small-scale habitat preferences between the sexes (Pirotta et al. 2020a ). In other areas where males and females co-occur, males usually have lower feeding success rates suggesting that females can outcompete them (Whitehead 2003 ).
Why and how did these extreme differences in morphology, behavior, distribution, and ecology arise? The origin of the extreme sexual dimorphism in sperm whales is most likely rooted in different sexual selection pressures on each sex (Whitehead 2003 ). Thus, the extraordinary sexual dimorphism in sperm whales highlights the interplay between mating strategies and all other aspects of behavior and ecology in a species’ evolution (Whitehead 1993 ). Here, we review the current knowledge of the sperm whales’ reproductive biology, mating system, and care system and how these strategies have contributed to shaping their lives through evolutionary time and in recent years. We consider how emerging field techniques can advance our understanding of these elusive social animals’ reproductive biology, mating tactics, and care systems.

19.2 Reproductive Biology
Most of what we know about sperm whale reproductive biology comes from research on whaling operations from the late 1900s, during which researchers collected data on reproductive parameters by inspecting the reproductive organs and body measurements of thousands of hunted whales, as well as the composition of groups observed in the field (e.g., Ohsumi 1965 ; Gaskin 1970 ; Best 1979 ; Best et al. 1984 ; Clarke et al. 2012 ). As a result, while the reproductive physiology of sperm whales is well established, their reproductive behaviors remain largely unknown and may only be revealed by studying live individuals.
Sperm whales are referred to as the quintessential K-selected species because of their slow reproductive rates and high investment in their offspring (Whitehead 2009 ). Females typically reach sexual maturity—indicated by the age of first ovulation—at 9 years (Rice 1989 ) and can conceive shortly after (10 years; Best et al. 1984 ). However, there is significant variation in female age of sexual maturity across regions (6–13 years; Rice 1989 ; Clarke et al. 2012 ). After sexual maturation, females continue to grow until they achieve physical maturity when they are 25–45 years old (Rice 1989 ). Individuals produce a single calf every 4–6 years with a 14–16-month gestation period (Ohsumi 1965 ; Best et al. 1984 ; Rice 1989 ).
Throughout their lifetime, female sperm whales experience a steady decrease in fecundity after they are 10–14 years old, which plummets after they are 40 (Ohsumi 1965 ; Best et al. 1984 ; Whitehead 2009 ). This pattern inspired the hypothesis that sperm whales may be one of the rare species—along with killer whales ( Orcinus orca ), short-finned pilot whales ( Globicephala macrorhynchus ), chimpanzees ( Pan troglodytes ), and humans (Croft et al. 2015 ; Betty et al. 2023 , this book; Wright et al. 2023 , this book)—to display menopause (Whitehead 2003 ). However, although reproductive rates drastically decrease over time, there is no support for a prolonged post-reproductive lifespan in sperm whales (Ellis et al. 2018 ). Old and less fertile females may increase their reproductive success by caring for their kin, as observed in pilot whales (Betty et al. 2023 , this book; Würsig et al. 2023 , this book). There is also evidence of geographic variation in pregnancy rates, which may be related to the effects of whaling directed on large males.
Like females, male sperm whales achieve sexual maturity at around age ten, as indicated by the presence of spermatozoa in their testes (Best 1979 ). Stable isotope analyses suggest males leave close to the onset of sexual maturity at 9–10 years of age and then move toward high latitudes in their 20s (Mendes et al. 2007 ). Males in their 20s experience a brief growth spurt followed by a decaying growth rate that can last until they are 60 (Rice 1989 ). Although males achieve sexual maturity at a similar age as females, it seems that males may not begin mating (i.e., reach sociosexual maturity) until they are 20 years old (Best et al. 1984 ). Throughout the text, when we refer to mature males, we refer to sociosexually mature males.
19.2.1 Copulation
Several events have been described as copulation among sperm whales (Dudley 1725 ; Best et al. 1984 ). Some describe the presence of large males among a group of females and juveniles displaying intense surface activity. In contrast, others describe belly-to-belly contact (in horizontal or vertical orientation) between a large whale assumed to be a male and a smaller one believed to be a female. However, these descriptions are inconsistent, and direct intromission has yet to be observed (Whitehead, 2003 ), so we caution against the interpretation of these interactions as copulation.
19.2.2 Seasonality of reproduction
Sperm whales breed most of the year (Rice 1989 ). In the Northern hemisphere, sperm whales breed between January and August, with a peak between March and June (Rice 1989 ). In the Southern hemisphere, sperm whales breed between July and March, with a peak between September and December (Rice 1989 ; Clarke et al. 2012 ). Not much is known about the seasonality of breeding in tropical waters. No discrete migration patterns associated with breeding have been described for sperm whales. Coinciding with this prolonged breeding season, female sperm whales have a prolonged estrous period, which lasts throughout the breeding season. Females enter estrus every 3–5 years, spending the interim time either gestating, lactating, or resting (Rice 1989 ; Clarke et al. 2012 ).
The factors influencing ovulation timing are unknown. Ovulation may be spontaneous or induced by mating or the presence of a mature male (Best et al. 1984 ). There is some evidence of synchronized ovulation among groups of females (Best et al. 1984 ). However, it is uncertain whether synchrony of estrus is achieved by induction between females, food availability, or the presence of males. While there is no evidence of seasonality in male fertility, the abundance of mature males in female-dominated areas may vary within a year (Whitehead 1993 ). However, there is no evidence of a direct link between this seasonality pattern and female reproductive availability.
19.3 Mating Strategies and Tactics
The male was the focus of intense attention from all group members, who crowded in on him, rolling themselves along his huge body. They just seemed delighted that he was there. For his part, the male was all calm serenity and gentleness. Gordon ( 1998 , 22–25).
Historically, descriptions of the sperm whale mating system centered on male–male competition for control over groups of females. Mature males were called “schoolmasters” that controlled access to “harems” (groups of females; e.g., Beale 1835 ). Accounts from open-boat whaling years (late nineteenth century) of intense fights between males involving head-butting, jaw-locking, and tooth-shattering (Clarke and Paliza 1988 ) may have influenced such portrayals. It was later found that associations between males and groups of females are ephemeral (Best 1979 ). Moreover, the association between groups of females and individual males seems to be determined by female choice rather than male aggression (Whitehead 1993 ). Here, we describe the mating strategies and tactics of male and female sperm whales.
19.3.1 Male Roving
Mature males visit the warm waters inhabited by groups of females and stay in a given region for a short period within a year (Whitehead 1993 ; Gero et al. 2014 ), but exactly how long they stay until heading poleward remains unknown. In warm water, males rove between groups of females and associate with specific females over a few days to weeks (Whitehead 1993 ; Coakes and Whitehead 2004 ; Gero et al. 2014 ).
The same males are rarely re-sighted with the same group of females or in the same breeding ground over different years (Whitehead 1993 ; Jaquet and Gendron 2009 ; Gero et al. 2014 ). Yet, some degree of male philopatry exists; studies in various ocean basins have documented mature males returning to the same areas over the years (Christal 1998 ; Gero et al. 2014 ; van der Linde and Eriksson 2020 ; Girardet et al. 2022 ). For example, an 8-year study around the Azores found that, while the majority of males were sighted only during 1 or 2 years, one individual was re-sighted every year (Van der Linde and Eriksson, 2020 ). It is unclear whether the scarcity of re-sightings of mature males across years results from the challenge of finding them because of their low abundance on breeding grounds (<5%; Whitehead 1993 ; Gero et al. 2014 ) and fleeting presence in a given area or from a lack of male geographic or social philopatry. Additionally, there may be individual variations in the movement patterns of mature males among breeding grounds.
When males cannot defend territories or females, roving among groups of females is a better mating strategy than staying with a group if the benefits of encountering new females outweigh the benefits of staying (Whitehead 1990 ). A model predicted that roving would be favored over residency when the time to find a new female group is shorter than a female’s typical estrous cycle and that roving would be more favorable in species with variation in the competitive ability of males (Whitehead 1990 ). Non-territorial species with high sexual dimorphism often have a roving strategy, suggesting that this behavior is linked with some form of size-dependent male–male competition, which may lead to delayed sexual maturity (Whitehead 1994 ). Males would thus benefit from delaying reproduction until they are large enough to be competitive (Whitehead 1994 ). However, it is not apparent how male size is involved in male–male competition in sperm whales despite the remarkable sexual dimorphism.
19.3.2 Male Contest Competition
In species with pronounced sexual dimorphism, aggression between males is frequent. However, observations of aggression between male sperm whales within breeding grounds are rare (Whitehead 2003 ; Gero et al. 2014 ). In 11 years across four decades of study around the Galápagos Islands, only one instance of aggression between males was documented (Whitehead 1993 ). Similar work off Dominica, spanning nearly 20 years, reported no aggression among males (Gero et al. 2014 ). However, males sometimes have parallel tooth scars, presumably acquired during fights with other individuals (Kato 1984 ). Because these scars are more frequent among larger males than younger ones, they may happen during intra-sexual contest competition (Best 1979 ; Kato 1984 ). As these tooth rake scars are most often acquired at higher latitudes, in male-only feeding grounds (Kato 1984 ; Whitehead 2003 ), scarring in male sperm whales, as in other odontocetes, is hypothesized to have evolved as a signal of male quality, rather than resulting from direct competition for individual females (MacLeod 1998 ). Sperm whales do not develop teeth until sexual maturity (and until then use suction to feed without teeth), which supports the hypothesis that sperm whale teeth evolved in the context of intra-sexual competition (MacLeod 1998 ). Indirect evidence of male–male combats is occasional missing or broken teeth (Clarke and Paliza 1988 ). However, the frequency of broken teeth is similar among sexes and age classes, providing weak evidence of intra-sexual fighting as the primary cause (Whitehead 2003 ).
The anatomy of the male sperm whale’s head may also support male contest competition. Open-boat whaling records described males using their heads as a “battering ram” against other males and whaling vessels (Berzin 1972 ; Carrier et al. 2002 ). Mechanical models indicate that the organs within the sperm whale nose could act as shock absorbers during male–male head-butting (Carrier et al. 2002 ; Panagiotopoulou et al. 2016 ). Mature males have disproportionally larger heads than females, which protrude up to 1.5 m from their lower mandible (Cranford 1999 ). Most of this difference is accounted for by the hypertrophy of two underlying organs, the spermaceti and the junk (homologous to the odontocete melon), which facilitate sound production and processing (Fig. 19.3 ; Cranford 1999 ). The spermaceti organ accounts for most of the relative size dimorphism between the sexes and can account for up to 30% of male body weight compared to 20% in females (Whitehead 2003 ). Among odontocetes, the relative size of the melon and the degree of sexual dimorphism are positively correlated, suggesting that enlarged melons may aid male–male competition (Carrier et al. 2002 ), but for sperm whales, this competition may be acoustic rather than physical.
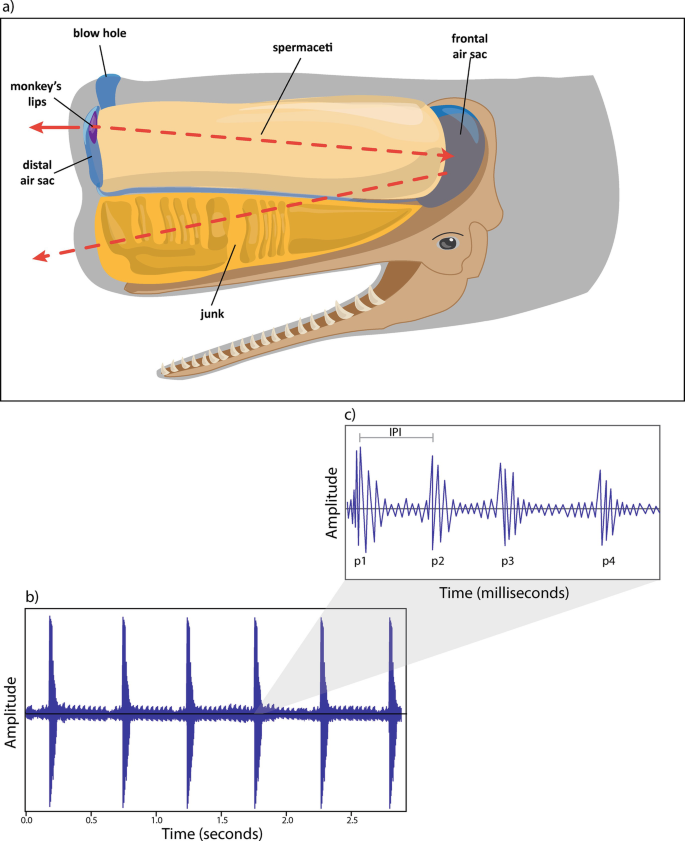
Illustration of the sperm whale’s nose anatomy ( a ). Red arrows show the trajectory of sound produced in the “monkey’s lips.” The solid arrow indicates the trajectory of the first pulse in a click, while the dashed line shows the trajectory followed by subsequent pulses (modified from Norris and Harvey 1972 ; Huggenberger et al. 2016 ). Waveforms of the multipulse structure of a sperm whale click: ( b ) the waveform of a sequence of clicks and ( c ) a single click are shown. Individual pulses within a click are indicated as p1–p4, and the inter-pulse interval (IPI) is shown in the inset ( c ) (modified from Norris and Harvey 1972 ). The IPI is thus proportional to the size of the sperm whale’s nose
There is considerable criticism against the hypothesis that the enlarged male sperm whale head functions primarily as a weapon for intrasexual contest competition. First, the scarcity of documented fights involving head-ramming between males mentioned above raises whether this behavior is typical enough to result in strong sexual selection (Whitehead 2003 ). However, it could be that fights between males are rarely observed because they take place very quickly or below the surface (Clarke and Paliza 1988 ). Secondly, the sound production organs involved in echolocation and communication are in the proposed collision area, making head-butting potentially dangerous (Huggenberger et al. 2016 ).
19.3.3 Male Signal Competition
Alternatively, sperm whale head sexual dimorphism may be used for acoustically signaling male quality. Physiologically constrained acoustic signals can act as indicators of male quality (Fitch and Hauser 2003 ; Wyman et al. 2012 ; Orbach 2019 ). Females may use acoustic displays to assess the quality of potential mates and males to gauge each other’s competitive potential (Fitch and Hauser 2003 ). Through their sound production mechanism, sperm whale vocalizations carry accurate information on the size of their producer. Sperm whale clicks contain multiple evenly spaced pulses (Backus and Schevill 1966 ), which result from the reverberations of a single click first produced at the front of the sperm whale head in the monkey lips and then bounce against the air sacs surrounding the spermaceti organ (Norris and Harvey 1972 ). The inter-pulse interval (IPI) of sperm whale clicks is therefore proportional to the size of the spermaceti organ (Gordon 1991 ). Thus, the male sperm whale head may advertise size and quality (Cranford 1999 ).
In the ocean, where sound travels long distances, acoustic displays can be an energetically effective way for males to advertise their size and assess potential competitors’ dominance while avoiding costly confrontations and minimizing travel (Orbach 2019 ). Sperm whale clicks may be audible to other whales in a 60 km radius, making these vocalizations an effective means to advertise the male’s presence to competing males and interested females (Madsen et al. 2002 ). Given that male sperm whales are very rarely observed together (<100 m) in breeding grounds, it seems likely that they use acoustic signals to avoid each other (Whitehead 1993 ; Christal and Whitehead 1997 ). Male sperm whales produce “clangs,” also referred to as “slow clicks,” which have longer inter-click intervals (6–8 s), lower directionality, and higher energy in low-frequency ranges than regular echolocation clicks (Weilgart and Whitehead 1988 ; Madsen et al. 2002 ). Males produce slow clicks most of their time on breeding grounds (75%) (Whitehead 1993 ), but they also make them at high latitudes (Madsen et al. 2002 ; Oliveira et al. 2013 ), suggesting slow clicks do not function exclusively in sexual competition but could also serve for communication with other males in the context of cooperation or competition for prey (Whitehead 2009 ; Cantor et al. 2019 ).
19.3.4 Female Mate Choice
In sperm whales, observations of interactions between females and mature males suggest that female mate choice plays a key role in mating (Whitehead 1993 ). Female groups’ reaction to a visiting male varies from actively traveling toward him and displaying a highly excited state to diving away from him (Whitehead 1993 ). Overall, females tend to aggregate around males (Gero et al. 2014 , Girardet et al. 2022 ), with some males being especially well received. The breadth of reactions of groups of females toward males and a perceived lack of aggression from males directed toward females and other males within breeding grounds (Whitehead 1993 ) indicates that female mate choice plays a central role in sperm whale reproduction. How exactly this female choice operates remains poorly understood.
Sperm whales likely can distinguish social membership (at the social unit and clan level) based on vocal repertoires (Hersh et al. 2022 ). Modeling efforts suggest there may be a greater affinity of certain mature males to certain clans or vice versa, but it remains inconclusive whether such association is between males and their natal clan or other clans (Rendell et al. 2005 ). Males sometimes mate with females from multiple vocal clans, as shown by second-degree relationships among vocal clans (Konrad et al. 2018a ) and no nuclear DNA differences among vocal clans (Whitehead 2003 ). Most genetic analyses assessing regional and global population structure have not included clan identity (Mesnick et al. 2011 ; Alexander et al. 2016 ; Day et al. 2021 ; Girardet et al. 2022 ; Palmer et al. 2022 ) or only examined maternally inherited mitochondrial DNA (mtDNA; Rendell et al. 2012 ). Thus, while clan membership could play a role in female mate choice, it remains unknown whether females prefer males from specific clans (Rendell and Whitehead 2005 ; Rendell et al. 2005 ).
Another possible female mating tactic is copying the mate choices of other females (Westneat et al. 2000 ; Laland 2004 ; Orbach 2019 ). This tactic is consistent with evidence of shared paternity within and between sympatric social units (e.g., Konrad et al. 2018b ; Girardet et al. 2022 ). However, this is not definitive because some shared paternity would be expected if females in a social unit are simultaneously receptive in the presence of an acceptable male (Westneat et al. 2000 ). Likewise, high levels of shared paternity could also be explained by some males having attractive characteristics or traits that enable outcompeting rival males, such as large body size.
Females may choose to mate with the same male in multiple years. This has been suggested by a few possible full sibling relationships (Pinela et al. 2009 ; Konrad et al. 2018b ; Girardet et al. 2022 ). However, more robust genetic analyses are required to rule out other potential relationships for these cases. Additionally, research in the Indian Ocean indicates that past mates may be well received in subsequent visits. Girardet et al. ( 2022 ) described a gathering of approximately 60 individuals from multiple social units that coincided with the arrival of a mature male, which was genetically identified as the likely father of a calf born the same year in that area. If females tend to mate again with past mates, this could induce males to return to the same areas.
19.4 Care System
The care of their young is very remarkable, they not only carrying them on their tails and suckling them, but often rising with them for the benefit of the air; and however they are chased or wounded, yet as long as they have sense, and perceive life in their young, they will never leave them (...). Paul Dudley ( 1725 )
19.4.1 Maternal Care of Offspring
Compared to other cetaceans, sperm whale females invest heavily in their offspring through prolonged gestation and post-partum calf care. These traits contribute to their slow reproductive rate.
Weaning occurs over a prolonged period, with substantial variation in the age of calves. In the Eastern Caribbean, calves appear to nurse from a minimum of 3 years up to 8 years (Gero et al. 2014 ). Whalers found milk in the stomach of a 13-year-old juvenile and solid food in the stomach of 1-year-old calves (Best et al. 1984 ). The latter observation could indicate that calves forage independently or receive food from older members of their social unit. While first-year calves seem to suckle 20–47% of the time based on tagging data, their echolocation and diving abilities (up to 662 m and 44 min) suggest that they may supplement their diet with foraging (Tønnesen et al. 2018 ). Thus, the transition from nursing to independent foraging likely occurs over several years. Maternal investment is likely greatest when calves are young, as females are less socially connected when their calf is less than a year old, presumably because they dedicate time to nursing and foraging to meet the energetic demands of lactating (Gero et al. 2013 ).
Sperm whale maternal investment may also include teaching their young, as in other odontocetes (Atlantic spotted dolphins, Stenella frontalis , Bender et al. 2009 ; killer whales, Guinet and Bouvier 1995 ). For a female’s behavior to qualify as teaching, she must modify her behavior in the presence of her calf in a way that does not directly benefit herself while enabling the calf to more efficiently acquire skills or knowledge (Caro and Hauser 1992 ). Although there is no evidence of sperm whale females teaching their calves, the sperm whale social system provides an opportunity for this behavior. Teaching can evolve in a species when appropriate mechanisms for social learning exist, and the teacher benefits from the learner’s learning (Hoppitt et al. 2008 ). Sperm whales meet these criteria, given their social learning ability (evidenced by their culture; Whitehead and Rendell 2014 ) and the potential benefits to the mother of both inclusive fitness and direct benefits by reducing the duration of calf dependency. Even if teaching does not occur, sperm whale calves have ample opportunities for social learning (Cantor et al. 2019 ; Rendell et al. 2019 ).
19.4.2 Alloparental Care
Communal care of calves is a key aspect of sperm whale reproductive strategies. Calf care, particularly for protection against predators, is a hypothesized driving factor of sperm whale social structure (Best 1979 ; Whitehead 1996 ; Gero et al. 2013 ). Unlike the infants of other cetacean species with close maternal attendance (Rendell et al. 2019 ), sperm whale calves spend time without their mother while she forages. Thus, alloparenting, defined as the behavior of a non-parent that benefits the calf and differs from what the caregiver would do if the calf were absent, is likely important for sperm whales (Woodroffe and Vincent 1994 ; Whitehead 1996 ). Sperm whales accompany each other’s calves, referred to as babysitting, and collectively defend against predators by gathering in defensive formations, such as the “rosette” (Fig. 19.4 ), with calves in the middle of the circle (Weller et al. 1996 ).

Illustration of a cooperative defense formation, referred to as a Rosette or Marguerite formation, based on the description by Weller et al. ( 1996 )
There is evidence that female sperm whales provide milk to calves that are not their own, i.e., allonurse. Calves attempting to suckle from multiple females in their social unit (Gordon 1987 ; Gero et al. 2009 ; Konrad et al. 2019 ) make short, shallow dives beside females (Fig. 19.5 ) and perform mammary bumps assumed to stimulate the milk let-down reflex (Gero and Whitehead 2007 ; Johnson et al. 2010 ). Suckling behavior may not result in milk intake (Cameron 1998 ), and it is uncertain whether calves receive any milk from other females. Yet, in the absence of receiving milk or other benefits (e.g., social or emotional; Lee 1987 ; Cameron 1998 ), it is unlikely a calf would invest energy attempting to suckle from allonurses. Further supporting the hypothesis of allonursing, lactating females consistently outnumbered calves within groups of sperm whales killed by whalers (Best et al. 1984 ).

A young calf (> 1 year old) performing dives associated with nursing next to an allonurse from his social unit (credit: Dominica Sperm Whale Project, 2016)
Although mature females and juveniles of both sexes provide allocare (Gero et al. 2009 ; Konrad et al. 2019 ), some individuals do so disproportionately. In the Eastern Caribbean, allonurses are often close maternal kin (Konrad et al. 2019 ). However, this may be context dependent. For example, in one Eastern Caribbean social unit all observed allonursing was performed by first-degree relatives of the mother, yet in social units where mothers had no close kin, unrelated female members provided allocare (Fig. 19.6 ; Konrad et al. 2019 ; Sarano et al. 2021 ). Reciprocation of alloparental behavior has also been observed in some cases (Gero et al. 2009 , 2013 ) but not in others (Konrad et al. 2019 ). Additionally, young females may provide a disproportionate amount of calf care, suggesting that they benefit from opportunities to gain maternal experience (Konrad et al. 2019 ). Older females may also be valuable sources of allocare within their social units, given the prevalence of lactating females over the age of 40 despite females of this age rarely having young calves (Best et al. 1984 ). Finally, the age of the calf appears to affect the amount of allocare it receives, with allonursing and babysitting prevalent for calves less than a year old (Konrad et al. 2019 ).
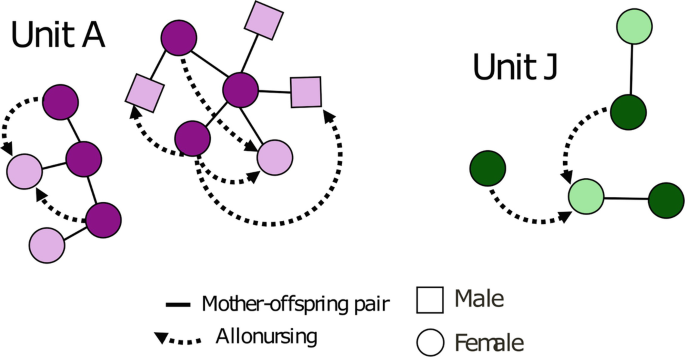
Differing patterns of allonursing in different social units from the Eastern Caribbean. Nodes represent individual whales from Unit A (purple) and Unit J (green), with darker shading indicating mature or juvenile individuals and light shading indicating calves. Solid edges between individuals denote mother–offspring relationships. Arrows point from mature or juvenile female to the calf observed attempting to nurse from her (figure based on results from Konrad et al. 2019 )
Some differences may occur in alloparenting among clans and regions. For example, differences in diving synchrony have been documented between two vocal clans in the Pacific Ocean, which may have implications for the patterns of calf care between clans (Cantor and Whitehead 2015 ). Additionally, a study of calf care within social units from the Sargasso Sea and the Eastern Caribbean noted differences in the patterns of allocare (Gero et al. 2009 ).
19.5 (An Odd) Convergent Evolution of Reproductive Strategies
The morphological, reproductive, and behavioral traits that make sperm whales unique among marine mammals reveal surprising similarities with the African elephant ( Loxodonta africana ; Weilgart et al. 1996 ; Whitehead 2003 ). Like sperm whales, African elephants have large bodies and brains and are the most sexually dimorphic terrestrial mammal, with mature males up to two times heavier than females (Sukumar, 2003 ). Females of both species form long-term matrilineal social units in which they cooperatively care for the young (Moss, 1983 ). Males of both species disperse from their natal units when juvenile, at 9–18 years of age, and either form bachelor groups or lead solitary lives (Whitehead 1994 , 2003 ). Mating is delayed well beyond puberty when male elephants are over 29 years old (Sukumar 2003 ). Elephant males also rove between groups of receptive females with marked preferences for older males, resulting in biased paternal patterns (Moss 1983 ). Mature male elephants also have distinct foraging strategies and home ranges that differ from groups of females and juveniles (Shannon et al. 2006 ). The remarkable parallels in the life histories and mating strategies of sperm whales and African elephants are coupled with similarly large home ranges, ecological success, and cognitive abilities, and likely reflect convergent evolution (Whitehead 2003 ).
Mating system evolution reflects predation pressure, habitat structure, resource availability, and sociality (Bowyer et al. 2020 ). The different reproductive strategies adopted by males and females within a species reflect different costs and benefits incurred by each sex (Orbach 2019 ). In sperm whales, there are significant differences in the energy budgets of males and females; while females dedicate considerable resources to the long-term care of their offspring, males invest in slow and continued growth to increase their chances of mating (Whitehead 2003 ). Thus, the female-centric societies in sperm whales and elephants likely emerged as a means of communal calf care against predation (Lee 1987 ; Sukumar 2003 ; Whitehead 2003 ; Rendell et al. 2019 ). The home ranges over which females of these species roam favor the development of longer-lasting social bonds in female social units compared with those of other social species that face similar predation pressures but have restricted home ranges (Whitehead 2003 ). The female investment in offspring is associated with a high incentive to choose a high-quality male (Whitehead 2003 ). Among sperm whales and African elephants, high-quality males are large and old (Moss 1983 ; Whitehead 1994 , 2003 ; Sukumar 2003 ). Males grow to outcompete rivals and rove between groups of receptive females to maximize mating opportunities (Whitehead 1990 , 1994 ). Thus, the distinct mating strategies of both sexes of sperm whales and elephants produce differences in morphology, sociality, and ecology (Sukumar 2003 ; Whitehead 2003 ).
19.6 Effects of Whaling on Sperm Whale Reproduction
Whaling operations have yielded insights into sperm whale reproductive biology. Sperm whales were the main target of the whaling industry between the eighteenth and nineteenth centuries and the 1950s–1980s (Whitehead 2002 ; Whitehead and Shin 2022 ). The global sperm whale population declined by approximately 57% over the past 300 years (Whitehead and Shin 2022 ). Although commercial whaling was banned in 1986, recovery has been slow or non-existent among sperm whale populations (Whitehead and Shin 2022 ). This results from the intrinsically low reproductive potential of sperm whales and the lingering effect of whaling on the sex ratio and the transmission of social information (Whitehead 2003 ).
While open-boat whaling targeted male and female sperm whales indiscriminately, modern whaling operations in some regions primarily targeted mature males (Hope and Whitehead 1991 ; Whitehead 2003 ). The proportion of mature males caught off Perú and Chile declined drastically, from 35% between 1958 and 1961 to 2% between 1979 and 1982 (Ramirez 1989 ; Whitehead et al. 1997 ). The removal of mature males from the Eastern Tropical Pacific Ocean was associated with a decrease of ~ 15% in pregnancy rates (Ramirez 1989 ). The effects of whaling on sperm whale reproductive potential lingered long after the whaling moratorium took effect. Between 1985 and 1995, the ratio of mature males to females around the Galápagos Islands remained significantly lower than expected (Cantor et al. 2017 ). Likewise, indicators of female fecundity were considerably less than those reported in regions where mature males were not as aggressively targeted in recent decades (Whitehead et al. 1997 ). In the 2010s, the proportion of males and calves off the Galápagos had slightly increased, suggesting a slow recovery of mature males that visit Eastern Tropical Pacific waters, corresponding with higher calving rates (Cantor et al. 2017 ). The population of Antarctic sperm whales—exclusively composed of mature males—has had the highest estimated rate of increase in recent decades (Whitehead and Shin 2022 ). While promising, it may take up to two decades after the cessation of whaling for the reproductive potential of sperm whale populations to be restored to inherent levels (Whitehead and Shin 2022 ).
The reproductive success of sperm whales may have also been affected by whaling through social disruption (Whitehead and Shin 2022 ). Female sperm whales acquire much of their behavior through social learning, including foraging strategies, movement patterns, and social behaviors (Whitehead and Rendell 2004 ; Marcoux et al. 2007a , b ; Cantor and Whitehead 2015 ; Eguiguren et al. 2019 ). Whalers may have removed individuals who were vital knowledge holders, which can have lasting effects across generations (Whitehead 2003 ; Whitehead and Shin 2022 ), as seen among African elephants. The impact of poaching on elephant social structure and reproductive rates was detected up to 15 years after the end of poaching for ivory (Gobush et al. 2008 ). Accounting for the effects of social disruption in population models of sperm whales has helped explain why the recovery of their populations has not been as fast as expected (Whitehead and Shin 2022 ).
19.7 Conclusions and Future Directions
Until recently, sperm whales were among the most hunted species of large mammal. Today, sperm whales are recognized for their ecological role, strong familial ties, rich learned cultures, and complex societies. Still, much remains to be discovered about their reproductive strategies, mating systems, and care systems. We suspect that most answers will come from accumulating long-term observational data and non-invasive technological advances, which record behavior, acoustics, and at-sea movements with unprecedented detail.
One gap in our understanding of sperm whales’ lives is the long-distance movements of mature males between feeding and breeding grounds. Photo-identification from scientific expeditions and citizen science (Levenson et al. 2015 ) may reveal the movement patterns of individual males. In addition, telemetry can provide insights into individual movements across the vast three-dimensional oceanic realm (Palacios et al. 2022 ). Although more invasive and logistically demanding, tracking individuals with tags can provide data on long-distance displacement within and across ocean basins (Lefort et al. 2022 ), as well as body orientation and fine-scale movements in the water column (Palacios et al. 2022 ), which could reveal inter-individual interactions. Moreover, attaching recording devices to whales can also provide information on acoustic communication (Johnson and Tyack 2003 ; Andreas et al. 2022 ) and long-term social interactions (Ortega-Ortiz et al. 2012 ). This high-resolution communication and social information can shed light on another poorly understood aspect of sperm whale reproductive strategies: mate selection.
Understanding mating choices will require an improved understanding of male–female interactions, likely using a multi-platform approach (Andreas et al. 2022 ; King and Jensen 2022 ). For instance, underwater imaging can reveal interactions between females and males as they approach social units (Girardet et al. 2022 ) and aerial drones can quantify behavioral interactions at the surface (Weiss et al. 2021 ). Behavioral data will be best interpreted in the context of mate choice if knowledge of reproductive status is available (e.g., via hormonal analyses, Dunstan et al. 2012 ; Hunt et al. 2013 ). Understanding mate choice also requires an understanding of traits that are attractive to the opposite sex. To investigate whether size affects mate choice, genetic analyses can be coupled with body size estimates obtained by passive acoustic monitoring using inter-pulse intervals of male echolocation clicks (Beslin et al. 2018 ) or morphometrics from drones (Dickson et al. 2021 ; Glarou et al. 2022 ).
Combining multiple sampling platforms will also advance our understanding of sperm whale calf care. In addition to nursing and defending calves, maternal and alloparental behaviors could include provisioning with solid food and teaching. Studies on how calves acquire coda dialects and foraging behavior may illuminate how they learn these skills. Additionally, extending studies across ocean basins will help provide a fuller picture of sperm whale calf care. As we improve the ability to record and understand multiple behavioral and physiological data types, we will gain insights into how sperm whales mate, reproduce, and care for the next generation as they slowly recover in the post-whaling era.
Alexander A, Steel D, Hoekzema K, Mesnick SL, Engelhaupt D, Kerr I, Payne R, Baker CS (2016) What influences the worldwide genetic structure of sperm whales ( Physeter macrocephalus )? Mol Ecol 25(12):2754–2772. https://doi.org/10.1111/mec.13638
Article CAS PubMed Google Scholar
Andreas J, Beguš G, Bronstein MM, Diamant R, Delaney D, Gero S, Goldwasser S, Gruber DF, de Haas S, Malkin P, Pavlov N, Payne R, Petri G, Rus D, Sharma P, Tchernov D, Tønnesen P, Torralba A, Vogt D, Wood RJ (2022) Toward understanding the communication in sperm whales. iScience 25(6):104393. https://doi.org/10.1016/j.isci.2022.104393
Article PubMed PubMed Central Google Scholar
Backus R, Schevill WE (1966) Physeter clicks. In: Norris KS (ed) Whales, porpoises and dolphins. University of California, Berkeley, CA, pp 510–528
Chapter Google Scholar
Beale T (1835) Chapter VI. Herding and other particulars, of the sperm whale. In: A few observations on the natural history of the sperm whale, Van Voorst, London, pp 51–54
Google Scholar
Bender CE, Herzing DL, Bjorklund DF (2009) Evidence of teaching in Atlantic spotted dolphins ( Stenella frontalis ) by mother dolphins foraging in the presence of their calves. Anim Cogn 12(1):43–53. https://doi.org/10.1007/s10071-008-0169-9
Article PubMed Google Scholar
Berzin AA (1972) The sperm whale. Israel Program for Scientific Translations, Israel
Beslin WAM, Whitehead H, Gero S (2018) Automatic acoustic estimation of sperm whale size distributions achieved through machine recognition of on-axis clicks. J Acoust Soc Am 144(6):3485–3495. https://doi.org/10.1121/1.5082291
Best PB (1979) Social organization in sperm whales, Physeter macrocephalus. In: Norris KS (ed) Behavior of marine animals, University of California Press, vol 3. Berkeley, CA, pp 227–290
Best PB (1999) Food and feeding of sperm whales Physeter macrocephalus off the west coast of South Africa. S Afr J Mar Sci 21(1):393–413. https://doi.org/10.2989/025776199784126033
Article Google Scholar
Best PB, Canham PAS, Macleod N (1984) Patterns of reproduction in sperm whales, Physeter macrocephalus . Rep Int Whal Comm 5(special issue):51–79
Betty EL, Elizabeth MJ, Zwamborn EMJ, Mieke Weyn M, Emma Luck E, Filipe Alves F (2023) Reproductive parameters, sociobiology, and mating strategies of pilot whales. In: Würsig B, Orbach DN (eds) Sex in cetaceans. Springer Nature, Cham
Bowyer RT, McCullough DR, Rachlow JL, Ciuti S, Whiting JC (2020) Evolution of ungulate mating systems: Integrating social and environmental factors. Ecol Evol 10(11):5160–5178. https://doi.org/10.1002/ece3.6246
Cameron EZ (1998) Is suckling behaviour a useful predictor of milk intake? A review. Anim Behav 56(3):521–532. https://doi.org/10.1006/anbe.1998.0793
Cantor M, Whitehead H (2015) How does social behavior differ among sperm whale clans? Mar Mamm Sci 31(4):1275–1290. https://doi.org/10.1111/mms.12218
Cantor M, Shoemaker LG, Cabral RB, Flores CO, Varga M, Whitehead H (2015) Multilevel animal societies can emerge from cultural transmission. Nat Commun 6(1):8091. https://doi.org/10.1038/ncomms9091
Cantor M, Eguiguren A, Merlen G, Whitehead H (2017) Galápagos sperm whales ( Physeter macrocephalus ): waxing and waning over three decades. Can J Zool 95(9):645–652. https://doi.org/10.1139/cjz-2016-0266
Cantor M, Gero S, Whitehead H, Rendell L (2019) Sperm whale: the largest toothed creature on earth. In: Würsig B (ed) Ethology and behavioral ecology of odontocetes. Springer Nature, Cham, pp 261–280
Caro TM, Hauser MD (1992) Is there teaching in nonhuman animals? Q Rev Biol 67(2):151–174. https://doi.org/10.1086/417553
Carrier DR, Deban SM, Otterstrom J (2002) The face that sunk the Essex: potential function of the spermaceti organ in aggression. J Exp Biol 205:1755–1763
Christal J (1998) An analysis of sperm whale social structure: patterns of association and genetic relatedness. PhD thesis, Dalhousie University
Christal J, Whitehead H (1997) Aggregations of mature male sperm whales on the Galápagos Islands breeding ground. Mar Mamm Sci 13(1):59–69. https://doi.org/10.1111/j.1748-7692.1997.tb00612.x
Christal J, Whitehead H (2001) Social affiliations within sperm whale ( Physeter macrocephalus ) groups. Ethology 107(18):323–340
Christal J, Whitehead H, Letteval E (1998) Sperm whale social units: variation and change. Can J Zool 76:1431–1440
Clarke R, Paliza O (1988) Intraspecific fighting in sperm whales. Rep Int Whal Comm 38:235–241
Clarke R, Paliza O, Aguayo A (1988) Sperm whales of the southeast Pacific, Part IV: fatness, food and feeding. In: Pilleri G (ed) Investigations on cetacea, Privately published by G, vol XXI. Pilleri, Berne, pp 53–195
Clarke R, Paliza O, Waerebeek KV (2012) Sperm whales of the Southeast Pacific. Part VII. Reproduction and growth in the female. Lat Am J Aqua Mamm 10(1):8–39. https://doi.org/10.5597/lajam00172
Coakes AK, Whitehead H (2004) Social structure and mating system of sperm whales off northern Chile. Can J Zool 82(8):1360–1369. https://doi.org/10.1139/z04-115
Cranford TW (1999) The sperm whale’s nose: sexual selection on a grand scale? Mar Mamm Sci 15(4):1133–1157. https://doi.org/10.1111/j.1748-7692.1999.tb00882.x
Croft DP, Brent LJN, Franks DW, Cant MA (2015) The evolution of prolonged life after reproduction. Trends Ecol Evol 30(7):407–416. https://doi.org/10.1016/j.tree.2015.04.011
Day J, Power D, Gales R, Bannister J, Piggott MP, Bilgmann K, Harcourt R, Beheregaray LB, Möller LM (2021) Australian sperm whales from different whaling stocks belong to the same population. Aqua Conserv Mar Freshw Ecosyst 31(6):1452–1465. https://doi.org/10.1002/aqc.3494
Dickson T, Rayment W, Dawson S (2021) Drone photogrammetry allows refinement of acoustically derived length estimation for male sperm whales. Mar Mamm Sci 37(3):1150–1158. https://doi.org/10.1111/mms.12795
Dudley P (1725) An essay upon the natural history of whales, with a particular account of the ambergris found in the spermaceti whale. Philos Trans R Soc B 33:256–269
Dunstan J, Gledhill A, Hall A, Miller P, Ramp C (2012) Quantification of the hormones progesterone and cortisol in whale breath samples using novel, non-invasive sampling and analysis with highly-sensitive ACQUITY UPLC and Xevo TQ-S. Waters Corporation, Milford, MA
Eguiguren A, Pirotta E, Cantor M, Rendell L, Whitehead H (2019) Habitat use of culturally distinct Galápagos sperm whale Physeter macrocephalus clans. Mar Ecol Prog Ser 609:257–270. https://doi.org/10.3354/meps12822
Ellis S, Franks DW, Nattrass S, Currie TE, Cant MA, Giles D, Balcomb KC, Croft DP (2018) Analyses of ovarian activity reveal repeated evolution of post-reproductive lifespans in toothed whales. Sci Rep 8(1):12833. https://doi.org/10.1038/s41598-018-31047-8
Article CAS PubMed PubMed Central Google Scholar
Engelhaupt D, Rus Hoelzel A, Nicholson C, Frantzis A, Mesnick S, Gero S, Whitehead H, Rendell L, Miller P, De Stefanis R, Cañadas A, Airoldi S, Mignucci-Giannoni AA (2009) Female philopatry in coastal basins and male dispersion across the North Atlantic in a highly mobile marine species, the sperm whale ( Physeter macrocephalus ). Mol Ecol 18(20):4193–4205. https://doi.org/10.1111/j.1365-294X.2009.04355.x
Fitch WT, Hauser MD (2003) Unpacking “honesty”: vertebrate vocal production and the evolution of acoustic signals. In: Simmons AM, Fay RR, Popper AN (eds) Acoustic communication. Springer, New York, NY, pp 65–137
Gaskin DE (1970) Composition of schools of sperm whales Physeter catodon Linn. east of New Zealand. N Z J Mar Freshw Res 4(4):456–471. https://doi.org/10.1080/00288330.1970.9515359
Gero S, Whitehead H (2007) Sucking behavior in sperm whale calves: observations and hypotheses. Mar Mamm Sci 23(2):398–413. https://doi.org/10.1111/j.1748-7692.2007.00113.x
Gero S, Engelhaupt D, Whitehead H (2008) Heterogeneous social associations within a sperm whale, Physeter macrocephalus , unit reflect pairwise relatedness. Behav Ecol Sociobiol 63(1):143–151. https://doi.org/10.1007/s00265-008-0645-x
Gero S, Engelhaupt D, Rendell L, Whitehead H (2009) Who cares? Between-group variation in alloparental caregiving in sperm whales. Behav Ecol 20(4):838–843. https://doi.org/10.1093/beheco/arp068
Gero S, Gordon J, Whitehead H (2013) Calves as social hubs: dynamics of the social network within sperm whale units. Proc R Soc Lond B 280(1763):20131113. https://doi.org/10.1098/rspb.2013.1113
Gero S, Milligan M, Rinaldi C, Francis P, Gordon J, Carlson C, Steffen A, Tyack P, Evans P, Whitehead H (2014) Behavior and social structure of the sperm whales of Dominica, West Indies. Mar Mamm Sci 30(3):905–922. https://doi.org/10.1111/mms.12086
Girardet J, Sarano F, Richard G, Tixier P, Guinet C, Alexander A, Sarano V, Vitry H, Preud’homme A, Heuzey R, Garcia-Cegarra AM, Adam O, Madon B, Jung J-L (2022) Long distance runners in the marine realm: new insights into genetic diversity, kin relationships and social fidelity of Indian Ocean male sperm whales. Front Mar Sci 9:815684. https://doi.org/10.3389/fmars.2022.815684
Glarou M, Gero S, Frantzis A, Brotons JM, Vivier F, Alexiadou P, Cerdà M, Pirotta E, Christiansen F (2022) Estimating body mass of sperm whales from aerial photographs. Mar Mamm Sci 39(1):251–273. https://doi.org/10.1111/mms.12982
Gobush KS, Mutayoba BM, Wasser SK (2008) Long-term impacts of poaching on relatedness, stress physiology, and reproductive output of adult female African elephants. Biol Conserv 22(6):1590–1599. https://doi.org/10.1111/j.1523-1739.2008.01035.x
Article CAS Google Scholar
Gordon JCD (1987) Sperm whale groups and social behavior observed off Sri Lanka. Rep Int Whal Comm 37:205–217
Gordon JCD (1991) Evaluation of a method for determining the length of sperm whales ( Physeter catodon ) from their vocalizations. J Zool 224(2):301–314. https://doi.org/10.1111/j.1469-7998.1991.tb04807.x
Gordon JCD (1998) Sperm whales. Colin Baxter, Grantown-on-Spey, Scotland
Guinet C, Bouvier J (1995) Development of intentional stranding hunting techniques in killer whale ( Orcinus orca ) calves at Crozet Archipelago. Can J Zool 73(1):27–33. https://doi.org/10.1139/z95-004
Hersh TA, Gero S, Rendell L, Cantor M, Weilgart L, Amano M, Dawson SM, Slooten E, Johnson CM, Kerr I, Payne R, Rogan A, Antunes R, Andrews O, Ferguson EL, Hom-Weaver CA, Norris TF, Barkley YM, Merkens KP, Oleson EM, Doniol-Valcroze T, Pilkington JF, Gordon J, Fernandes M, Guerra M, Hickmott L, Whitehead H (2022) Evidence from sperm whale clans of symbolic marking in non-human cultures. Proc Natl Acad Sci USA 119(37):e2201692119. https://doi.org/10.1073/pnas.2201692119
Hope P, Whitehead H (1991) Sperm whales off the Galápagos Islands from 1830-1850 and comparisons with modern studies. Rep Int Whal Comm 41:273–283
Hoppitt W, Brown G, Kendal R, Rendell L, Thornton A, Webster M, Laland K (2008) Lessons from animal teaching. Trends Ecol Evol 23(9):486–493. https://doi.org/10.1016/j.tree.2008.05.008
Huggenberger S, André M, Oelschläger HHA (2016) The nose of the sperm whale: overviews of functional design, structural homologies and evolution. J Mar Biol Ass 96(4):783–806. https://doi.org/10.1017/S0025315414001118
Hunt KE, Moore MJ, Rolland RM, Kellar NM, Hall AJ, Kershaw J, Raverty SA, Davis CE, Yeates LC, Fauquier DA, Rowles TK, Kraus SD (2013) Overcoming the challenges of studying conservation physiology in large whales: a review of available methods. Conserv Physiol 1(1):1–24. https://doi.org/10.1093/conphys/cot006
Jaquet N, Gendron D (2009) The social organization of sperm whales in the Gulf of California and comparisons with other populations. J Mar Biol Ass 89(5):975–983. https://doi.org/10.1017/S0025315409001507
Johnson MP, Tyack PL (2003) A digital acoustic recording tag for measuring the response of wild marine mammals to sound. IEEE J Oceanic Eng 28(1):3–12. https://doi.org/10.1109/JOE.2002.808212
Johnson G, Frantzis A, Johnson C, Alexiadou V, Ridgway S, Madsen PT (2010) Evidence that sperm whale ( Physeter macrocephalus ) calves suckle through their mouth. Mar Mamm Sci 26(4):990–996. https://doi.org/10.1111/j.1748-7692.2010.00385.x
Kato H (1984) Observation of tooth scars on the head of male sperm whale, as an indication of intra-sexual fighting. Sci Rep Whales Res Inst 35:39–46
Kawakami T (1980) A review of sperm whale food. Sci Rep Whales Res Inst 32:199–218
King SL, Jensen FH (2022) Rise of the machines: Integrating technology with playback experiments to study cetacean social cognition in the wild. Methods Ecol Evol. https://doi.org/10.1111/2041-210X.13935
Kobayashi H, Whitehead H, Amano M (2020) Long-term associations among male sperm whales ( Physeter macrocephalus ). PLoS One 15(12):e0244204. https://doi.org/10.1371/journal.pone.0244204
Konrad CM, Frasier TR, Rendell L, Whitehead H, Gero S (2018a) Kinship and association do not explain vocal repertoire variation among individual sperm whales or social units. Anim Behav 145:131–140. https://doi.org/10.1016/j.anbehav.2018.09.011
Konrad CM, Gero S, Frasier T, Whitehead H (2018b) Kinship influences sperm whale social organization within, but generally not among, social units. R Soc Open Sci 5(8):180914. https://doi.org/10.1098/rsos.180914
Konrad CM, Frasier TR, Whitehead H, Gero S (2019) Kin selection and allocare in sperm whales. Behav Ecol 30(1):194–201. https://doi.org/10.1093/beheco/ary143
Laland KN (2004) Social learning strategies. Anim Learn Behav 32(1):4–14. https://doi.org/10.3758/BF03196002
Lee PC (1987) Allomothering among African elephants. Anim Behav 35(1):278–291. https://doi.org/10.1016/S0003-3472(87)80234-8
Lefort KJ, Hussey NE, Jones JM, Johnson KF, Ferguson SH (2022) Satellite-tracked sperm whale migrates from the Canadian Arctic to the subtropical western North Atlantic. Mar Mamm Sci 38(3):1242–1248. https://doi.org/10.1111/mms.12909
Levenson J, Gero S, Van Oast J, Holmberg J (2015) Flukebook: a cloud-based photo-identification analysis tools for marine mammal research. Accessible at: https://www.flukebook.org
Lyrholm T, Gyllensten U (1998) Global matrilineal population structure in sperm whales as indicated by mitochondrial DNA sequences. Proc R Soc Lond B 265(1406):1679–1684. https://doi.org/10.1098/rspb.1998.0488
Lyrholm T, Leimar O, Johanneson B, Gyllensten U (1999) Sex-biased dispersal in sperm whales: contrasting mitochondrial and nuclear genetic structure of global populations. Proc R Soc Lond B 266(1417):347–354. https://doi.org/10.1098/rspb.1999.0644
MacLeod CD (1998) Intraspecific scarring in odontocete cetaceans: an indicator of male “quality” in aggressive social interactions? J Zool 244(1):71–77. https://doi.org/10.1111/j.1469-7998.1998.tb00008.x
Madsen PT, Whalberg M, Møhl B (2002) Male sperm whale ( Physeter macrocephalus ) acoustics in a high-latitude habitat: implications for echolocation and communication. Behav Ecol Sociobiol 53(1):31–41. https://doi.org/10.1007/s00265-002-0548-1
Marcoux M, Rendell L, Whitehead H (2007a) Indications of fitness differences among vocal clans of sperm whales. Behav Ecol Sociobiol 61(7):1093–1098. https://doi.org/10.1007/s00265-006-0342-6
Marcoux M, Whitehead H, Rendell L (2007b) Sperm whale feeding variation by location, year, social group and clan: evidence from stable isotopes. Mar Ecol Prog Ser 333:309–314. https://doi.org/10.3354/meps333309
Mendes S, Newton J, Reid RJ, Zuur AF, Pierce GJ (2007) Stable carbon and nitrogen isotope ratio profiling of sperm whale teeth reveals ontogenetic movements and trophic ecology. Oecologia 151(4):605–615. https://doi.org/10.1007/s00442-006-0612-z
Mesnick SL (2001) Genetic relatedness in sperm whales: evidence and cultural implications. Behav Brain Sci 24(2):346–347. https://doi.org/10.1017/S0140525X01463965
Mesnick SL, Taylor BL, Archer FI, Martien KK, Treviño SE, Hancock-Hanser BL, Moreno Medina SC, Pease VL, Robertson KM, Straley JM, Baird RW, Calambokidis J, Schorr GS, Wade P, Burkanov V, Lunsford CR, Rendell L, Morin PA (2011) Sperm whale population structure in the eastern and central North Pacific inferred by the use of single-nucleotide polymorphisms, microsatellites and mitochondrial DNA. Molec Ecol Resour 11(Suppl. 1):278–298. https://doi.org/10.1111/j.1755-0998.2010.02973.x
Mizroch SA, Rice DW (2013) Ocean nomads: distribution and movements of sperm whales in the North Pacific shown by whaling data and discovery marks. Mar Mamm Sci 29(2):E136–E165. https://doi.org/10.1111/j.1748-7692.2012.00601.x
Moss CJ (1983) Oestrous behaviour and female choice in the African elephant. Behav 86(3–4):167–195. https://doi.org/10.1163/156853983X00354
Norris KS, Harvey GW (1972) A theory for the function of the spermaceti organ of the sperm whale ( Physeter catodon L. ). In: Galler SR, Schmidt-Koenig K, Jacobs GJ, Belleville RE (eds) Animal orientation and navigation. NASA Scientific and Technical Office, Washington, DC
Ohsumi S (1965) Reproduction of the sperm whale in the North-West Pacific. Sci Rep Whales Res Inst 19:1–35
Oliveira C, Wahlberg M, Johnson M, Miller PJO, Madsen PT (2013) The function of male sperm whale slow clicks in a high latitude habitat: communication, echolocation, or prey debilitation? J Acoust Soc Am 133(5):3135–3144. https://doi.org/10.1121/1.4795798
Orbach DN (2019) Sexual strategies: male and female mating tactics. In: Würsig B (ed) Ethology and behavioral ecology of odontocetes. Springer Nature, Cham, pp 75–93
Ortega-Ortiz JG, Engelhaupt D, Winsor M, Mate BR, Rus Hoelzel A (2012) Kinship of long-term associates in the highly social sperm whale. Mol Ecol 21(3):732–744. https://doi.org/10.1111/j.1365-294X.2011.05274.x
Palacios DM, Irvine LM, Lagerquist BA, Fahlbusch JA, Calambokidis J, Tomkiewicz SM, Mate BR (2022) A satellite-linked tag for the long-term monitoring of diving behavior in large whales. Anim Biotelem 10(1):26. https://doi.org/10.1186/s40317-022-00297-9
Palmer E, Alexander A, Liggins L, Guerra M, Bury S, Hendriks H, Stockin K, Peters K (2022) A piece of the puzzle: analyses of recent strandings and historical records reveal new genetic and ecological insights on New Zealand sperm whales. Mar Ecol Prog Ser 690:201–217. https://doi.org/10.3354/meps14051
Panagiotopoulou O, Spyridis P, Mehari Abraha H, Carrier DR, Pataky TC (2016) Architecture of the sperm whale forehead facilitates ramming combat. PeerJ 4:e1895. https://doi.org/10.7717/peerj.1895
Pinela AM, Quérouil S, Magalhães S, Silva MA, Prieto R, Matos JA, Santos RS (2009) Population genetics and social organization of the sperm whale ( Physeter macrocephalus ) in the Azores inferred by microsatellite analyses. Can J Zool 87(9):802–813. https://doi.org/10.1139/Z09-066
Pirotta E, Brotons JM, Cerdà M, Bakkers S, Rendell LE (2020a) Multi-scale analysis reveals changing distribution patterns and the influence of social structure on the habitat use of an endangered marine predator, the sperm whale Physeter macrocephalus in the Western Mediterranean Sea. Deep Sea Res Part I Oceanogr Res Pap 155:103169. https://doi.org/10.1016/j.dsr.2019.103169
Pirotta E, Vighi M, Brotons J, Dillane E, Cerdà M, Rendell L (2020b) Stable isotopes suggest fine-scale sexual segregation in an isolated, endangered sperm whale population. Mar Ecol Prog Ser 654:209–218. https://doi.org/10.3354/meps13502
Ramirez P (1989) Captura de cachalote en Paita: 1976–1981. Boletín de Lima 63:81–88
Rendell LE, Whitehead H (2003) Vocal clans in sperm whales ( Physeter macrocephalus ). Proc R Soc Lond B 270(1512):225–231. https://doi.org/10.1098/rspb.2002.2239
Rendell L, Whitehead H (2005) Spatial and temporal variation in sperm whale coda vocalizations: stable usage and local dialects. Anim Behav 70(1):191–198. https://doi.org/10.1016/j.anbehav.2005.03.001
Rendell L, Whitehead H, Coakes A (2005) Do breeding male sperm whales show preferences among vocal clans of females? Mar Mamm Sci 21(2):317–322. https://doi.org/10.1111/j.1748-7692.2005.tb01231.x
Rendell L, Mesnick SL, Dalebout ML, Burtenshaw J, Whitehead H (2012) Can genetic differences explain vocal dialect variation in sperm whales, Physeter macrocephalus ? Behav Genet 42(2):332–343. https://doi.org/10.1007/s10519-011-9513-y
Rendell L, Cantor M, Gero S, Whitehead H, Mann J (2019) Causes and consequences of female centrality in cetacean societies. Philos Trans R Soc B 374(1780):20180066. https://doi.org/10.1098/rstb.2018.0066
Rice DW (1989) Sperm whale. Physeter macrocephalus Linnaeus, 1758. In: Ridgway SH, Harrison R (eds) Handbook of marine mammals. Academic Press, London, pp 177–233
Sarano F, Girardet J, Sarano V, Vitry H, Preud’homme A, Heuzey R, Garcia-Cegarra AM, Madon B, Delfour F, Glotin H, Adam O, Jung J-L (2021) Kin relationships in cultural species of the marine realm: Case study of a matrilineal social group of sperm whales off Mauritius island, Indian Ocean. R Soc Open Sci 8(2):rsos.201794. https://doi.org/10.1098/rsos.201794
Shannon G, Page BR, Duffy KJ, Slotow R (2006) The role of foraging behaviour in the sexual segregation of the African elephant. Oecologia 150(2):344–354. https://doi.org/10.1007/s00442-006-0521-1
Sukumar R (2003) The living elephants: evolutionary ecology, behaviour, and conservation. Oxford University Press, Oxford
Tønnesen P, Gero S, Ladegaard M, Johnson M, Madsen PT (2018) First-year sperm whale calves echolocate and perform long, deep dives. Behav Ecol Sociobiol 72(10):165. https://doi.org/10.1007/s00265-018-2570-y
van der Linde ML, Eriksson IK (2020) An assessment of sperm whale occurrence and social structure off São Miguel Island, Azores using fluke and dorsal identification photographs. Mar Mamm Sci 36(1):47–65. https://doi.org/10.1111/mms.12617
Weilgart LS, Whitehead H (1988) Distinctive vocalizations from mature male sperm whales ( Physeter macrocephalus ). Can J Zool 66:1931–9937
Weilgart L, Whitehead H, Payne K (1996) A colossal convergence. Am Sci 84(3):278–287
Weiss MN, Franks DW, Giles DA, Youngstrom S, Wasser SK, Balcomb KC, Ellifrit DK, Domenici P, Cant MA, Ellis S, Nielsen MLK, Grimes C, Croft DP (2021) Age and sex influence social interactions, but not associations, within a killer whale pod. Proc R Soc Lond B 288(1953):20210617. https://doi.org/10.1098/rspb.2021.0617
Weller DW, Würsig B, Whitehead H, Norris JC, Lynn SK, Davis RW, Clauss N, Brown P (1996) Observations of an interaction between sperm whales and short-finned pilot whales in the Gulf of Mexico. Mar Mamm Sci 12(4):588–594. https://doi.org/10.1111/j.1748-7692.1996.tb00071.x
Westneat DF, Walters A, McCarthy TM, Hatch MI, Hein WK (2000) Alternative mechanisms of nonindependent mate choice. Anim Behav 59(3):467–476. https://doi.org/10.1006/anbe.1999.1341
Whitehead H (1990) Rules for roving males. J Theor Biol 145(3):355–368. https://doi.org/10.1016/S0022-5193(05)80115-8
Whitehead H (1993) The behaviour of mature male sperm whales on the Galápagos Islands breeding grounds. Can J Zool 71(4):689–699. https://doi.org/10.1139/z93-093
Whitehead H (1994) Delayed competitive breeding in roving males. J Theor Biol 166(2):127–133. https://doi.org/10.1006/jtbi.1994.1011
Whitehead H (1996) Babysitting, dive synchrony, and indications of alloparental care in sperm whales. Behav Ecol Sociobiol 38(4):237–244. https://doi.org/10.1007/s002650050238
Whitehead H (2002) Estimates of the current global population size and historical trajectory for sperm whales. Mar Ecol Prog Ser 242:295–304
Whitehead H (2003) Sperm whales: social evolution in the ocean. The University of Chicago Press, Chicago, IL
Whitehead H (2009) Sperm whale Physeter macrocephalus . In: Würsig B, Perrin WF, Thewissen JGM (eds) Encyclopedia of marine mammals, 2nd edn. Academic Press, Amsterdam, pp 1091–1096
Whitehead H, Rendell L (2004) Movements, habitat use and feeding success of cultural clans of South Pacific sperm whales. J Anim Ecol 73:190–196
Whitehead H, Rendell L (2014) The cultural lives of whales and dolphins. University of Chicago Press, Chicago, IL
Book Google Scholar
Whitehead H, Shin M (2022) Current global population size, post-whaling trend and historical trajectory of sperm whales. Sci Rep 12(1):19468. https://doi.org/10.1038/s41598-022-24107-7
Whitehead H, Christal J, Dufault S (1997) Past and distant whaling and the rapid decline of sperm whales off the Galápagos Islands. Conserv Biol 11(6):1387–1396. https://doi.org/10.1046/j.1523-1739.1997.96246.x
Whitehead H, Antunes R, Gero S, Wong SNP, Engelhaupt D, Rendell L (2012) Multilevel societies of female sperm whales ( Physeter macrocephalus ) in the Atlantic and Pacific: why are they so different? Int J Primatol 33(5):1142–1164
Woodroffe R, Vincent A (1994) Mother’s little helpers: patterns of male care in mammals. Trends Ecol Evol 9(8):294–297. https://doi.org/10.1016/0169-5347(94)90033-7
Wright BM, Stredulinsky EH, Ford JKB (2023) Sex in killer whales: behavior, exogamy, and the evolution of sexual strategies in the ocean’s apex predator. In: Würsig B, Orbach DN (eds) Sex in cetaceans. Springer Nature, Cham
Würsig B, Rich J, Orbach DN (2023) Sex and behavior. In: Würsig B, Orbach DN (eds) Sex in cetaceans. Springer Nature, Cham
Wyman MT, Mooring MS, McCowan B, Penedo MCT, Reby D, Hart LA (2012) Acoustic cues to size and quality in the vocalizations of male North American bison, Bison bison . Anim Behav 84(6):1381–1391. https://doi.org/10.1016/j.anbehav.2012.08.037
Download references
Acknowledgments
We are grateful to the many researchers who have provided opportunities and logistical support, especially Hal Whitehead, Shane Gero, and Luke Rendell. We thank Shane Gero and Marina Milligan from the Dominica Sperm Whale Project for allowing us to reproduce their photos. We thank Randall W. Davis and editors for valuable comments and suggestions, which helped us improve the quality of the manuscript. AE was supported by the Killam Scholarship and the Nova Scotia Graduate Research Scholarship at Dalhousie University. MC was supported by the Marine Mammal Research Program Fund, the Jungers Faculty Development and Research Fund, the Marine Studies Initiative, and the College of Agricultural Sciences, all at Oregon State University.
Author information
Authors and affiliations.
Department of Biology, Dalhousie University, Halifax, NS, Canada
Ana Eguiguren
Fisheries and Oceans Canada, Pacific Science Enterprise Centre, West Vancouver, BC, Canada
Christine M. Konrad Clarke
Department of Fisheries, Wildlife and Conservation Sciences, Oregon State University, Newport, OR, USA
Mauricio Cantor
Marine Mammal Institute, Oregon State University, Newport, OR, USA
You can also search for this author in PubMed Google Scholar
Corresponding authors
Correspondence to Christine M. Konrad Clarke or Mauricio Cantor .
Editor information
Editors and affiliations.
Department of Marine Biology, Texas A&M University at Galveston, Galveston, TX, USA
Bernd Würsig
Department of Life Sciences, Texas A&M University- Corpus Christi, Corpus Christi, TX, USA
Dara N. Orbach
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Reprints and permissions
Copyright information
© 2023 The Author(s)
About this chapter
Cite this chapter.
Eguiguren, A., Konrad Clarke, C.M., Cantor, M. (2023). Sperm Whale Reproductive Strategies: Current Knowledge and Future Directions. In: Würsig, B., Orbach, D.N. (eds) Sex in Cetaceans. Springer, Cham. https://doi.org/10.1007/978-3-031-35651-3_19
Download citation
DOI : https://doi.org/10.1007/978-3-031-35651-3_19
Published : 26 September 2023
Publisher Name : Springer, Cham
Print ISBN : 978-3-031-35650-6
Online ISBN : 978-3-031-35651-3
eBook Packages : Biomedical and Life Sciences Biomedical and Life Sciences (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research

Incident with sperm whale stranded at Florida beach leaves experts puzzled: 'It's really unusual'
An endangered sperm whale that was recently stranded on a Florida beach died after officials were unable to rescue it.
What happened?
In March, a sperm whale became stranded on a shallow sandbar off Florida's Gulf Coast in the city of Venice. High winds and surf prevented rescuers from helping the animal make its way back into the ocean, and it started experiencing labored breathing and signs of suffering, according to the Guardian. Officials ultimately decided to euthanize the animal.
At 50,000 to 70,000 pounds, the whale was considered to be severely underweight and struggling to survive, the Guardian also reported.
Laura Engleby, branch chief with the marine mammal division at the National Oceanic and Atmospheric Administration, told local news outlet WTVT that it's rare for a sperm whale to get stranded in this area.
"It's really unusual," Engleby s aid . "The last one I remember being at was in 2008. We get about two sperm whales stranded a year in the southeastern US along the Gulf Coast – [which is] not as frequently."
Why is this stranding concerning?
Sperm whales are listed as endangered under the U.S. Endangered Species Act, meaning the species is in danger of extinction throughout all or a significant portion of its range.
Watch now: Alex Honnold test drives his new Rivian
These mammals face a barrage of threats , including collisions with water vessels, entanglement with fishing gear, ocean noise, marine debris, oil spills and contaminants, and a warming world. According to NOAA , our planet's changing climate can affect whale migration, feeding, and breeding.
Whales play an important role in the ecosystem, enabling tourism opportunities and supporting a web of life. They can even help us fight a warming world, as they play an important role in carbon sequestration .
Though rare in this area, around 2,000 strandings like this happen each year. For instance, in July 2023 , dozens of pilot whales were beached in Western Australia. That same month, 55 whales were stranded on a beach in Scotland — only one survived.
Beachings are rare enough that they do not pose a significant threat to any species, according to the Fish & Wildlife Foundation of Florida . However, experts have also said that an overheating planet could increase the number of whale beachings.
What's being done to conserve whales?
A number of organizations are dedicated to the conservation of whales and other marine wildlife, including Whale & Dolphin Conservation USA, the National Marine Sanctuary Foundation, Ocean Alliance, World Wildlife Fund, and others.
The Caribbean island of Dominica also recently created the world's first protected area for sperm whales. The sanctuary spans nearly 300 square miles, and scientists say the reserve will serve a hidden benefit of helping to fight our climate crisis, the Guardian reported .
Join our free newsletter for cool news and cool tips that make it easy to help yourself while helping the planet.
Incident with sperm whale stranded at Florida beach leaves experts puzzled: 'It's really unusual' first appeared on The Cool Down .

Adult male dwarf sperm whale stranded on Sullivan's Island beach euthanized: LMMN
by Ian Kayanja

SULLIVAN’S ISLAND, S.C. (WCIV) — An adult dwarf sperm whale stranded on Sullivan's Island had to be euthanized to "prevent further suffering," the Lowcountry Marine Mammal Network said in a social media post.
The Facebook status, posted on April 9, details the efforts made to save a stranded dwarf sperm whale on Thursday, April 4.
When the 7-foot whale was reported, officials with the Lowcountry Marine Mammal Networked worked with the Sullvan's Island Fire Department and veterinarians with the South Carolina Aquarium to attempt to save the animal.
READ MORE: "Dead sperm whale discovered near Charleston: LMMN."
Despite the expert efforts on site, it was determined that the animal would need to be sedated and euthanized.
It was then brought back to the Hollings Marine Lab where a necropsy was performed Friday, April 5.
At the time of the posting, the necropsy report was still pending and the Lowcountry Marine Mammal Network hasn't immediately made available the results – yet. However, the organization promises to share details once the samples from the necropsy are analyzed.
"It is always sad when the outcome of a live stranding results in the humane euthanasia of an animal, but the data and samples that can be collected from a deceased animal will make significant contributions to research that can help better inform us on how to protect our marine mammal populations," the Lowcountry Marine Mammal Network said in a social media post.
READ MORE: "Investigation into 42-foot sperm whale death near Charleston plans to continue: LMMN."
- Articles >
The Moscow Metro Museum of Art: 10 Must-See Stations
There are few times one can claim having been on the subway all afternoon and loving it, but the Moscow Metro provides just that opportunity. While many cities boast famous public transport systems—New York’s subway, London’s underground, San Salvador’s chicken buses—few warrant hours of exploration. Moscow is different: Take one ride on the Metro, and you’ll find out that this network of railways can be so much more than point A to B drudgery.
The Metro began operating in 1935 with just thirteen stations, covering less than seven miles, but it has since grown into the world’s third busiest transit system ( Tokyo is first ), spanning about 200 miles and offering over 180 stops along the way. The construction of the Metro began under Joseph Stalin’s command, and being one of the USSR’s most ambitious building projects, the iron-fisted leader instructed designers to create a place full of svet (radiance) and svetloe budushchee (a radiant future), a palace for the people and a tribute to the Mother nation.
Consequently, the Metro is among the most memorable attractions in Moscow. The stations provide a unique collection of public art, comparable to anything the city’s galleries have to offer and providing a sense of the Soviet era, which is absent from the State National History Museum. Even better, touring the Metro delivers palpable, experiential moments, which many of us don’t get standing in front of painting or a case of coins.
Though tours are available , discovering the Moscow Metro on your own provides a much more comprehensive, truer experience, something much less sterile than following a guide. What better place is there to see the “real” Moscow than on mass transit: A few hours will expose you to characters and caricatures you’ll be hard-pressed to find dining near the Bolshoi Theater. You become part of the attraction, hear it in the screech of the train, feel it as hurried commuters brush by: The Metro sucks you beneath the city and churns you into the mix.
With the recommendations of our born-and-bred Muscovite students, my wife Emma and I have just taken a self-guided tour of what some locals consider the top ten stations of the Moscow Metro. What most satisfied me about our Metro tour was the sense of adventure . I loved following our route on the maps of the wagon walls as we circled the city, plotting out the course to the subsequent stops; having the weird sensation of being underground for nearly four hours; and discovering the next cavern of treasures, playing Indiana Jones for the afternoon, piecing together fragments of Russia’s mysterious history. It’s the ultimate interactive museum.
Top Ten Stations (In order of appearance)
Kievskaya station.

Kievskaya Station went public in March of 1937, the rails between it and Park Kultury Station being the first to cross the Moscow River. Kievskaya is full of mosaics depicting aristocratic scenes of Russian life, with great cameo appearances by Lenin, Trotsky, and Stalin. Each work has a Cyrillic title/explanation etched in the marble beneath it; however, if your Russian is rusty, you can just appreciate seeing familiar revolutionary dates like 1905 ( the Russian Revolution ) and 1917 ( the October Revolution ).
Mayakovskaya Station
Mayakovskaya Station ranks in my top three most notable Metro stations. Mayakovskaya just feels right, done Art Deco but no sense of gaudiness or pretention. The arches are adorned with rounded chrome piping and create feeling of being in a jukebox, but the roof’s expansive mosaics of the sky are the real showstopper. Subjects cleverly range from looking up at a high jumper, workers atop a building, spires of Orthodox cathedrals, to nimble aircraft humming by, a fleet of prop planes spelling out CCCP in the bluest of skies.
Novoslobodskaya Station

Novoslobodskaya is the Metro’s unique stained glass station. Each column has its own distinctive panels of colorful glass, most of them with a floral theme, some of them capturing the odd sailor, musician, artist, gardener, or stenographer in action. The glass is framed in Art Deco metalwork, and there is the lovely aspect of discovering panels in the less frequented haunches of the hall (on the trackside, between the incoming staircases). Novosblod is, I’ve been told, the favorite amongst out-of-town visitors.
Komsomolskaya Station
Komsomolskaya Station is one of palatial grandeur. It seems both magnificent and obligatory, like the presidential palace of a colonial city. The yellow ceiling has leafy, white concrete garland and a series of golden military mosaics accenting the tile mosaics of glorified Russian life. Switching lines here, the hallway has an Alice-in-Wonderland feel, impossibly long with decorative tile walls, culminating in a very old station left in a remarkable state of disrepair, offering a really tangible glimpse behind the palace walls.
Dostoevskaya Station

Dostoevskaya is a tribute to the late, great hero of Russian literature . The station at first glance seems bare and unimpressive, a stark marble platform without a whiff of reassembled chips of tile. However, two columns have eerie stone inlay collages of scenes from Dostoevsky’s work, including The Idiot , The Brothers Karamazov , and Crime and Punishment. Then, standing at the center of the platform, the marble creates a kaleidoscope of reflections. At the entrance, there is a large, inlay portrait of the author.
Chkalovskaya Station
Chkalovskaya does space Art Deco style (yet again). Chrome borders all. Passageways with curvy overhangs create the illusion of walking through the belly of a chic, new-age spacecraft. There are two (kos)mosaics, one at each end, with planetary subjects. Transferring here brings you above ground, where some rather elaborate metalwork is on display. By name similarity only, I’d expected Komsolskaya Station to deliver some kosmonaut décor; instead, it was Chkalovskaya that took us up to the space station.
Elektrozavodskaya Station

Elektrozavodskaya is full of marble reliefs of workers, men and women, laboring through the different stages of industry. The superhuman figures are round with muscles, Hollywood fit, and seemingly undeterred by each Herculean task they respectively perform. The station is chocked with brass, from hammer and sickle light fixtures to beautiful, angular framework up the innards of the columns. The station’s art pieces are less clever or extravagant than others, but identifying the different stages of industry is entertaining.
Baumanskaya Statio
Baumanskaya Station is the only stop that wasn’t suggested by the students. Pulling in, the network of statues was just too enticing: Out of half-circle depressions in the platform’s columns, the USSR’s proud and powerful labor force again flaunts its success. Pilots, blacksmiths, politicians, and artists have all congregated, posing amongst more Art Deco framing. At the far end, a massive Soviet flag dons the face of Lenin and banners for ’05, ’17, and ‘45. Standing in front of the flag, you can play with the echoing roof.
Ploshchad Revolutsii Station

Novokuznetskaya Station
Novokuznetskaya Station finishes off this tour, more or less, where it started: beautiful mosaics. This station recalls the skyward-facing pieces from Mayakovskaya (Station #2), only with a little larger pictures in a more cramped, very trafficked area. Due to a line of street lamps in the center of the platform, it has the atmosphere of a bustling market. The more inventive sky scenes include a man on a ladder, women picking fruit, and a tank-dozer being craned in. The station’s also has a handsome black-and-white stone mural.
Here is a map and a brief description of our route:
Start at (1)Kievskaya on the “ring line” (look for the squares at the bottom of the platform signs to help you navigate—the ring line is #5, brown line) and go north to Belorusskaya, make a quick switch to the Dark Green/#2 line, and go south one stop to (2)Mayakovskaya. Backtrack to the ring line—Brown/#5—and continue north, getting off at (3)Novosblodskaya and (4)Komsolskaya. At Komsolskaya Station, transfer to the Red/#1 line, go south for two stops to Chistye Prudy, and get on the Light Green/#10 line going north. Take a look at (5)Dostoevskaya Station on the northern segment of Light Green/#10 line then change directions and head south to (6)Chkalovskaya, which offers a transfer to the Dark Blue/#3 line, going west, away from the city center. Have a look (7)Elektroskaya Station before backtracking into the center of Moscow, stopping off at (8)Baumskaya, getting off the Dark Blue/#3 line at (9)Ploschad Revolyutsii. Change to the Dark Green/#2 line and go south one stop to see (10)Novokuznetskaya Station.
Check out our new Moscow Indie Travel Guide , book a flight to Moscow and read 10 Bars with Views Worth Blowing the Budget For
Jonathon Engels, formerly a patron saint of misadventure, has been stumbling his way across cultural borders since 2005 and is currently volunteering in the mountains outside of Antigua, Guatemala. For more of his work, visit his website and blog .

Photo credits: SergeyRod , all others courtesy of the author and may not be used without permission
Environment | A frolicking humpback, 3 minkes and dolphins…
Share this:.
- Click to share on Facebook (Opens in new window)
- Click to share on Twitter (Opens in new window)
- Click to print (Opens in new window)
- Click to email a link to a friend (Opens in new window)
- Click to share on Reddit (Opens in new window)
Today's e-Edition
- Latest News
Environment
- Transportation
Breaking News
Environment | congressional recount: 16 ballots being challenged in san mateo county — some of which went uncounted due to a ‘simple oversight’, environment | a frolicking humpback, 3 minkes and dolphins put on a show off california coast.

A frolicking juvenile humpback repeatedly breached from waters among the coves off South Laguna, drawing cheers not only from passengers aboard whale-watching vessels but also from residents in homes along the steep hills overlooking the shoreline.
Also spotted were fin whales , minke whales and dolphins on Sunday, April 21 – a display that couldn’t have been better choreographed to celebrate Earth Day, several people commented.

A juvenile humpback whale breaches for more than an hour off South Laguna on Sunday, April 21, 2024, drawing cheers from people on charter boats and from homes above the beaches. (Photo courtesy of danawharf.com and Laura Lopez)

Whales put on a show off South Laguna on Sunday, April 21. At least three species of whales were seen: humpback, minke and fin whales. (Photo courtesy of Capt. Dave’s Dolphin and Whale Watching Safari, Matt Stumpf)

For a week now, whale watch charters out of the Dana Point Harbor have cheered the bountiful amount of sea life passengers have spotted.
“Our ocean is alive with so much wildlife,” said Donna Kalez, who operates Dana Wharf Sportfishing and Whale Watching . “As Earth Day approaches, we are reminded of the significance of a clean ocean and protecting its diverse marine life.”
Kalez said captains and naturalists aboard her charter boats have recorded 80 fin whale sightings and multiple humpbacks, minke, and gray whales accompanied by their calves in the last week. Nearly every day, she said, they’ve also seen at least one humpback whale breaching from the water.
“This year’s (Earthy Day) theme, ‘Planet versus Plastics,’ emphasizes the importance of combatting plastic pollution,” she said, adding that people can help out by using reusable water bottles and minimizing using single-use plastics. “It’s crucial to ensure that trash does not end up in the water. And, avoiding the release of balloons is essential as they often find their way into the ocean or in the waterways.”
Laguna Beach is at the forefront of preventing both from reaching the ocean with a ban on balloon purchases and use in public places and a ban on single-use plastics.
Also, the city’s coastline is mostly a Marine Protected Area , meaning fishing or taking anything from the tidepools is prohibited. The 10-year-plus closure has helped the fish population rebound and has made the cliff-protected lagoons a popular spot for whales to feed and also bring their calves.
“It kept jumping in the same location and it looked like there was a lot of food there,” he said. “It was a really healthy whale, a juvenile.”
Sansalone said he and the other boat captains kept their distance as the whale moved back and forth, leaping from the water at least 10 times.
“Everybody was yelling and screaming and having a good time.” he said of the passengers aboard the boats.
On the hillside, too, there were screams and shouts from residents overlooking the beach watching the display from their decks.
“I have been to Laguna many times and have even been on a couple whale-watching tours, but never have I experienced what I did today,” said Kimberly Munoz, who was in town for her brother, Laguna Beach artist Chris Richter’s gallery showing . “It was a spectacular sight seeing the whales splash in the sea. Such a treat for this Texas girl.”
For another neighbor, the sight made Earth Day even more important.
“This is the best Earth Day I’ve ever experienced,” said Lisa Yamasaki, who was feverishly taking photos from her deck. “Every time one of the whales jumped out of the water, you could hear people on the boats and along the hill gasping and cheering and then a beat later, you felt and heard the slap as its body or tail slammed into the water.”
- Report an error
- Policies and Standards
More in Environment

World News | Athens skies turn orange as dust clouds engulf the Greek capital

Environment | Firefighter, forester, trail builder: The first US Climate Corps jobs are here

Weather | When red-hot isn’t enough: New government heat risk tool sets magenta as most dangerous level

Environment | Gov. Newsom announces first new state park in a decade and sets climate goals for natural lands
Claudia Looi
Touring the Top 10 Moscow Metro Stations
By Claudia Looi 2 Comments

Komsomolskaya metro station looks like a museum. It has vaulted ceilings and baroque decor.
Hidden underground, in the heart of Moscow, are historical and architectural treasures of Russia. These are Soviet-era creations – the metro stations of Moscow.
Our guide Maria introduced these elaborate metro stations as “the palaces for the people.” Built between 1937 and 1955, each station holds its own history and stories. Stalin had the idea of building beautiful underground spaces that the masses could enjoy. They would look like museums, art centers, concert halls, palaces and churches. Each would have a different theme. None would be alike.
The two-hour private tour was with a former Intourist tour guide named Maria. Maria lived in Moscow all her life and through the communist era of 60s to 90s. She has been a tour guide for more than 30 years. Being in her 60s, she moved rather quickly for her age. We traveled and crammed with Maria and other Muscovites on the metro to visit 10 different metro stations.

Arrow showing the direction of metro line 1 and 2

Moscow subways are very clean
To Maria, every street, metro and building told a story. I couldn’t keep up with her stories. I don’t remember most of what she said because I was just thrilled being in Moscow. Added to that, she spilled out so many Russian words and names, which to one who can’t read Cyrillic, sounded so foreign and could be easily forgotten.
The metro tour was the first part of our all day tour of Moscow with Maria. Here are the stations we visited:
1. Komsomolskaya Metro Station is the most beautiful of them all. Painted yellow and decorated with chandeliers, gold leaves and semi precious stones, the station looks like a stately museum. And possibly decorated like a palace. I saw Komsomolskaya first, before the rest of the stations upon arrival in Moscow by train from St. Petersburg.
2. Revolution Square Metro Station (Ploshchad Revolyutsii) has marble arches and 72 bronze sculptures designed by Alexey Dushkin. The marble arches are flanked by the bronze sculptures. If you look closely you will see passersby touching the bronze dog's nose. Legend has it that good luck comes to those who touch the dog's nose.

Touch the dog's nose for good luck. At the Revolution Square station

Revolution Square Metro Station
3. Arbatskaya Metro Station served as a shelter during the Soviet-era. It is one of the largest and the deepest metro stations in Moscow.

Arbatskaya Metro Station
4. Biblioteka Imeni Lenina Metro Station was built in 1935 and named after the Russian State Library. It is located near the library and has a big mosaic portrait of Lenin and yellow ceramic tiles on the track walls.

Lenin's portrait at the Biblioteka Imeni Lenina Metro Station

5. Kievskaya Metro Station was one of the first to be completed in Moscow. Named after the capital city of Ukraine by Kiev-born, Nikita Khruschev, Stalin's successor.

Kievskaya Metro Station
6. Novoslobodskaya Metro Station was built in 1952. It has 32 stained glass murals with brass borders.

Novoslobodskaya metro station
7. Kurskaya Metro Station was one of the first few to be built in Moscow in 1938. It has ceiling panels and artwork showing Soviet leadership, Soviet lifestyle and political power. It has a dome with patriotic slogans decorated with red stars representing the Soviet's World War II Hall of Fame. Kurskaya Metro Station is a must-visit station in Moscow.

Ceiling panel and artworks at Kurskaya Metro Station

8. Mayakovskaya Metro Station built in 1938. It was named after Russian poet Vladmir Mayakovsky. This is one of the most beautiful metro stations in the world with 34 mosaics painted by Alexander Deyneka.

Mayakovskaya station

One of the over 30 ceiling mosaics in Mayakovskaya metro station
9. Belorusskaya Metro Station is named after the people of Belarus. In the picture below, there are statues of 3 members of the Partisan Resistance in Belarus during World War II. The statues were sculpted by Sergei Orlov, S. Rabinovich and I. Slonim.

10. Teatralnaya Metro Station (Theatre Metro Station) is located near the Bolshoi Theatre.

Teatralnaya Metro Station decorated with porcelain figures .

Taking the metro's escalator at the end of the tour with Maria the tour guide.
Have you visited the Moscow Metro? Leave your comment below.
January 15, 2017 at 8:17 am
An excellent read! Thanks for much for sharing the Russian metro system with us. We're heading to Moscow in April and exploring the metro stations were on our list and after reading your post, I'm even more excited to go visit them. Thanks again 🙂
December 6, 2017 at 10:45 pm
Hi, do you remember which tour company you contacted for this tour?
Leave a Reply Cancel reply
You must be logged in to post a comment.
Please go to the Instagram Feed settings page to create a feed.

Turn Your Curiosity Into Discovery
Latest facts.
8 Facts About National Make Lunch Count Day April 13th
12 Facts About National Tie Dye Day April 30th
40 facts about elektrostal.
Written by Lanette Mayes
Modified & Updated: 02 Mar 2024
Reviewed by Jessica Corbett

Elektrostal is a vibrant city located in the Moscow Oblast region of Russia. With a rich history, stunning architecture, and a thriving community, Elektrostal is a city that has much to offer. Whether you are a history buff, nature enthusiast, or simply curious about different cultures, Elektrostal is sure to captivate you.
This article will provide you with 40 fascinating facts about Elektrostal, giving you a better understanding of why this city is worth exploring. From its origins as an industrial hub to its modern-day charm, we will delve into the various aspects that make Elektrostal a unique and must-visit destination.
So, join us as we uncover the hidden treasures of Elektrostal and discover what makes this city a true gem in the heart of Russia.
Key Takeaways:
- Elektrostal, known as the “Motor City of Russia,” is a vibrant and growing city with a rich industrial history, offering diverse cultural experiences and a strong commitment to environmental sustainability.
- With its convenient location near Moscow, Elektrostal provides a picturesque landscape, vibrant nightlife, and a range of recreational activities, making it an ideal destination for residents and visitors alike.
Known as the “Motor City of Russia.”
Elektrostal, a city located in the Moscow Oblast region of Russia, earned the nickname “Motor City” due to its significant involvement in the automotive industry.
Home to the Elektrostal Metallurgical Plant.
Elektrostal is renowned for its metallurgical plant, which has been producing high-quality steel and alloys since its establishment in 1916.
Boasts a rich industrial heritage.
Elektrostal has a long history of industrial development, contributing to the growth and progress of the region.
Founded in 1916.
The city of Elektrostal was founded in 1916 as a result of the construction of the Elektrostal Metallurgical Plant.
Located approximately 50 kilometers east of Moscow.
Elektrostal is situated in close proximity to the Russian capital, making it easily accessible for both residents and visitors.
Known for its vibrant cultural scene.
Elektrostal is home to several cultural institutions, including museums, theaters, and art galleries that showcase the city’s rich artistic heritage.
A popular destination for nature lovers.
Surrounded by picturesque landscapes and forests, Elektrostal offers ample opportunities for outdoor activities such as hiking, camping, and birdwatching.
Hosts the annual Elektrostal City Day celebrations.
Every year, Elektrostal organizes festive events and activities to celebrate its founding, bringing together residents and visitors in a spirit of unity and joy.
Has a population of approximately 160,000 people.
Elektrostal is home to a diverse and vibrant community of around 160,000 residents, contributing to its dynamic atmosphere.
Boasts excellent education facilities.
The city is known for its well-established educational institutions, providing quality education to students of all ages.
A center for scientific research and innovation.
Elektrostal serves as an important hub for scientific research, particularly in the fields of metallurgy, materials science, and engineering.
Surrounded by picturesque lakes.
The city is blessed with numerous beautiful lakes, offering scenic views and recreational opportunities for locals and visitors alike.
Well-connected transportation system.
Elektrostal benefits from an efficient transportation network, including highways, railways, and public transportation options, ensuring convenient travel within and beyond the city.
Famous for its traditional Russian cuisine.
Food enthusiasts can indulge in authentic Russian dishes at numerous restaurants and cafes scattered throughout Elektrostal.
Home to notable architectural landmarks.
Elektrostal boasts impressive architecture, including the Church of the Transfiguration of the Lord and the Elektrostal Palace of Culture.
Offers a wide range of recreational facilities.
Residents and visitors can enjoy various recreational activities, such as sports complexes, swimming pools, and fitness centers, enhancing the overall quality of life.
Provides a high standard of healthcare.
Elektrostal is equipped with modern medical facilities, ensuring residents have access to quality healthcare services.
Home to the Elektrostal History Museum.
The Elektrostal History Museum showcases the city’s fascinating past through exhibitions and displays.
A hub for sports enthusiasts.
Elektrostal is passionate about sports, with numerous stadiums, arenas, and sports clubs offering opportunities for athletes and spectators.
Celebrates diverse cultural festivals.
Throughout the year, Elektrostal hosts a variety of cultural festivals, celebrating different ethnicities, traditions, and art forms.
Electric power played a significant role in its early development.
Elektrostal owes its name and initial growth to the establishment of electric power stations and the utilization of electricity in the industrial sector.
Boasts a thriving economy.
The city’s strong industrial base, coupled with its strategic location near Moscow, has contributed to Elektrostal’s prosperous economic status.
Houses the Elektrostal Drama Theater.
The Elektrostal Drama Theater is a cultural centerpiece, attracting theater enthusiasts from far and wide.
Popular destination for winter sports.
Elektrostal’s proximity to ski resorts and winter sport facilities makes it a favorite destination for skiing, snowboarding, and other winter activities.
Promotes environmental sustainability.
Elektrostal prioritizes environmental protection and sustainability, implementing initiatives to reduce pollution and preserve natural resources.
Home to renowned educational institutions.
Elektrostal is known for its prestigious schools and universities, offering a wide range of academic programs to students.
Committed to cultural preservation.
The city values its cultural heritage and takes active steps to preserve and promote traditional customs, crafts, and arts.
Hosts an annual International Film Festival.
The Elektrostal International Film Festival attracts filmmakers and cinema enthusiasts from around the world, showcasing a diverse range of films.
Encourages entrepreneurship and innovation.
Elektrostal supports aspiring entrepreneurs and fosters a culture of innovation, providing opportunities for startups and business development.
Offers a range of housing options.
Elektrostal provides diverse housing options, including apartments, houses, and residential complexes, catering to different lifestyles and budgets.
Home to notable sports teams.
Elektrostal is proud of its sports legacy, with several successful sports teams competing at regional and national levels.
Boasts a vibrant nightlife scene.
Residents and visitors can enjoy a lively nightlife in Elektrostal, with numerous bars, clubs, and entertainment venues.
Promotes cultural exchange and international relations.
Elektrostal actively engages in international partnerships, cultural exchanges, and diplomatic collaborations to foster global connections.
Surrounded by beautiful nature reserves.
Nearby nature reserves, such as the Barybino Forest and Luchinskoye Lake, offer opportunities for nature enthusiasts to explore and appreciate the region’s biodiversity.
Commemorates historical events.
The city pays tribute to significant historical events through memorials, monuments, and exhibitions, ensuring the preservation of collective memory.
Promotes sports and youth development.
Elektrostal invests in sports infrastructure and programs to encourage youth participation, health, and physical fitness.
Hosts annual cultural and artistic festivals.
Throughout the year, Elektrostal celebrates its cultural diversity through festivals dedicated to music, dance, art, and theater.
Provides a picturesque landscape for photography enthusiasts.
The city’s scenic beauty, architectural landmarks, and natural surroundings make it a paradise for photographers.
Connects to Moscow via a direct train line.
The convenient train connection between Elektrostal and Moscow makes commuting between the two cities effortless.
A city with a bright future.
Elektrostal continues to grow and develop, aiming to become a model city in terms of infrastructure, sustainability, and quality of life for its residents.
In conclusion, Elektrostal is a fascinating city with a rich history and a vibrant present. From its origins as a center of steel production to its modern-day status as a hub for education and industry, Elektrostal has plenty to offer both residents and visitors. With its beautiful parks, cultural attractions, and proximity to Moscow, there is no shortage of things to see and do in this dynamic city. Whether you’re interested in exploring its historical landmarks, enjoying outdoor activities, or immersing yourself in the local culture, Elektrostal has something for everyone. So, next time you find yourself in the Moscow region, don’t miss the opportunity to discover the hidden gems of Elektrostal.
Q: What is the population of Elektrostal?
A: As of the latest data, the population of Elektrostal is approximately XXXX.
Q: How far is Elektrostal from Moscow?
A: Elektrostal is located approximately XX kilometers away from Moscow.
Q: Are there any famous landmarks in Elektrostal?
A: Yes, Elektrostal is home to several notable landmarks, including XXXX and XXXX.
Q: What industries are prominent in Elektrostal?
A: Elektrostal is known for its steel production industry and is also a center for engineering and manufacturing.
Q: Are there any universities or educational institutions in Elektrostal?
A: Yes, Elektrostal is home to XXXX University and several other educational institutions.
Q: What are some popular outdoor activities in Elektrostal?
A: Elektrostal offers several outdoor activities, such as hiking, cycling, and picnicking in its beautiful parks.
Q: Is Elektrostal well-connected in terms of transportation?
A: Yes, Elektrostal has good transportation links, including trains and buses, making it easily accessible from nearby cities.
Q: Are there any annual events or festivals in Elektrostal?
A: Yes, Elektrostal hosts various events and festivals throughout the year, including XXXX and XXXX.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
Share this Fact:

IMAGES
VIDEO
COMMENTS
The sperm whale or cachalot (Physeter macrocephalus) is the largest of the toothed whales and the largest toothed predator. ... A social unit is a group of sperm whales who live and travel together over a period of years. Individuals rarely, if ever, join or leave a social unit. There is a huge variance in the size of social units.
Whaling Target. Sperm whales were mainstays of whaling's 18th and 19th century heyday. A mythical albino sperm whale was immortalized in Herman Melville's Moby Dick, though Ahab's nemesis was ...
The sperm whale is dark blue-gray or brownish, with white patches on the belly. It is thickset and has small paddlelike flippers and a series of rounded humps on its back. Males attain a maximum length of about 24 metres (78.7 feet) and weigh up to 50 metric tons (55.1 tons). Females are smaller, usually measuring less than about 14 metres (45. ...
Sperm Whale Migration. Female sperm whales ( Physeter macrocephalus) travel in groups with their young, circling the oceans to find food, they may travel a million miles in a lifetime. Inhabiting warmer waters than the males who thrive in the Arctic, they must meet up in the middle near the Azores.
About. Sperm whales are the largest of the toothed whales! They are so big that they are longer than the average transit bus, with males growing to be on average 60 feet long and females growing to 37 feet. Their heads are huge, making up one-third of their bodies. Despite their big heads, their eyes are quite small and unrecognizable to most ...
Sperm whales can be found around the world but typically stay away from the extremely cold waters near the polar ice in the north and the south. Females usually remain in temperate and tropical waters within 45-55° latitude, whereas males travel in temperate waters. In California, sperm whales can be seen in waters off the continental slope ...
Sperm whales are the largest member of the toothed whale Family, Odontoceti. This species is sexually dimorphic by size and weight. Females can reach a length of 36 feet (11 meters) and a weight of 15 tons (13,607 kilograms), while males grow up to 52 feet (15.9 meters) and 45 tons (40,823 kilograms). Sperm whales can be distinguished from ...
Schnöller notes that sperm whales tend to travel in the same direction when feeding. On the surface, they take 5-10 breathes before diving, which they signal by the bobbing of their heads before ...
Sperm Whale. Physeter marcrocephalus. Sperm whales are the largest toothed cetacean. Although they are the size of most large baleen whales, they actually have teeth on their bottom jaw, used to grasp large squid or fish, unlike other large whales that filter smaller denser prey through their baleen. Almost mythical creatures, sperm whales were ...
Biology and Ecology Feeding. Sperm whales generally feed at depth in search of their preferred prey which consists of a variety of squid species, including the giant squid, Architeuthis.Female sperm whales almost exclusively eat squid while males have been documented to prey on bottom dwelling fish, including sharks, rays, cod and hake 1.Sperm whales typically dive to an average depth of 800 ...
The most notable physical feature of the Sperm whale is its robust, square-shaped head, which takes up about 1/3 of its body length. The sides of the body have a bumpy or wrinkled appearance starting behind the head. The signature of sperm whales is a relatively small, narrow bottom jaw lined with the largest teeth of any predator in the world.
Pico Island is one of the 9 islands in the archipelago of the Azores, located in the Atlantic Ocean. Over 20 species of whales and dolphins either inhabit or pass by our waters along their migratory routes, making Pico one of the best spots in the world for whale watching. Every year these waters host about 20 species of cetaceans, including ...
Sperm whales are the largest of all toothed whales and can grow to a maximum length of 52 feet (15.8 m) and weight of 90,000 pounds (40 metric tons), with males growing much larger than females. 2. Sperm whales live for up to 60 years. 3. Sperm whales have one of the widest distributions of all marine mammals, living everywhere from the Arctic ...
Polidoro: How they travel, how they feed and reproduce, ... Sperm whales' basic social unit, the pod, comprises about 10 females and their offspring. Clans are much larger. On average ...
Sperm whale males reach sexual maturity around 18 years old and females at 9 years old. Males battle for mating rights, then breed with multiple females. Male sperm whales do not create harems of females like other animals. The sperm whale pregnancy term lasts about 15 months, resulting in a single calf.
Sperm whale trumpets are sounds only occasionally documented, with a well recognisable and stereotyped acoustic arrangement. ... It is widely recognised that sperm whales travel through the whole ...
The adult male sperm whales travel to the colder climates during the off-season and return to the warmer climates during the mating season, which occurs during the colder winter months. In fact, some male sperm whales can travel throughout all of the world's major oceans over the course of their 70-year lifespan , allowing them to travel the ...
The sperm whale (Physeter macrocephalus) is a unique creature shaped by the deep ocean—a dark desert where these whales live highly social lives (Whitehead 2003).Their distinctiveness is showcased in their morphology; sperm whales are the largest toothed predators, their massive heads contain the world's biggest brains in absolute size, and they possess the most powerful biological sonar.
Custom and curated expeditions around the world for photographers, adventurers, and travellers alike. Focusing on genuine wildlife encounters, environmentally conscience travel, and real stories in far away places. Swim with humpbacks whales, swim with sperm whales, swim with sharks.
An endangered sperm whale that was recently stranded on a Florida beach died after officials were unable to rescue it. In March, a sperm whale became stranded on a shallow sandbar off Florida's ...
In 2004, a sperm whale that had washed ashore in Taiwan exploded while it was being transported for a post-mortem examination. The explosion sent a 10-foot (3-meter) wide column of blubber and flesh into the air, injuring several bystanders. Several cases reported of exploding whales
Photo of an adult male dwarf sperm whale found on Sullivan's Island beach and posted on Lowcountry Marine Mammal Network's Facebook page on April 9, 2024.
Have a look (7)Elektroskaya Station before backtracking into the center of Moscow, stopping off at (8)Baumskaya, getting off the Dark Blue/#3 line at (9)Ploschad Revolyutsii. Change to the Dark Green/#2 line and go south one stop to see (10)Novokuznetskaya Station. Check out our new Moscow Indie Travel Guide, book a flight to Moscow and read 10 ...
At least three species of whales were seen: humpback, minke and fin whales. (Photo courtesy of Capt. Dave's Dolphin and Whale Watching Safari, Matt Stumpf) Whales put on a show off South Laguna ...
6. Novoslobodskaya Metro Station was built in 1952. It has 32 stained glass murals with brass borders. Novoslobodskaya metro station. 7. Kurskaya Metro Station was one of the first few to be built in Moscow in 1938. It has ceiling panels and artwork showing Soviet leadership, Soviet lifestyle and political power.
Elektrostal is a city in Moscow Oblast, Russia, located 58 kilometers east of Moscow. Elektrostal has about 158,000 residents. Mapcarta, the open map.
40 Facts About Elektrostal. Elektrostal is a vibrant city located in the Moscow Oblast region of Russia. With a rich history, stunning architecture, and a thriving community, Elektrostal is a city that has much to offer. Whether you are a history buff, nature enthusiast, or simply curious about different cultures, Elektrostal is sure to ...