- Skip to main content
- Skip to FDA Search
- Skip to in this section menu
- Skip to footer links

The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.

U.S. Food and Drug Administration
- Search
- Menu
- Recalls, Market Withdrawals, & Safety Alerts
Abbott Initiates Voluntarily Recall of Specific Lots of Three Coronary Catheters
COMPANY ANNOUNCEMENT
This recall has been completed and FDA has terminated this recall.
When a company announces a recall, market withdrawal, or safety alert, the FDA posts the company's announcement as a public service. FDA does not endorse either the product or the company.
Company Announcement
Abbott has initiated a voluntary recall of specific lots of three catheters: NC Trek RX Coronary Dilatation Catheter, NC Traveler Coronary Dilatation Catheter, and NC Tenku RX PTCA Balloon Catheter.
This recall does not affect patients who have successfully undergone cardiac procedures using these devices. Abbott has already implemented corrective actions to ensure the products perform as intended.
Products from the identified lots may exhibit difficulty in removing the protective balloon sheath, which could cause problems with inflating or deflating the balloon. Potential risks associated with balloon inflation and deflation difficulties include air embolism, additional intervention, thrombosis, and myocardial infarction. In one reported case, failure to deflate the balloon necessitated surgery, which resulted in multiple post-surgical complications leading to death. The FDA has classified this as a Class I recall, where exposure to a device presents a reasonable likelihood of serious adverse health consequences or death. The cumulative frequency of reported events in difficulty of removing the sheath, and inflation and deflation of the balloon, is 0.12 percent worldwide.
Abbott began contacting customers in March who received coronary catheters from the affected lots, and is arranging the return and replacement of all remaining products. The total number of distributed units from identified lots potentially affected is 449,661. Global Health Authorities have been notified of the voluntary recall.
Specific lots of affected product were manufactured between Jan. 1, 2015 – Jan. 2, 2017, and were distributed between Jan. 13, 2015 – March 14, 2017. For more information, please see Abbott’s field safety notice .
For Important Safety Information on NC Trek Catheters visit Abbott's home page .
Consumers with questions may contact the company via telephone at (800) 227-9902 between the hours of 5 a.m. and 5 p.m. PST.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online
- Regular Mail or Fax: Download form or call 1- 800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.
Company Contact Information

Create Free Account or
- Acute Coronary Syndromes
- Anticoagulation Management
- Arrhythmias and Clinical EP
- Cardiac Surgery
- Cardio-Oncology
- Cardiovascular Care Team
- Congenital Heart Disease and Pediatric Cardiology
- COVID-19 Hub
- Diabetes and Cardiometabolic Disease
- Dyslipidemia
- Geriatric Cardiology
- Heart Failure and Cardiomyopathies
- Invasive Cardiovascular Angiography and Intervention
- Noninvasive Imaging
- Pericardial Disease
- Pulmonary Hypertension and Venous Thromboembolism
- Sports and Exercise Cardiology
- Stable Ischemic Heart Disease
- Valvular Heart Disease
- Vascular Medicine
- Clinical Updates & Discoveries
- Advocacy & Policy
- Perspectives & Analysis
- Meeting Coverage
- ACC Member Publications
- ACC Podcasts
- View All Cardiology Updates
- Earn Credit
- View the Education Catalog
- ACC Anywhere: The Cardiology Video Library
- CardioSource Plus for Institutions and Practices
- ECG Drill and Practice
- Heart Songs
- Nuclear Cardiology
- Online Courses
- Collaborative Maintenance Pathway (CMP)
- Understanding MOC
- Image and Slide Gallery
- Annual Scientific Session and Related Events
- Chapter Meetings
- Live Meetings
- Live Meetings - International
- Webinars - Live
- Webinars - OnDemand
- Certificates and Certifications
- ACC Accreditation Services
- ACC Quality Improvement for Institutions Program
- CardioSmart
- National Cardiovascular Data Registry (NCDR)
- Advocacy at the ACC
- Cardiology as a Career Path
- Cardiology Careers
- Cardiovascular Buyers Guide
- Clinical Solutions
- Clinician Well-Being Portal
- Diversity and Inclusion
- Infographics
- Innovation Program
- Mobile and Web Apps
FDA Update | Recall of Abbott NC Trek RX and NC Traveler RX Coronary Dilatation Catheters
Cardiology Magazine
Abbott Vascular is recalling the NC Trek RX Coronary Dilatation Catheter and NC Traveler RX Coronary Dilatation Catheter, due to reports that the balloons from the impacted lots "may not deflate as intended" because of weaker material close to the balloon bond, resulting from excessive exposure to heat during manufacturing.
This recall impacts balloon diameters 4.0 mm, 4.5 mm and 5.0 mm.
The U.S. Food and Drug Administration has stated that use of these devices may cause "serious adverse health consequences, such as prolonged cardiac ischemia, air embolism, thrombosis, myocardial infarction and additional surgery that could lead to post-operative complications, including death."
Abbott Vascular has received 13 complaints related to this issue, with one death being reported.
Tweet #CardiologyMag
Keywords: ACC Publications, Cardiology Magazine
You must be logged in to save to your library.

The Latest From Cardiology
Editor's Corner | Dear Colleagues, A Guest Editorial by ACC President Richard J. Kovacs, MD, FACC
Coronavirus Disease 2019 (COVID-19) Provides Potent Reminder of the Risk of Infectious Agents
Feature | Telehealth: Rapid Implementation For Your Cardiology Clinic Updated!
Feature | Adopting Telemedicine During the COVID-19 Pandemic: A Return to Patient-Focused Care
Feature | Pediatric Cardiology: A Specialty Spurred by the Groundbreaking Work of Women – The Legacies of Maude Abbott, MD, and Helen B. Taussig, MD, FACC
Cover Feature | ACC’s Cardiovascular Summit a Primer on the Business of CV Medicine
Cover Story | Primer For the Economics in Cardiovascular Practices and Cardiovascular Service Lines
Cover Story | Site Neutrality: What Does It Mean to My Service Line?
Cover Story | MedAxiom’s Top Five Takeaways
Cover Story | Flexible Career Schedules: How Do We Work Them In?
Cover Story | Seven Tips to Recognize the Value of Team-Based Care
Cover Story | 12 Strategies For Creating a Successful Team
Cover Story | Building and Implementing Team-Based Care in a Successful Financial Model
Cover Story | What Does Cardiology Look Like in 2025?
Feature | ACC Cardio-Oncology Course Drives Discourse, Innovation in Fast-Growing Field
Innovation at ACC | Screenshot Software in Medical Imaging
Peripheral Matters | Chronic Thromboembolic Pulmonary Hypertension: Evolving Therapeutic Strategies?
Quality Improvement For Institutions | Lean Thinking: Changing Culture. Improving CV Care.
Feature | Has Employment of Cardiologists Been a Successful Strategy?
Interoperability and Data Blocking Final Rules Released
FDA Update | Bempedoic Acid Approved for Treatment of Adults With HeFH or Established ASCVD
FDA Update | Recall of Carestation 600 Series Anesthesia Systems
Number Check Information Graphic Feature | Nutrition and Health: Insights For National Nutrition Month
Just One More | Planting Seeds: Growing the Next Generations of Women In Cardiology
JACC in a Flash
Journal Wrap
Quick Reads Feature | The Pulse of ACC
JACC Journals on ACC.org
- JACC: Advances
- JACC: Basic to Translational Science
- JACC: CardioOncology
- JACC: Cardiovascular Imaging
- JACC: Cardiovascular Interventions
- JACC: Case Reports
- JACC: Clinical Electrophysiology
- JACC: Heart Failure
- Current Members
- Campaign for the Future
- Become a Member
- Renew Your Membership
- Member Benefits and Resources
- Member Sections
- ACC Member Directory
- ACC Innovation Program
- Our Strategic Direction
- Our History
- Our Bylaws and Code of Ethics
- Leadership and Governance
- Annual Report
- Industry Relations
- Support the ACC
- Jobs at the ACC
- Press Releases
- Social Media
- Book Our Conference Center
Clinical Topics
- Chronic Angina
- Congenital Heart Disease and Pediatric Cardiology
- Diabetes and Cardiometabolic Disease
- Hypertriglyceridemia
- Invasive Cardiovascular Angiography and Intervention
- Pulmonary Hypertension and Venous Thromboembolism
Latest in Cardiology
Education and meetings.
- Online Learning Catalog
- Products and Resources
- Annual Scientific Session
Tools and Practice Support
- Quality Improvement for Institutions
- Accreditation Services
- Practice Solutions
Heart House
- 2400 N St. NW
- Washington , DC 20037
- Email: [email protected]
- Phone: 1-202-375-6000
- Toll Free: 1-800-253-4636
- Fax: 1-202-375-6842
- Media Center
- ACC.org Quick Start Guide
- Advertising & Sponsorship Policy
- Clinical Content Disclaimer
- Editorial Board
- Privacy Policy
- Registered User Agreement
- Terms of Service
- Cookie Policy
© 2024 American College of Cardiology Foundation. All rights reserved.
- Beds & Mattresses
- Equipments & Furnishings
- Patient ID Wristbands
- Infection Prevention
- Office Supplies
- Labeling Products
- Medical Records
- Equipment Protection
- Advanced Wound Care
- Operating Room and Surgery
- Respiratory
- Vascular Access
- Diagnostics
- Lab Supplies
- Mammography Products
- Medical Imaging Supplies
- Durable Medical Equipment (DME)
- Bath Safety
- Transfer Equipment
- Wheelchairs
- Incontinence
- Nursing Supplies
- Urology & Ostomy
- Shop by Brand
Abbott NC Trek Coronary Dilatation Catheters - NC Trek Catheter, Rapid, 3.5 mm x 20 mm - 1012451-20

Questions? Speak with a specialist!

This item may require 5-7 days to ship out from our facility.
Product Information: NC Trek Catheter, Rapid, 3.5 mm x 20 mm
Manufacturer Part # 1012451-20
Description
- Coronary dilatation catheter with multilayer CrossFlex balloon helps provide flexibility and trackability for crossing stents and lesions
- Flexible dual tungsten markers help enable smooth, precise tracking while providing controlled and confident placement
- Smooth, rounded tip designed for ease of maneuverability through calcified lesions and stent struts
- Short, steep tapers for focused dilatation on lesion and not surrounding healthy tissue
- Limited longitudinal growth helps predict dilatation within treatment area
Customer Reviews
Related products, shenzhen dongdixin technology co lt digital noncontact forehead thermometer - digital noncontact forehead thermometer - th5001n, shenzhen dongdixin technology co lt.
This item may require 1-2 days to ship out from our facility.Product Information: Digital Noncontact Forehead ThermometerManufacturer Part # TH5001...
SunMed Precordial Chest Pieces - Wegner Chest Piece for Precordial Stethoscope, Pediatric - 6-0025-02
This item may require 1-2 days to ship out from our facility.Product Information: Wegner Chest Piece for Precordial Stethoscope, PediatricManufactu...
Omrom Healthcare Inc. ComFit Cuffs - CUFF, EASY WRAP, COMFIT, 9" TO 17" - HEM-FL31-B
Omron healthcare inc.
This item may require 5-7 days to ship out from our facility.Product Information: CUFF, EASY WRAP, COMFIT, 9" TO 17"Manufacturer Part # HEM-FL31-BD...
Abbott BinaxNOW RSV Tests - Nasopharyngeal Swab for BinaxNOW - 400-065
This item may require 1-2 days to ship out from our facility. Product Information: Nasopharyngeal Swab for BinaxNOW Manufacturer Part # 400-065 Thi...
Medline RightTemp Tympanic Thermometer - RightTemp Tympanic Thermometer Probe Cover - MDS8701
This item is currently out of stock and unavailable to purchase.Product Information: RightTemp Tympanic Thermometer Probe CoverManufacturer Part # ...
You recently viewed
- Facility & Personnel Supplies
- Medical Supplies
- Lab & Diagnostic Supplies
- Patient Care
License Requirement
Certain items may require either a prescription, medical license or a business facility license to process. If your purchase falls in that category, we will reach out to you to obtain proper documentation
Order Inquiries
+1(415) 683-7878
Warehouse Location
14791 Carmenita Road
Norwalk CA 90650
(Not open to public visits)
Hours of Operation
Mon-Sat 8AM - 8PM
U.S. Pacific Standard Time
- Privacy Policy
- Refund policy
- Terms of service
- Shipping Policy
- Payment Policy
- Contact Information
Copyright © 2024 Grayline Medical.
- American Express
- Diners Club
Added to your cart:
What's your email.
Advertisement
Fda: abbott recalls nc trek rx and nc traveler coronary catheters, some of the dilatation catheters are not deflating properly. one patient has died from postprocedural complications..

- Created with Sketch.

Abbott has voluntarily recalled specific lots of two coronary angioplasty catheters—the NC Trek RX coronary dilatation catheter and the NC Traveler coronary dilatation catheter—in three balloon diameters: 4.0, 4.5, and 5.0 mm.
The US Food and Drug Administration has classified the action as a Class I recall, its most serious category.
Affected products may deflate slowly, partially, or not at all, potentially causing “prolonged cardiac ischemia, air embolism, thrombosis, myocardial infarction, and additional intervention, such as surgery that could lead to postoperative complications which include death,” FDA’s MedWatch alert stated.
According to the manufacturer, the frequency of reported events is 0.12% worldwide, and at the time the field notice was issued, there had been no patient deaths. Since then, said the FDA, “Abbott has become aware of one reported case in which the inability to deflate the balloon necessitated intervention, which resulted in postprocedural complications leading to a patient death.”
The FDA action follows a Field Safety Notice , issued by Abbott on January 29, making hospitals and physicians aware of the affected lots—estimated at 40,429—that were distributed between August 16, 2019, and January 3, 2020. Any hospitals that have the defective products in stock are asked to stop using them immediately and return any that are unused to the company.
Providers are urged to read the field notice and report any problems to the manufacturer.

Shelley Wood is Managing Editor of TCTMD and the Editorial Director at CRF. She did her undergraduate degree at McGill…
- Disclosures
US Food and Drug Administration. Abbott initiates voluntary recall of specific lots of two coronary catheters . Published on: February 20, 2020. Accessed on: February 24, 2020.
Never Miss a Beat
Stay up-to-date with breaking news, conference slides, and topical videos covering the spectrum of CVD. Join our newsletter!
New at TCTMD? Register today!
Forgot Password
Forgot Your Password?
Enter the email you used to register to reset your password.
Search TCTMD
Sign up for our newsletter.
Sign up to receive the most important cardiovascular news, research, and major meeting presentations.
Submit an Event
Become a premium member or log in to view exclusive content.
This content is available for meeting attendees and/or Platinum Members
REGISTER for free or LOG IN to view this content
You are using an outdated browser. Please upgrade your browser to improve your experience.
- Skip to Main Content

- Customer Support & FAQ
- FDA UDI Home
- FDA Medical Devices Home
- Report a Device Problem (MedWatch)
- Device Recalls
- Device Safety Communications

DEVICE: NC TREK NEO™ (08717648232831)
Device identifier (di) information, device characteristics.
GMDN© Term Code, Names and Definitions ( * Terms of Use ): GMDN® is a registered trademark of The GMDN Agency. All rights reserved. Used under licence from The GMDN Agency Ltd.
FDA Product Code
Fda premarket submission, sterilization, storage and handling, clinically relevant size, device record status, alternative and additional identifiers additional identifiers, secondary di, unit of use di, direct marking (dm), production identifier(s) in udi, customer contact, device record history (6775e526-e175-407a-8dd1-bc8587841733).
NC Trek RX and NC Traveler Coronary Dilatation Catheters by Abbott
Pharmacy services.
- Mountain States Conference
- Investigational Drug Service
- Medical Services Rep
Recall of NC Trek RX Coronary Dilatation Catheter and the NC Traveler Coronary Dilatation Catheter by Abbott due to possible balloon malfunction.
More on the FDA website .

- En Español

- Medical Devices
- Radiation-Emitting Products
- Vaccines, Blood & Biologics
- Animal & Veterinary
- Tobacco Products
510(k) Premarket Notification
This website has been translated to Spanish from English, and is updated often. It is possible that some links will connect you to content only available in English or some of the words on the page will appear in English until translation has been completed (usually within 24 hours). We appreciate your patience with the translation process. In the case of any discrepancy in meaning, the English version is considered official. Thank you for visiting esp.fda.gov/tabaco.
User Profile
- Forgot Password

NC TREK NEO™ Coronary Dilatation Catheter 3.25 mm x 12 mm / Rapid-Exchange

Add to Favorites
Have A Rep Call Me
Send Information
Send Pricing
Request A Demo
NC TREK NEO™ Coronary Dilatation Catheter 3.25 mm x 12 mm / Rapid NC TREK NEO™ - 1400325-12
A flexible tube designed for percutaneous transluminal coronary angioplasty (PTCA) to dilate a stenotic coronary artery by controlled inflation of a distensible balloon(s) at its distal tip. It is typically available as: 1) an over-the-wire (OTW) type that has a double or triple-lumen, one for the guidewire and one or two for single- or double-balloon inflation; and 2) a rapid exchange (RX) type with a single-lumen. It is available in various sizes for the dilatation of small, narrowed, or obstructed coronary arteries or bypass grafts. It may also be intended for pre- or post-dilatation of a balloon-expandable stent (not included) in the coronary arteries. This is a single-use device.
- Although these devices have been tested for proper function after exposure to excursions of extreme temperatures (55 °C to -20 °C), storage is recommended in a dry, dark, cool place.
- Balloon Diameter: 3.2500 Millimeter
- Balloon Length: 12.0000 Millimeter
Device Sterile: False
Sterilization Prior To Use: False
Sterilization Methods: No Data Available
Device Name: Catheters, Transluminal Coronary Angioplasty, Percutaneous
Device Class: 2
Physical State: N/A
Definition: A PTCA catheter is a device that operates on the principle of hydraulic pressurization applied through an inflatable balloon attached to the distal end.
Submission Type ID: 1
Review Panel: CV
Review Code: N/A
Technical Method: N
Gmp Exempt Flag: N/A
Life Sustain Support Flag: N
Unclassified Reason: N/A
Implant Flag: N
Target Area: N/A
Regulation Number: 870.5100
Third Party Flag: N
Medical Specialty: CV
Device Id: 08717648232572
Device Type: Primary
DeviceId Issuing Agency: GS1
Contains DI Number: N/A
Package Quantity: N/A
Package Discontinue Date: N/A
Package Status: N/A
Package Type: N/A
Your Request
- United States eng
- Australia eng
- Germany deu
CARDIOVASCULAR
Trek™ & mini trek™ coronary dilatation catheters.

Designed with Smooth Transitions for Challenging Anatomy
TREK™ Coronary Dilatation Catheter’s unique design provides smooth transitions from hub to tip.
- Transitionless tip
- Flexible distal shaft
- Multi-layer CrossFlex²* balloon and Slim Seal** technology
Mini Trek™ Coronary Dilatation Catheter Indicated for Treatment of De Novo Chronic Total Occlusions
MINI TREK™ Coronary Dilatation Catheter’s ultra low profile design enables lesion access.
- Small chassis designed distinctively for MINI TREK™ Coronary Dilatation Catheter
- Available in sizes as small as 1.20 mm
Hypotube Design
Skive design provides smooth transition between hypotube and distal shaft.
Transitionless Tip
Confident crossing in resistant lesions.
Slim Seal ** Technology
Provides reduced wall thickness for flexibility and low tip crossing profiles.
Flexible Tungsten Markers †
Radiopaque flexible markers for confident placement.
Multi-layer Crossflex²* Balloon
Thin, multi-layer balloon technology provides low crossing profiles for access to complex lesions and a tri-fold design †† for excellent re-wrap.
Technical Information

Ordering Information
Broad size matrix with 77 rx sizes and 75 otw sizes.
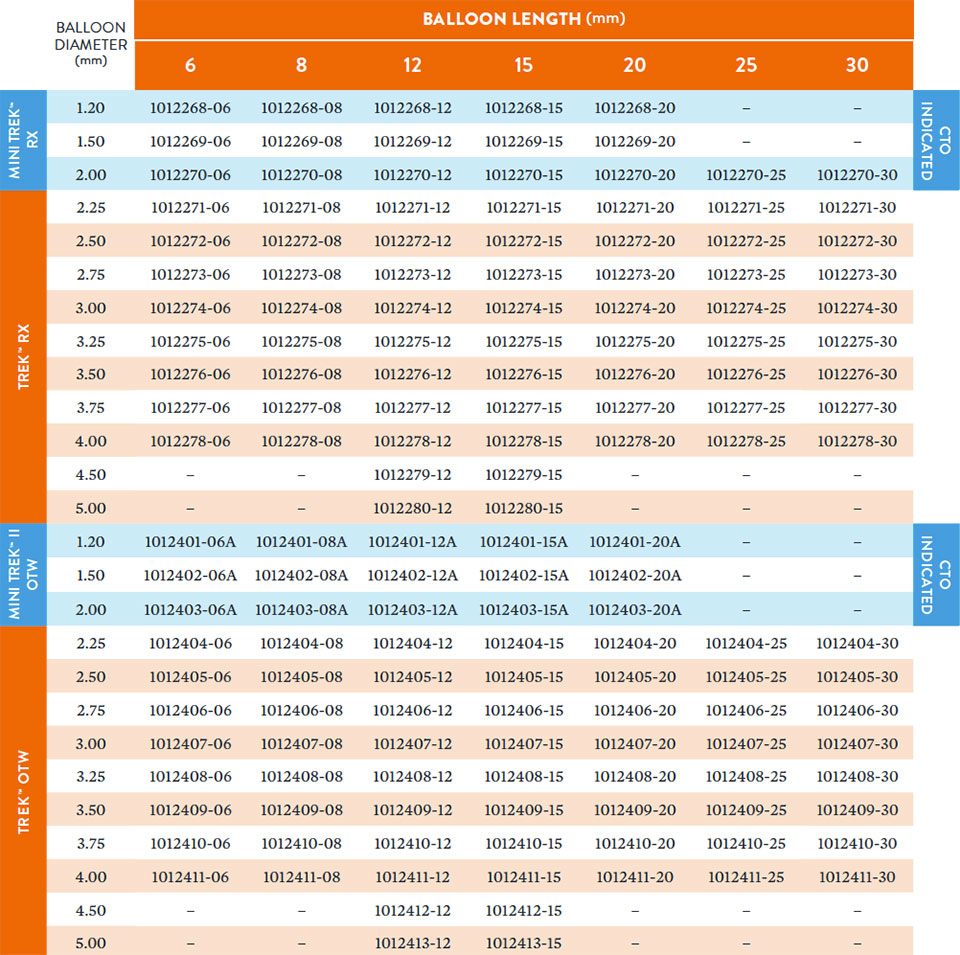
Compliance Chart
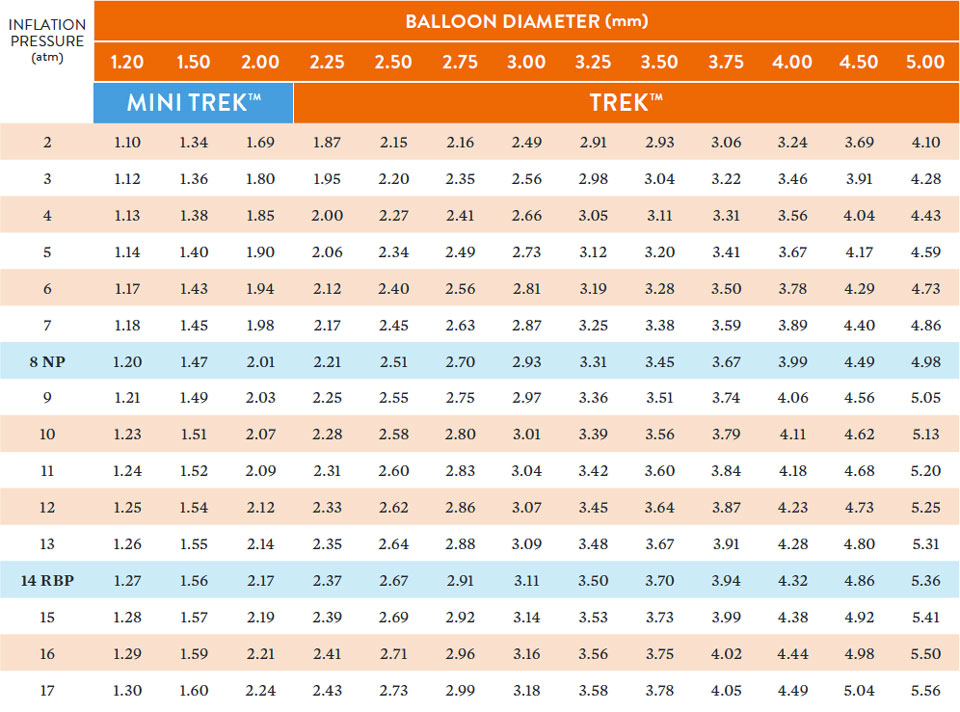
Data on file at Abbott. **Slim Seal technology available on 2.0 mm – 3.75 mm balloon sizes. † Single marker on all 6 mm lengths and 1.20 – 1.50 mm diameters (all lengths). Dual markers on all other sizes. *CrossFlex 2 technology available on 2.25 mm – 5.0 mm balloon sizes. †† Tri-fold design for 2.00 mm – 4.00 mm diameters. Bi-fold design for 1.20 mm & 1.50 mm diameters.
MAT-2111436 v1.0
POLICIES & ADVISORIES
- Customer Service
- Product Advisories
- Terms and Conditions
- Privacy Policy
HEALTHCARE PROFESSIONALS
- Disease Management
- Education & Training
- Reimbursement
- Manuals & Technical Resources
- Product Performance Reports
- Declarations of Conformity
PATIENTS & CAREGIVERS
- Treatments & Therapies
- Traveling with Your Device
Information contained herein for distribution outside the U.S. only. CAUTION: These products are intended for use by or under the direction of a physician. Prior to use, reference the Instructions for Use, inside the product carton (when available) or online for more detailed information on Indications, Contraindications, Warnings, Precautions and Adverse Events. Illustrations are artist's representations only and should not be considered as engineering drawings or photographs. Unless otherwise specified, all product names appearing in this Internet site are trademarks owned by or licensed to Abbott, its subsidiaries or affiliates. No use of any Abbott trademark, trade name, or trade dress in this site may be made without the prior written authorization of Abbott, except to identify the product or services of the company. ™ Indicates a trademark of the Abbott group of companies. ‡ Indicates a third party trademark, which is property of its respective owner. © 2024 Abbott. All Rights Reserved. MAT-2001111 v7.0
You are about to enter an Abbott country- or region-specific website.
Please be aware that the website you have requested is intended for the residents of a particular country or countries, as noted on that site. As a result, the site may contain information on pharmaceuticals, medical devices and other products or uses of those products that are not approved in other countries or regions
Do you wish to continue and enter this website?
MAT-2305078 v1.0
The following content is intended for Healthcare Professionals except for those in France. Some of the content is not in compliance with the French Advertising law N°2011-2012 dated 29th December 2011, article 34.
Si vous êtes un professionnel de santé exerçant en France ou dans les territoires Français, veuillez visiter notre site en français .
Do you want to continue?
MAT-2211958 v1.0
- Bone & Bone Marrow Biopsy
- Endomyocardial Biopsy
- HSG & Prostate
- Introducer Sets & Arterial needles
- Mammography
- Thrombus Management
- Bard Access
- Blood Pressure Accesories
- Balloon Dilatation
- Guide Wires
- Microcathters
- Not in Original Packaging
- Sheath Introducers
- HEINE ENT Diagnostic Instruments
- Magnifying Lamp
- Angiodynamics
- Edwards Lifesciences
- Health Care Logistics
- Izi Medical
- Sterilization Supplies
- Bard Magnum Biopsy Instrument and Needles
- BARD Vacora Vacuum Biopsy
- CareFusion Achieve Soft Tissue Biopsy
- Disposable Core Biopsy Systems
- MaxCore Biopsy Instruments
- Laryngoscopes
- Diagnostic Tests
- ETHICON Sutures
- Exam Room Supplies
- Acutus Medical
- Balt neurovascular
- Baylis Medical
- Boston Scientific
- Cardiac Cath Lab Kits
- Edwards LifeSciences
- Inari Medical
- Intact Vascular
- Johnson & Johnson
- Radiology Power Injector Syringes
- Spectranetics
- ST. JUDE MEDICAL
- Stryker Neurovascular
- Terumo Medical
- VASCULAR SOLUTIONS
- Infection Control
- Instrument Disinfectants
- Manager Specials
- IV Products
- Univent Tubes
- Sony Medical Grade Monitors
- Sony Black & White Print Packs
- Sony Color Print Packs
- Ultrasound supplies
- Cryosurgery
- Stryker Neuro Spine ENT
- Suction Supplies
- Surgical Accessories
- Sutures/Suture Removal
- Valleylab Covidien
- Weck Horizon ligation clip Hem-o-lok
- COATED VICRYL
- Becton Dickinson
- View all brands
Additional Information
- Customer Service
- Terms & Condiotions
Account Navigation
Currency - all prices are in aud.

- Sign in or Create an account

- Interventional Cardiology / Radiology / Electrophy
Abbott 1400300-15 NC TREK NEO™ Coronary Dilatation Catheter, 3.00 mm X 15 mm, 145 cm. Box of 01

Out of Stock
Sorry but this item is currently unavailable.
Please check back at a later stage.

Add to Wish List
Click the button below to add the Abbott 1400300-15 NC TREK NEO™ Coronary Dilatation Catheter, 3.00 mm X 15 mm, 145 cm. Box of 01 to your wish list.

Product Description
Abbott 1400300-15 NC TREK NEO™ Coronary Dilatation Catheter, 3.00 mm X 15 mm, 145 cm. Box of 01
NC TREK NEO™ Balloon is the newest generation non-compliant balloon re-engineered for challenging anatomy
NC TREK NEO™ Balloon delivers a broad size matrix, excellent pushability and exceptional balloon performance for challenging anatomy.
Product Reviews
Write your own review.
We promise to never spam you, and just use your email address to identify you as a valid customer.
This product hasn't received any reviews yet. Be the first to review this product!
Find Similar Products by Category
Related products.

Newsletter signup
Further info.
All prices are in USD . © 2024 MedicalEcart | Sitemap | Powered by BigCommerce
- Microdilatation
Videos with NC Trek

Live Case #3 - Dr Leibundgut & Dr Büttner
Swiss cto summit 2019.

4C 2019 : Live Case 5 - Le calcaire, c'est mon affaire !
Dr hakim benamer et dr erwan bressollette.
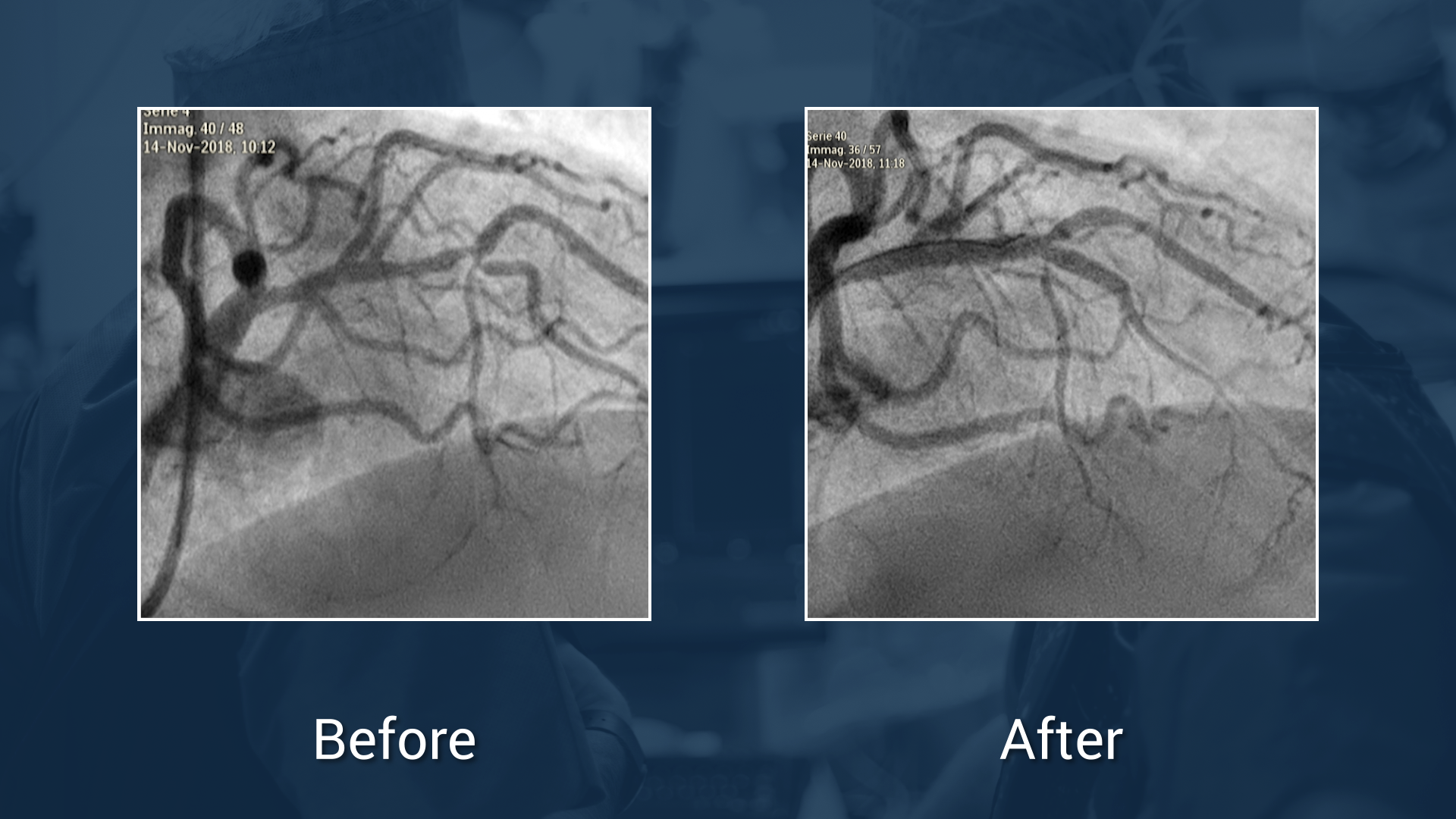
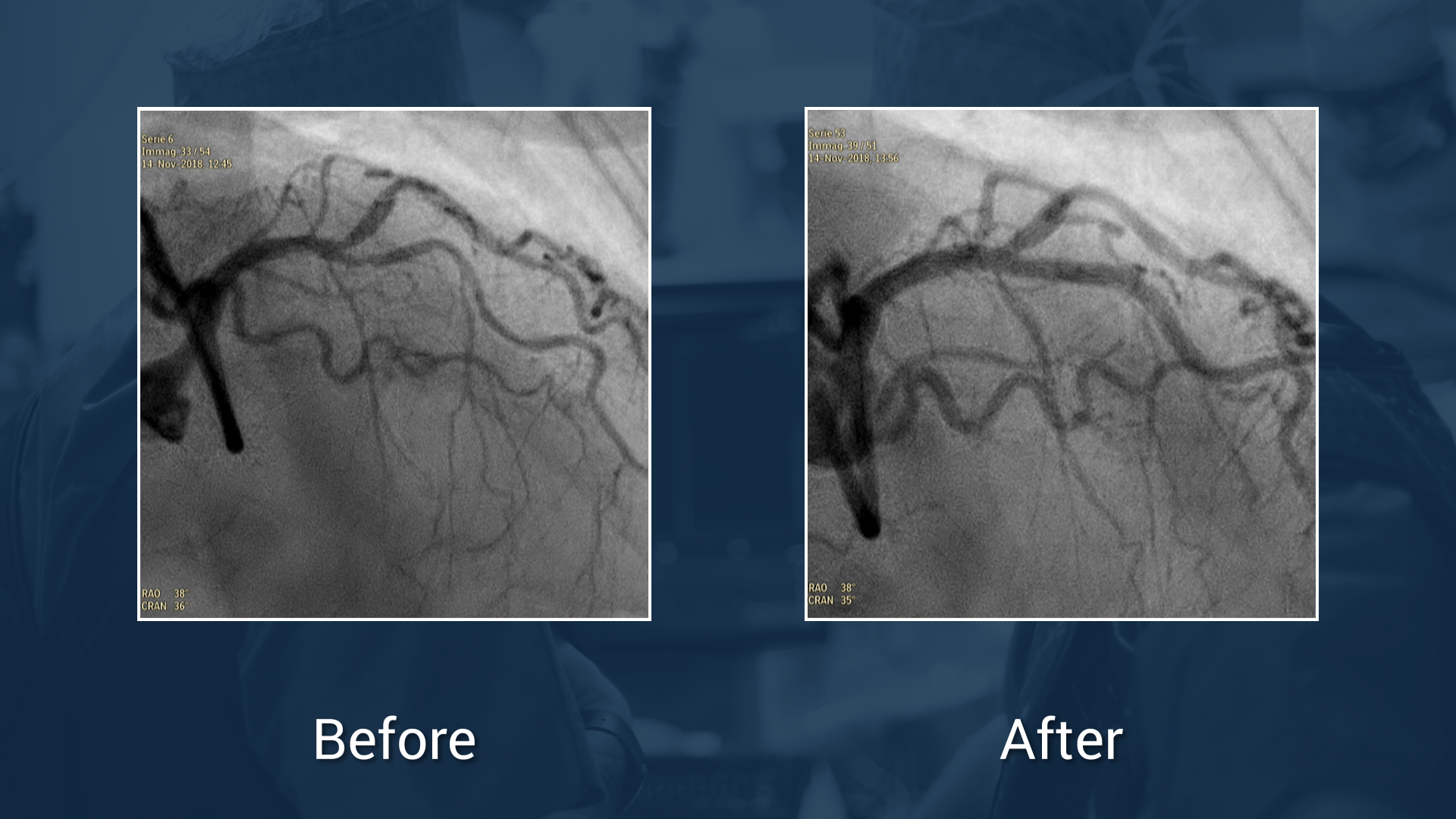
4C 2019 : Live Case 1 - Abord complexe
Dr marie-jeanne bertrand, dr nicolas amabile & dr rené koning.

4C 2019 : Live Case 3 - Les bifurcations… je gère
Dr lionel mangin, dr marion chatot & dr matthieu godin.
LAD / diagonal bifurcation with intramural distal LAD
Dr trani, dr burzotta & dr williams.
LAD diagonal bifurcation in tortous calcified vessels
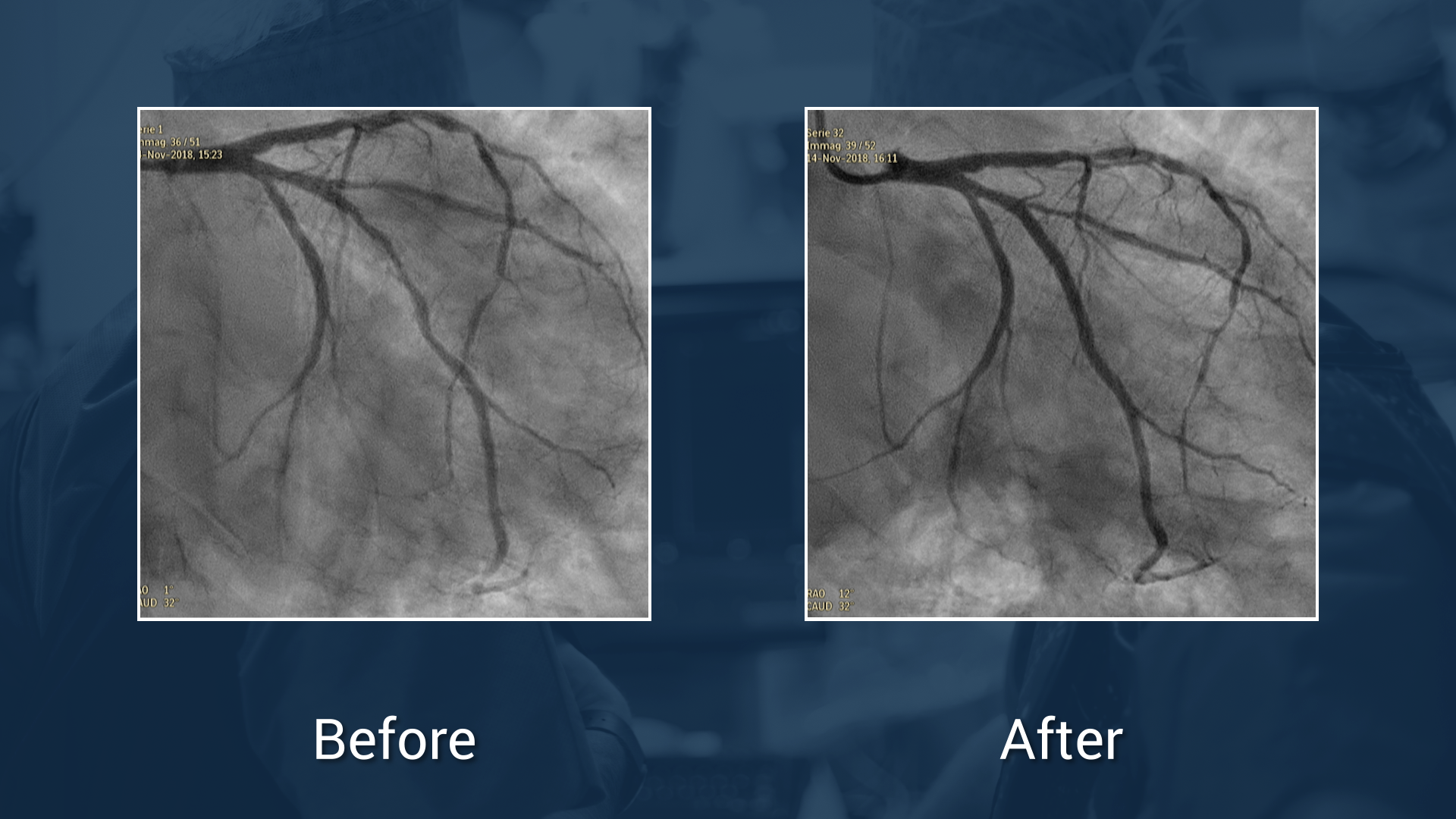
OCT guided PCI on a false bifurcation lesion
Restenose intrastent active CD proximale - scoring balloon - ballon actif - contrôle OCT
Dr georgios sideris & dr chakib benajiba.
IVUS guided recanalization of CTO of CX
Long in stent RCA CTO failure of previous attempt
Dr avran, dr barnay & dr pansieri.

IMAGES
VIDEO
COMMENTS
The NC TREK™ RX & OTW Coronary Dilatation Catheters are indicated for: a) balloon dilatation of the stenotic portion of a coronary artery or bypass graft stenosis, for the purpose of improving myocardial perfusion. b) balloon dilatation of a coronary artery occlusion, for the purpose of restoring coronary flow in patients with ST-segment ...
The NC TREK NEO™ Coronary Dilatation Catheters are indicated for: a) balloon dilatation of the stenotic portion of a coronary artery or bypass graft stenosis, for the purpose of improving myocardial perfusion. b) balloon dilatation of a coronary artery occlusion, for the purpose of restoring coronary flow in patients with ST-segment elevation ...
Balloon dilatation of a stent after implantation. Applies to MINI TREK™ RX and MINI TREK™ II OTW 1.50 mm - 2.00 mm sizes only: The TREK™ RX & OTW Coronary Dilatation Catheters are indicated for: Balloon dilatation of the stenotic portion of a coronary artery or bypass graft stenosis, for the purpose of improving myocardial perfusion.
Alicia Swanson. (408) 845-3427. Abbott has voluntarily recalled specific lots of two catheters used in coronary angioplasty procedures: the NC Trek RX Coronary Dilatation Catheter and the NC ...
For Important Safety Information on NC Trek Catheters visit Abbott's home page. Consumers with questions may contact the company via telephone at (800) 227-9902 between the hours of 5 a.m. and 5 p ...
The Food and Drug Administration (FDA) has announced that Abbott Vascular is recalling the NC Trek RX Coronary Dilatation Catheter and NC Traveler RX Coronary Dilatation Catheters, due to reports that the balloons from the impacted lots "may not deflate as intended" because of weaker material close to the balloon bond, resulting from excessive exposure to heat during manufacturing.
Abbott Vascular is recalling the NC Trek RX Coronary Dilatation Catheter and NC Traveler RX Coronary Dilatation Catheter, due to reports that the balloons from the impacted lots "may not deflate as intended" because of weaker material close to the balloon bond, resulting from excessive exposure to heat during manufacturing.
Manufacturer: Abbott. Description; Disclaimers; Catheter provides concentrated dilation force and flat compliance; Includes flexible, dual tungsten markers; ... NC Trek Catheter, Rapid, 3.75 mm x 20 mm, MSPV / Government Only: 1 EA: Stock Allocated QTY: / Remaining QTY: / Allocation Reset Date:
Start typing, then use the up and down arrows to select an option from the list. Depending on the user selection user can also view top products related to the selection.
NC TREK NEO Coronary Dilatation Catheter: Applicant: Abbott Vascular: 3200 Lakeside Drive: Santa Clara, CA 95054 Applicant Contact: Nishi Singh: Correspondent: Abbott Vascular: 3200 Lakeside Drive: Santa Clara, CA 95054 Correspondent Contact: Nishi Singh: Regulation Number ...
NC TREK ™ Coronary Balloon Dilatation Catheter ... or at medical.abbott/manuals for more detailed information on Indications, Contraindications, Warnings, Precautions and Adverse Events. Information contained herein for DISTRIBUTION outside of the U.S. only. Check the regulatory status of the device in areas where CE marking is not the ...
NC TREK™ RX: 1.50: 1012444-06 ... Unless otherwise specified, all product names appearing in this Internet site are trademarks owned by or licensed to Abbott, its subsidiaries or affiliates. No use of any Abbott trademark, trade name, or trade dress in this site may be made without the prior written authorization of Abbott, except to identify ...
Abbott NC Trek Coronary Dilatation Catheters - NC Trek Catheter, Rapid, 3.5 mm x 20 mm - 1012451-20 by Abbott. In Stock. $350.99 SKU 1012451-20 Discount Code: Use code TAKE5 for 5% OFF Total Purchase. Free Shipping Orders Over $100 Packaging. Quantity Quantity. Add to cart ...
By Shelley Wood. Abbott has voluntarily recalled specific lots of two coronary angioplasty catheters—the NC Trek RX coronary dilatation catheter and the NC Traveler coronary dilatation catheter—in three balloon diameters: 4.0, 4.5, and 5.0 mm. The US Food and Drug Administration has classified the action as a Class I recall, its most ...
AccessGUDID - NC TREK NEO™ (08717648232831)- NC TREK NEO™ Coronary Dilatation Catheter 5.50 mm X 12 mm / Rapid-Exchange. Skip to Main Content; National Library of Medicine NLM Tools and Resources FDA UDI Home FDA Medical Devices Home ... ABBOTT VASCULAR INC. Primary DI Number: 08717648232831 Issuing Agency: GS1 Commercial Distribution End ...
Recall of NC Trek RX Coronary Dilatation Catheter and the NC Traveler Coronary Dilatation Catheter by Abbott due to possible balloon malfunction. More on the FDA website . Recalls
The NC TREK NEO™ Coronary Dilatation Catheter is contraindicated for treatment of the unprotected left main coronary artery and for coronary artery spasm in the absence of a significant stenosis ... No use of any Abbott trademark, trade name, or trade dress in this site may be made without the prior written authorization of Abbott, except to ...
Device Classification Name: catheters, transluminal coronary angioplasty, percutaneous: 510(k) Number: K180040: Device Name: NC TREK™ RX Coronary Dilatation Catheter; NC TREK™ OTW Coronary Dilatation Catheter; TREK™ RX Coronary Dilatation Catheter; TREK™ OTW Coronary Dilatation Catheter; MINI TREK™ RX Coronary Dilatation Catheter; MINI TREK™ OTW Coronary Dilatation Catheter; MINI ...
NC TREK NEO™ Coronary Dilatation Catheter 3.25 mm x 12 mm / Rapid NC TREK NEO™ - 1400325-12. Device Description. A flexible tube designed for percutaneous transluminal coronary angioplasty (PTCA) to dilate a stenotic coronary artery by controlled inflation of a distensible balloon(s) at its distal tip.
Abbott 1012453-20 NC TREK Coronary Dilatation Catheter RX 4.00 mm X 20 mm, 143 cm, Box of 01 . NDICATIONS. The NC TREK RX & OTW Coronary Dilatation Catheters are indicated for: a) balloon dilatation of the stenotic portion of a coronary artery or bypass graft stenosis, for the purpose of improving myocardial perfusion.
Technical Information. Trek™ & Mini Trek™ Coronary Dilatation Catheters. Nominal Pressure (NP) 8 atm. Rated Burst Pressure (RBP) 14 atm. Tip Entry Profile. 0.017 in (3.00 mm Ø) Tip Crossing Profile.
Abbott 1400300-15 NC TREK NEO™ Coronary Dilatation Catheter, 3.00 mm X 15 mm, 145 cm. Box of 01. NC TREK NEO™ Balloon is the newest generation non-compliant balloon re-engineered for challenging anatomy. NC TREK NEO™ Balloon delivers a broad size matrix, excellent pushability and exceptional balloon performance for challenging anatomy. ...
Coronary Dilatation Catheter • Abbott Equipment; CATHETERS Microdilatation Abbott; NC Trek ... Videos with NC Trek. 12 Videos . Videos with NC Trek. 44:08. Watch. Live Case #3 - Dr Leibundgut & Dr Büttner Swiss CTO Summit 2019. Cardiology / Coronary / Cto / Live Case. View more . Share.