- Art & Science
- Coffee Break
- Case Studies
- Continuing Education
- Esophageal Dysphagia
- Evidence Based Practice
- Head Neck Cancer
- Instrumental Assessment
- International
- Neurogenic Dysphagia
- Rehabilitation
- Telepractice
- ENRICHMENT WEBINARS


Current and emerging evidence-based strategies for targeting the laryngeal elevators
Introduction.
During the pharyngeal stage of swallowing contraction of the geniohyoid, mylohyoid, thyrohyoid and anterior digastric muscles (laryngeal elevators) facilitates hyolaryngeal excursion and assists upper esophageal sphincter (UES) dilation. 1–3 When decreased hyolaryngeal excursion results in dysphagia, clinicians might choose to increase function in laryngeal elevators by incorporating rehabilitative exercises that promote neuromuscular adaptation. Adaptation occurs when the structural and functional properties of muscles change secondary to some form of stimulation. 4 As an example, clinical goals for a patient with dysphagia related to reduced hyolaryngeal excursion might include increasing the strength, range, or timing of submandibular muscle function during swallowing. When implementing clinical exercises targeting neuromuscular adaptation it is important for the clinician to choose those tasks which employ principles of strength training and motor learning, including resistance (overload), repetition, and specificity. 5
Mendelsohn Maneuver
A widely known exercise is the Mendelsohn maneuver (MM), which requires the patient to volitionally maintain contraction of the submandibular and other muscles during the pharyngeal stage of swallowing, with the aim of holding the larynx as high as possible for as long as possible. 2,6 Even though the MM can be applied as a direct (with food) or indirect (without food) exercise, in both contexts it adheres to the principle of specificity as the exercise is conducted during the act of swallowing. There may be some doubt as to whether this muscular contraction is made against a resistive load (to provide an overload to contraction). However, if one considers the ligaments and muscles originating below the hyoid as a band of elastic tissues providing a degree of resistance to contraction, anterior and vertical excursion of the hyoid must pull and maintain contraction against this resistive band. When an individual is sitting or standing, hyolaryngeal excursion must also act against another form of resistance – gravity. Although progressive loads cannot be applied in this context, the duration of contraction against the resistance can be increased progressively throughout an exercise protocol. Clinical outcome studies investigating the MM have demonstrated improvement in laryngeal excursion (extent of movement and duration of movement) in addition to swallow timing and bolus flow. 6,7
Effortful Swallow
The Effortful Swallow (ES) requires a patient to produce maximum effort when initiating a pharyngeal swallow. To achieve this they are typically prompted to “bear down” or “swallow hard”. It has been suggested that the mechanism for overload during production of ES is this increased volitional effort. 8 Accordingly, this exercise promotes an overload in the laryngeal elevators through amplified neurological drive (theoretically, increased motor unit recruitment) compared to normal swallowing. Although this exercise has been found to influence tongue-base retraction, measures of surface electromyography (sEMG) have found activity in the laryngeal elevators to be significantly greater during ES compared to normal swallows. 2,9 When combined with electrical stimulation, ES has also been found to significantly increase the degree of laryngeal excursion during swallowing in normal and dysphagic populations. 10,11 As with MM, ES adheres to the specificity principle as it is employed during the act of swallowing.
Shaker exercises, named after the original author of the approach and also referred to as head-lift exercises, utilize a protocol consisting of isometric and isotonic head-lifting movements while lying supine. 12 The activation of the hyolaryngeal elevators using this exercise has been confirmed using sEMG. 13 Published research has demonstrated a positive clinical effect of this exercise on hyolaryngeal excursion, UES dilation, and diet level of dysphagic individuals. 12,14 Head lift exercises do not adhere to the principle of specificity, although it is likely that overload is applied to the laryngeal elevators through effort applied to lifting the head towards the toes while lying supine. This is supported by sEMG data which has confirmed a significant increase in laryngeal elevator activity during the head-lift compared to resting baseline measurements. 15
Chin-to-Chest (CtC)
In the last few years clinical scientists have attempted to develop additional resistance-based exercises which target function of the laryngeal elevators, with the aim that these might be further developed as a potential rehabilitative exercises for patients with dysphagia related to decreased hyolaryngeal excursion. Our lab recently published a study which investigated the effect of a resistance-based exercise requiring an individual to open their jaw while pressing their chin into a semi-rigid brace fixed against their chest. 15 We coined this exercise “Chin-to-Chest” (CtC) to reflect the trajectory of movement. Because laryngeal elevators can also serve as depressors of the mandible, our theory was this exercise would require significant activation in the laryngeal elevators compared to rest, and that resistance to the contraction would apply a sufficient degree of overload so that motor unit recruitment was maximized. We also compared activation of the laryngeal elevators during CtC to activation during the head-lift exercise. We found significant increases in laryngeal elevator activity during CtC compared to rest, and significantly greater activation compared to the head-lift exercise. These results supported the incorporation of resistance against contraction to maximize the individual’s recruitment of motor units when performing the task. CtC does not adhere to the principle of specificity, although it does allow the clinician to incorporate the principle of progressive overload by asking the individual to modify the degree of contraction force when performing the exercise.
Chin Tuck Against Resistance (CTAR)
Yoon and colleagues recently published a study which also incorporated resistance against contraction, referred to as the Chin Tuck Against Resistance (CTAR). 16 These authors used a rubber ball placed between the chin and sternum as the resistive load. As with CtC, the authors’ implied that the rubber ball adds sufficient resistance to overload the laryngeal elevators during contraction. Participants were asked to squeeze the ball by tucking their chin as hard as possible so that it was compressed underneath their chin. Data from sEMG revealed significantly greater activation of the laryngeal elevators during CTAR compared to the head-lift exercise. These findings were consistent for both isometric and isotonic movements, and suggested that CTAR has the potential for serving as an alternative or supplementary exercise to the head-lift.
Transcutaneous Neuromuscular Stimulation
Although there are divergent opinions and equivocal evidence for the effect of transcutaneous neuromuscular stimulation (NMES) on laryngeal muscle activity, enough evidence exists (at least in this author’s opinion) to support the clinical theory that NMES applied transcutaneously to the submandibular muscles can facilitate contraction of the laryngeal elevators and influence hyolaryngeal excursion. Kim & Han demonstrated that NMES applied to the suprahyoid muscles resulted in vertical and anterior excursion of 9.6mm and 1.9mm, respectively, during electrical stimulation. 17 Toyama et al. recently compared submandibular NMES along with traditional therapy (Mendelsohn, thermal-tactile stimulation, and tongue exercises) to traditional therapy alone in groups of patients with dysphagia related to reduced hyolaryngeal excursion. The NMES + traditional therapy group exhibited greater post-treatment excursion of the hyoid along greater improvement in ratings of swallow function from videoflouroscopic studies. 18 NMES applied to the submandibular muscles during the act of swallowing is specific to the trajectory of the muscles but is facilitative rather than resistive, and thus does not provide an overload. However, Park et al. suggested that stimulation to the infrahyoid muscles during swallowing can act as a resistive load to the laryngeal elevators. In a randomized controlled trial they found that NMES applied transcutaneously to the infrahyoid muscles at a level that elicited a motor response resulted in significantly greater laryngeal elevation than a control group where NMES was only applied at a sensory level. 10
Newer exercises such as CtC and CTAR show promise but are in need of further research in clinical populations before they can be considered evidence-based strategies for clinical application. Small sample clinical trials and larger controlled trials will be needed, and evidence for the physiological effect of these exercises needs to be further clarified (e.g., via structural imaging during exercise performance). The good news for clinicians is that existing and emerging options do exist for targeting reduced hyolaryngeal excursion. Evidence for MM, ES, Shaker exercises, and NMES has been reported from clinical populations. Studies applying CtC and CTAR to clinical populations are on the horizon. As the research evidence for strategies targeting the laryngeal elevators becomes more robust, clinicians will have additional tools for tailoring plans of treatment to the needs and abilities of the individual patient.
- Thexton a J, Crompton a W, German RZ. Electromyographic activity during the reflex pharyngeal swallow in the pig: Doty and Bosma (1956) revisited. J Appl Physiol . 2007;102(2):587–600. doi:10.1152/japplphysiol.00456.2006.
- Logemann JA. Evaluation and Treatment of Swallowing Disorders . 2nd ed. Austin: Pro Ed; 1998.
- Palmer PM, Luschei ES, Jaffe D, McCulloch TM. Contributions of individual muscles to the submental surface electromyogram during swallowing. J Speech, Lang Hear Res . 1999;42:1378–1391.
- Steele CM. Exercise-Based Approaches to Dysphagia Rehabilitation. Nestle Nutr Inst Work Ser . 2012;72:109–117.
- Burkhead LM, Sapienza CM, Rosenbek JC. Strength-training exercise in dysphagia rehabilitation: principles, procedures, and directions for future research. Dysphagia . 2007;22(3):251–65. doi:10.1007/s00455-006-9074-z.
- McCullough GH, Kim Y. Effects of the Mendelsohn maneuver on extent of hyoid movement and UES opening post-stroke. Dysphagia . 2013;28(4):511–9. doi:10.1007/s00455-013-9461-1.
- McCullough G, Kamarunas E, Mann G, Schmidley J, Robbins J, Crary M. Effects of mendelsohn maneuver on measures of swallowing duration post-stroke. Top Stroke Rehabil . 2012;19(March 2009):234–243. doi:10.1310/tsr1903-234.Effects.
- Clark HM, Shelton N. Training effects of the effortful swallow under three exercise conditions. Dysphagia . 2014;29(5):553–63. doi:10.1007/s00455-014-9544-7.
- Wheeler-Hegland KM, Rosenbek JC, Sapienza CM. Submental sEMG and hyoid movement during Mendelsohn maneuver, effortful swallow, and expiratory muscle strength training. J Speech Lang Hear Res . 2008;51(5):1072–87. doi:10.1044/1092-4388(2008/07-0016).
- Park J-W, Kim Y, Oh J-C, Lee H-J. Effortful swallowing training combined with electrical stimulation in post-stroke dysphagia: a randomized controlled study. Dysphagia . 2012;27(4):521–7. doi:10.1007/s00455-012-9403-3.
- Park J-W, Oh J-C, Lee HJ, Park S-J, Yoon T-S, Kwon BS. Effortful swallowing training coupled with electrical stimulation leads to an increase in hyoid elevation during swallowing. Dysphagia . 2009;24(3):296–301. doi:10.1007/s00455-008-9205-9.
- Shaker R, Easterling C, Kern M, et al. Rehabilitation of swallowing by exercise in tube-fed patients with pharyngeal dysphagia secondary to abnormal UES opening. Gastroenterology . 2002;122(5):1314–1321. doi:10.1053/gast.2002.32999.
- Yoshida M, Groher ME, Crary MA, Mann GC, Akagawa Y. Comparison of surface electromyographic ( sEMG ) activity of submental muscles between the head lift and tongue press exercises as a therapeutic exercise for pharyngeal dysphagia. Gerodontology . 2007;24(2):111–116.
- Logemann JA, Rademaker A, Pauloski B, et al. A randomized study comparing the Shaker exercise with traditional therapy: a preliminary study. Dysphagia . 2009;24(4):403–411. doi:10.1007/s00455-009-9217-0.A.
- Watts CR. Measurement of hyolaryngeal muscle activation using surface electromyography for comparison of two rehabilitative dysphagia exercises. Arch Phys Med Rehabil . 2013;94(12):2542–8. doi:10.1016/j.apmr.2013.04.013.
- Yoon WL, Khoo JKP, Rickard Liow SJ. Chin tuck against resistance (CTAR): new method for enhancing suprahyoid muscle activity using a Shaker-type exercise. Dysphagia . 2014;29(2):243–8. doi:10.1007/s00455-013-9502-9.
- Kim SJ, Han TR. Effect of surface electrical stimulation of suprahyoid muscles on hyolaryngeal movement. Neuromodulation . 2009;12(2):134–40. doi:10.1111/j.1525-1403.2009.00200.x.
- Toyama K, Matsumoto S, Kurasawa M, et al. Novel Neuromuscular Electrical Stimulation System for Treatment of Dysphagia after Brain Injury. Neurol Med Chir (Tokyo) . 2014:1–8. doi:10.2176/nmc.oa.2013-0341.
RELATED ARTICLES MORE FROM AUTHOR
When Dementia and Dysphagia Co-Occur: The Role of the SLP
A Long Way Round: The Journey from Brain to Muscle to Your sEMG Sensor
Where we fall with ARDS (Part 1): The Rabbit Hole

We apologize for the inconvenience...
To ensure we keep this website safe, please can you confirm you are a human by ticking the box below.
If you are unable to complete the above request please contact us using the below link, providing a screenshot of your experience.
https://ioppublishing.org/contacts/
Assessing Hyolaryngeal Excursion: Comparing Quantitative Methods to Palpation at the Bedside and Visualization During Videofluoroscopy
Affiliations.
- 1 Department of Communicative Sciences and Disorders, NYU Steinhardt, 665 Broadway, 9th Floor, New York, NY, 10012, USA.
- 2 Division of Otolaryngology-Head and Neck Surgery, University of Wisconsin School of Medicine and Public Health, Wisconsin Institutes for Medical Research (WIMR), BLDG. 1485, 1111 Highland Avenue, Madison, WI, 53705-2275, USA.
- 3 Division of Otolaryngology-Head and Neck Surgery, University of Wisconsin School of Medicine and Public Health, Wisconsin Institutes for Medical Research (WIMR), BLDG. 1485, 1111 Highland Avenue, Madison, WI, 53705-2275, USA. [email protected].
- PMID: 30043080
- PMCID: PMC6345616
- DOI: 10.1007/s00455-018-9927-2
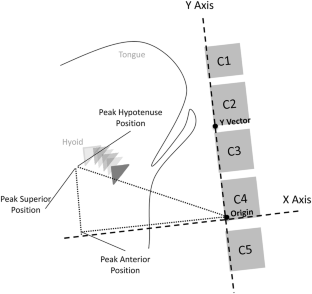
Purpose: Hyolaryngeal excursion (HE) is typically assessed via palpation during clinical swallowing exams (CSE) or visually during videofluoroscopy (VFSS). Minimal evidence exists to support the use of these perceptual methods for judging HE. We investigated whether binary judgment of HE differentiates quantitative measures of hyoid movement, using frame-by-frame VFSS analysis to measure anatomically scaled peak hyoid positions.
Methods: Medical records of patients who received a CSE and VFSS within a 24-h period were reviewed. Clinician ratings of HE ('reduced' or 'normal') were collected from CSE and VFSS reports, along with rater experience. Five ml puree swallows were extracted from each VFSS for randomized, blinded analysis. Peak hyoid position from C4 was captured in anterior, superior, and hypotenuse positions and expressed relative to C2-C4 length. T-test comparisons of hyoid positions between patients judged to have reduced versus normal HE on palpation and VFSS were conducted.
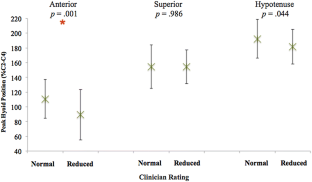
Results: Eighty-seven patients (56 male, mean age 61) met criteria. Peak anterior hyoid position was significantly different between patients judged to have reduced (mean = 89.2% C2-C4) and normal (mean = 110.6% C2-C4) HE on palpation (p = 0.001). Further analysis revealed no effect of clinician experience on differentiation of objective measures based on palpation. No differences were found across any objective measures when compared to clinician VFSS ratings.
Conclusions: Clinicians appeared to be able to differentiate peak anterior hyoid movement but not superior or hypotenuse movement on palpation. On VFSS visualization, no significant differences were found between swallows judged to have reduced versus normal HE in any directional dimension. While perceptual methods may contribute to clinical decision-making, clinicians should remain cautious when making judgments about HE using these methods.
Keywords: Clinical swallowing evaluation; Deglutition; Deglutition disorders; Dysphagia; Hyolaryngeal excursion; Palpation.
Publication types
- Comparative Study
- Research Support, N.I.H., Extramural
- Research Support, Non-U.S. Gov't
- Cineradiography / statistics & numerical data*
- Clinical Decision-Making / methods*
- Decision Support Techniques
- Deglutition
- Deglutition Disorders / diagnosis*
- Hyoid Bone / diagnostic imaging
- Hyoid Bone / pathology
- Larynx / diagnostic imaging
- Larynx / pathology
- Middle Aged
- Observer Variation
- Palpation / statistics & numerical data*
- Point-of-Care Testing / statistics & numerical data*
Grants and funding
- R01 DC004336/DC/NIDCD NIH HHS/United States

Clinical Archives of Communication Disorders 2019; 4(3): 177-184.
Published online: December 31, 2019
DOI: http://dx.doi.org/10.21849/cacd.2019.00052
Hyoid excursion during the swallow in stroke survivors
1 Communication Sciences and Disorders, Ohio University, Athens, Ohio, USA
2 Communication Sciences and Disorders, Illinois State University, Normal, IL, USA
Correspondence: Youngsun Kim, Communication Sciences and Disorders, Ohio University, W233 Grover Center, Athens, OH 45701-2979, USA, Tel: 1-740-597-1286, Fax: 1-740-593-0287, E-mail: [email protected]
© 2019 The Korean Association of Speech-Language Pathologists
( open-access ):
Conclusions
Keywords : Swallowing ; Dysphagia ; Aspiration ; Hyoid excursion ; Stroke
INTRODUCTION
Videofluoroscopic swallowing examination (vfse), experimental procedures, procedures of temporal measurement of hyoid excursion (duration), procedures of biomechanical measurement of hyoid excursion (distance), procedures for hyoid velocity during swallowing, statistical analysis, reliability, duration of maximum hyoid excursion, distance of maximum hyoid excursion, velocity of maximum hyoid excursion, bolus volumes on hyoid excursion, clinical implications, limitations and future study.
Assessing Hyolaryngeal Excursion: Comparing Quantitative Methods to Palpation at the Bedside and Visualization During Videofluoroscopy
- Original Article
- Published: 24 July 2018
- Volume 34 , pages 298–307, ( 2019 )
Cite this article

- Danielle Brates ORCID: orcid.org/0000-0003-3829-2865 1 , 2 ,
- Sonja M. Molfenter 1 &
- Susan L. Thibeault 2
3001 Accesses
11 Citations
202 Altmetric
24 Mentions
Explore all metrics
A Correction to this article was published on 16 September 2020
This article has been updated
Hyolaryngeal excursion (HE) is typically assessed via palpation during clinical swallowing exams (CSE) or visually during videofluoroscopy (VFSS). Minimal evidence exists to support the use of these perceptual methods for judging HE. We investigated whether binary judgment of HE differentiates quantitative measures of hyoid movement, using frame-by-frame VFSS analysis to measure anatomically scaled peak hyoid positions.
Medical records of patients who received a CSE and VFSS within a 24-h period were reviewed. Clinician ratings of HE (‘reduced’ or ‘normal’) were collected from CSE and VFSS reports, along with rater experience. Five ml puree swallows were extracted from each VFSS for randomized, blinded analysis. Peak hyoid position from C4 was captured in anterior, superior, and hypotenuse positions and expressed relative to C2–C4 length. T -test comparisons of hyoid positions between patients judged to have reduced versus normal HE on palpation and VFSS were conducted.
Eighty-seven patients (56 male, mean age 61) met criteria. Peak anterior hyoid position was significantly different between patients judged to have reduced (mean = 89.2% C2–C4) and normal (mean = 110.6% C2–C4) HE on palpation ( p = 0.001). Further analysis revealed no effect of clinician experience on differentiation of objective measures based on palpation. No differences were found across any objective measures when compared to clinician VFSS ratings.
Conclusions
Clinicians appeared to be able to differentiate peak anterior hyoid movement but not superior or hypotenuse movement on palpation. On VFSS visualization, no significant differences were found between swallows judged to have reduced versus normal HE in any directional dimension. While perceptual methods may contribute to clinical decision-making, clinicians should remain cautious when making judgments about HE using these methods.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Reproduced with permission from Molfenter and Steele [ 24 ]
Similar content being viewed by others
Cervical Vertebral Height Approximates Hyoid Displacement in Videofluoroscopic Images of Healthy Adults
Ultrasound: Validity of a Pocket-Sized System in the Assessment of Swallowing

An Exploratory Study of Hyoid Visibility, Position, and Swallowing-Related Displacement in a Pediatric Population
Change history, 16 september 2020.
This letter notifies the readers of the Dysphagia journal of an error in the original published version of this manuscript, for which a previously available open source spreadsheet tool had been used to calculate the position of the hyoid bone or larynx on lateral view videofluoroscopic images. An error in the mathematical formula built into the spreadsheet resulted in a reversal of the results for the X and Y planes of measurement. This erratum provides corrections to the results and interpretations of the original manuscript.
Dodds WJ, Stewart ET, Logemann JA. Physiology and radiology of the normal oral and pharyngeal phases of swallowing. AJR Am J Roentgenol. 1990;154(5):953–63. https://doi.org/10.2214/ajr.154.5.2108569 .
Article CAS PubMed Google Scholar
Matsuo K, Palmer JB. Anatomy and physiology of feeding and swallowing: normal and abnormal. Phys Med Rehabil Clin N Am. 2008;19(4):691–707. https://doi.org/10.1016/j.pmr.2008.06.001 .
Article PubMed PubMed Central Google Scholar
Paik NJ, Kim SJ, Lee HJ, Jeon JY, Lim JY, Han TR. Movement of the hyoid bone and the epiglottis during swallowing in patients with dysphagia from different etiologies. J Electromyogr Kines. 2008;18(2):329–35. https://doi.org/10.1016/j.jelekin.2006.09.011 .
Article Google Scholar
Logemann JA, Kahrilas PJ, Cheng J, Pauloski BR, Gibbons PJ, Rademaker AW, Lin S. Closure mechanisms of laryngeal vestibule during swallow. Am J Physiol. 1992;262(2 Pt 1):G338–44. https://doi.org/10.1152/ajpgi.1992.262.2.G338 .
Jacob P, Kahrilas PJ, Logemann JA, Shah V, Ha T. Upper esophageal sphincter opening and modulation during swallowing. Gastroenterology. 1989;97(6):1469–78.
Article CAS Google Scholar
McCullough GH, Rosenbek JC, Wertz RT, McCoy S, Mann G, McCullough K. Utility of clinical swallowing examination measures for detecting aspiration post-stroke. J Speech Lang Hear Res. 2005;48(6):1280–93. https://doi.org/10.1044/1092-4388(2005/089) .
Ramsey DJC, Smithard DG, Kalra L. Early assessments of dysphagia and aspiration risk in acute stroke patients. Stroke. 2003;34(5):1252–7. https://doi.org/10.1161/01.Str.0000066309.06490.B8 .
Article PubMed Google Scholar
Logemann JA. Evaluation and treatment of swallowing disorders. 2nd ed. Austin, TX: Pro-Ed; 1998.
Google Scholar
McCullough GH, Martino R. Clinical evaluation of patients with dysphagia: importance of history taking and physical exam. In: Shaker EC, Belafsky PC, Postma GN, editors. Manual of diagnostic and therapeutic techniques for disorders of deglutition. New York: Springer; 2013. p. 11–30.
Chapter Google Scholar
Ishida R, Palmer JB, Hiiemae KM. Hyoid motion during swallowing: factors affecting forward and upward displacement. Dysphagia. 2002;17(4):262–72. https://doi.org/10.1007/s00455-002-0064-5 .
Kim Y, McCullough GH. Maximum hyoid displacement in normal swallowing. Dysphagia. 2008;23(3):274–9. https://doi.org/10.1007/s00455-007-9135-y .
Chi-Fishman G, Sonies BC. Effects of systematic bolus viscosity and volume changes on hyoid movement kinematics. Dysphagia. 2002;17(4):278–87. https://doi.org/10.1007/s00455-002-0070-7 .
Nagy A, Molfenter SM, Peladeau-Pigeon M, Stokely S, Steele CM. The effect of bolus volume on hyoid kinematics in healthy swallowing. Biomed Res Int. 2014;2014:738971. https://doi.org/10.1155/2014/738971 .
Rofes L, Arreola V, Martin A, Clave P. Natural capsaicinoids improve swallow response in older patients with oropharyngeal dysphagia. Gut. 2013;62(9):1280–7. https://doi.org/10.1136/gutjnl-2011-300753 .
Leow LP, Huckabee ML, Sharma S, Tooley TP. The influence of taste on swallowing apnea, oral preparation time, and duration and amplitude of submental muscle contraction. Chem Senses. 2007;32(2):119–28. https://doi.org/10.1093/chemse/bjl037 .
Leonard RJ, Kendall KA, McKenzie S, Goncalves MI, Walker A. Structural displacements in normal swallowing: a videofluoroscopic study. Dysphagia. 2000;15(3):146–52. https://doi.org/10.1007/s004550010017 .
Logemann JA, Pauloski BR, Rademaker AW, Colangelo LA, Kahrilas PJ, Smith CH. Temporal and biomechanical characteristics of oropharyngeal swallow in younger and older men. J Speech Lang Hear Res. 2000;43(5):1264–74.
Molfenter SM, Steele CM. Physiological variability in the deglutition literature: hyoid and laryngeal kinematics. Dysphagia. 2011;26(1):67–74. https://doi.org/10.1007/s00455-010-9309-x .
McCullough GH, Wertz RT, Rosenbek JC, Dinneen C. Clinicians’ preferences and practices in conducting clinical-bedside and videofluoroscopic swallowing examinations in an adult, neurogenic population. Am J Speech Lang Pathol. 1999;8(2):149–63.
Mann G. MASA: the Mann assessment of swallowing ability. Boston: Cengage Learning; 2002.
McCullough GH, Wertz RT, Rosenbek JC, Mills RH, Ross KB, Ashford JR. Inter- and intrajudge reliability of a clinical examination of swallowing in adults. Dysphagia. 2000;15(2):58–67. https://doi.org/10.1007/s004550010002 .
Rangarathnam B, McCullough GH. Utility of a clinical swallowing exam for understanding swallowing physiology. Dysphagia. 2016;31(4):491–7. https://doi.org/10.1007/s00455-016-9702-1 .
Lee JW, Randall DR, Evangelista LM, Kuhn MA, Belafsky PC. Subjective assessment of videofluoroscopic swallow studies. Otolaryngol Head Neck Surg. 2017;156(5):901–5. https://doi.org/10.1177/0194599817691276 .
Schneider CA, Rasband WS, Eliceiri KW. NIH image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671–5.
Molfenter SM, Steele CM. Use of an anatomical scalar to control for sex-based size differences in measures of hyoid excursion during swallowing. J Speech Lang Hear Res. 2014;57(3):768–78. https://doi.org/10.1044/2014_Jslhr-S-13-0152 .
Perlman AL, VanDaele DJ, Otterbacher MS. Quantitative assessment of hyoid bone displacement from video images during swallowing. J Speech Hear Res. 1995;38(3):579–85.
Thompson TZ, Obeidin F, Davidoff AA, Hightower CL, Johnson CZ, Rice SL, Sokolove RL, Taylor BK, Tuck JM, Pearson WG Jr. Coordinate mapping of hyolaryngeal mechanics in swallowing. J Vis Exp. 2014. https://doi.org/10.3791/51476 .
Fleiss JL. The design and analysis of clinical experiments. New York, NY: Wiley; 1986.
Steele CM, Bailey GL, Chau T, Molfenter SM, Oshalla M, Waito AA, Zoratto DC. The relationship between hyoid and laryngeal displacement and swallowing impairment. Clin Otolaryngol. 2011;36(1):30–6. https://doi.org/10.1111/j.1749-4486.2010.02219.x .
Article CAS PubMed PubMed Central Google Scholar
World Health Organization. Physical status: the use and interpretation of anthropometry. Technical Report Series. Geneva: WHO; 1995.
Molfenter SM, Steele CM. Use of an anatomical scalar to control for sex-based size differences in measures of hyoid excursion during swallowing. J Speech Lang Hear Res. 2014;57(3):768–78. https://doi.org/10.1044/2014_JSLHR-S-13-0152 .
Benoist M. Natural history of the aging spine. Eur Spine J. 2003;12(Suppl 2):S86–9. https://doi.org/10.1007/s00586-003-0593-0 .
Malcolm GP. Surgical disorders of the cervical spine: presentation and management of common disorders. J Neurol Neurosurg Psychiatry. 2002;73(Suppl 1):i34–41.
PubMed PubMed Central Google Scholar
Ezra D, Masharawi Y, Salame K, Slon V, Alperovitch-Najenson D, Hershkovitz I. Demographic aspects in cervical vertebral bodies’ size and shape (C3–C7): a skeletal study. Spine J. 2017;17(1):135–42. https://doi.org/10.1016/j.spinee.2016.08.022 .
Palmer JB, Tanaka E, Ensrud E. Motions of the posterior pharyngeal wall in human swallowing: a quantitative videofluorographic study. Arch Phys Med Rehabil. 2000;81(11):1520–6. https://doi.org/10.1053/apmr.2000.17829 .
Martin-Harris B, Brodsky MB, Michel Y, Castell DO, Schleicher M, Sandidge J, Maxwell R, Blair J. MBS measurement tool for swallow impairment—MBSImp: establishing a standard. Dysphagia. 2008;23(4):392–405. https://doi.org/10.1007/s00455-008-9185-9 .
Download references
Acknowledgements
The authors would like to thank James C. Borders for his assistance during data collection, Glen Leverson and Sharon Weinberg for their assistance during data analysis, and the speech-language pathologists at UW-Madison Voice and Swallow clinics. Portions of this work were presented at the 2018 Dysphagia Research Society meeting.
Diane M. Bless Endowed Chair in Otolaryngology, University of Wisonsin-Madison.
Author information
Authors and affiliations.
Department of Communicative Sciences and Disorders, NYU Steinhardt, 665 Broadway, 9th Floor, New York, NY, 10012, USA
Danielle Brates & Sonja M. Molfenter
Division of Otolaryngology-Head and Neck Surgery, University of Wisconsin School of Medicine and Public Health, Wisconsin Institutes for Medical Research (WIMR), BLDG. 1485, 1111 Highland Avenue, Madison, WI, 53705-2275, USA
Danielle Brates & Susan L. Thibeault
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Susan L. Thibeault .
Ethics declarations
Conflict of interest.
The research conducted by the first author was supported by funding from the Diane M. Bless Endowed Chair in Otolaryngology. The authors declare no conflict of interest to disclose.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Institutional Review Board and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was waived with IRB approval for retrospective medical chart review data collection.
Rights and permissions
Reprints and permissions
About this article
Brates, D., Molfenter, S.M. & Thibeault, S.L. Assessing Hyolaryngeal Excursion: Comparing Quantitative Methods to Palpation at the Bedside and Visualization During Videofluoroscopy. Dysphagia 34 , 298–307 (2019). https://doi.org/10.1007/s00455-018-9927-2
Download citation
Received : 26 March 2018
Accepted : 17 July 2018
Published : 24 July 2018
Issue Date : 15 June 2019
DOI : https://doi.org/10.1007/s00455-018-9927-2

Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Deglutition
- Deglutition disorders
- Hyolaryngeal excursion
- Clinical swallowing evaluation
- Find a journal
- Publish with us
- Track your research
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Ann Rehabil Med
- v.39(2); 2015 Apr
Changes in Hyolaryngeal Movement and Swallowing Function After Neuromuscular Electrical Stimulation in Patients With Dysphagia
Hoo young lee.
1 Department of Rehabilitation Medicine, Gangnam Severance Hospital, Seoul, Korea.
2 Rehabilitation Institute of Neuromuscular Disease, Yonsei University College of Medicine, Seoul, Korea.
Ji Seong Hong
3 Department of Rehabilitation Medicine, National Health Insurance Service Ilsan Hospital, Goyang, Korea.
Kil Chan Lee
4 Department of Rehabilitation Medicine and Research Institute of Rehabilitation Medicine, Yonsei University College of Medicine, Seoul, Korea.
Yoon-Kyum Shin
5 Brain Korea 21 PLUS Project for Medical Science, Yonsei University College of Medicine, Seoul, Korea.
Sung-Rae Cho
6 Avison Biomedical Research Center, Yonsei University College of Medicine, Seoul, Korea.
To investigate immediate changes in hyolaryngeal movement and swallowing function after a cycle of neuromuscular electrical stimulation (NMES) on both submental and throat regions and submental placement alone in patients with dysphagia.
Fifteen patients with dysphagia were recruited. First, videofluoroscopic swallowing study (VFSS) was performed before NMES. All patients thereafter received a cycle of NMES by 2 methods of electrode placement: 1) both submental and throat regions and 2) submental placement alone concomitant with VFSS. The Penetration-Aspiration Score (PAS) and the NIH-Swallowing Safety Scale (NIH-SSS) were measured for swallowing function.
During swallowing, hyolaryngeal descent significantly occurred by NMES on both submental and throat regions, and anterior displacement of hyolaryngeal complex was significant on submental placement alone. NMES on submental placement alone did not change the PAS and NIH-SSS. However, NMES on both submental and throat regions significantly reduced the NIH-SSS, although it did not change the PAS. Patients with no brainstem lesion and with dysphagia duration of <3 months showed significantly improved the NIH-SSS.
Immediate hyolaryngeal movement was paradoxically depressed after NMES on both submental and throat regions with significant reductions in the NIH-SSS but not the PAS, suggesting improvement in pharyngeal peristalsis and cricopharyngeal functions at the esophageal entry rather than decreased aspiration and penetration. The results also suggested that patients with dysphagia should be carefully screened when determining motor-level NMES.
INTRODUCTION
Swallowing is a complex function that requires elevation and anterior excursion of the hyolaryngeal complex in the pharyngeal phase, which aids laryngeal vestibule closure and serves to prevent aspiration into the respiratory tract [ 1 ]. In recent years, surface electrical stimulation has been gaining attention for its muscle strengthening effect by motor stimulation and facilitation of swallowing reflex by sensory stimulation [ 2 ]. The effects of neuromuscular electrical stimulation (NMES) on the physiology of swallowing have been well studied but remain unclear. The emerging hypothesis on the physiological effects of NMES on swallowing is that motor stimulation produces a resistance to hyolaryngeal elevation and induces effortful swallowing [ 3 ]. Shaw et al. [ 4 ] retrospectively analyzed patients who received NMES with electrodes placed in both submental and throat regions, and demonstrated an improved swallowing function in patients with mild to moderate dysphagia. A cohort study by Blumenfeld et al. [ 5 ] suggested that dysphagia therapy with NMES on throat regions with a motor-level stimulation is superior to traditional dysphagia therapy alone in individuals in the acute care facility. On the other hand, Humbert et al. [ 6 ] designed a study with electrodes placed in different ways including both submental and throat regions and submental placement alone. Their results showed a significant hyolaryngeal descent with stimulation at rest and significant reduction in hyolaryngeal peak elevation during swallowing when the electrodes were placed in both submental and laryngeal regions with intensity at the motor level. It was suggested that in those patients who had the ability to raise their hyolaryngeal complex, hyoid depression with stimulation might serve as resistance exercise during therapy. However, if patients were unable to produce hyolaryngeal elevation and were unable to resist the NMES-induced hyoid depression, the stimulation might increase the risk of aspiration, as the hyolaryngeal complex would remain held down during swallowing. Stimulated swallows were less safe than non-stimulated swallows according to the National Institutes of Health-Swallowing Safety Scale (NIH-SSS). Other studies showed that NMES had no additional improvement, as compared to traditional swallowing treatments [ 7 , 8 ]. Thus, the mechanism underlying NMES therapy for dysphagia is still unclear.
Of the electrode placement methods of NMES, two different methods such as both submental and throat regions and submental placement alone have been widely used to investigate the actual strengthening effect [ 2 , 6 ]. NMES on both submental and throat regions has been theoretically expected to strengthen thyrohyoid muscle that contributes to elevation of hyolarynx. NMES with submental placement alone reinforces muscle complexes such as anterior belly of the digastric and mylohyoid that are anatomically responsible for hyoid elevation. However, more superficially located sternohyoid and omohyoid muscles that depress hyoid are also affected by NMES on both submental and throat regions, and geniohyoid muscle that pulls the hyoid anteriorly rather than hyoid elevation might be simultaneously affected by NMES with submental placement alone, respectively [ 2 , 6 ].
We comparatively investigated immediate changes in hyolaryngeal movement and swallowing function induced by a cycle of NMES on both submental and throat regions and submental placement alone in patients with dysphagia. Furthermore, we investigated the changes before and after NMES according to the presence of brainstem lesion and duration of dysphagia.
MATERIALS AND METHODS
Participants.
Fifteen patients were recruited for evaluation and management of their dysphagia. Because NMES is reportedly beneficial for swallowing in heterogeneous patient etiologies such as stroke, cancer, head trauma, and respiratory failure [ 9 , 10 ], the inclusion criteria were dysphagia patients of variable etiologies including medical deconditioning. Patients with severe cognitive dysfunction or apraxia that precluded one command obey, exhibition of nonstop verbalization, significant reflux from the use of a feeding tube, dysphagia due to drug toxicity, agitation, decreased level of consciousness or otherwise noncompliant, and pregnancy were excluded [ 7 ]. Patients with poor sitting balance, or unstable medical condition were also excluded.
General characteristics of subjects were described in Table 1 . There were 9 male and 6 female patients. The mean age was 58 years old. Causes of dysphagia were diverse, i.e., 4 brain tumor, 3 stroke, 2 cerebral palsy, 1 traumatic brain injury, 3 Parkinsonism, and 2 medical deconditioning without any brain lesion. Among the 13 participants who had a brain lesion, 4 patients had a brainstem lesion and 9 patients had no brainstem lesion. Six patients had dysphagia duration of <3 months and 9 patients had duration of >3 months. The mean duration of dysphagia was 3 months.

PAS, Penetration-Aspiration Scale; NIH-SSS, National Institutes of Health-Swallowing Safety Scale; CPD, cricopharyngeal dysfunction; D, delayed; I, intact; IC, incomplete; C, complete.
Since the aim of this study was to investigate the NMES-induced immediate changes of the hyolaryngeal movement, a subject who complained of dysphagia but did not show any abnormal findings in videofluorographic swallowing study (VFSS) before NMES and a subject who showed just delayed swallowing reflex and incomplete laryngeal elevation in VFSS were also included in the study.
All VFSS procedures were conducted by 2 physiatrists and a radiologic technologist. First, VFSS was performed to measure the hyolaryngeal movement during non-stimulated swallow before NMES. The participants were seated upright laterally, and cough-induced head movements were stabilized with the examiner's hands. Distance from the X-ray tube to the laryngeal prominence was maintained at 0.5 m. The videofluoroscopic image was recorded on a videocassette recorder at 10 frames per second. They were instructed to check movement of tongue and lip and elevation of velum, and to hold the 5 mL thin liquid of barium sulfate suspension in their mouth until told to swallow [ 11 ]. Subjects were then told to swallow without NMES. Particular attention was paid to the pharyngeal phase including presence of penetration and aspiration.
Each participant was subsequently familiarized with the sensations from the surface electrical stimulation unit (VitalStim; Chattanooga Group, Hixson, TN, USA). The electrical stimulation unit provided 2 channels of bipolar electrical stimulation at a fixed 80 Hz pulse rate and a fixed biphasic pulse duration of 700 µs. Each channel was independently adjustable between 0 and 25 µA stimulation intensity. The skin in the submental and laryngeal regions was cleaned with alcohol and wiped to increase its adherence to the electrodes. Adult sized electrodes with a 2.1-cm round active area were used.
Next, all patients received a cycle of repetitive NMES (10 times per 1 cycle) with two methods of electrode placement: 1) both submental and throat regions and 2) submental placement alone [ 12 ]. Since the goal of this study was to investigate the immediate effect of NMES on the hyolaryngeal movement and swallowing function, only a cycle of NMES was provided in random order. VFSS was conducted while NMES was applied with the two methods.
Two pairs of electrodes were used for placement in both submental and throat regions, with the top pair placed horizontally in the submental region over the region of the mylohyoid muscle above the hyoid bone. The bottom pair was placed on the skin over the thyroid cartilage on either side of the midline over the region of the thyrohyoid muscle medial to the sternocleidomastoid muscle ( Fig. 1A ). For the submental placement alone method, both pairs of horizontally arranged electrode were placed in the skin overlying the submental region ( Fig. 1B ). The edge of the hyoid bone was detected by palpation. Prior to data recording, each electrode pair was placed on the skin and the stimulation intensity was gradually raised in a 1-mA step-wise fashion until the participant could first feel a tingling sensation. Then, the stimulation level was gradually increased to the maximum level that the participant could tolerate. The maximum tolerance levels, which were at least 10 mA in all participants, were determined and recorded for all electrode pairs in a placement simultaneously. The stimulator contained 2-sets of bipolar electrodes, and automatically cycled at on for 59 seconds and off for 1 second.

This procedure was repeated twice if aspiration was not observed. The consistency of hyolaryngeal movement and swallowing safety scores were confirmed in such subjects. The stimulation level was set at the maximum tolerance level, as instructed in the training manual for the use of electrical stimulation in the treatment of dysphagia [ 12 ].
Data analysis
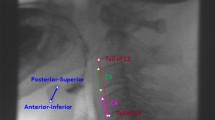
The movement of the hyolaryngeal complex such as elevation, depression, or anterior displacement was measured in non-stimulated and stimulated trials. In brief, the anterior-superior margin of the hyoid bone, the anterior margin of the subglottic airway column that represents the larynx, and the anterior-inferior margin of the second and fourth cervical vertebral bodies were measured by the ImageJ (Wayne Rasband, National Institutes of Health, Bethesda, MD, USA) application tool that utilizes various metric measurements. ImageJ is a public domain Java image processing and analysis program with widespread academic application [ 13 ]. We defined the zero point as the anterior-inferior margin of the fourth cervical vertebral body, and the y-axis as the straight line connecting the zero point and the anterior-inferior margin of the second cervical vertebral body. The x-axis was drawn at a 90° angle to the y-axis through the point on the fourth cervical vertebra. The maximal excursion point of the hyoid bone and the larynx during the swallowing reflex were analyzed in the x- and y-axis by the ImageJ software.
We observed relative elevation or depression by subtracting the non-stimulated swallow peak shown as the absolute pixel number in y-axis from the stimulated swallow peak shown as another absolute pixel number in the same axis. Positive value meant relative elevation and negative value meant relative depression of the hyolaryngeal complex. The peak anterior displacement for the hyolaryngeal complex on the x-axis was determined for non-stimulated and stimulated swallows for each subject. Relative anterior displacement of the hyolarynx was checked by subtracting the non-stimulated swallow peak shown as the absolute pixel number in x-axis from the stimulated swallow peak shown as another absolute pixel number in the same axis. Negative value meant relative anterior movement of the hyolaryngeal complex. In this study, relative change in horizontal and vertical position of the hyolaryngeal complex during swallowing reflex was analyzed. Swallowing trials were also assessed for safety by two physiatrists using the Rosenbek Penetration-Aspiration Score (PAS) [ 14 ] and NIH-SSS ( Table 2 ).

PAS, Penetration-Aspiration Scale; NIH-SSS, National Institutes of Health-Swallowing Safety Scale.
Statistical analysis
The primary goal of the study was to assess the immediate change in the hyolaryngeal movement and swallowing function in response to the NMES stimulation placements. McNemar test was used to compare changes between pre- and post-NMES on both submental and throat regions and submental placement alone, in order to test the hypothesis that surface electrical stimulation would cause descent or anterior displacement of hyolaryngeal complex. Wilcoxon signed-rank test for paired samples was also used to compare the PAS and NIH-SSS scores between non-stimulated and stimulated swallows. All statistical analyses were performed using the SPSS ver. 19.0 (IBM SPSS Inc., Armonk, NY, USA). A value of p<0.05 was considered statistically significant.
Findings of videofluoroscopic swallowing study
Among 15 patients who complained of dysphagia, 12 patients (80%) showed delayed swallowing reflex and 11 patients (73.3%) showed incomplete pharyngeal peristalsis. Regarding the laryngeal movement, 6 patients (40%) showed incomplete elevation of larynx and 10 patients (66.7%) showed incomplete closure of larynx. In addition, 3 patients (20%) showed cricopharyngeal dysfunction. Regarding the penetration and aspiration, 11 patients (73.3%) showed penetration and 6 patients (40%) showed aspiration ( Table 1 ).
Change in hyolaryngeal position after NMES
Based on the previous findings that electrical stimulation applied to the anterior neck did not elevate hyolaryngeal complex but rather decreased hyolaryngeal excursion [ 2 ], we determined the comparative change in hyolaryngeal position during swallowing after NMES on both submental and throat regions versus submental placement alone.
When NMES was applied to both submental and throat regions (n=15), a significant depression of the hyolaryngeal complex was observed during swallowing (p=0.016) ( Table 3 ). However, there was no significant change in elevation or anterior excursion of the hyolaryngeal complex after NMES on both submental and throat regions. On the other hand, in the submental placement alone group (n=15), there was a significant increase in the anterior excursion of hyolaryngeal complex (p=0.031) ( Table 3 ). However, laryngeal elevation or depression was not significantly observed in the submental placement alone group. Taken together, neither NMES placements showed significant elevation of the hyolaryngeal complex.

* p<0.05 in comparison between pre- and post-NMES on both submental and throat regions and submental placement alone by McNemar test.
Change in dysphagia rating scale after NMES
We compared the PAS and NIH-SSS after NMES on both submental and throat area stimulation versus submental placement alone to evaluate the risk for aspiration and swallowing safety scale in motor-level stimulation with two different placements.
The results indicated that there was no significant difference in the PAS after NMES on both submental and throat area stimulation and submental placement alone ( Fig. 2A ). However, application to both submental and throat regions significantly decreased the NIH-SSS from 4.47±0.84 to 3.40±0.96 (p=0.027), while submental placement alone did not change the dysphagia rating scores ( Fig. 2B ).

Change in dysphagia rating scale according to the presence of brainstem lesion
Swallowing reflex depends on swallowing centers in the brainstem, while initiation of swallowing is a voluntary action that involves the integrity of motor areas of the cerebral cortex [ 15 , 16 ]. Therefore, we compared the PAS and NIH-SSS on both submental and throat area stimulation and submental placement alone between patients with and without brainstem lesion.
There was no significant difference in the PAS after NMES on both submental and throat area stimulation and submental placement alone regardless of the brainstem lesion ( Fig. 3A, B ). In addition, patients with brainstem lesion did not show a significant change in the NIH-SSS after NMES with both placement methods ( Fig. 3D ). However, patients with no brainstem lesion in whom NMES was applied to both submental and throat regions showed a significant decrease in the NIH-SSS from 4.36±0.98 to 2.82±1.10 (p=0.026), whereas submental placement alone did not change the scores in the same patients ( Fig. 3C ).

Change in dysphagia rating scale according to the duration of dysphagia
We then determined whether the duration of dysphagia would affect the change in the dysphagia rating scale after NMES on both submental and throat area and submental placement alone. Given that chronic phase was defined as onset duration of >3 months [ 17 ], PAS and NIH-SSS were compared in patients with dysphagia duration that was <3 and >3 months.
As a result, there was no significant difference in the PAS after NMES on both submental and throat area and submental placement alone, irrespective of the duration of dysphagia ( Fig. 4A, B ). Additionally, subjects whose duration of dysphagia was >3 months did not show significant change in the NIH-SSS after NMES with both placement methods ( Fig. 4D ). On the other hand, patients whose dysphagia duration was <3 months showed a significant decrease in the NIH-SSS from 6.83±1.45 to 2.83±1.52 after NMES on both submental and throat area (p=0.043), whereas submental placement alone did not change the scores in the same patients ( Fig. 4C ).

We investigated the immediate changes in hyolaryngeal movement and risk for aspiration and swallowing safety scale in response to NMES with placements on both submental and throat regions and the submental region alone in patients with dysphagia. In particular, this study showed that NMES on both submental and throat region significantly reduced the NIH-SSS, but not the PAS score, suggesting that pharyngeal peristalsis and cricopharyngeal functions at the esophageal entry might be improved rather than the decrease of aspiration and penetration.
During swallowing, the hyoid bone and larynx elevate by approximately 20 mm and the hyoid bone moves anteriorly by approximately 5 mm in healthy young males [ 18 ]. The suprahyoid muscles involved in hyolaryngeal elevation include the mylohyoid, geniohyoid, and anterior belly of the digastric muscles. The only muscle that elevates the larynx to the hyoid is the thyrohyoid muscle, which lies beneath the strap muscles such as sternohyoid and omohyoid [ 19 ].
In previous studies by Ludlow et al. [ 2 ], hyoid depression was observed with motor-level stimulation on placement of surface electrodes over the anterior neck region. With respect to the relationship between the clinical safety rating scale and the depression of hyolaryngeal movement, no group change in aspiration scale was noted. Rather, levels of electrical stimulation just above the sensory threshold for detecting a tingling skin sensation, caused significant improvement during swallowing on the NIH-SSS.
Likewise, our results showed hyolaryngeal descent after motor-level stimulation on both submental and throat regions. This implies that sternohyoid and omohyoid stimulation exceeded the hyolaryngeal elevation effects. In other words, electrodes over the anterior neck might activate the sternohyoid and omohyoid rather than thyrohyoid that underlies the strap muscles and suprahyoid muscles such as geniohyoid and mylohyoid.
Submental stimulation alone on the surface of the skin produced no elevation of the hyolaryngeal complex but significant anterior movement. The anterior belly of the digastric muscle raises the hyoid if the jaw is clenched. Further below, the mylohyoid muscle moves the hyoid upwards to the mandible. Geniohyoid muscle lies deeper and pulls the hyoid bone forward towards the inside of the mandible [ 2 ]. If the currents run deep beneath the skin, NMES with submental placement alone may elevate the hyoid upwards in an anterior direction. However, the stimulation of the platysma without simultaneous stimulation of the thyrohyoid would leave the larynx in an anterior direction, possibly resulting in further opening of the vestibule and increased aspiration risk. In this study, the PAS and NIH-SSS results indicated no significant change during swallowing with motor-level NMES on submental placement alone.
None of the previous studies have compared immediate changes in swallowing function between both submental and throat stimulation and submental stimulation alone. Our results showed that motor-level stimulation on the anterior neck area would lower the hyolaryngeal complex. Nevertheless, NMES on both submental and throat regions improved the NIH-SSS, although the PAS was unchanged. Especially, patients with no brainstem lesion and with dysphagia duration of <3 months had significantly improved NIH-SSS. This result suggested that the improved pharyngeal peristalsis and cricopharyngeal function at the esophageal entry would have more beneficial effects from NMES, rather than the decreased penetration and aspiration risks caused by elevation of the hyolaryngeal complex ( Table 3 ).
A limitation of this study was that it included a small number of subjects with dysphagia of heterogeneous etiologies. Recruitment of more subjects from a homogenous disease population is required to statistically compare immediate changes in hyolaryngeal movement by NMES with both submental and throat regions versus submental placement alone. We also need to investigate the effects of long-term NMES on the hyolaryngeal movement and swallowing function for the treatment of dysphagia. In addition, as only relative change of hyolaryngeal movement rather than absolute distance to the maximal excursion was measured, further study measuring absolute distance is required. Moreover, as a single food preparation was used rather than foods with diverse viscosities, further study with diverse food textures needs to be conducted.
In conclusion, the study showed that a cycle of motor-level NMES on the submental and throat regions paradoxically caused immediate hyolaryngeal descent; and submental stimulation alone produced significant anterior displacement of the hyolaryngeal complex. However, NMES on both submental and throat regions significantly reduced the NIH-SSS, but not the PAS, suggesting pharyngeal peristalsis and cricopharyngeal functions at the esophageal entry might be improved rather than the decrease of aspiration and penetration. The results also suggested that patients with dysphagia should be carefully screened before planning motor-level NMES for dysphagia treatment.
ACKNOWLEDGMENTS
The work was supported by the National Research Foundation (NRF-2010-0020408, 2014R1A2A1A11052042) funded by the Ministry of Education, Science and Technology, Republic of Korea.
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.
- Search Menu
- The Journals of Gerontology, Series A (1995-present)
- Journal of Gerontology (1946-1994)
- Advance Articles
- Editor's Choice
- Translational articles
- Supplements
- Special Issues
- Calls for Papers
- Author Guidelines
- Biological Sciences Submission Site
- Medical Sciences Submission Site
- Why Submit to the GSA Portfolio?
- Advertising and Corporate Services
- Advertising
- Reprints and ePrints
- Sponsored Supplements
- Journals Career Network
- About The Journals of Gerontology, Series A
- About The Gerontological Society of America
- Editorial Board - Biological Sciences
- Editorial Board - Medical Sciences
- Self-Archiving Policy
- Dispatch Dates
- Terms and Conditions
- GSA Journals
- Journals on Oxford Academic
- Books on Oxford Academic

Article Contents
D iscussion.
- < Previous
Swallow Respiratory Patterns and Aging: Presbyphagia or Dysphagia?
- Article contents
- Figures & tables
- Supplementary Data
Paula Leslie, Michael J. Drinnan, Gary A. Ford, Janet A. Wilson, Swallow Respiratory Patterns and Aging: Presbyphagia or Dysphagia?, The Journals of Gerontology: Series A , Volume 60, Issue 3, March 2005, Pages 391–395, https://doi.org/10.1093/gerona/60.3.391
- Permissions Icon Permissions
Background . Assessment referrals are increasing for unexpected dysphagia, particularly for older people. It is unclear if this is due to more impaired swallows or healthy age-related changes. Swallow respiration coordination prevents aspiration, and may deteriorate with age. Nonpathological features of the swallow in healthy aging and the factors that influence an individual's ability to eat and drink safely need greater understanding. Some changes might predispose an older person to dysphagic complications in the event of an insult such as a stroke. We investigated the effects of healthy aging on resting and swallow respiratory patterns.
Methods . Fifty healthy volunteers (aged 20–78 years) were recruited to have swallow respiration patterns recorded on a computer. Bolus volume and consistency variations were studied: 5 and 20 ml of water and 5 ml of yogurt.
Results . Measurable swallows significantly decreased with age for water boluses. Swallow apnea increased with age (5 ml of water r = 0.433, p =.002; 5 ml of yogurt r = 0.367, p =.023). Independent of age were: breathing out (occurred after 98% of boluses); multiple swallowing (occurred with all bolus types); postswallow respiration reset pattern (more irregular after yogurt, Wilcoxon signed rank Z = −2.236, p =.025); and resting respiration.
Conclusions . Subtle changes occur in swallow respiration coordination with age. These changes may be compensatory protective mechanisms rather than the result of decreased muscle mobility or reaction times, and not indicative of impairment. Misattributing healthy age-related changes to impairment affects patient care and the use of healthcare resources.
AN increasing number of older people referred for swallowing assessment would not be expected to have dysphagia as part of their primary disorder—for example, patients admitted with hip fractures. It is unclear whether these patients have pathologically disordered swallows or healthy age-associated changes to function.
Dysphagia is a serious consequence of neurological disorders such as stroke ( 1 ). In 2000/2001 there were over 76,000 admissions for stroke in the United Kingdom ( 2 ). Persons aged 75 years or older accounted for 60% of finished consultant episodes ( 2 ), showing the serious result of secondary aging and the increase in prevalence of diseases that influence swallowing. Presbyphagia, changes in the swallow mechanism with age, may compound the risk for aspiration; this risk will increase as the mean age of the population shifts upwards.
Age-related changes occur in swallowing physiology. Primary aging causes change in the structure, motility, coordination, and sensitivity of the swallow process ( 3–7 ). Some of these changes may contribute to dysphagia, or perhaps more accurately presbyphagia. Respiration and swallowing are inextricably linked, using the same structures requiring fine coordination of the two processes. Efficient transport of food and drink to the esophagus has to co-occur with maintenance of a safe airway and prevention of material entering the lower respiratory tract. There is less evidence on the influence of age on swallow respiration patterns, but it does appear to contribute to an increased risk of aspiration ( 8 , 9 ). Complications due to aspiration are just one facet of nutrition and/or hydration issues in elderly persons. Increased knowledge is needed on what represents a pathological impairment and what is a harmless feature of aging to ensure appropriate management. The aim of this study was to investigate the effects of healthy aging on respiratory patterns at rest and surrounding the swallow.
Study Group
Fifty healthy volunteers were recruited (31 women) with as wide an age range as possible (20–78 years). Although we are interested in swallow features in older people, we must have young normative data for comparison. The group comprised hospital staff, relatives, and friends. Exclusion criteria were history of dysphagia/eating/drinking difficulties, neurological impairment, current medical conditions requiring medication, or structural abnormalities that affect the swallowing or respiratory systems.
Ethical Approval and Consent
Written informed consent was obtained for all participants in the study. The Newcastle and North Tyneside Joint Ethics Committee granted ethical approval for the study.
Our system is based on a Pentium notebook computer (Toshiba, Tokyo, Japan), using an analogue-digital converter (DAQcard 700; National Instruments, Austin, TX) and C++ programming language. Respiratory airflow was measured via a nasal cannula and an external pressure transducer (Gaeltec, Dunvegan, Scotland) ( 10 ). Sound was recorded from a throat microphone (Medical Physics, Freeman Hospital) ( Figure 1 ). Both signals were sampled at 1000 Hz, and then down-sampled to maximum and minimum values at 100 Hz. The easily portable system fits into a computer case.
Volunteers were seen in the hospital's Speech-Language Department or in their homes. The same researcher conducted all studies whether hospital or home based. The apparatus is noninvasive, and no overt signs of tension were observed during any recording. Resting respiration was recorded for 10 minutes following 5 minutes of familiarization with the equipment and quiet breathing. Ten boluses each of 5 ml of water, 20 ml of water, and 5 ml of yogurt were presented to the participants.
Water was measured by graduated syringe into a small plastic cup; the participant was asked to drink the entire contents in one swallow to mimic real drinking as closely as possible. Injecting water into the mouth may affect the timing of the swallow within the respiratory cycle, whether or not you are then allowed to swallow at will. We were trying to make the situation as normal as possible, so it was important not to make the person too conscious of their swallow and affect the natural respiratory pattern.
Yogurt was measured using a 5 ml medicine spoon calibrated with a graduated syringe. Training was provided in how to obtain a standard 5 ml, and then participants fed themselves in the same way as for the water, with the researcher checking that bolus size was standard. For all bolus types, some values could not be measured. If, for example, a person stopped breathing for a period around the actual swallow apnea or they consciously modified swallow-respiration as one or two reported, data could not be recorded or used. Initially we intended to use 20 ml of yogurt, but participants found it difficult to swallow in one mouthful, so 5 ml of yogurt was used.
Respiratory Analyses
Measurable swallows.
In most studies, data are “lost” because they cannot be analyzed. Some swallows were not measurable because of confounding factors, piecemeal swallows (where the bolus is ingested as several small bits), or because the participant was not breathing out through the nose (so breath direction could not be assigned). Initial examination of the data suggested a relationship between age and the number of boluses that could have their features “measured.” We investigated the number of swallows that could be characterized as a proportion of the total number of boluses given; data are presented as percentages.
Swallow apnea
The respiratory trace was recorded before, during, and after swallowing. Apnea duration was measured by the length of zero airflow on the respiratory trace ( Figure 2 ). The small apparently inspiratory “breath,” which often accompanies the apnea, was included in the timing. This is rarely reported in studies of swallow respiration, probably due to the respiration recording technique rather than to the infrequency of the occurrence. Changes in the position of the structures postswallow cause a partial vacuum, and pressure drops ( 11 ). Measuring breath direction with a pressure-sensitive device can give an apparent “airflow” reading when the patient is in fact apneic. If a confounding event occurred within five cycles of the start of a swallow, then it was not analyzed.
Postswallow breath direction
Airflow direction postswallow was noted for all boluses. Breathing in after a swallow is rare in the healthy population ( 8 ), but there is evidence that this changes with age ( 12 ). Results are presented as percentage of boluses after which the person breathed out (for each bolus type). A person who breathed out after 7 of 10 yogurt boluses was classified as having 70% postswallow expiration.
Multiple swallowing
A bolus was classified as having multiple swallows if one or more swallows occurred within four respiratory cycles after the main apnea (excluding piecemeal swallows or swallows during which some of the bolus remained in the cup/spoon). Results are presented as percentages of boluses after which the person swallowed multiple times (for each bolus type). A person who swallowed multiple times after 7 of 10 yogurt boluses was classified as being 70% multiple swallowing.
Breathing reset pattern
The reset pattern for respiration was classified as normal or abnormal for each person. Respiration generally switched back to a typical sine-type wave very soon after the end of apnea ( Figure 2 ). This was assessed for 5 ml of water and 5 ml of yogurt. If the reset pattern did not return to a typical sine wave, it was classified as abnormal.
Data Collection/Statistical Analysis
Data were entered into an Excel 2000 (Microsoft, Redmond, WA) spreadsheet and analyzed with SPSS for Windows (Release 11.0; Chicago, IL). Parametric tests including Pearson's correlation ( r ) were used, because the results were normally distributed. For the categorical breathing reset pattern, nonparametric Spearman's correlation ( r s ) and Wilcoxon signed rank test were used. Results were accepted as statistically significant at the 5% level of probability.
Two people were excluded because they were mouth breathing, and the direction of nasal airflow could not be measured. In the remainder of the participants, age was not significantly correlated with any of the resting respiration characteristics ( Table 1 ).
Measurable Swallows
As age increased, fewer swallows could be analyzed. The number of swallows during which airflow direction postbolus could be measured had a significant negative correlation with age for the 5 ml water bolus, but was only approaching a trend with 20 ml of water ( Table 2 ). With multiple swallowing postbolus, there was a significant negative correlation between the number of analyzable swallows and age for the 5 ml and 20 ml water boluses ( Table 2 ).
Swallow Apnea
Mean apnea increased significantly from 0.74 to 0.86 seconds ( p =.001) with increased volume but not with change in consistency ( p =.520). A significant, positive correlation was found between age and duration of swallow apnea for both the 5 ml water boluses and 5 ml yogurt boluses ( Table 3 ). The statistical significance of this relationship was not maintained with 20 ml of water, which had a wider range and standard deviation of apnea duration. Each individual showed a significant correlation between apnea duration on 5 ml of water and each of the other bolus types; these data have been reported previously ( 13 ).
Postswallow Breath Direction
Most people always breathed out after swallowing: 1095 of the 1113 boluses (98%) across the three types were followed by an out breath, with no significant difference between bolus types. For each bolus type, only 2 or 3 of the 40+ people ever breathed in after swallowing, and it was not the same people. There was a significant correlation between a person's breath direction on 5 ml of water and each of the other bolus types but, given the considerable positive skew toward breathing out postswallow, this is unsurprising. Age was not correlated with airflow direction postswallow.
Multiple Swallowing
The proportion of boluses followed by multiple swallowing increased as the bolus type changed from 5 ml of water to 20 ml of water to 5 ml of yogurt (χ 2 = 20.8, p =.00003, Figure 3 ). The proportion of people multiple swallowing also increased (χ 2 = 6.0, p =.05, Figure 4 ). Each individual had a strong correlation between multiple swallowing on 5 ml of water and 20 ml of water ( n = 44, r = 0.684, p <.001) and between 5 ml of water and 5 ml of yogurt ( n = 40, r = 0.753, p <.001). Age was not significantly correlated with multiple swallowing.
Breathing Reset Pattern
Some people had an unusual respiration reset pattern: irregular or prolonged. The multiple swallowing judgment sometimes precluded that of the reset pattern. A normal reset pattern ( Figure 2 ) was shown by 41 of 49 (84%) people after 5 ml of water compared with 30 of 42 (71%) people after 5 ml of yogurt. Individuals had a high correlation of reset pattern between 5 ml of water and 5 ml of yogurt with r S = 0.641, p <.001 ( n = 42). The Wilcoxon signed rank test gave a significant difference for the reset pattern, depending on bolus type with Z = −2.236, p =.025 ( n = 42). There was no evidence of age affecting respiration reset patterns.
This is one of the largest studies to investigate the effects of healthy aging on swallow respiratory patterns across a wide age range of men and women. Subtle but distinctive changes in swallow function occur with age. To our knowledge, this is the first study to investigate the effect of advancing age on the “measurability” of swallow respiration features. The increase in unclassifiable swallows suggests delicate changes in swallow respiration coordination. Subtle variation in the swallow process due to aging (presbyphagia rather than dysphagia) exists, but the overall coordination of the swallow is preserved ( 14 ).
This group is one of the largest to provide supporting evidence of increasing apnea duration with age ( 8 , 15 ). Such a change may be a protective mechanism rather than solely a result of decreased muscle mobility or reaction times. This increased duration of apnea may enable the system to compensate for other age-related changes such as longer oropharyngeal and hypopharyngeal transit times ( 3 ) and delayed initiation of maximum hyolaryngeal excursion ( 16 ). Vocal cord closure is one of the primary airway-protective mechanisms against aspiration of foreign material. Airway protection must be maintained while the bolus is passing through the pharynx, i.e., bolus transit time is less than that of vocal cord adduction ( 17 ). Reducing the margin of safety between the two increases aspiration risk. The lack of correlation between age and apnea duration on 20 ml of water may indicate that the bolus is approaching a size at which respiration patterns become unstable or that a larger bolus is required to maintain an age-independent swallow mechanism. This would fit with sensation decreasing as we age, and so older people need larger, more flavorsome boluses (not the opposite, as has been traditionally thought).
Some gross features of the swallow respiration pattern, such as breath direction and multiple swallowing postbolus, are unaffected by age. Multiple swallowing is more common in general with yogurt ( 13 ), which may mask age effects. Resting respiration patterns do not appear to change with age. This may be constant for an individual, independent of age. The observed changes seem confined to swallow respiration patterns. Yogurt bolus characteristics may also hide age effects on measurability: subtle changes with age concealed by the powerful reactions of multiple swallowing and increased salivation to a sticky, acidic substance.
Changes in the swallow process with age may predispose individuals to be at risk of dehydration, malnutrition, dysphagia, and aspiration. Malnutrition is increasingly being identified in the community and in patients on admission to hospital ( 18 ), and is interlinked with dysphagia in a vicious cycle ( 19 ). Dysphagia obviously affects nutrition, but malnutrition can exacerbate dysphagia or cause a borderline swallow to decompensate.
Changes with age may not be pathological. What we categorize as a poststroke impairment may simply be a feature of increased age. Clinically, it is important to distinguish these. Older people are at risk of malnutrition for a variety of reasons, including low socioeconomic status and difficulties with activities of daily living ( 20 ). They may simply be too weak to eat the meal or so slow it becomes unpalatably cold ( 21 ). Restricting oral intake is a serious step in terms of nutrition and cost (for example, that of food supplements). Clinicians should be very cautious about taking such action with a population already at risk of malnutrition, especially considering that the evidence for poor outcomes with aspiration is far more limited than that for the effects of malnutrition.
This study contributes to the small body of knowledge on how the healthy swallow changes with age. We have established some features that remain constant and some that are age dependent. These may be confused with pathological sequelae. Clinicians need to increase their awareness of what is normal as the patient gets older to identify problems earlier and to prevent decline. The field of healthy swallowing is an under-researched area, particularly with respect to the effects of aging. We need to modify our current thinking on the swallow: are all of the changes we observe in the 85-year-old poststroke patient due to dysphagia or simply oropharyngeal presbyphagia? We must increase our knowledge of the effects of presbyphagia on the increasing healthy older population and the effects of dysphagia in these people as they succumb to age related illnesses.
Decision Editor: John E. Morley, MB, BCh
Schematic illustration of equipment
Respiration trace as seen on notebook screen
Boluses followed by multiple swallowing by bolus type
People exhibiting multiple swallowing by bolus type
Healthy Resting Respiration Parameters Correlated With Age.
Note : SD = standard deviation; CI = confidence interval.
Proportion of Swallows During Which Feature Could Be Measured Correlated With Age.
Note : SD = standard deviation; CI = confidence interval; PS = postswallow.
Health Swallow Apnea Correlated With Age.
This work was supported by The Stroke Association grant number TSA 10/98.
We thank the staff, friends, relatives, and members of the League of Friends at the Freeman Hospital.
Presented in part at the American Speech-Language-Hearing Association Convention, 2000, Washington, D.C.
This study was performed at the Freeman Hospital, Newcastle-upon-Tyne, United Kingdom.
Gordon C, Langton Hewer R, Wade DT. Dysphagia in acute stroke. Br Med J. 1987 ; 295 : 411 -414.
Department of Health. Hospital In-Patient Data. Department of Health . Available at: http://www.doh.gov.uk/PublicationsAndStatistics/Statistics/HospitalEpisodeStatistics/HESFreeData/HESFreeDataList/fs/en?CONTENT_ID=4097375&chk=HLM%2BM4 (Last accessed March 7, 2005).
Yokoyama M, Mitomi N, Tetsuka K, Tayama N, Niimi S. Role of laryngeal movement and effect of aging on swallowing pressure in the pharynx and upper esophageal sphincter. Laryngoscope. 2000 ; 110 : 434 -439.
Rademaker AW, Pauloski BR, Colangelo LA, Logemann JA. Age and volume effects on liquid swallowing function in normal women. J Speech Lang Hear Res. 1998 ; 41 : 275 -284.
McKee GJ, Johnston BT, McBride GB, Primrose WJ. Does age or sex affect pharyngeal swallowing? Clin Otolaryngol. 1998 ; 23 : 100 -106.
Nilsson H, Ekberg O, Olsson R, Hindfelt B. Quantitative aspects of swallowing in an elderly nondysphagic population. Dysphagia. 1996 ; 11 : 180 -184.
Aviv JE. Effects of aging on sensitivity of the pharyngeal and supraglottic areas. Am J Med. 1997 ; 103 : 74S -76S.
Selley WG, Flack FC, Ellis RE, Brooks WA. Respiratory patterns associated with swallowing: Part 1. The normal adult pattern and changes with age. Age Ageing. 1989 ; 18 : 168 -172.
Shaker R, Li Q, Ren J, Townsend WF, Dodds WJ, Martin BJ, et al. Co-ordination of deglutition and phases of respiration, effect of aging, tachypnea, bolus volume and chronic obstructive pulmonary disease. Am J Physiol: Gastrointest Liver Physiol. 1992 ; 26 : G750 -G755.
Tarrant SC, Ellis RE, Flack FC, Selley WC. Comparative review of techniques for recording respiratory events at rest and during deglutition. Dysphagia. 1997 ; 12 : 24 -38.
Paydafar D, Gilbert RJ, Poppel CS, Nassab PF. Respiratory phase resetting and airflow changes induced by swallowing in humans. J Physiol. 1995 ; 483 : 273 -288.
Nilsson H, Ekberg O, Olsson R, Kjellin O, Hindfelt B. Quantitative assessment of swallowing in healthy adults. Dysphagia. 1996 ; 11 : 110 -116.
Leslie P, Drinnan MJ, Ford GA, Wilson JA. Swallow respiration patterns in dysphagic patients following acute stroke. Dysphagia. 2002 ; 17 : 202 -207.
Zamir Z, Ren J, Hogan WJ, Shaker R. Coordination of deglutative vocal cord closure and oral-pharyngeal swallowing events in the elderly. Eur J Gastroenterol Hepatol. 1996 ; 8 : 425 -429.
Hiss SG, Treole K, Stuart A. Effects of age, gender, bolus volume, and trial on swallowing apnea duration and swallow/respiratory phase relationships of normal adults. Dysphagia. 2001 ; 16 : 128 -135.
Robbins J, Hamilton JW, Lof GL, Kempster GB. Oropharyngeal swallowing in normal adults of different ages. Gastroenterology. 1992 ; 103 : 823 -829.
Ren J, Shaker R, Zamir Z, Dodds WJ, Hogan WJ, Hoffmann RG. Effect of age and bolus variables on the coordination of the glottis and upper esophageal sphincter during swallowing. Am J Gastroenterol. 1993 ; 88 : 665 -669.
Finestone HM, Greene-Finestone LS, Wilson ES, Teasell RW. Malnutrition in stroke patients on the rehabilitation service and at follow-up: prevalence and predictors. Arch Phys Med Rehabil. 1995 ; 76 : 310 -316.
Hudson HM, Daubert CR, Mills RH. The interdependency of protein-energy malnutrition, aging, and dysphagia. Dysphagia. 2000 ; 15 : 31 -38.
Gazewood J, Mehr D. Diagnosis and management of weight loss in the elderly. J Fam Pract. 1998 ; 47 : 19 -25.
Westergren A, Unosson M, Ohlsson O, Lorefält B, Hallberg I. Eating difficulties, assisted eating and nutritional status in elderly (≥65 years) patients in hospital rehabilitation. Int J Nurs Stud. 2002 ; 39 : 341 -351.
Email alerts
Citing articles via, looking for your next opportunity.
- Recommend to Your Librarian
Affiliations
- Online ISSN 1758-535X
- Copyright © 2024 The Gerontological Society of America
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.

IMAGES
VIDEO
COMMENTS
It is believed that inadequate hyolaryngeal excursion (HE) may result in incomplete airway closure and subsequent penetration, aspiration , ... Lastly, we lacked rater training or a standard definition for what constituted "reduced" versus "functional" HE. Ratings were made via clinical decision-making based on past experience and training.
Introduction. During the pharyngeal stage of swallowing contraction of the geniohyoid, mylohyoid, thyrohyoid and anterior digastric muscles (laryngeal elevators) facilitates hyolaryngeal excursion and assists upper esophageal sphincter (UES) dilation. 1-3 When decreased hyolaryngeal excursion results in dysphagia, clinicians might choose to ...
The goal of a bedside swallowing examination, including laryngeal palpation, is to assess a patient's degree of swallowing impairment, aspiration risk, and need for further testing. When palpating swallows, you can only feel hyolaryngeal movement. Laryngeal palpation can't differentiate between elevation and excursion.
Purpose Hyolaryngeal excursion (HE) is typically assessed via palpation during clinical swallowing exams (CSE) or visually during videofluoroscopy (VFSS). Minimal evidence exists to support the use of these perceptual methods for judging HE. We investigated whether binary judgment of HE differentiates quantitative measures of hyoid movement, using frame-by-frame VFSS analysis to measure ...
Hyolaryngeal excursion amplitude, duration, and velocity of movement during swallowing were measured via VFSS. The findings of maximal hyoid displacement varied among the investigations. One study reported that the effortful swallow reduced maximal hyoid excursion ( Bülow et al., 1999 ), whereas another showed a significant increase in this ...
The hyolaryngeal complex consists of the hyoid muscle, the laryngeal cartilages, and the muscles intrinsic to the hyoid and larynx, including the thyrohyoid. The cricopharyngeus, the most inferior portion of the pharyngeal constrictor muscles, attaches to the hyolaryngeal complex and forms the upper esophageal sphincter . Proper hyolaryngeal ...
The hyolaryngeal complex includes the hyoid bone, thyrohyoid membrane, and laryngeal cartilages serving as an attachment site for the cricopharyngeus that forms the upper esophageal sphincter. ... the long pharyngeal muscles may be more responsible for laryngeal excursion based on their length advantage (Fig. 3). Further functional studies are ...
Hyolaryngeal excursion as the physiological source of swallowing accelerometry signals. D C B Zoratto 1,2,3, T Chau 1,2,3,4 and C M Steele 1,2,3,4,5. Published 18 May 2010 • 2010 Institute of Physics and Engineering in Medicine Physiological Measurement, Volume 31, Number 6 Citation D C B Zoratto et al 2010 Physiol. Meas. 31 843.
Purpose Hyolaryngeal excursion (HE) is typically assessed via palpation during clinical swallowing exams (CSE) or visually during videofluoroscopy (VFSS). Minimal evidence exists to support the ...
Superior hyolaryngeal excursion during swallowing is thought to contribute to airway protection prevented aspiration. Anterior hyolaryngeal excursion is thought to be more related to upper esophageal sphincter opening [16], [17], [19], [20]. The mylohyoid and geniohyoid muscles attach to the body of the hyoid [21].
Excursion velocities were calculated from excursion magnitude and duration measures. Successful treatment for dysphagia facilitated increased hyolaryngeal excursion magnitude, duration and velocity. These changes were most prominent for the hyoid and most often observed with thin liquids.
Purpose: Hyolaryngeal excursion (HE) is typically assessed via palpation during clinical swallowing exams (CSE) or visually during videofluoroscopy (VFSS). Minimal evidence exists to support the use of these perceptual methods for judging HE. We investigated whether binary judgment of HE differentiates quantitative measures of hyoid movement, using frame-by-frame VFSS analysis to measure ...
Anterior hyolaryngeal excursion is related to the UES opening and its crucial for the safe transition of bolus to esophagus without aspiration. Our study hypothesized that the long sarcomere length of mylohyoid muscle could be a contributing or responsible factor to the reduction in the hyolaryngeal elevation. There is no study on the ...
Dysphagia is a swallowing disorder involving the oral cavity, pharynx, esophagus, or gastroesophageal junction. Consequences of dysphagia include malnutrition and dehydration, aspiration pneumonia, compromised general health, chronic lung disease, choking, and even death. Adults with dysphagia may also experience disinterest, reduced enjoyment ...
This is known as hyolaryngeal excursion. Closure of the larynx is needed to form a seal to the entrance of the trachea in order to protect the respiratory airways. This closure is achieved through hyolaryngeal excursion. Another purpose of hyoid movement is the opening of the esophagus. This event allows the bolus to pass through the upper ...
This paper compares the results of clinician perception of hyolaryngeal excursion, both on tactile palpation during clinical swallowing examination (CSE) and visual judgement on videofluoroscopy, with objective measurement of hyoid excursion on videofluoroscopy. Hyoid excursion was measured using both peak position measures and displacement ...
Purpose Hyolaryngeal excursion (HE) is typically assessed via palpation during clinical swallowing exams (CSE) or visually during videofluoroscopy (VFSS). Minimal evidence exists to support the use of these perceptual methods for judging HE. We investigated whether binary judgment of HE differentiates quantitative measures of hyoid movement,
However, there was no significant change in elevation or anterior excursion of the hyolaryngeal complex after NMES on both submental and throat regions. On the other hand, in the submental placement alone group (n=15), there was a significant increase in the anterior excursion of hyolaryngeal complex (p=0.031) (Table 3). However, laryngeal ...
Such a change may be a protective mechanism rather than solely a result of decreased muscle mobility or reaction times. This increased duration of apnea may enable the system to compensate for other age-related changes such as longer oropharyngeal and hypopharyngeal transit times and delayed initiation of maximum hyolaryngeal excursion . Vocal ...
Use your voice to slide up the pitch scale as high as you can, to a high, squeaky voice. Hold the high note for several seconds with as much strength as possible. While you do this, you can gently pull up on your Adam's apple. Both of these exercises help lift the larynx, which may improve your swallowing.
difficulty moving food from the mouth to the stomach. dysphagia can result from three main causes. some congenital causes of dysphagia. some structural causes of dysphagia. some medical causes of dyaphagia. dysphagia may present acutely from. dysphagia may present over time from. *some are very aware and even compensate; others are unaware ...