- Random article
- Teaching guide
- Privacy & cookies

by Chris Woodford . Last updated: March 24, 2022.
Photo: Now that's what I call heat! The temperature of the SpaceX Falcon 9 space rocket exhaust you can see here is around 3000°C (5500°F) —hot enough to melt most everyday materials! Photo by Keegan Barber courtesy of NASA .

What is heat anyway?
Artwork: Hotter things have more heat energy than colder things. That's because the atoms or molecules move around faster in hot things (red, right) than they do in cold things (blue, left). This idea is called the kinetic theory.
What happens when something has no heat at all?
Photo: Ice may look cold but it's an awful lot hotter than absolute zero. Picture by Erich Regehr courtesy of US Fish & Wildlife Service .
What's the difference between heat and temperature?
Artwork: An iceberg is much colder than a cup of coffee but it contains more heat energy because it's so much bigger.
How can we measure temperature?
How does heat travel.
Animation: When you hold an iron bar in a fire, heat travels along the metal by conduction (red arrow). Why? Atoms at the hot end move more quickly as they absorb the fire's heat. They gradually pass their energy further along the bar, eventually warming the whole thing up.
Animation: How convection pumps heat into a saucepan. The pattern of warming, rising soup (red arrows) and falling, cooling soup (blue arrows) works like a conveyor that carries heat from the stove into the soup (orange arrows).
Picture: Infrared thermal images (sometimes called thermographs or thermograms) show that all objects give off some heat energy by radiation. In these two photos, you can see a rocket on a launch pad photographed with a normal camera (above) and an infrared thermal camera (below). The coldest parts are purple, blue, and black; the hottest areas are red, yellow, and white. Photo by R. Hurt, NASA/JPL-Caltech, courtesy of NASA.
Why do some things take longer to heat up than others?
Chart: Everyday materials have very different specific heat capacities. Metals (blue) have low specific heat capacities: they conduct heat well and store it badly, so they feel cold to the touch. Ceramic/mineral materials (orange) have higher specific heat capacitors: they don't conduct heat as well as metals, store it better, and feel slightly warmer when you touch them. Organic insulating materials (green), such as wood and leather, conduct heat very poorly and store it well, so they feel warm to the touch. With very high specific heat capacity, water (yellow) is in a class of its own.
Photo: The wooden spoon feels much warmer than the metal one, even though both are the same temperature. The metal spoon conducts heat more readily from your hand, and it's this that makes it feel colder.
Latent heat
Artwork: Normally things get hotter (their temperature rises) as you supply more heat energy. That doesn't happen at the points when things melt (change from solid to liquid) and vaporize (turn from liquid to gas). Instead, the energy you supply is used to change the state of matter . The energy doesn't vanish: it's stored as latent heat.
If you liked this article...
Find out more, on this website.
- Heat exchangers
- Heat insulation
For younger readers
- Heat by Ian Mahaney. Rosen, 2019. A 24-page, basic introduction for ages 8–10. It covers much the same scope as this article (where heat comes from, conduction, convection and radiation, measuring heat, heat capacity, and a few basic experiments).
- Investigating Heat by Sally M. Walker. Lerner Publications, 2017. This one is about 40 pages and also suitable for ages 8–10.
- Secrets of Heat and Cold by Andrew Solway. Encyclopedia Britannica, 2015. A question-and-answer-style introduction to the science of heat. Best for ages 8–10.
- Energy by Chris Woodford. Dorling Kindersley, 2007. My own book about energy includes a short section on heat energy. Suitable for ages 9–12.
For older readers
- Theory of Heat by James Clerk Maxwell. Longmans, 1871. Read Maxwell's statistical ideas about heat and the kinetic theory in his own words. The full text is available here in various electronic formats.
- Atoms under the Floorboards by Chris Woodford. Bloomsbury, 2015. One of my books for older readers. Chapter 13 is a simple introduction to heat and thermodynamics.
Text copyright © Chris Woodford 2009, 2022. All rights reserved. Full copyright notice and terms of use .
Rate this page
Tell your friends, cite this page, more to explore on our website....
- Get the book
- Send feedback
- Book Dr. Bailes

Heat Rises…and Falls — Stack Effect, Air Movement, & Heat Flow
- Allison Bailes

Heat rises. Everyone knows that, right? It’s absolutely true. Heat does rise. The problem is that sometimes people say this as if the flow of heat is driven by its wanting to rise. It’s not. Heat can move up, down, or sideways, depending on the situation. What the laws of thermodynamics tell us is that heat moves from areas of higher temperature to areas of lower temperature. Put a torch to the top of a steel pole, and heat will travel downward by conduction. So, temperature difference is really what drives heat to move in any given direction.
When you’re dealing with fluids, you have to account for density and buoyancy as well. Air is the fluid we live in, and this time of year we spend a lot of money pumping heat into it in our homes and workplaces. When we heat air, the molecules jiggle and zip around faster, which causes them to spread out. When a mass of air takes up more space, it has a lower density. When you have a lower density fluid immersed in a higher density fluid, the lower density fluid rises and the higher density fluid falls.
Think of air bubbles in water, as shown in the photo above. Think of a helium balloon. Think of a hot air balloon. Now, imagine an object with higher density immersed in a fluid. Put Wile E. Coyote’s anvil in the air above his head, and it turns him into a pancake.
The point here is that it’s easy to get confused by heat in the building science of air movement. Warm air rises when it’s surrounded by cold air because of its lower density. Yes, that’s due to heat, but density is the main factor causing the movement here. The name for this phenomenon is stack effect . Two factors affect how much stack effect a building experiences:
- Temperature difference between inside and out (because density depends on temperature)
- Height of the building
The problem with stack effect in buildings is that buildings aren’t vacuum chambers. They leak. Obviously, a house isn’t going to start floating up into the air like a balloon (although I recall with great fondness the Disney movies of my childhood that showed such magical events). But the low density air inside the house will move up and out into the cold, dense winter air when given the chance.
Try this experiment if you don’t believe me. Open your pull-down stairs or scuttle hole to the attic on a cold day when your home is warm. Climb up into the attic and then put your face over the hole. You’ll feel the stack effect pushing lots of warm air into the attic.
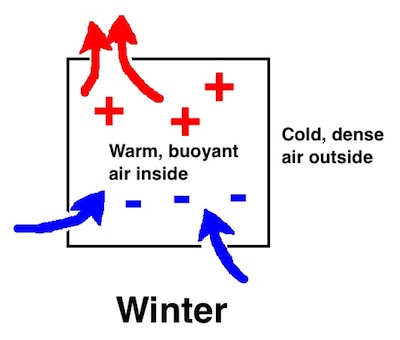
So, in winter, the warm, low density air inside your house wants to rise…if it can. If your house has no leaks, the warm air can’t escape and do its thing. There’s still a pressure difference across the building envelope, but that’s OK if the air barrier’s good. Positive pressure inside the house with nowhere to go because there are no pathways .
What happens in reality is that homes leak. Your nice warm air finds way to leak out ( exfiltration ) and cold air leaks in (infiltration). Because of its lower density, the warm air will leak out the top of the house if there are leaks there. When a cubic foot leaks out, however, it has to be made up by a cubic foot leaking in. As the warm air leaks out at the top, cold air leaks in at the bottom. The leakier your house is, the more temperature difference you’ll notice between the top and bottom of the house.
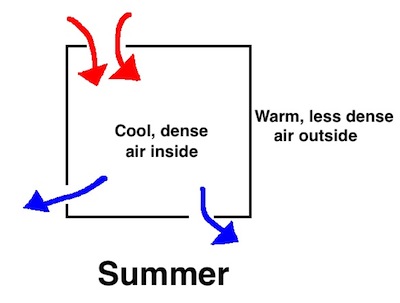
All this happens because the warm air inside your home in winter is less dense than the cold air outside. In summer, the dense air is inside your home because that’s where the temperature is lower, especially if you’re air conditioning your home. What that means is that leaks in your house bring warm air in at the top and allow cool air to fall out at the bottom.
Ah, warm air falls! Heat sinks. That old expression, “Heat rises,” is not a basic truth after all. As with many aspects of building science, you have to look at the full context to understand what’s going on.
Followup Article
Who Knew the Stack Effect Could Be So Controversial?
Related Articles
What Is Pressure? – Understanding Air Leakage
Rats to You, Daniel Bernoulli! – Understanding Air Pressure (with a cool video!)
Infiltration Occurs at the Surface, Not in the Volume
It’s the Hole – Understanding What a Blower Door Is for
Building Science 101
Photo of water bubbles by Christian Haugen from flickr.com, used under a Creative Commons license.
This Post Has 27 Comments
Terry Brennan and Bill Turner Terry Brennan and Bill Turner (Camroden Associates and Turner Engineering) have a fantastic reference document written about 10 years ago on Stack Effect and Planned and Unplanned Airflow. Email me a request and I will send you the document. Keep in mind that these same energy principles are the reasoning behind where the vapor barrier goes in an exterior assembly based on climate zone. The proclivity for which side of the assembly the highest concentration of heat and moisture most typically can be found dictates where the point of condensation in the assembly should be located.
Nicely written, clear Nicely written, clear explanatory post. BTW, I used to scuba dive, and we’d spend much time practicing how to make those air bubble rings during boring decompressions 🙂
Matthew C. Matthew C. : I don’t think I’ve seen that paper, but since Terry Brennan is one of the most knowledgeable people in the world when it comes to air movement, I’d certainly like to see it. John P. : Thanks! So the ring bubble is a scuba thing, eh?
Alison, nicely put. To be Alison, nicely put. To be picky I try to avoid saying that heat rises for other reasons. I say that heat moves toward cold. This is particularly obvious with conduction and convection. I do say that “Warmed Air” has a tendency to rise because it is lighter (convection). Your observation that in the warm months convection currents can draw warm air into the conditioned space from an attic is an important and often neglected point. I for one will be more careful with my use of the phrase “Warm Air Rises.” Thanks for the post.
Hi Alison, Hi Alison, Nice blog, but I would like to join Armand in being concerned about the picky details. The issue of warm air rising has created much confusion among energy professionals and home owners. In an effort to clear the air, so to speak, I would like to discuss better wording that may help to correct this long ignored science. Warm air moving up is indeed what we see, but warm air has no internal energy that makes it move up or down. It is entirely controlled by gravity. Much more to be said. Bud
Armand M. : Armand M. : I don’t have a problem with saying that heat rises because I usually follow that up with “and heat falls, too.” What are your other reasons for avoiding it? Bud P. : Yes, we can quibble about details and semantics on this topic for a long time. If warm air moves upward, we can say that it’s rising. Because it’s warmer than the surroundings in winter and leaves behind cooler, denser air at the bottom of the house, we can say heat rises. I don’t have a problem with that. Buoyancy is the relevant principle here, and Archimedes figured it out thousands of years ago. What is the better wording that you suggest?
Thanks for the prompt reply, Thanks for the prompt reply, One in particular is when the statement “warm air rises” is followed with “it then pulls in its replacement air”. Since warm cannot rise of its own accord, there is no pulling effect behind it and thus the implied negative pressure. Attic ventilation is an example where you often hear that the warm air rises to exit the upper vents and pulls in its replacement air. Wording for our new energy correct language is just being put together, but one initial thought is to avoid the “pull” word and use “displacement air” to replace “replacement air” when describing the inflow of colder air. The objective here is to dispel the notion that warm air is leading the way in the process of convection, when in fact it is the cold air that initiates the process. It seems like semantics until you try to calculate the moving forces behind stack effect and find there is no equation for the mythical upward force from the warm air. Buoyancy is indeed the principle involved and it is the difference in the weigh of warm vs cold air. You did use the term “push” when describing the airflow into the attic, which is correct. Bud
Bud, I like your words… Bud, I like your words…”it is the cold air that initiates the process” it reminds me of Julius Sumner Miller: “the less dense air is pushed up by the colder air” http://www.youtube.com/watch?v=S57nIs503fA&feature;=results_video&playnext;=1&list;=PL4795B8F0927BE8B9  ;
Allison, well put. I always Allison, well put. I always make the point that they are not “heat balloons.” They’re “hot air balloons” and try to apply my mentor’s adage that in summertime (in some climates) you have to turn your building science upside down and inside out. Also, I have a link to an excellent photo of a house-shaped hot air balloon. E-mail me a request if you’re interested.
Allison, how can I make the Allison, how can I make the links that I enter “hot”
Bud : I’m Bud : I’m not sure why you think that “cold air that initiates the process” when it’s really pressure differences that move air across the building envelope. At the top of the house, there’s a pressure difference across the ceiling, and air moves from the area of high pressure (inside the house) to the area of low pressure (the attic). At the bottom, the pressure difference is reversed (positive outside, negative inside). Leaks at both the top and bottom are necessary for air to keep moving. If the top is airtight, the pressure difference at the bottom goes to zero. If the bottom is airtight, the pressure difference at the top goes to zero. Regarding the word ‘pull,’ I don’t see a problem with it. If air leaks out through the top of the house, more warm air rises inside the house to take its place. That makes the pressure at the bottom of the house more negative, thus ‘pulling’ in more air from outside.
Great article Allison. I will Great article Allison. I will share it, so some of my green building buddies can read it. Funny thing about the semantics conversation is that usually when this is brought up, it leaves out the comprehension level of the average home owner. Which is whom I serve. I already see the blank stares on their face when I try to explain air infiltration based on pressure planes and differentials and the stack effect. If I didn’t know what you were talking about, I would have had a hard time understanding the article, let alone the following conversations.
I disagree. I just finished I disagree. I just finished reading Dr. Bailes’ article. I’m not the most technical person and I know next to nothing about building science. But I found the article clear and easy to understand. I’m a visual person and the drawings helped me to grasp a concept I’ve never even thought about. I don’t think even my husband (David) could have done a better job explaining this to a lay person.
Allison, you said: I Allison, you said: I’m not sure why you think that “cold air that initiates the process” When you insert a mass of cold air into the middle of a warm room, the stack of air passing down through the cold air will have a greater pressure at the bottom than an adjacent stack of air passing through the warm room. The pressures at the top will be identical because each will have an identical amount of air above them. As the cold air moves due to the difference in pressure at the bottom it creates a reduced pressure at the top resulting in the warm air being pulled in the replace it. It is obvious that both must move, but the warm air cannot rise by itself and therefore cannot be credited with pulling the cold air in behind it. Convection is also the movement of air and since it is the increased pressure that determines the direction of that movement, I give credit to the cold air. You said, and this is an example of the conventional thinking we must change: “If air leaks out through the top of the house, more warm air rises inside the house to take its place. That makes the pressure at the bottom of the house more negative, thus ‘pulling’ in more air from outside.” This is not correct. The negative pressure we see at the bottom of our homes is due to the reduced pressure from a stack of air passing down through the house that now includes the warm air inside. Since the colder air outside our homes is heavier, it is applying a positive pressure on the bottom of our homes which makes the inside look negative. That difference in pressure then forces cold air into our homes pushing the warm air up and forcing it out the top with a positive inside pressure. Here is my test page where I’m trying to create a working explanation of stack effect which walks through the creation of these positive and negative pressures. http://myenergyworkshop.homestead.com/hot-air.html Bud
Bud : You Bud : You said: “…this is an example of the conventional thinking we must change.” I’m still waiting to be convinced that there’s reason or need to change.
The change is that we need to The change is that we need to recognize that warm air does not rise and “pull” in its replacement air. A good example of a misconception that has evolved from this pulling concept is the short-circuit theory when gable vents are left in place along with soffit and ridge vents. As the theory goes, the exiting warm air will pull its replacement air from the gables instead of from the soffits where we want it. Thus the soffit air flow will have been short circuited. The reality is, the air that flows in the soffits is going to happen regardless of where it flows out. It is not a pulling effect that is creating the pressure across the soffits but the barometric differences between inside and out. The link provided does get a little more involved as I am putting numbers on the stack effect pressures, but it does illustrate that the negative pressures we see in our basements are the result of barometric differences and not a vacuum created by exiting air. Since this is getting long, you are welcome to use my email and we can continue this over a longer period of time. This energy correctness is just getting started and will require time to find changes that will be comfortable to all. Your contributions would be greatly appreciated. Bud
Bud, Thanks for posting your Bud, Thanks for posting your unconventional thoughts…I like what you are saying…and I like the “test page” that you posted
Thanks for the video, he is Thanks for the video, he is very entertaining and does make a definite statement about cold air is the force behind warm air moving up. Bud
The comment by Jon LaMonte The comment by Jon LaMonte brushes closest to what should be the point: demonstrating to clients a basic understanding and importance to controlling the air flow in their buildings and homes. Technical precision of the processes occuring is not needed hear and would interfere with the intended COMMUNICATION. I(the client) don’t care what came first, the chicken or the egg. I need the eggs to make french toast.
Clarence: Thanks for the Clarence: Thanks for the comment. I love reading people’s blogs on these types of subjects. Often the writer forgets who the ultimate entity is that is being served, the home or building owner. That is why I appreciate Allison’s blogs so much because they not only educate and create a useful forum for us energy nerds, but they are also usually clear enough for the lay person to understand and learn from as well. I always get a kick out of the comment sections because it is often obvious that that ultimate purpose is lost in translation or semantics.
Nice job on keeping it simple Nice job on keeping it simple Allison!
Bud & others are Bud & others are welcome to comment here: What must we say?
We could call this thread We could call this thread positve pressure.dp
Allison, You always seem to Allison, You always seem to choose your words very carefully. I am curious if Bud Poll’s November blog at Home Energy Pros prompted or influenced the wording in this blog?
John B. : I John B. : I did see Bud’s discussion on HEP and wasn’t swayed by his argument. I’ve got another article on this topic coming out tomorrow morning, so check back then.
If I said “Cars are blue If I said “Cars are blue…” I would certainly not be ‘wrong’ either, but I would also not be helpful. If I qualified that by further stating “… and cars are other colors too.” would perhaps be even less helpful. It would however, in the world of BS, provoke comments and drive blog traffic and prompt some individuals who want to be helpful to make comments where they would otherwise not. Mission accompished Allison. 😉 I try to never say “heat rises” but instead here’s how I choose to handle it. I say “We know there are three forms of heat – convective, conductive and radiant – and they are all working together. When warm air rises, that is primarily concerning the convective form. We’re first aiming to get a handle on that one because it can dominate heat loss if not effectively controlled.” Its helpful, I find, to quickly and up front take the time with a client to explain the 3 forms. I find that most people ‘get it’ and it provides foundation for a host of other explanations that eventually follow. If I said “Heat rises”, that is the only part anyone would remember (no matter what qualification you append to it). The proof is likely that in your own mind, as in mine, it gained a strong foothold in my earliest childhood memories (long before I knew how much BS I was in for). Please know that I have long ago curtailed my desire to police so called improper use of words like “concrete vs. cement” or “irrespective vs irregardless” or “fuel vs gas” because they don’t really affect the understanding of a concept. On the otherhand, when it comes to BS, clarity and understanding has along way to go and needs support by eliminating that unhelpful phrase. Long live “Warm air rises.” Nice blog. Since it serves to clarfy more than confuse the end sum is always positive. 🙂
Lol… seems like the point Lol… seems like the point is “one leaves and the other comes in at the same time.” Is there a balance, always? If it is cold in a building and hot outside, that cold air will join the heat. And the opposite. Where is the heat if an enclosure is 32f and so is the outside?
Comments are closed.
- previous post: Energy and Power and Confusion and Consternation
- next post: You Do NOT Talk About Building Science Fight Club
It’s a wonderful world — and universe — out there.
Come explore with us!
Science News Explores
Explainer: how heat moves.
Here are the three processes by which energy can be transferred from one place to another

Heat is being transferred from the hot end of this rod to the cold end via conduction, but the hot end of the rod is also radiating heat via that orange glow.
Dvoinik/iStockphoto
Share this:
- Google Classroom
By Sid Perkins
September 30, 2016 at 6:15 am
Throughout the universe, it’s natural for energy to flow from one place to another. And unless people interfere, thermal energy — or heat — naturally flows in one direction only: from hot toward cold.
Heat moves naturally by any of three means. The processes are known as conduction, convection and radiation. Sometimes more than one may occur at the same time.
First, a little background. All matter is made from atoms — either single ones or those bonded in groups known as molecules. These atoms and molecules are always in motion. If they have the same mass, hot atoms and molecules move, on average, faster than cold ones. Even if atoms are locked in a solid, they still vibrate back and forth around some average position.
In a liquid, atoms and molecules are free to flow from place to place. Within a gas, they are even more free to move and will completely spread out within the volume in which they are trapped.
Some of the most easily understood examples of heat flow occur in your kitchen.
Put a pan on a stovetop and turn on the heat. The metal sitting over the burner will be the first part of the pan to get hot. Atoms in the pan’s bottom will start to vibrate faster as they warm. They also vibrate farther back and forth from their average position. As they bump into their neighbors, they share with that neighbor some of their energy. (Think of this as a very tiny version of a cue ball slamming into other balls during a game of billiards. The target balls, previously sitting still, gain some of the cue ball’s energy and move.)
As a result of collisions with their warmer neighbors, atoms start moving faster. In other words, they are now warming. These atoms, in turn, transfer some of their increased energy to neighbors even farther from the original source of heat. This conduction of heat through a solid metal is how the handle of a pan gets hot even though it may be nowhere near the source of heat.
Convection occurs when a material is free to move, such as a liquid or a gas. Again, consider a pan on the stove. Put water in the pan, then turn on the heat. As the pan gets hot, some of that heat transfers to the molecules of water sitting on the bottom of the pan via conduction. That speeds up the motion of those water molecules — they are warming.

As the water warms, it now begins to expand. That makes it less dense. It rises above denser water, carrying away heat from the bottom of the pan. Cooler water flows down to take its place next to the hot bottom of the pan. As this water warms, it expands and rises, ferrying its newly-gained energy with it. In short order, a circular flow of rising warm water and falling cooler water sets up. This circular pattern of heat transfer is known as convection .
It’s also what largely warms food in an oven. Air that’s warmed by a heating element or gas flames at the top or bottom of the oven carries that heat to the central zone where the food sits.
Air that’s warmed at Earth’s surface expands and rises just like the water in the pan on the stove. Large birds such as frigate birds (and human flyers riding engineless gliders) often ride these thermals — rising blobs of air — to gain altitude without using any energy of their own. In the ocean, convection caused by heating and cooling helps to drive ocean currents. These currents move water around the globe.
The third type of energy transfer is in some ways the most unusual. It can move through materials — or in the absence of them. This is radiation.
Consider visible light, a form of radiation. It passes through some types of glass and plastic. X-rays, another form of radiation, readily pass through flesh but are largely blocked by bone. Radio waves pass through the walls of your home to reach the antenna on your stereo. Infrared radiation, or heat, passes through the air from fireplaces and light bulbs. But unlike conduction and convection, radiation doesn’t require a material to transfer its energy. Light, X-rays, infrared waves and radio waves all travel to Earth from the far reaches of the universe. Those forms of radiation will pass through plenty of empty space along the way.
X-rays, visible light, infrared radiation, radio waves are all different forms of electromagnetic radiation . Each type of radiation falls into a particular band of wavelengths. Those types differ in the amount of energy they have. In general, the longer the wavelength, the lower the frequency of a particular type of radiation and the less energy it will carry.
To complicate things, it’s important to note that more than one form of heat transfer may occur at the same time. A stove’s burner not only heats a pan but also the nearby air and makes it less dense. That carries warmth upward via convection. But the burner also radiates heat as infrared waves, making things nearby warm up. And if you’re using a cast-iron skillet to cook a tasty meal, be sure to grab the handle with a potholder: It’s gonna be hot, thanks to conduction!
More Stories from Science News Explores on Physics
Scientists Say: Semiconductor

Turning jeans blue with sunlight might help the environment
Explainer: what is the solar cycle.

Forests could help detect ‘ghost particles’ from space
Explainer: Sprites, jets, ELVES and other storm-powered lights

Here’s why blueberries aren’t blue — but appear to be

The weird sky glow called STEVE is really confusing scientists

Physics explains what happens when a lawn sprinkler sucks in water
- Skip to main content
- Keyboard shortcuts for audio player
How hot is too hot? New weather forecasting tool can help figure that out
Alejandra Borunda

People rest at a cooling station in Portland, Oregon during the deadly Northwest heat dome of 2021. Climate change has made heat risks more dangerous across the country. A new heat forecasting tool could help people stay safe. KATHRYN ELSESSER/AFP via Getty Images hide caption
People rest at a cooling station in Portland, Oregon during the deadly Northwest heat dome of 2021. Climate change has made heat risks more dangerous across the country. A new heat forecasting tool could help people stay safe.
This summer, people across the U.S. will have a new way to keep track of dangerous heat headed their way through a new heat warning system called HeatRisk . The tool, developed by the Centers for Disease Control (CDC) and the National Oceanic and Atmospheric Administration (NOAA), will be used by National Weather Service offices across the country to give people an understanding of when heat goes from uncomfortable to dangerous.
HeatRisk incorporates a host of factors that make heat dangerous to human health , beyond just temperature . It considers elements like humidity, which reduces people's ability to cool by sweating, and whether a 90-degree day comes in April versus July — hot weather is more dangerous early in the season before people's bodies have adjusted.
"For the first time, we'll be able to know how hot is too hot for health, and not just today, but for the coming weeks," says Dr. Aaron Bernstein, director of the National Center for Environmental Health and a pediatrician.
Hopefully, he says, the new tool will be easy to understand. It uses a color-coded scale from zero (green) to five (magenta). At zero, the heat conditions are likely not risky for most people. At 2, or yellow, risks are growing for those who are sensitive to heat—like children, or people with medical conditions that make them heat-sensitive. Four, or bright magenta, signals the heat could hurt nearly anyone. That threshold can be crossed when temperatures go above historical highs, or when extreme conditions stretch for several days in a row.
The National Weather Service (NWS) will be able to issue HeatRisk warnings a full week ahead of dangerous heat. Climate change, driven primarily by human burning of fossil fuels, has increased the intensity, duration, and danger of heat waves across North America .
That extra planning time "will be a game-changer," says John Balbus, director of the Office of Climate Change and Health Equity, an office within the Department of Health and Human Services. It will allow crucial extra time for cities to ramp up their emergency response plans and for individuals to think about how to protect themselves, he says.
Why is a heat warning useful?
When it gets hot, people end up in the emergency room—or even die. Last summer, the hottest ever recorded in many parts of the U.S., nearly 120,000 people went to the emergency room for heat-related concerns—nearly twice as many as in the previous two decades, on average.
High temperatures are a major factor, but only part of the puzzle, says Ambarish Vaidyanathan, a researcher at the CDC who helped develop HeatRisk. Humidity matters too: when the air is saturated with water, people still sweat—but sweat droplets can't evaporate, so people can't cool down.
Unusually high overnight temperatures prevent people from getting relief from the heat. People's past exposure to heat matters, too. The body can adjust to high heat up to a point, but that acclimatization takes time. So a 100-degree day in April poses more health risks than the same temperature in July because most people haven't had the time to adjust.
Where people live, and what heat conditions they're used to, also play a role in their vulnerability to heat. "90 degrees in Miami is not the same as 90 degrees in Portland, Maine," says Dr. Mandy Cohen, director of the CDC.
HeatRisk takes all of these factors into account. A town in Michigan, for example, might get a red, or level-3 warning, when the mercury reads 85 degrees Fahrenheit, but a town in Florida with similar conditions might only get a risk warning of yellow, or 1.
Paul Charlton is an emergency medicine physician who works with rural communities in New Mexico. He thinks HeatRisk could be useful to his patients, emergency managers, and clinicians.
"A lot of emergency departments would know how to care for one person that came in with heat stroke," he says. "But a lot of emergency departments would not be as well prepared to take care of ten or 50 or 100 or a thousand people that might be coming in." That could—and did—happen during really extreme heat, like the 2021 heat dome in the Pacific Northwest. Charlton says having a better risk forecast would give people like him invaluable time to plan and prepare for potentially catastrophic heat.
Where did HeatRisk come from?
Scientists at the National Weather Service and the CDC developed the tool. It was conceptualized a decade ago after some local weather bureaus in the western U.S. realized they needed a better way to warn people about upcoming heat waves.
HeatRisk has been tested and refined over the years across the West since its inception in 2013. Now, school systems in California use it to decide when outdoor activities are safe. Maricopa County, which includes the Phoenix metro, has incorporated its use into its heat management plans .
NWS and CDC scientists looked at heat-related deaths around the country and analyzed the weather conditions when people died. That allowed them to find links between people's risk of dying and heat-related factors like temperature, humidity, and how long heatwaves lasted for hundreds of places across the U.S. They used those relationships to predict how different hot-weather conditions will impact people's health in different parts of the country, at different times of year.
In Phoenix, a recent analysis showed that about two-thirds of heat-related deaths happened on red or purple HeatRisk days, says Michael Staudenmaier, chief of science for the NWS's Western Regional Headquarters. But more than 30% of the heat-related deaths occurred in the yellow and orange categories when heat conditions were bad but not anywhere near record-breaking extremes, he says. It shows there is a "wide range of temperatures where heat-related impacts can occur," even in places well-accustomed to it.
It shows that people can be vulnerable to heat illness or even death at levels much lower than they might think, Staudenmaier says.
What is heat? What could go wrong on DS1 if it gets too cold? What could go wrong on DS1 if there's too much heat?
Will DS1 get heated directly by the Sun? Does heat travel differently in space than it does on Earth? What role does the Sun play in space missions like DS1's?
What other forms of energy does a spacecraft releases into space? What makes EM radiation? Where does energy come from and go? What happens to the heat once it is released into space?
December 22, 2003
If heat rises, why does the temperature decrease at higher elevations?
Paul Shepson, professor of atmospheric chemistry at Purdue University's School of Science, explains.
In the earth¿s atmosphere, pressure, which is related to the number of molecules per unit volume, decreases exponentially with altitude. Thus, if a parcel of air from the surface rises (because of wind flowing up the side of a mountain, for example), it undergoes an expansion, from higher to lower pressure. When you allow air to expand, it cools. This phenomenon is familiar to everyone--stick your finger on the valve of a car tire, and let some air escape. It is not cool inside the tire, but as the air comes out it expands and thus cools.
Michael Tinnesand, associate director for academic programs at the American Chemical Society, provides the following explanation:
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing . By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
The basic answer is that the farther away you get from the earth, the thinner the atmosphere gets. The total heat content of a system is directly related to the amount of matter present, so it is cooler at higher elevations.
The heating of the earth itself also plays a role. The planet is warmed by incoming solar energy. Some of this heat bounces off the atmosphere and never reaches the lower atmosphere, and some is re-radiated back to space. In addition, the atmosphere acts like a greenhouse to reflect some of the heat back toward the earth's surface. At higher altitudes it is relatively harder to retain this energy as more heat is lost to space.
NOTIFICATIONS
Heat energy.
- + Create new collection
Most of us use the word ‘heat’ to mean something that feels warm, but science defines heat as the flow of energy from a warm object to a cooler object.
Actually, heat energy is all around us – in volcanoes, in icebergs and in your body. All matter contains heat energy.
Heat energy is the result of the movement of tiny particles called atoms, molecules or ions in solids, liquids and gases. Heat energy can be transferred from one object to another. The transfer or flow due to the difference in temperature between the two objects is called heat.
For example, an ice cube has heat energy and so does a glass of lemonade. If you put the ice in the lemonade, the lemonade (which is warmer) will transfer some of its heat energy to the ice. In other words, it will heat up the ice. Eventually, the ice will melt and the lemonade and water from the ice will be the same temperature. This is known as reaching a state of thermal equilibrium.

Moving particles
Matter is all around you. It is everything in the universe – anything that has both mass and volume and takes up space is matter. Matter exists in different physical forms – solids, liquids and gases.
All matter is made of tiny particles called atoms, molecules and ions. These tiny particles are always in motion – either bumping into each other or vibrating back and forth. It is the motion of particles that creates a form of energy called heat (or thermal) energy that is present in all matter.
The particles in solids are tightly packed and can only vibrate. The particles in liquids also vibrate but are able to move around by rolling over each other and sliding around. In gases, the particles move freely with rapid, random motion.
Transferring heat energy – particles in collision
At higher temperatures, particles have more energy. Some of this energy can be transmitted to other particles that are at a lower temperature. For example, in the gas state, when a fast moving particle collides with a slower moving particle, it transfers some of its energy to the slower moving particle, increasing the speed of that particle.
With billions of moving particles colliding into each other, an area of high energy will slowly transfer across the material until thermal equilibrium is reached (the temperature is the same across the material).
Changing states by heat transfer
Faster moving particles ‘excite’ nearby particles. If heated sufficiently, the movement of particles in a solid increases and overcomes the bonds that hold the particles together. The substance changes its state from a solid to a liquid (melting). If the movement of the particles increases further in the liquid, then a stage is reached where the substance changes into a gas (evaporation).
Three ways of transferring heat energy
All heat energy, including heat generated by fire, is transferred in different ways:
Convection transfers heat energy through gases and liquids. As air is heated, the particles gain heat energy allowing them to move faster and further apart, carrying the heat energy with them. Warm air is less dense than cold air and will rise. Cooler air moves in below to replace the air that has risen. It heats up, rises, and is again replaced by cooler air, creating a circular flow called a convection current. These currents circle and heat the room.
Conduction transfers heat energy in solids. The moving particles of a warm soild material can increase the heat energy of the particles in a cooler solid material by transferring it directly from one particle to the next. Since particles are closer together, solids conduct heat better than liquids or gases.
Radiation is a method of heat transfer that does not require particles to carry the heat energy. Instead, heat is transferred in infrared waves (part of the electromagnetic spectrum). Heat waves radiate out from hot objects in all directions, travelling at the speed of light, until they hit another object. When this happens, the heat energy carried by the waves can be either absorbed or reflected.
Fire illustrates the three different methods of heat transfer. For example, the firebox will heat up due to convection. The air above the fire will be warm due to convection. You can warm your hands near to the flames due to radiant heat transfer.
An effect of heat – expansion
When gases, liquids and solids are heated, they expand. As they cool, they contract or get smaller. The expansion of the gases and liquids is because the particles are moving around very fast when they are heated and are able to move further apart so they take up more room. If the gas or liquid is heated in a closed container, the particles collide with the sides of the container, and this causes pressure. The greater the number of collisions, the greater the pressure.
Sometimes when a house is on fire, the windows will explode outwards. This is because the air in the house has been heated and the excited molecules are moving at high speed around the room. They are pushing against the walls, ceiling, floor and windows. Because the windows are the weakest part of the house structure, they break and burst open, releasing the increased pressure.
Related content
To help understand more about heat, particularly in relation to fire, see these articles:
- Fire behaviour
- Fire behaviour in the outdoors
- Detecting fire
- What is fire?
- Using solar energy
Activity ideas
There are a number of activities that support student learning. Hands-on activities include:
- Drama in the microworld – using drama to model atoms, molecules, heat transfer and combustion.
- The great candle experiment – the oft-used inverted jar in a saucer of water but without the common misconceptions.
- The flying teabag – investigating convection.
In Alternative conceptions about fire discover some common misunderstandings about fire and keep them in mind while teaching – and address them as they come up.
See our newsletters here .
Would you like to take a short survey?
This survey will open in a new tab and you can fill it out after your visit to the site.

- Science Notes Posts
- Contact Science Notes
- Todd Helmenstine Biography
- Anne Helmenstine Biography
- Free Printable Periodic Tables (PDF and PNG)
- Periodic Table Wallpapers
- Interactive Periodic Table
- Periodic Table Posters
- How to Grow Crystals
- Chemistry Projects
- Fire and Flames Projects
- Holiday Science
- Chemistry Problems With Answers
- Physics Problems
- Unit Conversion Example Problems
- Chemistry Worksheets
- Biology Worksheets
- Periodic Table Worksheets
- Physical Science Worksheets
- Science Lab Worksheets
- My Amazon Books
Heat Transfer – Conduction, Convection, Radiation

Heat transfer occurs when thermal energy moves from one place to another. Atoms and molecules inherently have kinetic and thermal energy, so all matter participates in heat transfer. There are three main types of heat transfer, plus other processes that move energy from high temperature to low temperature.
What Is Heat Transfer?
Heat transfer is the movement of heat due to a temperature difference between a system and its surroundings. The energy transfer is always from higher temperature to lower temperature, due to the second law of thermodynamics . The units of heat transfer are the joule (J), calorie (cal), and kilocalorie (kcal). The unit for the rate of heat transfer is the kilowatt (KW).
The Three Types of Heat Transfer With Examples
The three types of heat transfer differ according to the nature of the medium that transmits heat:
- Conduction requires contact.
- Convection requires fluid flow.
- Radiation does not require any medium.
- Conduction is heat transfer directly between neighboring atoms or molecules. Usually, it is heat transfer through a solid. For example, the metal handle of a pan on a stove becomes hot due to convection. Touching the hot pan conducts heat to your hand.
- Convection is heat transfer via the movement of a fluid, such as air or water. Heating water on a stove is a good example. The water at the top of the pot becomes hot because water near the heat source rises. Another example is the movement of air around a campfire. Hot air rises, transferring heat upward. Meanwhile, the partial vacuum left by this movement draws in cool outside air that feeds the fire with fresh oxygen.
- Radiation is the emission of electromagnetic radiation. While it occurs through a medium, it does not require one. For example, it’s warm outside on a sunny day because solar radiation crosses space and heats the atmosphere. The burner element of a stove also emits radiation. However, some heat from a burner comes from conduction between the hot element and a metal pan. Most real-life processes involve multiple forms of heat transfer.
Conduction requires that molecules touch each other, making it a slower process than convection or radiation. Atoms and molecules with a lot of energy have more kinetic energy and engage in more collisions with other matter. They are “hot.” When hot matter interacts with cold matter, some energy gets transferred during the collision. This drives conduction. Forms of matter that readily conduct heat are called thermal conductors .
Examples of Conduction
Conduction is a common process in everyday life. For example:
- Holding an ice cube immediately makes your hands feel cold. Meanwhile, the heat transferred from your skin to the ice melts it into liquid water.
- Walking barefoot on a hot road or sunny beach burns your feet because the solid material transmits heat into your foot.
- Iron clothes transfers heat from the iron to the fabric.
- The handle of a coffee cup filled with hot coffee becomes warm or even hot via conduction through the mug material.
Conduction Equation
One equation for conduction calculates heat transfer per unit of time from thermal conductivity, area, thickness of the material, and the temperature difference between two regions:
Q = [K ∙ A ∙ (T hot – T cold )] / d
- Q is heat transfer per unit time
- K is the coefficient of thermal conductivity of the substance
- A is the area of heat transfer
- T hot is the temperature of the hot region
- T cold is the temperature of the cold region
- d is the thickness of the body
Convection is the movement of fluid molecules from higher temperature to lower temperature regions. Changing the temperature of a fluid affects its density, producing convection currents. If the volume of a fluid increases, than its density decreases and it becomes buoyant.
Examples of Convection
Convection is a familiar process on Earth, primarily involving air or water. However, it applies to other fluids, such as refrigeration gases and magma. Examples of convection include:
- Boiling water undergoes convection as less dense hot molecules rise through higher density cooler molecules.
- Hot air rises and cooler air sinks and replaces it.
- Convection drives global circulation in the oceans between the equators and poles.
- A convection oven circulates hot air and cooks more evenly than one that only uses heating elements or a gas flame.
Convection Equation
The equation for the rate of convection relates area and the difference between the fluid temperature and surface temperature:
Q = h c ∙ A ∙ (T s – T f )
- Q is the heat transfer per unit time
- h c is the coefficient of convective heat transfer
- T s is the surface temperature
- T f is the fluid temperature
Radiation is the release of electromagnetic energy. Another name for thermal radiation is radiant heat. Unlike conduction or convection, radiation requires no medium for heat transfer. So, radiation occurs both within a medium (solid, liquid, gas) or through a vacuum.
Examples of Radiation
There are many examples of radiation:
- A microwave oven emits microwave radiation, which increases the thermal energy in food
- The Sun emits light (including ultraviolet radiation) and heat
- Uranium-238 emits alpha radiation as it decays into thorium-234
Radiation Equation
The Stephan-Boltzmann law describes relationship between the power and temperature of thermal radiation:
P = e ∙ σ ∙ A· (Tr – Tc) 4
- P is the net power of radiation
- A is the area of radiation
- Tr is the radiator temperature
- Tc is the surrounding temperature
- e is emissivity
- σ is Stefan’s constant (σ = 5.67 × 10 -8 Wm -2 K -4 )
More Heat Transfer – Chemical Bonds and Phase Transitions
While conduction, convection, and radiation are the three modes of heat transfer, other processes absorb and release heat. For example, atoms release energy when chemical bonds break and absorb energy in order to form bonds. Releasing energy is an exergonic process, while absorbing energy is an endergonic process. Sometimes the energy is light or sound, but most of the time it’s heat, making these processes exothermic and endothermic .
Phase transitions between the states of matter also involve the absorption or release of energy. A great example of this is evaporative cooling, where the phase transition from a liquid into a vapor absorbs thermal energy from the environment.
- Faghri, Amir; Zhang, Yuwen; Howell, John (2010). Advanced Heat and Mass Transfer . Columbia, MO: Global Digital Press. ISBN 978-0-9842760-0-4.
- Geankoplis, Christie John (2003). Transport Processes and Separation Principles (4th ed.). Prentice Hall. ISBN 0-13-101367-X.
- Peng, Z.; Doroodchi, E.; Moghtaderi, B. (2020). “Heat transfer modelling in Discrete Element Method (DEM)-based simulations of thermal processes: Theory and model development”. Progress in Energy and Combustion Science . 79: 100847. doi: 10.1016/j.pecs.2020.100847
- Welty, James R.; Wicks, Charles E.; Wilson, Robert Elliott (1976). Fundamentals of Momentum, Heat, and Mass Transfer (2nd ed.). New York: Wiley. ISBN 978-0-471-93354-0.
Related Posts
Curious Kids: how does heat travel through space if space is a vacuum?
Senior Research Associate in Space and Planetary Physics, Lancaster University
Disclosure statement
Nathan Case receives funding from the Science and Technology Facilities Council.
Lancaster University provides funding as a founding partner of The Conversation UK.
View all partners

Curious Kids is a series by The Conversation , which gives children of all ages the chance to have their questions about the world answered by experts. All questions are welcome: you or an adult can send them – along with your name, age and town or city where you live – to [email protected]. We won’t be able to answer every question, but we’ll do our best.
How does heat travel through space if space is a vacuum? – Katerina, age ten, Norwich, UK.
What a great question!
First off, to understand what heat is, you need to know that everything you can touch or see is made up of tiny building blocks called atoms. Atoms are so small that you can’t even see them (except with some very special equipment ) – yet they make up all the matter in the universe.
If something is hot, it means that its atoms have lots of energy and are bouncing around. If something is cold, its atoms have much less energy and they stay quite still.
It’s true that space is a vacuum, which means that there isn’t much matter floating around out there. Space isn’t a perfect vacuum though. Even if we ignore the big stuff like stars, planets and comets, space is not completely empty.
In fact, the sun is constantly blowing matter, known as the solar wind , out into our solar system. This is part of what causes the beautiful light display we call the aurora.
Read more: Curious Kids: what causes the northern lights?
But the solar wind isn’t very dense - it has much, much fewer atoms in it than air, for example. This means it can’t carry much heat in it and so it can’t explain how the warmth from the sun reaches Earth.
There are three ways heat can be shared: conduction, convection and radiation. Let’s think about each of these in turn, to discover which one allows heat to travel through space.
Conduction is what scientists call the transfer of heat through touching. If you touch something warm, heat goes from it to you. If you touch something cold, heat goes from you to it.
Some materials, such as metals, are good conductors. Other materials, such as glass, are poor conductors, and are called insulators.
Heat can also be conducted in more than one step. For example, if you hold a metal spoon in a mug of hot tea, heat will be transferred from the tea to the spoon, and then from the spoon to your hand.

But we’re not touching the sun (and that’s a good thing too - its surface temperature is over 5,000°C!) and space is a vacuum so there isn’t anything to act as a spoon and conduct the heat. So we can rule out conduction.
Convection is the transfer of heat through the flow of fluids. Both liquids and gases can convect heat. Atoms will flow away from hot regions toward cooler regions, carrying their heat and energy with them.
If you’ve ever been in a bath that has started to go cold, and then turn the hot tap on, you’ll feel the hot water convect from the tap further into the bath.
The hot atoms will then bump into colder atoms, sharing their heat through conduction, until the bath becomes an even temperature.
But because space is a vacuum, there are no liquids or gases to convect heat away from the sun, all the way to Earth. So we can rule out convection.
Hot bodies of matter such as the sun – and even our own human bodies – give off heat. As the matter’s atoms move and vibrate they give off, or “radiate”, electromagnetic energy – this is called “thermal radiation”.
Electromagnetic energy comes in a range, or spectrum, of types - some of these we can see: they make up the rainbow of “visible light”. Other types that we cannot see exist too, such as the infrared energy our hot bodies radiate and microwave energy we use to cook food.

Unlike conduction and convection, radiation does not need matter to transfer heat. Energy is radiated from the sun, through the vacuum of space at the speed of light. When this energy arrives at Earth, some of it is transferred to the gases in our atmosphere.
Some of it passes through and heats up the atoms on the earth’s surface. Some will even be absorbed by your skin .
The ground soaks up the energy from the sun’s radiation, and this causes it to give off heat, too. Some of this heat is conducted – like when the hot sand on the beach burns your feet in the summer. Some is convected through wind and ocean currents , and some of it is radiated back into the atmosphere , or even outer space.
More Curious Kids articles, written by academic experts:
What makes a shooting star fall? - Katelyn, age seven, Adelaide, Australia.
What causes the northern lights? – Ffion, age 6.75, Pembrokeshire, UK.
How is water made? – Clara, age eight, Canberra, Australia
- Curious Kids
- Articles for young people

Executive Dean, Faculty of Health

Regional Engagement Officer - Shepparton

Lecturer/Senior Lecturer, Earth System Science (School of Science)

Sydney Horizon Educators (Identified)

Deputy Social Media Producer
Is It Better to Leave Your Heat at the Same Temperature All Day or Turn It Down?
Save money on your heating bill this winter with these tips.

We've been independently researching and testing products for over 120 years. If you buy through our links, we may earn a commission. Learn more about our review process.
Some people swear by turning the temperature down to a chillier degree during the workday, while others believe that lowering the heat means your furnace is going to have to work overtime to bring your house back up to a comfortable temperature later on. So what’s an eco-minded, cost-conscious homeowner to do?
According to Moody, this rule applies to all types of furnaces, and holds true even when the temperature outside is very cold. You should turn down the heat before you go to bed too. Besides saving energy, you’ll also be more comfortable when you’re sleeping.
The easiest, most efficient way to manage the temperature in your house is to use a programmable thermostat, which allows you to set different temperatures for different times and different days. For example, on weekdays you may want your temperature to drop to 58 degrees when you leave for work, and then go back up to 68 an hour before you get home so that your house is all nice and cozy when you walk in the door. If your thermostat is older, you may want to consider upgrading to a wi-fi enabled model . These allow you to set the temperature remotely from your smartphone if your schedule ever changes.
And if you want to get really fancy, there are also “smart” or “learning” thermostats, like Nest , which can actually learn your habits after several days of use and will automatically set the temperature according to your work schedule and bedtime. Smart thermostats even know when you leave the house, so if you go out for brunch on Sunday for two hours when you’d normally be at home, it will lower the temperature accordingly. Moody says they’ll also give you tailored suggestions on how to save even more energy.
Managing the temperature in your home isn’t the only way to slash your energy usage, however. Moody strongly recommends an annual furnace tune-up to make sure everything is running smoothly. "If your furnace isn’t working correctly, or if the equipment is outdated, then you won’t get the maximum energy savings no matter how mindfully you use your thermostat," he says. He adds that a yearly service can also help detect deadly carbon monoxide gas, which could be released by a malfunctioning furnace.

@media(max-width: 64rem){.css-o9j0dn:before{margin-bottom:0.5rem;margin-right:0.625rem;color:#ffffff;width:1.25rem;bottom:-0.2rem;height:1.25rem;content:'_';display:inline-block;position:relative;line-height:1;background-repeat:no-repeat;}.loaded .css-o9j0dn:before{background-image:url(/_assets/design-tokens/goodhousekeeping/static/images/Clover.5c7a1a0.svg);}}@media(min-width: 48rem){.loaded .css-o9j0dn:before{background-image:url(/_assets/design-tokens/goodhousekeeping/static/images/Clover.5c7a1a0.svg);}} Organic Life

The Anti-Aging Effects of Rosehip Oil

Design a Dreamy Small Garden

How to Grow an Avocado Plant Indoors

How to Grow and Care for a Christmas Cactus

8 Best Blue Light Glasses of 2024

How to Plant Mums and Keep Them Growing

15 Foods That Are Natural Diuretics

40 Picture-Perfect Flowers for a Fall Garden

What You Need to Know About Collagen Supplements

How to Plant, Grow and Harvest Sweet Potatoes

How to Safely Get Rid of Wasps

IMAGES
VIDEO
COMMENTS
Hot air has no problem going down if there is a fan blowing it that direction. In summary, heat can travel in all directions. The direction that heat is traveling depends strongly on the situation. Furthermore, even hot air can travel in all sorts of directions and not just up. Hot air only travels up when gravity is the dominant force at work.
Artwork: Hotter things have more heat energy than colder things. That's because the atoms or molecules move around faster in hot things (red, right) than they do in cold things (blue, left). This idea is called the kinetic theory. The kinetic theory helps us understand where the energy goes when we heat something up.
Heat can move up, down, or sideways, depending on the situation. What the laws of thermodynamics tell us is that heat moves from areas of higher temperature to areas of lower temperature. Put a torch to the top of a steel pole, and heat will travel downward by conduction. So, temperature difference is really what drives heat to move in any ...
3. Heat travels in three ways: Conduction, convection, and radiation. Conduction is when objects of different temp come into contact, and the vibrating molecules from the hotter object increase the vibration of the molecules in the cooler object, thereby cooling the hotter object and heating the cooler one.
Put water in the pan, then turn on the heat. As the pan gets hot, some of that heat transfers to the molecules of water sitting on the bottom of the pan via conduction. That speeds up the motion of those water molecules — they are warming. Lava lamps illustrate heat transfer via convection: Waxy blobs get warmed at the base and expand.
Why is a heat warning useful? When it gets hot, people end up in the emergency room—or even die. Last summer, the hottest ever recorded in many parts of the U.S., nearly 120,000 people went to ...
About. Transcript. There are three forms of thermal energy transfer: conduction, convection, and radiation. Conduction involves molecules transferring kinetic energy to one another through collisions. Convection occurs when hot air rises, allowing cooler air to come in and be heated. Thermal radiation happens when accelerated charged particles ...
Heat moves in three ways: Radiation, conduction, and convection. Radiation happens when heat moves as energy waves, called infrared waves, directly from its source to something else. This is how the heat from the Sun gets to Earth. In fact, all hot things radiate heat to cooler things. When the heat waves hits the cooler thing, they make the ...
Heat is sometimes called a process quantity, because it is defined in the context of a process by which energy can be transferred.We don't talk about a cup of coffee containing heat, but we can talk about the heat transferred from the cup of hot coffee to your hand. Heat is also an extensive property, so the change in temperature resulting from heat transferred to a system depends on how many ...
Heat has no weight or any physical properties of that sort. As a result, heat transfer in and of itself is not impacted by gravity at all. It is just as likely to be transferred in all direction, whether it be up, down, or to the side. Hot air does rise. This is because hot air is less dense than the cooler air around it.
The basic answer is that the farther away you get from the earth, the thinner the atmosphere gets. The total heat content of a system is directly related to the amount of matter present, so it is ...
When this happens, the heat energy carried by the waves can be either absorbed or reflected. Fire illustrates the three different methods of heat transfer. For example, the firebox will heat up due to convection. The air above the fire will be warm due to convection. You can warm your hands near to the flames due to radiant heat transfer.
Heating of the air can occur via conduction or convection - transferring heat to these air bubbles, and sharing it between them. The land surface heats up during the day because of solar ...
The Three Types of Heat Transfer With Examples. The three types of heat transfer differ according to the nature of the medium that transmits heat: Conduction requires contact. Convection requires fluid flow. Radiation does not require any medium. Conduction is heat transfer directly between neighboring atoms or molecules.
Heat can travel in all directions. The direction heat travels depends on the specifics of a system not in thermal equilibrium. In contrast, "hot air" on earth's surface tends to go up. The simple reason for this is that hot air is less dense than cold air and less dense objects are forced up by more dense objects falling down ("bouyancy").
The sun's radiation consists of small, massless packets of energy called photons. They travel seamlessly through space; whenever they strike any object, the object absorbs photons and its energy is increased, which then heats it up. So, these photons travel through a vacuum without any problem, but as soon as they collide with an object, like ...
Unlike conduction and convection, radiation does not need matter to transfer heat. Energy is radiated from the sun, through the vacuum of space at the speed of light. When this energy arrives at ...
Exploring H2O Molecules. DyslexicHobo. Apr 26, 2007. Heat. In summary, when heated, molecules gain more kinetic energy and collide with surrounding molecules, causing them to spread out and become less dense. This is why heated air or steam rises. However, for liquids with a higher molecular mass, the opposite occurs and they will sink when heated.
19. Air does indeed flow from high pressure to low pressure area (see the wind arrows on a weather chart), but in the case of two rooms the much more important effect is that of warm thinner air rising towards the ceiling when the air from the two rooms gets mixed. Thus, cold air from the cold room will be leaving the room close to the floor ...
That's a myth. No matter how cool the house has gotten, it will warm up at the same rate." According to Moody, this rule applies to all types of furnaces, and holds true even when the ...
Firstly, the air is cooler than your body so as it passes, your body heat transfers in part to the air, which is then carried away. Second, as the air passes, it evaporates moisture on your skin, which takes absorbs heat in the process. As for the propagation of cold temperatures in general. If you put an ice block out on the counter, the air ...