You are using an outdated browser. Upgrade your browser today or install Google Chrome Frame to better experience this site.
- Section 5 - Henipavirus Infections
- Section 5 - Hepatitis B

Hepatitis A
Cdc yellow book 2024.
Author(s): Noele Nelson, Mark Weng
Infectious Agent
Transmission, epidemiology, clinical presentation.
INFECTIOUS AGENT: Hepatitis A virus
High endemicity: parts of Africa and Asia
Intermediate endemicity: parts of Asia; also, Central and South America, and eastern Europe
Low endemicity: Western Europe, United States
TRAVELER CATEGORIES AT GREATEST RISK FOR EXPOSURE & INFECTION
PREVENTION METHODS
Follow safe food and water precautions
Hepatitis A is a vaccine-preventable disease
DIAGNOSTIC SUPPORT
Hepatitis A virus (HAV) is a nonenveloped RNA virus classified as a picornavirus.
HAV is transmitted through direct person-to-person contact (fecal–oral transmission) or through ingestion of contaminated food or water. HAV can survive in the environment for prolonged periods at low pH. Freezing does not inactivate the virus, and HAV can be transmitted through ice and frozen foods. Heat inactivation must occur at temperatures >185°F (>85°C) for 1 minute. HAV can be transmitted from raw or inadequately cooked foods contaminated during growing, processing, or distribution, and through contamination by an infected food handler. Recent large-scale outbreaks have been caused by common-source food exposures (e.g., frozen berries, fresh fruit and vegetables, seafood) and through person-to-person spread among people experiencing homelessness and people who use injection and non-injection drugs.
Infected people shed HAV in their feces. People are most infectious 1–2 weeks before the onset of clinical signs and symptoms of jaundice or elevation of liver enzymes, when virus concentration is greatest in the stool and blood. Viral excretion and the risk for transmission diminish rapidly after liver dysfunction or symptoms appear, which is concurrent with the appearance of circulating antibodies to HAV. Infants and children can shed virus for up to 6 months after infection.
Hepatitis A is among the most common vaccine-preventable infections acquired during travel. Cases of travel-related hepatitis A can occur in travelers to developed and developing countries and who have standard tourist accommodations, eating behaviors, and itineraries. Risk is greatest for those who live in or visit rural areas, trek in backcountry areas, or frequently eat or drink in settings with poor sanitation. Common-source food exposures are increasingly recognized as a risk for hepatitis A, and sporadic outbreaks have been reported in Australia, Europe, North America, and other regions with low levels of endemic transmission. Multinational hepatitis A outbreaks among men who have sex with men (MSM) have been described, including, since 2016, among MSM who travel to areas in European Union countries with ongoing HAV transmission among MSM.
Hepatitis A is common in areas with inadequate sanitation and limited access to clean water. In highly endemic areas (e.g., parts of Africa and Asia), a large proportion of adults in the population are infected as children, are immune to HAV, and epidemics are uncommon. In areas of intermediate endemicity (e.g., Central and South America, eastern Europe, parts of Asia), childhood transmission is less frequent, more adolescents and adults are susceptible to infection, and outbreaks are more likely. In areas of low endemicity (e.g., western Europe, the United States), infection is less common, but disease occurs among people in high-risk groups and as communitywide outbreaks. Determining HAV endemicity globally is complex, however, and limited data are available on subpopulation variation of HAV antibody seroprevalence within regions ( Map 5-06 ).
In the United States, the most frequently identified risk factors for HAV infection vary from year to year. The Advisory Committee on Immunization Practices (ACIP) recommends routine hepatitis A vaccination for all children, and vaccination for adults at increased risk for HAV infection or at increased risk for severe disease from HAV infection.
Map 5-06 Estimated age at midpoint of population immunity (AMPI) to hepatitis A, by country
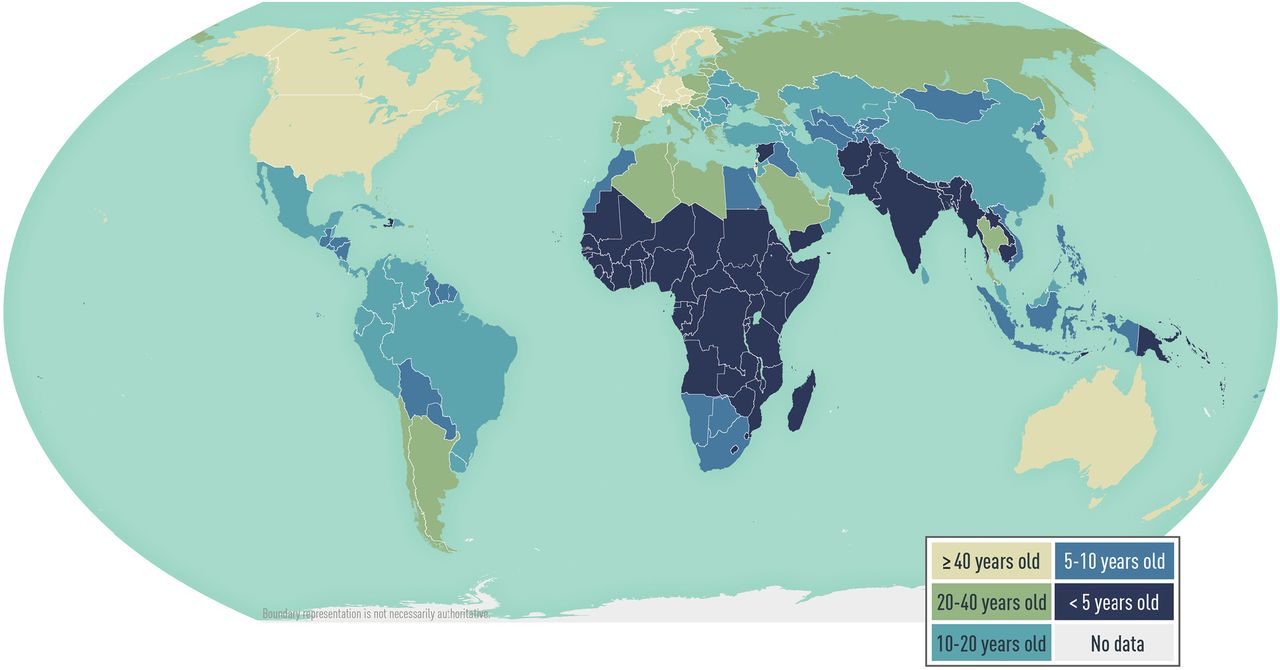
View Larger Figure
Adapted from Jacobsen KH. Globalization and the changing epidemiology of hepatitis A virus. Cold Spring Harb Perspect Med. 2018;8(10).a031716. AMPI is the youngest age at which half of the birth cohort has serologic evidence of prior exposure to hepatitis A virus. As the AMPI increases, the endemicity level of hepatitis A generally decreases.
The incubation period averages 28 days (range 15–50 days). Infection can range from mild illness lasting 1–2 weeks to severely disabling disease lasting several months. Clinical manifestations include abrupt onset of fever, malaise, anorexia, nausea, and abdominal discomfort, followed by jaundice within a few days. The likelihood of having symptoms with HAV infection is related to the age of the infected person. In children aged <6 years, most (70%) infections are asymptomatic; jaundice is uncommon in symptomatic young children. Among older children and adults, the illness usually lasts <2 months, but ≈10%–15% of infected people have prolonged or relapsing symptoms over 6–9 months.
Severe hepatic and extrahepatic complications, including fulminant hepatitis and liver failure, are rare but more common in older adults and people with underlying liver disease. Chronic infection does not occur. The overall case-fatality ratio varies according to the population affected.
Hepatitis A cannot be differentiated from other types of viral hepatitis based on clinical or epidemiologic features. Diagnosis requires a positive test for HAV IgM in serum, which is detectable 2 weeks before the onset of symptoms to ≈6 months after symptom onset.
Serologic total HAV IgG and IgM tests are available commercially. The combination of a positive total HAV result and a negative HAV IgM result indicates past infection or vaccination, and hence immunity. Presence of serum HAV IgM usually indicates current or recent infection and does not distinguish between immunity derived from infection versus vaccination. Hepatitis A is a nationally notifiable disease.
Information on how to obtain HAV diagnostic support from the Centers for Disease Control and Prevention (CDC), including contact information, which samples to send, and how to send them is available at CDC's Test Directory webpage. For research use and for outbreak investigations, select “Hepatitis A NAT and Genotyping.” For testing regulated by Clinical Laboratory Improvement Amendments, select “Hepatitis A Serology.”
Provide supportive care.
Travelers can prevent HAV through vaccination or immune globulin (IG), practicing food and water precautions, and maintaining standards of hygiene and sanitation.
Two single-antigen hepatitis A vaccines, Havrix (GlaxoSmithKline) and Vaqta (Merck), are approved for people ≥12 months of age in a 2-dose series. A combined hepatitis A and hepatitis B vaccine (Twinrix, GlaxoSmithKline) is approved for people ≥18 years of age in the United States ( Table 5-10 ). The immunogenicity of the combination vaccine is equivalent to that of the single-antigen hepatitis A and hepatitis B vaccines when tested after completion of the recommended schedule. Postvaccination testing for serologic response is not indicated for healthy people, but is recommended for people whose subsequent clinical management depends on knowledge of their immune status and people for whom revaccination might be indicated (e.g., people with HIV and other immunocompromised people).
Table 5-10 Vaccines used to prevent hepatitis A virus (HAV) infection
Abbreviations: ELU, ELISA units inactivated HAV; HBsAg, hepatitis B surface antigen; IM, intramuscular; U, units of HAV antigen
1 Combined hepatitis A and hepatitis B vaccine (Twinrix) should not be used for postexposure prophylaxis.
Indications for Use
All susceptible people traveling for any purpose, frequency, or duration to countries with high or intermediate hepatitis A endemicity should be vaccinated or receive IG before departure. Furthermore, prevalence patterns of HAV infection vary among regions within a country; in some areas, limited data result in uncertainty in endemicity maps, especially in low- and middle- income countries. Countries with decreasing prevalence of HAV infection have growing numbers of susceptible people and are at risk for hepatitis A outbreaks. In recent years, large hepatitis A outbreaks have been reported in high-income countries among people exposed to imported HAV-contaminated food, among MSM, among people who use drugs, and among people experiencing homelessness. Considering the complexity of interpreting hepatitis A risk maps and potential risk for foodborne hepatitis A in low-endemicity countries, some experts advise people traveling outside the United States to consider hepatitis A vaccination regardless of destination.
Vaccination is also recommended for unvaccinated household members and other people (e.g., regular babysitters) who anticipate close personal contact with an international adoptee from a high- or intermediate-endemicity country ≤60 days after the child’s arrival in the United States. The first dose of the 2-dose hepatitis A vaccine series should be administered as soon as adoption is planned, ideally ≥2 weeks before the arrival of the child (see Sec. 7, Ch. 5, International Adoption ).
Administration
All susceptible people (i.e., those unvaccinated or never infected) traveling to or working in countries with high or intermediate hepatitis A endemicity are at risk for HAV infection. Before departure, these travelers should be vaccinated, or receive IG if they are too young or have contraindications for hepatitis A vaccination. For travelers already partially vaccinated (i.e., did not receive a full series of hepatitis A-containing vaccine), administer a dose prior to travel according to the routine immunization schedule.
Infants Younger than 6 Months Old
Infants aged <6 months and travelers allergic to a vaccine component, or who elect not to receive vaccine, should receive IG, which provides effective temporary protection against HAV infection. For travel duration ≤1 month, the manufacturer recommends 1 dose of IG at 0.1 mL/kg; for travel >1 month but ≤2 months, 1 dose of IG at 0.2 mL/kg is recommended. A 0.2 mL/kg dose of IG should be repeated every 2 months for the duration of travel if the traveler remains in a high-risk setting; but encourage hepatitis A vaccination if not contraindicated.
Infants 6–11 Months Old
Administer hepatitis A vaccine to infants aged 6–11 months traveling outside the United States when protection against hepatitis A is recommended. Although hepatitis A vaccine is considered safe and immunogenic in infants, hepatitis A vaccine doses administered before 12 months of age could result in a suboptimal immune response, particularly in infants with passively acquired maternal antibody. Therefore, hepatitis A vaccine doses administered at <12 months of age are not considered to provide long-term protection, and the 2-dose hepatitis A vaccine series should be initiated at age 12 months according to the routine immunization schedule.
Adults Over 40 Years Old
Adults aged >40 years, immunocompromised people, and people with chronic liver disease should receive a single dose of hepatitis A vaccine as soon as travel is considered. People planning travel in <2 weeks can receive IG (0.1 mL/kg) in addition to vaccine at a separate injection site (i.e., separate limbs) based on provider risk assessment, including considerations of the traveler’s age, immune status, underlying conditions, risk for exposure, and availability of IG. The hepatitis A vaccine series should be completed according to the routine immunization schedule.
An alternative accelerated 4-dose schedule is available for Twinrix; doses can be administered at 0, 7, and 21–30 days, then a dose at 12 months. For more details, refer to NP Nelson et al. in the bibliography of this chapter. Although vaccinating an immune traveler is not contraindicated and does not increase the risk for adverse effects, screening for total HAV antibodies before travel can be useful in some circumstances to determine susceptibility and avoid unnecessary vaccination.
Safety & Adverse Reactions
Based on passive surveillance, the most frequently reported adverse events after single-antigen hepatitis A vaccination were fever, injection site reactions, and rash. The most frequently reported adverse events after Twinrix vaccination were dizziness, fever, headache, and injection site reactions. These findings are similar to those for other inactivated vaccines routinely administered among similar age groups.
Precautions & Contraindications
Hepatitis A vaccines should not be administered to travelers with a history of hypersensitivity to any vaccine component, including neomycin. Twinrix should not be administered to people with a history of hypersensitivity to yeast. The tip caps of prefilled syringes of Havrix and Twinrix and the vial stopper, syringe plunger stopper, and tip caps of Vaqta might contain dry natural rubber, which can cause allergic reactions in latex-sensitive people. Because hepatitis A vaccine consists of inactivated virus, and hepatitis B vaccine consists of a recombinant protein, no special precautions are needed for vaccination of immunocompromised travelers with single-antigen vaccines or Twinrix. Check precautions and contraindications before administering IG.
The ACIP recommends vaccinating selected groups of pregnant people if they have not been vaccinated previously. These include people (e.g., travelers) at increased risk for HAV infection during pregnancy as well as those at risk for having a severe outcome from HAV infection (e.g., those with chronic liver disease or HIV).
Other Considerations
The best approach is to administer hepatitis A vaccine according to the routine immunization schedule; however, an interrupted series does not need to be restarted. Over 90% of vaccinated people develop levels of antibodies to HAV that correlate with protection 1 month after the first dose of hepatitis A vaccines. Given their similar immunogenicity, a series that has been started with one brand of hepatitis A single-antigen vaccine can be completed with another brand of single-antigen vaccine. For children and adults who complete a primary series of hepatitis A-containing vaccine, booster doses of vaccine are not recommended. Measles-mumps-rubella and varicella vaccines should not be administered <6 months after IG administration.
Postexposure Prophylaxis
Travelers exposed to HAV who are asymptomatic and who have not received hepatitis A vaccine should receive 1 dose of single-antigen hepatitis A vaccine or IG (0.1 mL/kg) as soon as possible, ideally ≤2 weeks following exposure. The efficacy of IG or vaccine when administered >2 weeks after exposure has not been established.
Hepatitis A vaccines should be administered as postexposure prophylaxis (PEP) for all people aged ≥12 months who have been exposed to HAV ≤2 weeks and have not previously completed the hepatitis A vaccine series. In addition to hepatitis A vaccine, administer IG (0.1 mL/kg) to people who are immunocompromised or who have chronic liver disease, and to people aged >40 years, depending on the risk assessment, which should include consideration of the exposed person’s age, immune status, underlying conditions, exposure type (risk of transmission), and availability of IG.
Administer PEP as soon as possible. If giving both hepatitis A vaccine and IG (0.1 mL/kg), administer both simultaneously in different anatomic sites (i.e., separate limbs). If only 1 product is available, administer it as soon as possible and have the exposed person return for the other product if it becomes available ≤2 weeks following exposure. When the dose of hepatitis A vaccine given postexposure is the first dose the exposed person has ever received, administer a second dose 6 months after the first for long-term immunity; however, the second dose is not necessary for PEP.
Infants <12 months of age and people who are allergic to a vaccine component or who elect not to receive vaccine should receive a single dose of IG (0.1 mL/kg) as soon as possible ≤2 weeks of exposure.
Do not use Twinrix for PEP. Twinrix contains half of the single-antigen hepatitis A adult dose, and no data are available on the efficacy of combination vaccine for prophylaxis after exposure to HAV.
CDC website: Hepatitis A
The following authors contributed to the previous version of this chapter: Noele P. Nelson
Bibliography
Centers for Disease Control and Prevention. Viral Hepatitis Surveillance 2019. Atlanta: U.S. Department of Health and Human Services; 2021. Jacobsen KH. Globalization and the changing epidemiology of hepatitis A virus. Cold Spring Harb Perspect Med. 2018; 8(10):a031716.
Nelson NP. Updated dosing instructions for immune globulin (human) GamaSTAN S/D for hepatitis A virus prophylaxis. MMWR 2017;66(36):959–60.
Nelson NP, Weng MK, Hofmeister MG, Moore KL, Doshani M, Kamili S, et al. Prevention of hepatitis A virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices, 2020. MMWR Recomm Rep. 2020;69(5):1–38.
File Formats Help:
- Adobe PDF file
- Microsoft PowerPoint file
- Microsoft Word file
- Microsoft Excel file
- Audio/Video file
- Apple Quicktime file
- RealPlayer file
- Zip Archive file
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.
- GP practice services
- Health advice
- Health research
- Medical professionals
- Health topics
Advice and clinical information on a wide variety of healthcare topics.
All health topics
Latest features
Allergies, blood & immune system
Bones, joints and muscles
Brain and nerves
Chest and lungs
Children's health
Cosmetic surgery
Digestive health
Ear, nose and throat
General health & lifestyle
Heart health and blood vessels
Kidney & urinary tract
Men's health
Mental health
Oral and dental care
Senior health
Sexual health
Signs and symptoms
Skin, nail and hair health
Travel and vaccinations
Treatment and medication
Women's health
Healthy living
Expert insight and opinion on nutrition, physical and mental health.
Exercise and physical activity
Healthy eating
Healthy relationships
Managing harmful habits
Mental wellbeing
Relaxation and sleep
Managing conditions
From ACE inhibitors for high blood pressure, to steroids for eczema, find out what options are available, how they work and the possible side effects.
Featured conditions
ADHD in children
Crohn's disease
Endometriosis
Fibromyalgia
Gastroenteritis
Irritable bowel syndrome
Polycystic ovary syndrome
Scarlet fever
Tonsillitis
Vaginal thrush
Health conditions A-Z
Medicine information
Information and fact sheets for patients and professionals. Find out side effects, medicine names, dosages and uses.
All medicines A-Z
Allergy medicines
Analgesics and pain medication
Anti-inflammatory medicines
Breathing treatment and respiratory care
Cancer treatment and drugs
Contraceptive medicines
Diabetes medicines
ENT and mouth care
Eye care medicine
Gastrointestinal treatment
Genitourinary medicine
Heart disease treatment and prevention
Hormonal imbalance treatment
Hormone deficiency treatment
Immunosuppressive drugs
Infection treatment medicine
Kidney conditions treatments
Muscle, bone and joint pain treatment
Nausea medicine and vomiting treatment
Nervous system drugs
Reproductive health
Skin conditions treatments
Substance abuse treatment
Vaccines and immunisation
Vitamin and mineral supplements
Tests & investigations
Information and guidance about tests and an easy, fast and accurate symptom checker.
About tests & investigations
Symptom checker
Blood tests
BMI calculator
Pregnancy due date calculator
General signs and symptoms
Patient health questionnaire
Generalised anxiety disorder assessment
Medical professional hub
Information and tools written by clinicians for medical professionals, and training resources provided by FourteenFish.
Content for medical professionals
FourteenFish training
Professional articles
Evidence-based professional reference pages authored by our clinical team for the use of medical professionals.
View all professional articles A-Z
Actinic keratosis
Bronchiolitis
Molluscum contagiosum
Obesity in adults
Osmolality, osmolarity, and fluid homeostasis
Recurrent abdominal pain in children
Medical tools and resources
Clinical tools for medical professional use.
All medical tools and resources
Hepatitis A vaccine
Peer reviewed by Dr Colin Tidy, MRCGP Last updated by Dr Hayley Willacy, FRCGP Last updated 15 Feb 2023
Meets Patient’s editorial guidelines
In this series: Travel vaccinations Hepatitis B vaccine Rabies vaccine Tick-borne encephalitis vaccine Typhoid vaccine Yellow fever vaccine
You should consider vaccination against hepatitis A before you travel to certain countries, such as the Indian subcontinent.
In this article :
What is hepatitis a, who should be immunised against hepatitis a, are there any side-effects from the hepatitis a vaccine, who should not receive hepatitis a vaccine, other points.
Check with your practice nurse at least two weeks before you travel to see if you should have this vaccination.
Continue reading below
Hepatitis A is an illness caused by the hepatitis A virus. The virus mainly causes inflammation of the liver. Symptoms include:
Generally feeling unwell.
Yellowing of your skin or the whites of your eyes ( jaundice ).
Sometimes, being sick (vomiting).
A raised temperature (fever).
However, some people who are infected do not develop any symptoms (a subclinical illness). The illness is not usually serious and full recovery is usual but the symptoms can be quite unpleasant for a while. The hepatitis A virus is passed out in the stools (faeces) of infected people and infection is usually spread by eating dirty (contaminated) food or drink.
Hepatitis A infection can occur in the UK but it is more common in countries where there is poor sanitation or where disposal of sewage is poor. In the UK, most cases of hepatitis A are seen in people who have recently returned after travelling to such countries. If you catch hepatitis A, the illness is not usually serious but it may ruin a holiday or business trip. See the separate leaflet called Hepatitis A for more details .
This leaflet is just about vaccination to help prevent hepatitis A infection.
Travellers to countries outside Western Europe, North America and Australasia should consider being immunised. The highest-risk areas include the Indian subcontinent (in particular India, Pakistan, Bangladesh and Nepal), Africa, parts of the Far East (except Japan), South and Central America and the Middle East. Vaccination is generally recommended for anyone over the age of 1 year. Your doctor or practice nurse can advise if you should be immunised against hepatitis A for your travel destination.
You can find out if immunisation against hepatitis A is recommended for any countries you are planning to visit from the NHS website Fitfortravel.
Close contacts of someone with hepatitis A
Occasional outbreaks of hepatitis A occur in the UK within families or in institutions. Close contacts of someone found to have hepatitis A infection (for example, family members or other members of the institution) may be offered vaccination. This only happens rarely. The most important measure for anybody with hepatitis A is good personal hygiene. In particular, washing hands after going to the toilet or before eating.
People with chronic liver disease
If you have a persistent (chronic) liver disease (for example, cirrhosis ) it is suggested that you have the hepatitis A vaccine. Hepatitis A infection is not more common in those with chronic liver disease but, if infection does occur, it can cause a more serious illness.
People exposed to hepatitis A at work
For example, laboratory workers who are exposed to hepatitis A during their work and sewage workers are advised to be immunised against hepatitis A.
Staff of some large residential institutions
Outbreaks of hepatitis A have been associated with large residential institutions for people with learning difficulties, where standards of personal hygiene among clients or patients may be poor. Therefore, vaccination of staff and residents of some institutions may be recommended.
Injecting drug users
Drug users who share drug injecting equipment are also thought to have an increased risk of hepatitis A infection and so should consider vaccination.
People with blood clotting problems
If someone has certain blood clotting problems (such as haemophilia) and needs to receive blood clotting factors, they may have an increased risk of hepatitis A infection. This is because the hepatitis A virus may not be completely destroyed during the preparation of these blood products. Vaccination is therefore suggested for these people.

Oral-anal contact
Men who have sex with men, and other people whose sexual practices involve oral-anal contact, may also like to consider vaccination against hepatitis A.
Note : if you have been infected with hepatitis A in the past, you should be immune to further infection and therefore not need vaccination. A blood test can detect antibodies to check if you are already immune. This may be worthwhile doing if you have had a history of yellowing of your skin or the whites of your eyes (jaundice) or come from an area where hepatitis A is common.
There are a number of different hepatitis A vaccines available. Some just protect against hepatitis A, but there are also some combined vaccines for both hepatitis A and hepatitis B or hepatitis A and typhoid fever . A combined vaccine may be useful if you require protection against both diseases.
The hepatitis A single vaccine is given as two doses. The first dose of the vaccine protects against hepatitis A for about one year. The vaccine causes your body to make antibodies against the virus. These antibodies protect you from illness should you become infected with this virus. Ideally, you should have an injection at least two weeks before travel to allow immunity to develop. However, the vaccine may still be advised even if there is less than two weeks before you travel.
How long does a hepatitis A vaccine last?
A second dose of the vaccine 6-12 months after the first gives protection for about 20 years. If you are late with this second dose, you should have it as soon as possible but you don't need to start with the first dose again. Another booster dose of hepatitis A vaccine after 20 years can be given to those people still at risk of infection.
The doses of the combined vaccines against both hepatitis A and hepatitis B or hepatitis A and typhoid may need to be given at slightly different time intervals. Your doctor or practice nurse will be able to advise you in detail.
Some people develop a temporary soreness and redness at the injection site. Much less common are:
A mild raised temperature (fever).
Feeling sick (nauseated).
Feeling off your food for a few days.
Severe reactions are extremely rare.
There are a very few situations where the hepatitis A vaccine is not recommended. They include:
If you have an illness causing a high temperature. In this situation, it is best to postpone vaccination until after you have fully recovered from the illness.
If you have had an allergic reaction to the vaccine or to any of its components in the past.
One type of vaccine (Epaxal®) should not be given to anyone who is known to be allergic to eggs.
Children under the age of 1 year. However, the risk of hepatitis A in children under the age of 1 year is very low. The hepatitis A vaccine is not licensed for this age group.
The vaccine may be given if you are pregnant or breastfeeding and vaccination against hepatitis A is thought to be necessary.
Remember - vaccination for travellers is only one aspect of preventing illness. No vaccination is 100% effective. So when travelling to at-risk areas, you should have very good personal hygiene and also be careful about what you eat and drink.
You should avoid eating and drinking the following when travelling to areas where the risk of hepatitis A is higher:
Raw or inadequately cooked shellfish .
Raw salads and vegetables that may have been washed in unclean (contaminated) water. (Wash fruit and vegetables in safe water and peel them yourself.)
Other foods that may have been grown close to the ground , such as strawberries.
Untreated drinking water, including ice cubes made from untreated water. (Remember also to use only treated or bottled water when brushing your teeth.)
Unpasteurised milk, cheese, ice cream and other dairy products.
Also, be careful when buying food from street traders. Make sure that food has been recently prepared and that it is served hot and on clean serving plates. Food that has been left out at room temperature (for example, for a buffet) or food that may have been exposed to flies could also pose a risk.
Further reading and references
- Travel Health Pro ; National Travel Health Network and Centre (NaTHNaC)
- Immunisation against infectious disease - the Green Book (latest edition) ; UK Health Security Agency.
- Travellers' Health ; US Centers for Disease Control and Prevention
- Travel and Diabetes ; Diabetes UK
Article History
The information on this page is written and peer reviewed by qualified clinicians.
Next review due: 14 Feb 2028
15 feb 2023 | latest version.
Last updated by
Peer reviewed by

Feeling unwell?
Assess your symptoms online for free
- Skip to content
- Accessibility help
Immunizations - travel: Scenario: Rapid vaccination courses and vaccination at short notice
Last revised in July 2023
Covers advice for people who require travel vaccinations at short notice.
Scenario: Rapid vaccination courses and vaccination at short notice
From age 2 months onwards.
Rapid vaccination courses and vaccination at short notice
- Hepatitis B vaccine may be given as an accelerated course over 3 weeks. For further information, see the section on Hepatitis B vaccination .
- Tick-borne encephalitis vaccine may be given as two doses 2 weeks apart. For further information, see the section on Tick-borne encephalitis vaccination .
- Japanese encephalitis vaccine may be given to people over the age of 3 years as two doses 7 days apart (instead of the usual 28 days). For further information, see the section on Japanese encephalitis vaccination .
- Rabies vaccine can be given as an accelerated course over 1 week. For further information, see the section on Rabies vaccination .
- Previously unimmunized people who require tetanus and polio vaccination should be given the maximum number of doses that the travel departure date allows, and the course should be completed upon return. For further information, see the sections on Hepatitis A vaccination , Tetanus vaccination , and Polio vaccination .
- Typhoid vaccination can be considered up to the day of departure. Full immunity can take up to 1 month to develop, although a four-fold rise in antibody against Vi antigen has been detected 7 days following primary immunization with Vi vaccine. For further information, see the section on Typhoid vaccination .
- Meningococcal vaccine may be beneficial for some people up to the day of departure, depending on whether the area being visited is considered high risk, and their length of stay. In a study cited by the manufacturer of Menveo ® , bactericidal antibodies were observed in at least 64% of people at 1-week post-vaccination. Vaccination must be given not less than 10 days before arrival in Saudi Arabia for entry to be granted. For further information, see the section on Meningococcal vaccination .
- Yellow fever vaccine may be given up to the day of departure if there is a risk of contracting yellow fever. Full immunity develops 10 days after vaccine administration and anyone receiving the vaccine at short notice should therefore be counselled about the importance of insect bite avoidance. People requiring a valid yellow fever vaccination certificate at their destination need to be vaccinated at least 10 days prior to travel. For further information, see the section on Yellow fever vaccination .
- Telephone: 0845 602 6712, Monday to Friday, 13:00 to 15:00.
Basis for recommendation
The recommendations on vaccination at short notice are largely based on the relevant chapters in the Department of Health publication Immunisation against infectious disease (The Green Book) [ PHE, 2021 ], and from data provided by the manufacturer of Menveo ® [ ABPI, 2020b ].
Tetanus and polio vaccines
- The recommendation that previously unimmunized people who require tetanus and polio vaccination should be given the maximum number of doses of vaccines that the travel departure date allows, and the course should be completed upon return is pragmatic, based on what CKS considers to be good medical practice.
The content on the NICE Clinical Knowledge Summaries site (CKS) is the copyright of Clarity Informatics Limited (trading as Agilio Software Primary Care) . By using CKS, you agree to the licence set out in the CKS End User Licence Agreement .
Hepatitis A Vaccination
Pronounced (hep-ah-TY-tiss)
Hepatitis A is a liver disease caused by the hepatitis A virus (HAV). Hepatitis A can affect anyone. Vaccines are available for long-term prevention of HAV infection in persons 1 year of age and older. Good personal hygiene and proper sanitation can also help prevent the spread of hepatitis A.
Basic information for people interested in the vaccine...
Vaccine recommendations and contraindications; composition, dosage, and administration; handling and storage...
- Hepatitis A = HepA
- Hepatitis A Virus = HAV
- Vaccines & Immunizations
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.
Available travel vaccines
The following vaccinations are available for people travelling abroad.
Cholera vaccination
Vaccination against cholera isn't routinely needed for most travellers.
But in some cases it may be recommended for aid workers and people likely to have limited access to medical services – for example, people working in refugee camps or after natural disasters.
Most cases of cholera are confined to regions of the world with poor sanitation and water hygiene, such as parts of:
- South America
The vaccine is usually given as a drink in 2 separate doses, taken 1 to 6 weeks apart.
Children aged 2 to 6 years old should have a third dose taken 1 to 6 weeks after the second dose.
You should make sure you have the final dose of this vaccine at least a week before you travel.
A single booster dose or full revaccination is usually recommended if you have previously been vaccinated against cholera and you're planning to travel to an area where the infection is common.
Diphtheria vaccination
A combined vaccination that protects against diphtheria , polio and tetanus is routinely given to all children in the UK.
You should make sure you and your children are up-to-date with your routine vaccinations before travelling.
Further booster doses are usually only recommended if you're going to visit parts of the world where diphtheria is widespread and your last vaccination dose was more than 10 years ago.
Diphtheria is more common in parts of the world where fewer people are vaccinated, such as:
- Central and Southeast Asia
- Eastern Europe
Additional doses of the vaccination are given in a single 3-in-1 Td/IPV (tetanus, diphtheria and polio) injection.
Hepatitis A vaccination
Vaccination against hepatitis A is recommended if you're travelling to countries where there are poor levels of sanitation and hygiene, and hepatitis A is common.
Ask your GP, pharmacy or travel clinic if you should have the hepatitis A vaccine if you're travelling to:
- Sub-Saharan and North Africa
- the Middle East
- South and Central America
The vaccination against hepatitis A is usually given as a single initial injection, with a second dose 6 to 12 months later. Two doses should protect you for at least 25 years.
You should preferably have the initial dose at least 2 weeks before you leave, although it can be given up to the day of your departure if needed.
Jabs that offer combined protection against hepatitis A and hepatitis B or typhoid are also available if you're likely to also be at risk of these conditions.
Hepatitis B vaccination
Vaccination against hepatitis B is recommended if you're travelling in parts of the world where hepatitis B is common, especially if you'll be doing activities that increase your risk of developing the infection.
Hepatitis B is spread through blood and body fluids. Things like having sex, injecting drugs or playing contact sports on your travels can increase your risk.
Anyone travelling for long periods or who's likely to need medical care while abroad is also at increased risk.
Hepatitis B is found worldwide, but it's more common in parts of:
- Sub-Saharan Africa
- Southern and Eastern Europe
The hepatitis B vaccination generally involves a course of 3 injections. Depending on how quickly you need protection, these may be spread over a period as long as 6 months or as short as 3 weeks.
A combined hepatitis A and hepatitis B jab is also available if you're likely to be at risk of both these conditions while travelling.
Japanese encephalitis vaccination
Vaccination against Japanese encephalitis is usually recommended if you're planning a long stay (usually at least a month) in a country where you could get the condition.
It's particularly important if:
- you're visiting during the rainy season or there's a year-round risk because of a tropical climate
- you're going to visit rural areas, such as rice fields or marshlands
- you'll be taking part in any activities that may increase your risk of becoming infected, such as cycling or camping
Japanese encephalitis is found throughout Asia and beyond. The area it's found in stretches from the western Pacific islands in the east, across to the borders of Pakistan in the west.
It's found as far north as Northeastern China and as far south as the islands of the Torres Strait and Cape York in Northeastern Australia.
Despite its name, Japanese encephalitis is now relatively rare in Japan because of mass immunisation programmes.
Find out more about risk areas on the Travel Health Pro website
Vaccination against Japanese encephalitis usually consists of 2 injections, with the second dose given 28 days after the first.
Ideally, you need to have the second dose a week before you leave.
Meningococcal meningitis vaccination
Vaccination against some types of meningococcal meningitis is usually recommended if you're travelling to areas at risk and your planned activities put you at higher risk – for example, if you're a long-term traveller who has close contact with the local population.
High-risk areas for meningococcal meningitis include:
- parts of Africa
- Saudi Arabia during the mass gatherings of Hajj or Umrah
All travellers to Saudi Arabia for the Hajj or Umrah pilgrimages are required to show proof of vaccination.
If travelling to a high-risk area, you should be vaccinated against meningococcal meningitis with a MenACWY vaccine , also known as the quadrivalent meningococcal meningitis vaccine.
This is a single injection that should be given 2 to 3 weeks before you travel. Babies under a year old need 2 injections.
You should have the MenACWY vaccine before travelling to high-risk areas, even if you had the meningitis C vaccine as a child.
Read more about the meningococcal meningitis vaccines .
Measles, mumps and rubella (MMR) vaccination
The MMR vaccine that protects against measles , mumps and rubella is routinely given to all children in the UK.
You should make sure you and your children are up-to-date with routine vaccinations, including MMR, before travelling.
If you haven't been fully vaccinated against these conditions or you're not already immune, you should ask about MMR vaccination before you travel.
The MMR vaccine is given as 2 injections. These are usually given when a child is 3 years and 4 months old.
But if vaccination has been missed previously, adults can have the doses 1 month apart, and children can have them 3 months apart if necessary.
Read more about the MMR vaccine .
Polio vaccination
A combined vaccination that protects against diphtheria, polio and tetanus is routinely given to all children in the UK.
Further booster doses are usually only recommended if you're going to visit parts of the world where polio is, or has recently been, present and your last vaccination dose was more than 10 years ago.
Currently the condition is most common in Pakistan and Afghanistan, but it's also a risk in other regions of the world.
Read more about the Td/IPV (3-in-1) vaccine .
Rabies vaccination
Vaccination against rabies is advised if you're travelling to an area where you could get rabies, particularly if:
- you're staying for a month or more
- there's unlikely to be quick access to appropriate medical care
- you plan to do activities that could put you at increased risk of exposure to rabies, such as cycling or running
Rabies can be found in many parts of the world. GOV.UK provides a detailed list of countries that have rabies in domestic animals or wildlife .
Vaccination involves a course of 3 injections before you travel, usually given over a period of 28 days.
If you're bitten, licked or scratched by an animal in a country where rabies is a problem, further doses of rabies vaccine (with or without a special anti-rabies injection given around the wound) may be required as emergency treatment.
Find out more about the rabies vaccine
GOV.UK: Rabies risks for travellers
Tetanus vaccination
A combined vaccination that protects against diphtheria, polio and tetanus is routinely given to all children in the UK.
Further booster doses are usually only recommended if:
- you're travelling to areas where access to medical services is likely to be limited and your last vaccination dose was more than 10 years ago
- you've not had two booster doses
Read more about the Td/IPV (3-in-1) vaccine .
Tick-borne encephalitis vaccination
Vaccination against tick-borne encephalitis (TBE) is usually recommended for anyone who plans to live or work in a high-risk area, or hike and camp in these areas during late spring or summer.
The ticks that cause TBE are mainly found in forested areas of central, eastern and northern Europe, although at-risk areas also include eastern Russia and some countries in east Asia, including some regions of China and Japan.
The vaccination requires a course of 3 injections for full protection. The second dose is given 1 to 3 months after the first and provides immunity for about a year.
A third dose, given 5 to 12 months after the second, provides immunity for up to 3 years.
The course can sometimes be accelerated if necessary. This involves 2 doses being given 2 weeks apart.
Booster doses of the vaccine are recommended every 3 years, if necessary.
Tuberculosis (TB) vaccination
The BCG vaccine (which stands for Bacillus Calmette-Guérin vaccine) protects against tuberculosis , which is also known as TB.
The BCG vaccine isn't given as part of the routine NHS vaccination schedule. It's given on the NHS only when a child or adult is thought to have an increased risk of coming into contact with TB.
When preparing for travel abroad, the BCG vaccine is recommended for any unvaccinated people under 16 who'll be living or working with friends, family or local people for more than 3 months in a country where TB is common or the risk of multi-drug resistant TB is high.
The BCG vaccine is given as a single injection.
Areas of the world where the risk of TB is high enough to recommend BCG vaccination for previously unvaccinated travellers include:
- parts of South and Southeast Asia
Read more about the BCG vaccine .
Typhoid vaccination
Vaccination against typhoid fever is recommended if you're travelling to parts of the world where the condition is common, particularly if you'll:
- have frequent or prolonged exposure to conditions where sanitation and food hygiene are likely to be poor
- be staying or working with local people
High-risk areas include:
- parts of South and Central America
Two main vaccines are available for typhoid fever in the UK. One is given as a single injection, and the other is given as 3 capsules to take on alternate days.
It's also possible to have a combined hepatitis A and typhoid jab.
Ideally, the typhoid vaccine should be given at least 1 month before you travel, but it can be given closer to your travel date if necessary.
Booster vaccinations are recommended every 3 years if you continue to be at risk of infection.
Read more about the typhoid vaccine .
Yellow fever vaccination
Vaccination against yellow fever is advised if you're travelling to areas where there's a risk of getting yellow fever.
Some countries require a proof of vaccination certificate before they let you enter the country.
Yellow fever occurs in some areas of tropical Africa and Central and South America. More information about yellow fever and areas where it's found is available on Travel Health Pro .
A single dose of the yellow fever vaccine is thought to provide lifelong protection. For most people, a booster dose is no longer recommended.
You must have a yellow fever vaccination at least 10 days before you travel. You will also need to complete a yellow fever vaccination checklist to make sure you can have the vaccine.
Find out more about the yellow fever vaccination checklist on the Travel Health Pro website
You should be issued with an International Certificate of Vaccination or Prophylaxis when you have the vaccine. This certificate is valid for life.
Some people cannot have the yellow fever vaccine.
Read more about the yellow fever vaccine and who can have it .
When to get further advice
Speak to your GP before having any vaccinations if:
- you're planning to get pregnant
- you're pregnant
- you're breastfeeding
- you have an immune deficiency
- you have any allergies
Page last reviewed: 16 March 2023 Next review due: 16 March 2026
Cookies on GOV.UK
We use some essential cookies to make this website work.
We’d like to set additional cookies to understand how you use GOV.UK, remember your settings and improve government services.
We also use cookies set by other sites to help us deliver content from their services.
You have accepted additional cookies. You can change your cookie settings at any time.
You have rejected additional cookies. You can change your cookie settings at any time.
- Health and social care
- Public health
- Health protection
- Immunisation
Hepatitis B: the green book, chapter 18
Hepatitis B immunisation information for public health professionals.
PDF , 278 KB , 33 pages
This file may not be suitable for users of assistive technology.
Hepatitis B is an infection of the liver caused by the hepatitis B virus (HBV). Many individuals with a new infection with hepatitis B may have a sub-clinical or a flu-like illness. Jaundice only occurs in about 10% of younger children and in 30 to 50% of adults. Acute infection may occasionally lead to fulminant hepatic necrosis, which is often fatal.
Updated to remove the single booster dose in healthy immunocompetent adults who have completed a primary course, advice for pre-exposure vaccination of recipients of solid organ transplants, more detail on assessing occupational risk and inclusion of 2 new adult vaccines. Signposting to clinical guidance on management of the pregnant woman, including use of antiviral treatment in third trimester.
Updated table 18.4, description under table 18.1 and dosage section on page 5.
Removed previous edition of chapter.
Revised table 18 (August 2017 version): amended Hepatitis B prophylaxis for reported exposure incidents.
Revised hepatitis B green book chapter.
Chapter amended to clarify Thiomersal preservative is not used in hepatitis B vaccines available in the UK.
This chapter has been updated with minor editorial amends plus 1 change in policy: for travellers who have completed the primary course of vaccination, a booster at 5 years is no longer recommended.
Updated Body text to include link to Green Book chapter update patches on the National Archives website. Added link to NHS choices.
First published.
Related content
Is this page useful.
- Yes this page is useful
- No this page is not useful
Help us improve GOV.UK
Don’t include personal or financial information like your National Insurance number or credit card details.
To help us improve GOV.UK, we’d like to know more about your visit today. We’ll send you a link to a feedback form. It will take only 2 minutes to fill in. Don’t worry we won’t send you spam or share your email address with anyone.

IMAGES
COMMENTS
Hepatitis A: the green book, chapter 17. Hepatitis A immunisation information for public health professionals. From: UK Health Security Agency. Published. 20 March 2013. Last updated. 15 January ...
Hepatitis A virus can cause liver disease. Hepatitis A virus is found in the stool (poop) and blood of infected people. People infected with hepatitis A virus can spread it to others. You can be infected with hepatitis A virus if you. Symptoms can appear quickly and may include fever, fatigue, loss of appetite, nausea, vomiting, abdominal pain ...
The disease. Hepatitis A is an infection of the liver caused by hepatitis A virus. The disease is generally mild, but severity increases with age. Asymptomatic disease is common in children. Jaundice may occur in 70-80% of those infected as adults. Fulminant hepatitis can occur but is rare.
The Green Book has the latest information on vaccines and vaccination procedures, ... travel and living abroad; Visas and immigration; ... Hepatitis A: the green book, chapter 17.
Epidemiology. Hepatitis A is among the most common vaccine-preventable infections acquired during travel. Cases of travel-related hepatitis A can occur in travelers to developed and developing countries and who have standard tourist accommodations, eating behaviors, and itineraries.
The information on available types of hepatitis A vaccine and vaccination schedules is based on expert opinion in the Department of Health publication Immunisation against infectious disease (The Green Book) Chapter 17: Hepatitis A and on the manufacturer's Summary of Product Characteristics for VAQTA ® [ABPI, 2020a].
Hepatitis A is most commonly spread from person to person by infected faeces (stools) and poor hygiene. Transmission within households is very common. The faeces from infected people are ...
This guidance is split into 2 parts: Part 1 describes the recommendations and rationale for management of cases, close contacts and outbreaks. Part 2 gives the background for the guidance including the clinical features, epidemiology and laboratory testing of hepatitis A and the evidence for the recommendations.
Disease: "The Green Book" • NaTHNaC - Hepatitis A (travelhealthpro.org.uk) recommendations for hepatitis A vaccination for travel • Public health control and management of hepatitis A guidance Criteria for inclusion Adults and children over 1 year old who: • intend to travel, where hepatitis A vaccination is currently
Hepatitis A is an illness caused by the hepatitis A virus. The virus mainly causes inflammation of the liver. Symptoms include: Generally feeling unwell. Yellowing of your skin or the whites of your eyes ( jaundice ). Sometimes, being sick (vomiting). A raised temperature (fever). However, some people who are infected do not develop any ...
Good hygiene practices are the cornerstone of the prevention of hepatitis A infection. The index case and his or her family and other close contacts should receive verbal and written guidance (see appendix 1) on the importance of hand washing after using the toilet, changing nappies and before preparing food.
MMWR 2019; 68:153-156. Recommendations of the Advisory Committee on Immunization Practices for Use of Hepatitis A Vaccine for Postexposure Prophylaxis and for Preexposure Prophylaxis for International Travel. MMWR 2018;67 (43);1216-1220. Updated Dosing Instructions for Immune Globulin (Human) GamaSTAN S/D for Hepatitis A Virus Prophylaxis.
NONE. Hepatitis B. Hepatitis B vaccine alone is not remunerated by the NHS for the purposes of travel. Supplies may be limited due to shortages.2. Engerix B® 20mcg/ml prefilled syringe £12.99. Engerix B 10mcg/0.5ml prefilled syringe (15 years and under only) £9.67. Engerix B® 20mcg/ml vial £12.34.
Children need 2 doses of hepatitis A vaccine:. First dose: 12 through 23 months of age; Second dose: at least 6 months after the first dose; Infants 6 through 11 months old traveling outside the United States when protection against hepatitis A is recommended should receive 1 dose of hepatitis A vaccine. These children should still get 2 additional doses at the recommended ages for long ...
Hepatitis A primary doses and hepatitis A, tetanus, and polio boosters can be given up to the day of departure. Previously unimmunized people who require tetanus and polio vaccination should be given the maximum number of doses that the travel departure date allows, and the course should be completed upon return.
Hepatitis A Vaccination. Pronounced (hep-ah-TY-tiss) Hepatitis A is a liver disease caused by the hepatitis A virus (HAV). Hepatitis A can affect anyone. Vaccines are available for long-term prevention of HAV infection in persons 1 year of age and older. Good personal hygiene and proper sanitation can also help prevent the spread of hepatitis A.
Since the last edition of Immunisation against infectious disease (the Green Book), the immunisation programme has seen a number of changes, to both the vaccination schedule and to peoples' attitudes to vaccination. New vaccines have been introduced against meningococcal group C and pneumococcal infections which are the cause of serious diseases.
Disease: "The Green Book" • NaTHNaC recommendations for hepatitis A vaccination for travel • Public health control and management of hepatitis A guidance Criteria for inclusion Adults and children over 1 year old who: • intend to travel, where hepatitis A vaccination is currently
Against Infectious Disease: 'The Green Book'. Criteria for inclusion Individuals over 1 year of age requiring Hepatitis A and Hepatitis B pre-exposure prophylaxis including individuals who: • intend to travel, where hepatitis A and hepatitis B vaccination is currently recommended for travel by NaTHNaC (see the Travel
Hepatitis A vaccination. Vaccination against hepatitis A is recommended if you're travelling to countries where there are poor levels of sanitation and hygiene, and hepatitis A is common. Ask your GP, pharmacy or travel clinic if you should have the hepatitis A vaccine if you're travelling to: Sub-Saharan and North Africa; Asia; the Middle East
Green Book • NaTHNaC - Hepatitis A (travelhealthpro.org.uk) recommendations for hepatitis A vaccination for travel • Public health control and management of hepatitis A guidance Criteria for inclusion Adults and children over 1 year old who: • intend to travel, where hepatitis A vaccination is currently recommended for ...
Details. Hepatitis B is an infection of the liver caused by the hepatitis B virus (HBV). Many individuals with a new infection with hepatitis B may have a sub-clinical or a flu-like illness ...
Disease: the 'Green Book ' •NaTHNaCrecommendations for h epatitis A and typhoid vaccination . for travel. Criteria for inclusion . Individuals from 16 years of age requiring hepat itis A and typhoid vaccine who intend to travel, where typhoid and hepatitis A vaccination is currently recommended for travel by NaTHNaC (see the . Travel Health