Understanding The Journey: How Sperm Make Their Way From The Testes To The Egg
- Last updated May 26, 2024
- Difficulty Intemediate
- Category Travel

The journey of sperm from the testes to the egg is a fascinating and complex process, full of hurdles and challenges. From the moment they are produced in the testes to the moment they reach their destination, sperm navigate through a series of intricate pathways and face numerous obstacles. In this article, we will delve into the remarkable journey of sperm, exploring how they overcome the odds and ultimately succeed in fertilizing an egg. So, fasten your seatbelts and get ready to embark on a journey of discovery into the world of sperm and reproduction.

What You'll Learn
Sperm production and maturation in the testes, the journey of sperm through the reproductive system, factors affecting sperm motility and viability during travel, sperm-egg interaction and fertilization process.

The testes are the male reproductive organs responsible for the production and maturation of sperm. This complex process involves several steps and requires a delicate balance of hormones and cellular interactions.
Sperm production, also known as spermatogenesis, starts within the seminiferous tubules, which are the functional units of the testes. These tubules are lined with specialized cells called Sertoli cells, which provide the necessary support and nourishment for spermatogenesis to occur.
The process of spermatogenesis begins with the division of specialized cells known as spermatogonia. These cells are located on the outer lining of the seminiferous tubules and undergo a series of cell divisions to produce primary spermatocytes.
The primary spermatocytes then enter the first meiotic division, during which their genetic material is exchanged and recombined. This process, called genetic recombination or crossing over, results in the creation of genetic diversity among the sperm cells.
After completing the first meiotic division, the cells are now called secondary spermatocytes. These cells quickly undergo the second meiotic division, resulting in the formation of haploid spermatids. Haploid cells contain half the number of chromosomes compared to the original spermatogonia.
The spermatids are still immature and non-motile at this stage. They go through a process called spermiogenesis, during which they undergo extensive remodeling and maturation. This maturation process includes the formation of the sperm head, which contains the DNA, and the development of the tail, which allows the sperm to swim.
Sertoli cells play a crucial role during spermiogenesis. They provide physical support and secrete proteins and other factors that facilitate sperm maturation. Sertoli cells also engulf any excess cytoplasm from the spermatids, which helps in removing any unnecessary material and streamlining the sperm.
Once the spermatids have completed their maturation, they are released into the lumen of the seminiferous tubules as fully-formed spermatozoa, also known as sperm cells. These sperm cells then travel through a series of ducts within the testes, called the rete testis and the epididymis, where they undergo further maturation and acquire the ability to swim.
Finally, the mature sperm cells are stored in the epididymis until ejaculation. During ejaculation, the sperm cells are propelled out of the epididymis and through the vas deferens, which connects the epididymis to the urethra. From the urethra, the sperm cells can travel through the reproductive tract and ultimately reach the egg, if fertilization occurs.
In summary, the process of sperm production and maturation in the testes is a complex and highly regulated process. It involves the successive division and differentiation of spermatogonia into sperm cells, with the help of Sertoli cells and hormonal influences. The fully-formed sperm cells then undergo further maturation within the epididymis before reaching their final destination.
The Ultimate Guide: Traveling Through London, Paris, Amsterdam, Berlin, and Prague
You may want to see also
- Sperm Production: The journey begins in the testes, where sperm are produced through a process called spermatogenesis. Specialized cells called testicular germ cells undergo multiple divisions to form immature sperm cells. These cells then go through a process of maturation and differentiation to become fully functional spermatozoa.
- Epididymis: Once sperm are developed, they are transported to the epididymis. This coiled tube, located on the back of each testicle, serves as a storage and maturation site for sperm. Here, sperm undergo further changes, such as acquiring the ability to swim and gaining the capacity to fertilize an egg. Sperm can remain in the epididymis for several weeks until they are ready to be ejaculated.
- Vas Deferens: When sexual arousal occurs, the sperm are propelled into action. The vas deferens, also known as the sperm duct, is a muscular tube that carries sperm from the epididymis towards the urethra. During ejaculation, the muscles in the walls of the vas deferens contract, helping to propel the sperm forward.
- Seminal Vesicles and Prostate Gland: As the sperm pass through the vas deferens, they combine with fluids from the seminal vesicles and the prostate gland. These fluids nourish and protect the sperm, providing them with the energy they need for their journey. The seminal vesicles contribute around 60% of the volume of semen, while the prostate gland adds additional enzymes and substances that aid sperm motility.
- Urethra: The urethra serves as a common pathway for both urine and semen. When it is time for ejaculation, the muscles at the base of the bladder close off the passage to the bladder, preventing the mixture of urine and semen. This ensures that only semen is ejaculated through the urethra.
- Ejaculation and Ejaculatory Ducts: During orgasmic contractions, the sperm are forcefully expelled from the urethra. The ejaculatory ducts, which are located within the prostate gland, help to propel the semen forward and assist in the expulsion of sperm during ejaculation.
- Vagina and Cervix: Once ejaculated, the sperm need to find their way through the female reproductive tract. During sexual intercourse, semen is deposited into the vagina. The sperm then begin their challenging journey through the cervix, which is the lower part of the uterus that opens into the vagina. The cervical mucus provides a favorable environment for the sperm to swim through, helping them bypass the acidic vaginal environment.
- Uterus and Fallopian Tubes: From the cervix, the sperm continue their journey into the uterus and ultimately reach the fallopian tubes. The uterine contractions and the beating of cilia lining the fallopian tubes aid in moving the sperm along. If an egg is present in the fallopian tube, the sperm can swim towards it, attempting to fertilize it.
- Fertilization: If a sperm successfully penetrates and fertilizes the egg, conception occurs. The fertilized egg then implants itself into the lining of the uterus, leading to pregnancy.
The Ultimate Guide to Maintaining Birth Control While Traveling
Sperm, the reproductive cells in males, go through an incredible journey to reach the egg for fertilization. This journey is not an easy one, and many factors can affect the motility and viability of sperm during travel. Understanding these factors can help individuals and couples better understand fertility issues and take appropriate measures to increase their chances of successful conception. In this article, we will explore the various factors that can influence sperm motility and viability during their travel from the testes to the egg.
- Hormonal balance: Hormones play a crucial role in regulating sperm production and motility. Imbalances in hormone levels, such as low testosterone or high estrogen, can negatively affect sperm quality and movement. Maintaining a healthy hormonal balance is essential for optimal sperm function.
- Temperature: Sperm cells are highly sensitive to temperature changes. The testes are located outside the body because sperm production requires a slightly lower temperature than the rest of the body. If the testes become too warm, it can impair sperm production and motility. Heat from hot baths, saunas, tight underwear, or prolonged sitting can increase testicular temperature and negatively impact sperm function.
- Lifestyle factors: Several lifestyle factors can influence sperm motility and viability during travel. Smoking, excessive alcohol consumption, drug use, and exposure to certain environmental toxins can all affect sperm quality. It is crucial to maintain a healthy lifestyle and avoid these substances to support optimal sperm function.
- Age: Age can also impact sperm quality and motility. As men age, the quality of their sperm tends to decline. Sperm motility may decrease, and the chances of DNA damage in sperm cells may increase, leading to reduced fertility. It is important for couples to be aware of the potential impact of age on male fertility when planning for conception.
- Genetic factors: Genetic abnormalities can affect sperm production and function. Conditions such as Klinefelter syndrome, Y-chromosome deletions, and chromosomal translocations can result in lower sperm motility and viability. Genetic testing may be recommended for couples experiencing fertility issues to identify any underlying genetic factors that may be impacting sperm health.
- Infections and inflammations: Infections and inflammations of the reproductive system, such as prostatitis or epididymitis, can impair sperm motility. These conditions can cause blockages or scarring in the reproductive tract, hindering the movement of sperm. Treating any infections or inflammations promptly can help improve sperm quality and increase the chances of successful fertilization.
- Nutritional and dietary factors: Proper nutrition plays a vital role in maintaining sperm motility and viability. Nutritional deficiencies in vitamins, minerals, and antioxidants can negatively impact sperm health. A balanced diet that includes a variety of fruits, vegetables, lean proteins, and whole grains is essential for supporting optimal sperm function.
- Stress: Chronic stress can have a detrimental effect on overall reproductive health, including sperm motility. High levels of stress hormones can disrupt hormone balance and impair sperm production. Finding healthy ways to manage stress, such as exercise, meditation, or counseling, can help improve sperm quality and increase fertility potential.
In conclusion, several factors can affect sperm motility and viability during their journey from the testes to the egg. Hormonal balance, temperature, lifestyle factors, age, genetic factors, infections and inflammations, nutritional and dietary factors, and stress all play a role in sperm health. By understanding and addressing these factors, individuals and couples can optimize their chances of successful fertilization and achieve their desired pregnancy.
The Ultimate Guide: How to Travel from Clark Airport to Subic Bay
Fertilization is a complex process that involves the interaction between sperm and egg. Understanding how sperm travel from the testes to the egg can provide insights into the intricate mechanisms of fertilization. In this blog post, we will explore the journey of sperm and the steps involved in the fertilization process.
Sperm production in the testes:
Sperm production, also known as spermatogenesis, occurs in the testes. It starts with the division of spermatogonia, the male germ cells, which eventually develop into mature sperm cells. This process takes approximately 74 days from the initial division to the release of mature sperm into the seminiferous tubules.
Maturation and storage:
After production, mature sperm undergo a maturation process called spermiogenesis in the epididymis. The epididymis is a coiled tube located on the back of each testicle. The maturation process allows the sperm to develop their motility and acquire the ability to fertilize an egg. The epididymis also serves as a storage site for the sperm until they are ejaculated.
Ejaculation:
During sexual intercourse, the semen, which contains sperm, is ejaculated from the penis into the vagina. The ejaculatory fluid, including sperm, travels through the reproductive system towards the cervix, which is the entrance to the uterus.
Cervical mucus:
The cervical mucus plays a crucial role in the journey of sperm towards the egg. Around the time of ovulation, the cervical mucus becomes thinner and more slippery, providing a favorable environment for sperm to swim through. This mucus also acts as a filter, allowing only healthy sperm to pass through and reach the uterus.
Once inside the uterus, the sperm navigate through the uterine cavity towards the fallopian tubes. The uterine contractions help propel the sperm forward, increasing their chances of reaching the egg.
Journey through the fallopian tubes:
The fallopian tubes are the site where fertilization usually occurs. Sperm can survive in the female reproductive tract for several days, waiting for the egg to be released. Once the egg is released from the ovary and enters the fallopian tube, the race for fertilization begins.
Sperm-egg interaction:
When sperm encounter an egg, they undergo a process called capacitation, which involves changes in their membrane that enable them to penetrate the egg. The egg is enveloped by a protective layer called the zona pellucida. Sperm use enzymes to break down this layer and reach the egg's surface.
Fertilization:
Once a sperm reaches the egg's surface, it binds to specific receptors on the egg's membrane. This binding triggers the release of enzymes from the sperm, allowing it to penetrate the egg. Upon penetration, the egg undergoes a series of biochemical changes that prevent other sperm from fertilizing it. The genetic material from the sperm combines with the genetic material of the egg, forming a single-celled embryo.
Implantation:
After fertilization, the embryo travels down the fallopian tube towards the uterus. It undergoes multiple cell divisions and becomes a blastocyst. The blastocyst then implants itself into the uterine lining, marking the beginning of pregnancy.
In conclusion, the journey of sperm from the testes to the egg is a remarkable process that involves a series of steps, including sperm production, maturation, ejaculation, travel through the reproductive system, sperm-egg interaction, fertilization, and implantation. Understanding these intricate mechanisms can provide valuable insights into human reproduction and fertility.
Exploring Florida: What You Need to Know About Traveling with a Driver's License
Frequently asked questions.
Sperm travel from the testes to the egg through a series of ducts and organs collectively known as the male reproductive system. They start in the epididymis, where they mature and gain motility. Then, they travel through the vas deferens, which connects the epididymis to the urethra. During ejaculation, the sperm are propelled through the urethra and expelled from the penis, allowing them to potentially reach the egg during sexual intercourse.
Yes, there is a specific pathway that sperm follow to reach the egg. After they leave the testes, they pass through the epididymis, then move into the vas deferens. From there, they enter the ejaculatory ducts and travel through the urethra before being ejaculated. Once outside the male body, sperm can enter the female reproductive system through the vagina, cervix, uterus, and then reach the fallopian tubes, where fertilization can occur if an egg is present.
Yes, there are several obstacles and challenges that sperm face during their journey to the egg. First, they must navigate through the female reproductive system, which includes encountering the acidic environment of the vagina and the cervix's mucus barrier. Additionally, only a small percentage of the millions of sperm ejaculated during sexual intercourse will reach the fallopian tubes, where the egg is located. Finally, even if sperm reach the egg, they still need to penetrate the egg's protective outer layer before fertilization can occur.

- Kryms Kaya Author Traveller

- Elani Piper Author Editor Reviewer
It is awesome. Thank you for your feedback!
We are sorry. Plesae let us know what went wrong?
We will update our content. Thank you for your feedback!
Leave a comment
Travel photos, related posts.

The Must-Do Travels in Florence, Italy
- May 31, 2024

Tips for Traveling from Basel to Italy

Rapid PCR Test for Travel to Dubai: Is it Accepted?
- May 26, 2024

Unlocking Faster Security: Adding Known Traveler Number to Southwest

Exploring Payment Options at Kroger: Can You Use Travelers Checks?
- May 11, 2024

Can a Lateral Flow Test Be Used as an Antigen Test for Travel?
Request A Callback
Sperm, Meet Egg: The Process of Fertilisation

Turbo-charged sperm speed through a dimly lit canal, coursing feverishly through vast landscapes and titanic cavities. A lone egg desperately awaits her suitor, pledging her life if left unavowed. Then, the swiftest sperm rises from the depths of darkness, breaking through a fortress wall and presenting himself to his prospective partner. A union is formed. The end. This is the stuff of movies and drama. But it’s also the stuff of fertilisation. When sperm are released into the vaginal canal, an intricate performance ensues. With the ovaries serving as arc lights, the stage womb is set.
Understanding Fertilisation
Every month, one amongst a woman’s two ovaries releases a mature egg into the fallopian tube. This egg remains available for fertilisation for about 24 hours, and if met by a sperm, can lead to conception . Sperm can survive for up to 5 days within the uterine cavity, so it is possible that you will get pregnant if you’ve had intercourse in the 5 days leading up to ovulation .

It’s wise to maintain an ovulation calendar to preempt your fertile periods. Conception involves millions of sperm vying to outpace each other in order to reach the available egg. Only one sperm wins, achieving fertilisation after a long and arduous journey. The resultant embryo, still in the fallopian tube, then descends downward until it reaches the uterine cavity, where it implants itself.
The Steps of Fertilisation
Fertilisation is a complex process, with the female body working in mysterious ways to align the egg and the sperm. Detailed below, are the steps that go into it.

- Step 1. Ovulation
Ovulation refers to the emergence of a single mature egg from one of the ovarian follicles. The egg only has a 24-hour window to be fertilised . During this time, vaginal discharge becomes wet and slippery, a telling sign that fertility is at its peak . Other ovulation symptoms include bloating and abdominal pain . If unprotected intercourse is had during this time or in the 5 days prior, there is a good chance that it will result in a pregnancy.

- Step 2. Ejaculation
Semen is a versatile substance, providing both nourishment and protection for sperm . As soon as ejaculation happens, the semen left behind forms a wall across the vagina to save sperm from moving downward. This wall lasts only about half an hour before it starts to trickle out of the vagina. The sperm cells that do make it through after ejaculation begin a long journey up the cervical canal, each holding out hope to make it to the egg first.

Step 3. Journey Through the Cervical Canal
The cervical canal is a warm and conducive environment that allows sperm to thrive and push on in their journey. Generously lined with cervical mucus, the canal is tailored for sperm transportation , especially during the fertile window when mucus is at its maximum. Interestingly, the days before ovulation will herald molecular changes that you may not even be aware of. Microscopic threads of molecules line up along the cervical canal, to allow sperm to latch on as they pace through.
- Step 4. Biochemical Alterations + Accelerated Movement
Sperm that enter the cervical canal must change their structural form in order to survive. Their new environment triggers biochemical changes that allow them to travel at breakneck speeds through the uterus and fallopian tubes.

- Step 5. Branching Off
Once sperm reach the uterus, they have a critical decision to make. Do they go right or left? There’s a fallopian tube on either side and it’s anybody’s guess which tube has released an egg this time. Sperm tend to branch out at this point, some gravitating to the left and others to the right.
Must read - 7 Pregnancy Questions to Ask your Doctor
Sperm that pick the correct tube has a significant chance of reaching the egg in time. Now, with about half the competitors as before, sperm must power to the finish line in time.
- Step 6. Fertilisation
Only the most resilient sperm reach the egg and even the ones that do must cross another hurdle before they can reach their final destination. Every egg is covered with a tough outer layer and hundreds of sperm engage in a race to see who can penetrate first. When one sperm finally does manage to achieve fertilisation, the egg immediately experiences chemical changes that block other sperm from entering. Then, the chromosomes in the egg and sperm combine, giving rise to a zygote.
Must read - How to Handle the 1st Trimester of Pregnancy?

- Step 7. Implantation
The zygote divides repeatedly over the next few hours and days. It gradually rolls down through the fallopian tube, reaching the uterine cavity about a week later as a 100-cell ball. The zygote now implants itself into the uterine lining, going on to develop into a baby.
You can also join our Cloudnine Community to discuss and get more information about - Aspiring Parents , Fertility Treatments .
Want to consult a Doctor? Book Video Consultation Online with the best gynecologist in India.
Watch Video on PCOD affect Fertility
As frenzied as the fertilisation process is, it marks the beginning of a chapter that will unfold page by page over the next nine months. By being in the know of how fertilisation works, you can stay better prepared in planning your family. So that when the arc lights in your womb are turned on, you’re still the director of the show.
Explore Cloudnine Momeaze for all your shopping needs with wide range of products.
Our customers had a few questions regarding this blog, check them out now!
How to track your ovulation date?
Slight spotting while conceiving with PCOS
Have Similar Questions?
Get answers from our experts, browse through hundreds of q&a, attend live sessions, and much more.
Want to consult the best gynecologists in India? Please find the links below.
- Best Gynecologists in Bangalore
- Best Gynecologists in Chennai
- Best Gynecologists in Mumbai
- Best Gynecologists in Pune
- Best Gynecologists in Chandigarh
- Best Gynecologists in Gurgaon
- Best Gynecologists in Noida
Want to consult the best Maternity Packages in India? Please find the links below.
- Best Maternity Packages in Bangalore
- Best Maternity Packages in Chennai
- Best Maternity Packages in Mumbai
- Best Maternity Packages in Pune
- Best Maternity Packages in Chandigarh
- Best Maternity packages in Gurgaon
- Best Maternity Packages in Noida
- [email protected]
- +91 99728 99728
Registered Office
Corporate office.
- Bengaluru Chandigarh Chennai Gurugram Hyderabad Lucknow Ludhiana Mumbai Noida Panchkula Pune Mohali New Delhi Faridabad
- Specialities
- Parent Corner
- Media & News
- Home Services
- Virtual Tour
- Covid-19 Pregnancy Helpline
- Free Pregnancy Counseling
- Terms & Conditions
- Privacy Policy
- Company Policies
Best Gynecologists in India
- Best Gynecologists in Bengaluru
- Best Gynecologists in Bellandur
- Best Gynecologists in HRBR Layout
- Best Gynecologists in Jayanagar
- Best Gynecologists in Malleswaram
- Best Gynecologists in Old Airport Road
- Best Gynecologists in Whitefield
- Best Gynecologists in Sahakarnagar
- Best Gynecologists in Electronic City
- Best Gynecologists in Kanakapura Road
- Best Gynecologists in Sarjapur Road
- Best Gynecologists in Varthur Road
- Best Gynecologists in Thanisandra
- Best Gynecologists in Industrial Area Phase II
- Best Gynecologists in T Nagar
- Best Gynecologists in OMR
- Best Gynecologists in Sector 14
- Best Gynecologists in Sector 47
- Best Gynecologists in Golf Course Road
- Best Gynecologists in Hyderabad
- Best Gynecologists in Hitech City
- Best Gynecologists in Banjara Hills
- Best Gynecologists in Gachibowli
- Best Gynecologists in Ludhiana
- Best Gynecologists in Malad
- Best Gynecologists in Vashi
- Best Gynecologists in Nerul
- Best Gynecologists in Sector 51
- Best Gynecologists in Kalyani Nagar
- Best Gynecologists in SB Road
- Best Gynecologists in Pimple Saudagar
- Best Gynecologists in Panchkula
- Best Gynecologists in Sector 5 Swastik Vihar
- Best Gynecologists in New Delhi
- Best Gynecologists in Patparganj
- Best Gynecologists in Punjabi Bagh
- Best Gynecologists in Kailash Colony
- Best Gynecologists in Faridabad
- Best Gynecologists in New Industrial Town
Best Pediatricians in India
- Best Pediatricians in Bengaluru
- Best Pediatricians in Bellandur
- Best Pediatricians in HRBR Layout
- Best Pediatricians in Jayanagar
- Best Pediatricians in Malleswaram
- Best Pediatricians in Old Airport Road
- Best Pediatricians in Whitefield
- Best Pediatricians in Sahakarnagar
- Best Pediatricians in Electronic City
- Best Pediatricians in Kanakapura Road
- Best Pediatricians in Sarjapur Road
- Best Pediatricians in Varthur Road
- Best Pediatricians in Thanisandra
- Best Pediatricians in Chandigarh
- Best Pediatricians in Industrial Area Phase II
- Best Pediatricians in Chennai
- Best Pediatricians in T Nagar
- Best Pediatricians in OMR
- Best Pediatricians in Faridabad
- Best Pediatricians in New Industrial Township
- Best Pediatricians in Gurugram
- Best Pediatricians in Sector 14
- Best Pediatricians in Sector 47
- Best Pediatricians in Golf Course Road
- Best Pediatricians in Hyderabad
- Best Pediatricians in Hitech City
- Best Pediatricians in Banjara Hills
- Best Pediatricians in Gachibowli
- Best Pediatricians in Ludhiana
- Best Pediatricians in Mumbai
- Best Pediatricians in Malad
- Best Pediatricians in Vashi
- Best Pediatricians in Nerul
- Best Pediatricians in Noida
- Best Pediatricians in Pune
- Best Pediatricians in Kalyani Nagar
- Best Pediatricians in SB Road
- Best Pediatricians in Pimple Saudagar
- Best Pediatricians in Panchkula
- Best Pediatricians in Sector 5 Swastik Vihar
- Best Pediatricians in New Delhi
- Best Pediatricians in Patparganj
- Best Pediatricians in Punjabi Bagh
- Best Pediatricians in Kailash Colony
Best Physiotherapists in India
- Best Physiotherapists in Bengaluru
- Best Physiotherapists in Bellandur
- Best Physiotherapists in HRBR Layout
- Best Physiotherapists in Jayanagar
- Best Physiotherapists in Malleswaram
- Best Physiotherapists in Old Airport Road
- Best Physiotherapists in Whitefield
- Best Physiotherapists in Kanakapura Road
- Best Physiotherapists in Electronic City
- Best Physiotherapists in Sahakarnagar
- Best Physiotherapists in Chandigarh
- Best Physiotherapists in Industrial Area Phase II
- Best Physiotherapists in Chennai
- Best Physiotherapists in T Nagar
- Best Physiotherapists in Old Mahabalipuram Road (OMR)
- Best Physiotherapists in Faridabad
- Best Physiotherapists in New Industrial Township
- Best Physiotherapists in Gurugram
- Best Physiotherapists in Sector 14
- Best Physiotherapists in Sector 47
- Best Physiotherapists in Golf Course Road
- Best Physiotherapists in Hyderabad
- Best Physiotherapists in Hitech City
- Best Physiotherapists in Banjara Hills
- Best Physiotherapists in Gachibowli
- Best Physiotherapists in Ludhiana
- Best Physiotherapists in Mumbai
- Best Physiotherapists in Malad
- Best Physiotherapists in Vashi
- Best Physiotherapists in Noida
- Best Physiotherapists in Sector 51
- Best Physiotherapists in New Delhi
- Best Physiotherapists in Punjabi Bagh
- Best Physiotherapists in Patparganj
- Best Physiotherapists in Kailash Colony
- Best Physiotherapists in Pune
- Best Physiotherapists in Kalyani Nagar
- Best Physiotherapists in SB Road
- Best Physiotherapists in Pimple Saudagar
- Best Physiotherapists in Panchkula
- Best Physiotherapists in Sector 5 Swastik Vihar
Best Radiologist in India
- Best Radiologist in Bengaluru
- Best Radiologist in Bellandur
- Best Radiologist in HRBR Layout
- Best Radiologist in Jayanagar
- Best Radiologist in Malleswaram
- Best Radiologist in Old Airport Road
- Best Radiologist in Whitefield
- Best Radiologist in Kanakapura Road
- Best Radiologist in Electronic City
- Best Radiologist in Sahakarnagar
- Best Radiologist in Chandigarh
- Best Radiologist in Industrial Area Phase II
- Best Radiologist in Chennai
- Best Radiologist in T Nagar
- Best Radiologist in OMR
- Best Radiologist in Faridabad
- Best Radiologist in Gurugram
- Best Radiologist in Sector 14
- Best Radiologist in Sector 47
- Best Radiologist in Golf Course Road
- Best Radiologist in Hyderabad
- Best Radiologist in Banjara Hills
- Best Radiologist in Hitech City
- Best Radiologist in Gachibowli
- Best Radiologist in Ludhiana
- Best Radiologist in Mumbai
- Best Radiologist in Malad
- Best Radiologist in Vashi
- Best Radiologist in New Delhi
- Best Radiologist in Punjabi Bagh
- Best Radiologist in Kailash Colony
- Best Radiologist in Noida
- Best Radiologist in Sector 51
- Best Radiologist in Pune
- Best Radiologist in Kalyani Nagar
- Best Radiologist in SB Road
- Best Radiologist in Pimple Saudagar
- Best Radiologist in Panchkula
- Best Radiologist in Sector 5 Swastik Vihar
Best Dietitian in India
- Best Dietitian in Bengaluru
- Best Dietitian in Bellandur
- Best Dietitian in HRBR Layout
- Best Dietitian in Jayanagar
- Best Dietitian in Malleswaram
- Best Dietitian in Old Airport Road
- Best Dietitian in Whitefield
- Best Dietitian in Kanakapura Road
- Best Dietitian in Electronic City
- Best Dietitian in Sahakarnagar
- Best Dietitian in Chandigarh
- Best Dietitian in Industrial Area Phase II
- Best Dietitian in Chennai
- Best Dietitian in T Nagar
- Best Dietitian in OMR
- Best Dietitian in Faridabad
- Best Dietitian in New Industrial Township
- Best Dietitian in Gurugram
- Best Dietitian in Sector 14
- Best Dietitian in Sector 47
- Best Dietitian in Golf Course Road
- Best Dietitian in Hyderabad
- Best Dietitian in Banjara Hills
- Best Dietitian in Hitech City
- Best Dietitian in Gachibowli
- Best Dietitian in Ludhiana
- Best Dietitian in Mumbai
- Best Dietitian in Malad
- Best Dietitian in Vashi
- Best Dietitian in New Delhi
- Best Dietitian in Punjabi Bagh
- Best Dietitian in Patparganj
- Best Dietitian in Kailash Colony
- Best Dietitian in Noida
- Best Dietitian in Sector 51
- Best Dietitian in Pune
- Best Dietitian in Kalyani Nagar
- Best Dietitian in SB Road
- Best Dietitian in Pimple Saudagar
- Best Dietitian in Panchkula
- Best Dietitian in Sector 5 Swastik Vihar
Best Maternity Packages in India
- Best Maternity Packages in Bengaluru
- Best Maternity Packages in Faridabad
- Best Maternity Packages in Gurugram
- Best Maternity Packages in Hyderabad
- Best Maternity Packages in Ludhiana
- Best Maternity Packages in New Delhi
- Best Maternity Packages in Panchkula
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.

StatPearls [Internet].
Embryology, fertilization.
Rebecca Oliver ; Hajira Basit .
Affiliations
Last Update: April 17, 2023 .
- Introduction
Fertilization is a complex multi-step process that is complete in 24 hours. The sperm from a male meets an ovum from a female and forms a zygote; this is the point in which pregnancy begins and leads to a 280-day journey for a female. There are two ways to track this process, and they differ by the day counting begins. There are the post-ovulation age and the gestational age, calculated by adding two weeks to the last menstrual period. There are many steps that both the egg and sperm must go through for this process to be successful. Furthermore, the fertilized egg itself goes through drastic changes. This article will detail the process in the following sections.
- Development
In the first weeks after fertilization, the zygote makes many changes and develops rapidly. The first eight weeks of development is known as the organogenic period and is the embryonic stage of development. This period is a crucial phase of development for the embryo’s organs. During the first three weeks, teratogens have an all or nothing effect on the embryo. During the third through eighth-week growth and function are affected. Weeks nine to thirty-seven are known as the fetal period. This period is important for extensive growth in size and continuous differentiation of organ systems. The respiratory system completes development just prior to birth. An important part of embryology that does not complete during the embryonic or fetal phase is gametogenesis. In both males and females, these processes begin during the fetal period and continue into puberty. This process is a mitotic and meiotic process that results in the production of an ovum and sperm. [1] Before turning into gametogonia, the embryonic development of gametes is the same in males and females, and at week ten they can be differentiated. Primordial germ cells migrate from the dorsal endoderm of the yolk sac to the hindgut of the gonadal ridge where they go through mitotic divisions and become the gametogonia.
Once fertilization takes place, there are quick changes at the cellular level of the zygote. The zygote is a single cell, and it undergoes mitosis to create many cells. Once the zygote has reached the thirty-two cell stage, it becomes morula. Day four begins blastulation and cavities begin to form by first forming a hollow ball. Some studies suggest that the timing of this process may affect implantation. [2] There are now two different cell types, an inner and outer. The inner cells are called the inner cell mass, and the exterior is known as the trophoblast, which later helps form the placenta, and the inner cell mass becomes the embryo. The inner cell mass will further differentiate into the epiblast and the hypoblast. The hypoblast will become the primitive yolk sac, and the epiblast will become the amniotic sac. During this phase, the entity is a blastula, and the zona pellucida is now gone, allowing for growth and differentiation. During week three, tubes will form, and this is known as the gastrulation phase. Movements during gastrulation are dependent on differential cell adhesion, chemotaxis, chemokinesis, and planar polarity. [3] During this time, there are three layers of cells that will make up different organ systems. These are known as the endoderm, mesoderm, and ectoderm. The ectoderm forms the epidermis, nails, hair, peripheral nervous system, brain, and spinal cord. The mesoderm forms the muscle, bone, connective tissue, notochord, kidney, gonads, and circulatory system. The endoderm forms the epithelial lining of the digestive tract, stomach, colon, liver, bladder, and pancreas. At sixteen weeks the primitive streak forms. The primitive streak establishes the midline of the body. The next stage in development is neurulation. At this time the notochord induces the ectoderm to form the neural plate which eventually forms the neural tube. The neural tube will become the brain and spinal cord. The mesoderm divides into the axial, paraxial, intermediate, and lateral plate mesoderm, which give rise to different body parts — the paraxial mesoderm forms somites, which differentiate into cartilage, muscle, bone, and dermis. The intermediate mesoderm becomes the urogenital system, and the lateral plate mesoderm becomes the heart and vessels. The endoderm becomes the gastrointestinal tract, and the ectoderm will meet the endoderm forming the mouth and the anus. An important gene regulatory mechanism is Dkk1; the deletion of Dkk1 is known to cause an imperforate anus with a rectourinary fistula. [4]
- Biochemical
There are significant changes that the egg and sperm must undergo for the fertilization process to occur; this starts as soon as sperm gets deposited in the vagina. Sperm undergoes capacitation to have increased motility and metabolism to help it make the journey to the fallopian tube. Capacitation occurs due to the acidic environment of the vagina. It activates ATP enzymes in the cytosol of the sperm. The process of capacitation is important because it makes changes in the plasma membrane by altering the lipid and glycoprotein composition, which is one of two changes the sperm undergoes during this process. [5] The second change helps penetrate the matrix. The egg’s extracellular matrix is difficult to penetrate. The acrosome on the sperm contains important lysosomal enzymes. These enzymes are considered to be released by exocytosis and required for the penetration of the egg. [6] Hyaluronidase from the acrosome digests the cells embedded in hyaluronic acid surrounding the oocyte. This process exposes acrosin, which is in the inner membrane of the sperm. Acrosin is necessary to digest the zona pellucida. Once the acrosome reaction takes place, no other sperm may penetrate the zona pellucida; this is imperative so that the appropriate number of chromosomes is available and that there is not a trisomy zygote. The fusion of the acrosome of the sperm to the zona pellucida induces a rise in calcium. This rise in calcium stimulates secretory vesicles known as cortical granules to expel contents, which modifies the extracellular matrix. The cortical granules release enzymes that make it impenetrable to sperm entry by digesting sperm receptor glycoproteins ZP2 and ZP3 so that they can no longer bind spermatozoon. [7]
- Molecular Level
We have discussed in previous sections changes occurring at the biochemical and cellular levels. Here we will discuss the molecular changes taking place during the fertilization process. Before the actual fertilization process occurs, the sperm travels to the fallopian tubes where it will penetrate the egg. The spermatozoa in ejaculate vary, and the make-up of each spermatozoon contributes to its ability to get to the egg and fertilize it. [8] Spermatozoa have differences in DNA fragmentation status, motility, morphology, and sensitivity to signaling molecules. Diving even deeper into this topic, research has shown that spermatozoa with stable chromatin reach the fertilization site with greater ease and can bind well. [9] The sperm and egg are two haploid nuclei that join to form a diploid nucleus. Once the sperm and egg have joined their membranes and fused, the zygote undergoes mitotic divisions. As mentioned before, the changes the egg undergoes once one acrosome has penetrated its membrane to keep other sperm out to prevent triploidy is crucial from a molecular standpoint. The sperm has a vital role in providing a centriole, which helps organize and assemble the mitotic spindle. [10]
The function of fertilization is to create a diploid(2N) zygote. Fertilization by a sperm activates the ovum, which takes place in the distal third of the fallopian tube. Once the sperm has entered the ovum, immediate changes take place not to allow further penetration of the ovum. These exact changes are in the biochemical section of this article. Once the sperm has penetrated the ovum, the sex of the embryo will be determined based on the presence or absence of a Y chromosome, which contains the SRY gene known as the testis-determining factor and also known as sex-determining region Y.
A follicle must mature from an oocyte to a Graafian follicle to be ready for fertilization. During ovulation, the follicle will be released and swept into the fallopian tube. If the sperm were deposited in the last ten hours, the spermatozoa would have made their way to this location, and fertilization can occur. Fertilization must take place within twenty-four hours of ovulation; otherwise, the ovum will not be available to be fertilized and will end in menses. The follicle has two layers that the sperm must penetrate, both the corona radiate and zona pellucida. The first step is the penetration of the corona radiata. The acrosome of the spermatozoa then releases enzymes, which aid in the digestion of this layer and allows for access to the secondary oocyte.
Infertility is a common problem encountered in the medical community. If a female patient cannot become pregnant after one year of unprotected intercourse performed regularly, an evaluation is in order. An important consideration is the patient’s body mass index. If a patient is overweight, weight loss may improve her chances of becoming pregnant. [11] It is essential to consider labs in patients who are unable to become pregnant because abnormalities of the thyroid gland or androgen excess may indicate an endocrinopathy. A commonly encountered endocrinopathy that causes issues with fertility is polycystic ovarian syndrome. In PCOS, testosterone increases, which interferes with egg maturation. [12] A physical exam is also useful in evaluating infertility. Masses or tenderness in the adnexae or the pouch of Douglas is consistent with endometriosis or chronic pelvic inflammatory disease. Furthermore, structural abnormalities may suggest an infection, leiomyoma, malignancy, or Mullerian anomaly. In women who are not suspected to be ovulating, it is crucial to do a thorough hormone analysis to investigate if ovulation is occurring. A mid-luteal progesterone level should be tested one week before expected menstruation. To show proof of ovulation, a progesterone level greater than 3 ng/mL is the expectation. Another useful hormone to evaluate female fertility is the anti-Mullerian hormone. [13] This hormone is in the TGF-beta family and expresses itself by small early antral follicles. These levels reflect the size of the primordial follicle pool and are a good indicator of ovarian function.
- Clinical Significance
There are numerous clinical scenarios in which fertilization comes into play. Fertilization and the development that follows is a delicate and complex process that can result in defects. Hormones are important for preparing the female body to implant a fertilized egg and to grow and nourish it. FSH, which causes the release of estrogen from the ovaries, aids the cervical mucus in being more hospitable to sperm movement through the vaginal canal and cervix. An LH surge is necessary for the release of an egg from the ovary out of the follicle and into the fallopian tube where it can undergo fertilization. Progesterone produced by the corpus luteum and later by the placenta creates and maintains a thickened endometrium to allow a nourishing environment for implantation and growth. Pregnancy tests detect fertilization by measuring beta-human chorionic gonadotrophin released by the growing placenta after implantation. Another important clinical significance is neural tube defects, which are birth defects of the central nervous system that occurs when the neural tube fails to close completely. [14] Folic acid supplementation during pregnancy has been shown to help prevent neural tube defects, thus is a commonly recommended prenatal supplement. Another important clinical consideration is a group of genes known as Hox genes, which play a significant role in body plan and development along the cephalic to the caudal axis. If there are mutations in these genes, then body parts may develop in the incorrect location. [15]
- Review Questions
- Access free multiple choice questions on this topic.
- Comment on this article.
Disclosure: Rebecca Oliver declares no relevant financial relationships with ineligible companies.
Disclosure: Hajira Basit declares no relevant financial relationships with ineligible companies.
This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
- Cite this Page Oliver R, Basit H. Embryology, Fertilization. [Updated 2023 Apr 17]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
In this Page
Bulk download.
- Bulk download StatPearls data from FTP
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed

Similar articles in PubMed
- Volume changes during the preimplantation stages of mouse egg development. [Yonsei Med J. 1973] Volume changes during the preimplantation stages of mouse egg development. Chung SO. Yonsei Med J. 1973; 14:63-90.
- A study of hetero-specific sperm-egg interactions in the rat, mouse, and deer mouse using in vitro fertilization and sperm injection. [J Exp Zool. 1980] A study of hetero-specific sperm-egg interactions in the rat, mouse, and deer mouse using in vitro fertilization and sperm injection. Thadani VM. J Exp Zool. 1980 Jun; 212(3):435-53.
- Intracellular sperm/egg interactions in Drosophila: a three-dimensional structural analysis of a paternal product in the developing egg. [Mech Dev. 1991] Intracellular sperm/egg interactions in Drosophila: a three-dimensional structural analysis of a paternal product in the developing egg. Karr TL. Mech Dev. 1991 Jun; 34(2-3):101-11.
- Review The mode of action of IUDs. [Contraception. 1987] Review The mode of action of IUDs. Ortiz ME, Croxatto HB. Contraception. 1987 Jul; 36(1):37-53.
- Review Intracellular calcium signaling in the fertilized eggs of Annelida. [Biochem Biophys Res Commun. 2014] Review Intracellular calcium signaling in the fertilized eggs of Annelida. Nakano T, Deguchi R, Kyozuka K. Biochem Biophys Res Commun. 2014 Aug 1; 450(3):1188-94. Epub 2014 Jun 19.
Recent Activity
- Embryology, Fertilization - StatPearls Embryology, Fertilization - StatPearls
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers
What Is Fertilization?
Updates to text, information, guidelines, formatting and sources, and new medical review.
What is fertilization?

Where does fertilization occur?
Read this next, when does fertilization occur , how long does it take for a sperm to fertilize an egg, can you feel when an egg gets fertilized, how long can sperm live inside you to get pregnant.
As you've no doubt learned by now, the road to fertilization is a bumpy one, with plenty of twists and turns. It takes the right conditions and perfect timing for the egg and sperm to meet up and produce that baby you're hoping for. But once they do, you've embarked on your own amazing journey: the journey of motherhood.
What Our Community Is Talking About
All about sperm
Sperm Travel Path: Understanding the Route to Fertilization

Short answer sperm travel path: Sperm travel from the testes through the epididymis, vas deferens, and ejaculatory duct before being released through the urethra during ejaculation. The journey takes approximately 64-72 days to complete.
Exploring the Fascinating Journey: Sperm Travel Path
Understanding how sperm travel path affects conception: step by step guide, all you need to know about sperm travel path: frequently asked questions, the intriguing process of fertilization: an in-depth look at sperm travel path, breaking down the miracle of life: inside the male reproductive tract and its role in sperm travel path, discovering the unseen world of conception: the hidden secrets of sperm travel path.
Table of Contents
When it comes to the miracle of human life, there are plenty of fascinating facts and intricate details that often go unnoticed. One aspect of this journey that is particularly intriguing is the path that sperm travel during fertilization. Despite their tiny size, these cells embark on a complex and challenging journey in order to reach the egg.
To begin with, it’s important to understand the basic anatomy of sperm. Each one features a head, midpiece, and tail. The head contains genetic material (DNA) while the midpiece holds mitochondria needed for energy production. Finally, the tail – which resembles a whip-like structure – propels sperm towards its ultimate destination.
The first step in this journey begins when sperm cells are released from the testes. They enter into a part of the male reproductive system known as the epididymis where they mature over several weeks before being released during ejaculation. From here, they must travel through various ducts, including the vas deferens and urethra before exiting through the penis.
Once outside of the male body, sperm face further challenges as they navigate through cervix into uterine cavity via vaginal canal during intercourse or other artificial methods like IVF. Here, they must contend with acidic pH levels in female reproductive tract as well as immune cells that may see them as foreign invaders attempting to harm host (woman carrying embryo).
As if all this wasn’t difficult enough, sperm still have a long way yet to travel! They must then make their way through fallopian tube where fertilization occurs based on their fortunate meeting with an egg cell.
Sperm can remain viable for up to five days within female reproductive tract which provides extra opportunities for fertilization attempting at ovulation time – roughly midpoint menstrual cycle when ovary releases an egg cell into its respective Fallopian tube readying itself for fertilization.
Overall, exploring the fascinating journey undertaken by sperm cells provides us with insight into the incredible complexity involved in human reproduction. From their early maturation in the epididymis to their ultimate goal of reaching and fertilizing an egg, these tiny cells face a veritable obstacle course and yet still manage to achieve what amounts to nothing short of a miracle – when successful fertilization occurs! So let us honor this journey that we often overlooks as we get caught up with complexities of our lives!
When it comes to conception, understanding the process of sperm travel is essential. After all, it takes a single sperm to fertilize an egg and result in pregnancy. So, how does this little swimmer make its way up towards the egg? In this step-by-step guide, we’ll explore the complex journey that sperm must undertake in order to reach their ultimate destination.
The first step in understanding how sperm travel path affects conception is knowing where they come from. Sperm are produced in the testes of males and mature over approximately 72 days. Whenever a man ejaculates, he releases millions of these little swimmers which then navigate through several obstacles before they can reach their final goal.
The next critical step is for them to make their way into the cervix – the opening that separates a woman’s uterus from her vagina. This passage can prove challenging for many sperm as its narrow opening and acidic environment can be quite hostile. Only a relatively small proportion of these hardy cells will remain viable enough to make it through this initial challenge.
Once through the cervix, those swimmer’s that do survive are launched into even rougher waters, weaving their way upstream through thick mucus membranes located inside the female reproductive tract; One by one-many falling by wayside losing momentum along with movement- until only very few finally come close enough to take on and fertilize the coveted ovum or egg.
Fertilization happens when one lucky little guy makes it past all these hurdles and meets a waiting egg emerging around day 14 after ovulation within your fallopian tubes-enabling mothers await with pregnancy tests eagerly due for conception notice.
Overall factors such as age, lifestyle choices such as diet or smoking habits can play vital roles in altering what size or quality of sperms actually end up reaching these prized eggs much later on hence affecting fertility rates drastically causing recurring patterns of infertility rejections for some couples trying to conceive.
In conclusion, the journey of sperm travel is a complex, multi-step process that’s far from easy. However, understanding how it all works can help you boost your chances of conception. With proper planning regarding sexual timing and fertility wellness strategies like stress management or nutritional interventions advised by professionals, it is possible for many couples to overcome these hurdles and bring their family dream into fruition despite any challenges in the path~!
When it comes to reproduction, people often focus on the act of sex itself without giving much thought to what happens after ejaculation. However, understanding the journey that sperm cells take from release into the vagina to reaching their destination can be essential in ensuring successful conception. In this article, we’ll address some frequently asked questions about sperm travel path and give you all you need to know about reproductive biology.
Q: What is sperm? A: Sperm are tiny male reproductive cells that are produced by the testes and contain genetic material needed for fertilization. During sex, they swim through seminiferous tubules and gather in the epididymis before they’re ready for ejaculation.
Q: How does ejaculation work? A: When a man is sexually aroused, his parasympathetic nervous system takes over and signals his body to prepare for ejaculation. Once he reaches orgasm, the muscles surrounding his urethra contract and push semen out of his penis. The average man releases around 2-5 milliliters of semen per ejaculate, containing millions of sperm cells.
Q: Where do sperm go after ejaculation? A: After being released during ejaculation, sperm enter the vagina through semen. While their journey towards their final destination may seem short, it’s actually very intricate due to several variables including acidity levels in cervical mucus as well as vaginal fluctuation in pH.
Q: How long does it take for sperm to reach an egg? A: On average, it takes up anywhere between 30 minutes to three days for a single sperm cell to travel from the cervix up into one of a woman’s fallopian tubes where fertilization can occur with an available egg cell – but depending on conditions like distance between partners’ bodies or time since ovulation conception might never happen at all!
Q: What requirements do sperms have to meet so that they can successfully fertilize an egg? A: Before fertilizing an egg, sperm have to go through a series of tests such as the cervical mucus test and the penetration test. From there, if sperm find their way into the fallopian tubes where an egg is present then they can potentially meet up with waiting eggs for fertilization.
Q: What are some factors that could decrease sperm count or make them less mobile? A: There are several lifestyle habits that could worsen sperm health like smoking or excessive drinking, sedentary behavior and stress which reduces levels of testosterone in males. Poor nutrition, chronic illness, sexually transmitted infections (STIs), genetics, medications you take regularly for other conditions – such as antidepressants- poor sleep hygiene also negatively impacts reproductive function in both men and women.
In conclusion, there’s more to reproductive biology than just intercourse. Understanding the journey that sperms travel from ejaculation to fertilization is essential in ensuring successful conception. While various variables can come into play at times making it challenging for some couples to conceive according to expectations; taking care of yourself and reducing harmful lifestyle choices
Fertilization is a miraculous process that has fascinated researchers since the beginning of time. The combination of genetic material from two different organisms results in the creation of a totally unique being—a blend of traits and characteristics that make each individual distinct and special.
For conception to occur, a male’s sperm must travel through a complex maze before it meets with the female’s egg. This journey starts from the moment an ejaculation occurs and ends when fertilization takes place.
The path sperm take is nothing short of remarkable. It can take anywhere from five minutes to several days for them to reach their destination—a feat, given that sperm cells are minuscule compared to human beings and have to overcome several obstacles along the way.
Once released into the vagina during intercourse, sperm begin their arduous journey upwards into the fallopian tubes, where the female’s egg awaits fertilization. Sperm swim against gravity through multiple barriers such as cervical mucus, which can be difficult to penetrate even for healthy sperm cells.
Sperm’s motility, or ability to move quickly towards their destination, is also crucial in fertilization. They move like tiny propellers driven by a whip-like tail called flagellum, which helps them traverse through different kinds of fluids present throughout their journey.
As they progress further towards fallopian tubes, they encounter various natural filters such as immunity system cells protecting against foreign invaders trying to access eggs leading up to white blood cell towers defending these pathways along their long course “mountains”.
When finally reaching mature ovum awaiting them in its zone at the end of this journey in ampulla regions or points (depending on woman cycle), millions come close but only one makes it inside and fertilize egg marking beginning stages an embryo genesis phase; all others either die or get lost along this path filled with numerous biochemical obstacles preventing successful fertilization at every step (Incredible!).
The process of fertilization carried out by sperm is truly intriguing; it’s a testament to the amazing power and resilience of these tiny, yet mighty cells. It’s a fascinating journey that spans several days and involves crossing numerous barriers and obstacles.
It’s no wonder that researchers and scientists continue to study this complex process, hoping to unravel its mysteries further. Just imagine what other secrets could yet be hidden within the intricate processes of human pregnancy – who knows what new discoveries await us as we delve deeper into the world of reproductive biology!
The miracle of life is a fascinating phenomenon that never ceases to amaze us. We all know that the human reproductive system plays a vital role in bringing new life into this world, but have you ever wondered about the intricacies of the male reproductive tract and its role in sperm travel path? Let’s dive deeper and explore this miraculous journey.
The Male Reproductive Tract
The male reproductive system mainly comprises two organs, the testes and the penis. The testes are responsible for producing sperm cells, which are then transported out of the body by way of the penis. However, it is not as simple as it sounds; several structures and mechanisms ensure that sperm travel along their designated path.
Sperm Production
Sperm production takes place inside a network of tiny tubes called seminiferous tubules located within each testicle. Within these tubes, cells undergo meiosis – a special cell division mechanism – to produce mature, functional sperm cells with half the genetic material required for reproduction.
Transportation
Once produced, mature sperm cells move from seminiferous tubules toward epididymis (a duct situated above each testis). During that journey, they acquire motility from surrounding fluids secreted by accessory glands like prostate gland and seminal vesicle. The fluid also provides nutrients to sustain their energy needs while they swim to reach their ultimate destination — an egg inside female reproductive tract.
Ejaculation
When sexual stimulation or arousal occurs, muscles surrounding the epididymis contract, forcing sperm into vas deferens – muscular ducts that carry them upwards towards prostate gland. Here they mix with seminal fluid when ejaculation occurs—from here; millions of swimming soldiers commence their incredible race to find familiar eggs.
Travel Path
When semen shoots out through penis during ejaculation inevitably makes some contact with outsides surfaces before it enters female genitalia. Inclined vaginal walls help channel movement up toward cervix opening leading deeper into reproductive tract. There are more hurdles to overcome inside a female’s body than one would expect as the path is full of obstacles. This includes acidic environments in the vagina, and barriers produced by mucus on the cervix.
Final Thoughts
The male reproductive tract and its role in sperm travel can be viewed as a remarkable example of evolution at work. The closely coordinated response between organs & proteins secretion, advanced muscular contractions, transportation throughout several ducts – all these mechanisms adapted to enhance chance of fertilization success showcases nature’s brilliance. As humans, we must appreciate how our bodies have evolved over millennia to bring new life into this world. And now that you know just how incredible and fascinating the male reproductive system is let us take a moment to marvel at it.
Conception is one of nature’s most fascinating and intricate processes. It represents the merging of two cells, the sperm and the egg, which leads to a new life. This process has fascinated scientists for years, leading to numerous studies and research in an attempt to uncover all its mysteries. One particular area of discovery that continues to intrigue researchers is the path travelled by sperm during conception.
Sperm travel through a series of complex environments within the female reproductive system on their journey to fertilize an egg. To understand this journey, it’s essential first to know what happens when a male ejaculates.
When males ejaculate, semen – a mixture made up of sperm, enzymes, proteins and other substances – is released from the penis into the female reproductive system via intercourse or direct injection techniques such as artificial insemination. After entering into this environment created by a woman’s body temperature and hormones, some sperm die instantly due to unfriendly conditions like acidity.
The surviving sperms start their journey through different parts of the female reproductive system such as cervix-uterus-fallopian tubes over several hours or days depending on individuals’ anatomy and physiology before finding themselves in contact with an egg cell. This trip requires them first navigating against gravity’s pull entirely; once they reach the uterus at about 45mins/1hour after ejaculation, contractions of cervical mucus carry them further up into fallopian tubes where most successful healthy fertilisations take place under ideal ovulation timing.
While this process seems straightforward enough in theory: get from point A (ejaculation) to point B (an egg cell), there are no guarantees that any given sperm will reach its destination successfully fertilizing an egg cell ultimately. In fact, only about 300 million out of billions released sperm amount actually get close enough for chance encounter with unprotected eggs for natural conceptions: The vast majority wastes existing energy resources lingering around due unfavourable conditions and possible sperm anomalous health issues.
Overall, for conception to occur, the intricate symphony of events leading up to fertilization must work seamlessly together. Even slight disruptions in any of the stages could result in infertility or no viable pregnancies.
Discovering the secrets of sperm travel path is only part of our knowledge pool about reproductive health sciences- as there are still many things we do not know yet; however, it’s an exciting area of study that has vast potential implications for infertility treatments and fertility preservation efforts. It’s an undiscovered universe that presents much promise for improving human reproduction management in spite of its challenging terrain- uncovering more about this unseen world wouldn’t hurt!

Save my name, email, and website in this browser for the next time I comment.

Diagram of the journey of sperm to egg
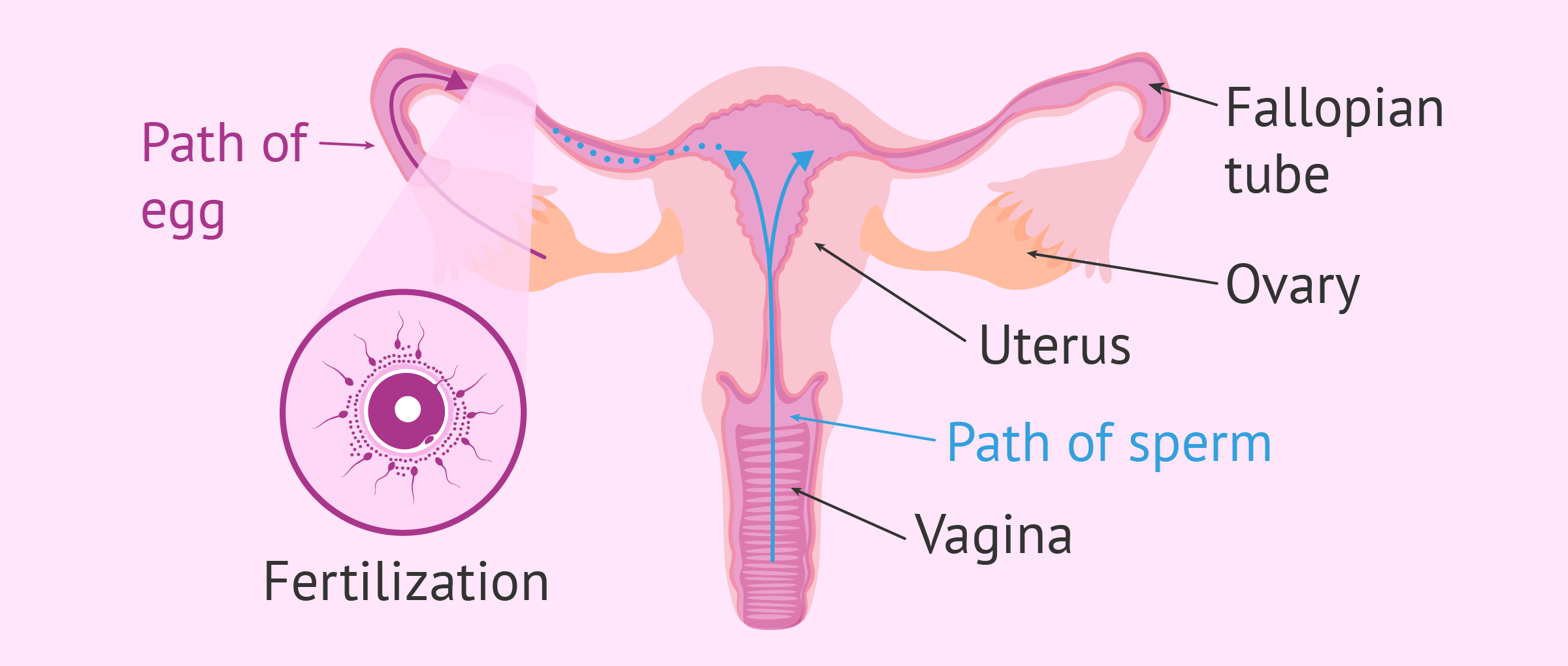
This diagram shows the entire process of fertilization inside the woman's body. The ultimate goal is to meet the egg in the Fallopian tube.
Leave a Reply
Privacy Overview

What Is IVF? Here's Why People Choose It and How It Works
T he Alabama Supreme Court ruled (PDF) in February that embryos created through in vitro fertilization have the same legal rights as children , moving the national spotlight on a medical procedure many people use to become pregnant and grow their families.
Some fertility clinics in Alabama paused their services , out of new legal concern for the handling of embryos, though the state legislature has quickly moved to protect IVF providers . The wider effect on infertility services following the overturning of Roe v. Wade in 2022 remains to be seen.
IVF is the most common form of assisted reproductive technology , which describes the handling of eggs and embryos for fertility treatment. In a nutshell, IVF is the process of fertilizing an egg and sperm in a medical lab and then implanting the resulting embryo into someone's uterus, with the hope it will continue to develop into a successful pregnancy and baby.
A little more than 2% of all babies born in the US each year are conceived through IVF or a similar treatment, according to the US Centers for Disease Control and Prevention, and use of it has "more than doubled" in the last decade. But despite its growing popularity, IVF remains a complicated procedure, full of financial and sometimes emotional burden for hopeful parents. It also highlights the nuances of human reproduction, whether it be natural conception or conception assisted by a little bit of science.
"I think it's important for people to understand that we, as humans, are not that efficient with reproduction," Dr. Asima Ahmad, chief medical officer of Carrot Fertility , wrote in an email. Many embryos created in IVF will not make it to a live birth because they won't continue to grow, or they'll fail to implant into the uterus and continue as a pregnancy, for example.
"There are many things that have to align the right way for a live birth to occur," Ahmad said.
Read more: For These Personal Finance Influencers, Infertility Wasn't Part of the Plan
Why would someone need IVF?
People use IVF for many different reasons. These include instances of medical problems that make it difficult for someone to get pregnant naturally, saving childbearing for an age when pregnancy becomes more difficult, and the need for donor eggs or sperm to have a child.
In other cases, people may use IVF or freeze embryos to preserve their fertility if they have a medical procedure that can affect it, like cancer treatment, or if they're undergoing gender affirmation surgery.
How does IVF work?
Many steps go into IVF treatment -- one of the reasons why it's so expensive -- but it's basically taking a process that has traditionally occurred in the fallopian tube out into the world, with additional reproductive assistance from doctors and embryologists when required by the patient or doctor running the IVF procedure.
Here's how the process breaks down, according to information from NYU Langone , Johns Hopkins Medicine and Penn Medicine .
Step 1: Collect the eggs
In the month leading up to a natural conception, only one egg is released (sometimes two) during ovulation for potential fertilization and pregnancy. But when conception takes place outside the body, you need more eggs to work with, so you'll go through an egg retrieval.
Egg retrieval requires whoever is using their eggs for the pregnancy to be given a hormone injection that causes multiple eggs to mature instead of just one. (Many people who are using IVF will use their own eggs to become pregnant, but some patients may find an egg donor or even a surrogate to carry the pregnancy.)
To get the mature eggs from the ovary, you'll go through a process called follicular aspiration, where a very thin needle is guided through the vagina during an ultrasound. The device will suction out the eggs. You'll be given medication for this part. (Fun fact: Human egg cells are gigantic compared to the other cells in our body.)
How many times someone will need to go through egg retrieval depends on a few factors, including their reasons for infertility and their age. The number of healthy eggs your body releases declines with age, so an IVF patient in their 40s, for example, may need more eggs and cycles to have a chance at a successful pregnancy. But some people have higher or lower egg quality and quantity due to medical history or individual biology, as is true for sperm, or step No. 2.
Step 2: Collect the sperm
Because much of the sperm-production part of conception takes part outside the body, getting sperm for IVF is less medically involved than retrieving the eggs. Whoever is using their sperm in the pregnancy will probably be asked to abstain from ejaculating for a few days before the collection to ensure a higher sperm count, and most clinics prefer your sample be given in-office.
For those who don't produce sperm in their ejaculation, contributing to a case of infertility, sperm can sometimes be retrieved in a minor surgical procedure, similar in theory to egg retrieval. Whether you donate sperm before or after the egg donation will be up to your case and your doctor.
Step 3: Lab fertilizes the egg
Once you have eggs and sperm, the next step in IVF (and how the procedure gets its name) is the fertilization of the egg in a lab.
Quickly after the eggs have been retrieved, an embryologist will fertilize the eggs, either by letting a sperm find its own way to the egg, or by injecting a sperm cell directly into the egg (a process called intracytoplasmic sperm injection ). Whichever method is used for you will depend on your reasons for IVF or your lab.
Then, everyone waits. It takes about 18 hours to determine whether an egg has been successfully fertilized, and then a couple more days to see if the embryo is developing properly. In many cases, genetic testing of the embryos is done to determine its health or its likelihood of resulting in a baby.
Regardless of fertility status, not every fertilized egg will make it to a healthy embryo. Estimates on how many are lost during a natural conception seem to vary , but as many as one half of fertilized eggs are lost quickly after conception . During IVF, these early losses and failed conceptions are under a microscope, literally.
According to Ahmad, the chance of getting pregnant even in "the most fertile age range" is about 25% to 30% per cycle.
Step 4: Healthy embryo implanted into uterus
Once an embryo is selected and starts to develop, it will be transferred into a uterus for pregnancy to continue normally during a time in the menstrual cycle when the uterine lining can successfully receive it. This is a relatively short procedure where the embryo is released directly into the uterus, passing the cervix.
After some time , patients will know whether the embryo was able to successfully go through the " hatching " process of embed itself into the uterine lining, which then triggers someone's body to go into pregnancy mode , hopefully resulting in a baby nine months later.
Step 5: Remaining embryos stored if requested
The Alabama court case centered on embryos that were stored by patients at a fertility clinic.
People often have "extra" embryos if they went through the first parts of the IVF procedure and were able to become pregnant. If someone wants additional children in the future, they may keep those embryos frozen in a protective fluid with the use of liquid nitrogen for whenever they're ready to try to become pregnant again. The price of embryo storage varies based on the clinic, but may cost at least a few hundred dollars per year, according to an estimate by ReproTech.
What happens when someone doesn't use their remaining embryos, or they're frozen for a while?
People may be left with stored or additional embryos they don't know what to do with, have more embryos left than they initially planned for, they're unable to go through pregnancy again for medical reasons, or they decide not to implant embryos that testing deemed genetically abnormal or those who don't have a good chance of resulting in a healthy pregnancy.
If embryos are not used or frozen, embryos are discarded as medical waste , or they're transferred into someone's womb during a time in the menstrual cycle when pregnancy wouldn't be possible . They can also be donated to science for research, and even to another person.
Whatever the choice, deciding the fate of remaining embryos created during IVF can be difficult for patients for a variety of reasons. The American Society for Reproductive Medicine, an organization in the field of reproductive science and medical practice, acknowledges the "ethical concerns" unused embryos pose for patients and the clinics that store them. The ASRM calls patient choice a "core value" of reproductive medicine and that patient wishes about their embryos should be "respected if practical and within legal limits." The legal limits of what you're able to do as a patient depend on state laws, as well as individual protocols at fertility clinics.
In general, patients have the wheel in deciding how to proceed in their IVF journey, according to Ahmad, which includes whether to only transfer "fresh" embryos and not store any (transferring frozen embryos may be recommended by physicians for different reasons, including a higher chance of pregnancy), and whether or not to do genetic testing. She notes that some fertility clinics may have individual protocols for freezing all embryos for testing or another reason, but choices in IVF should involve a risk-benefit discussion between patients and their doctors.
"Ultimately," Ahmad said, "the goal is for the best possible outcome for the patient."


A Journey of Sperm
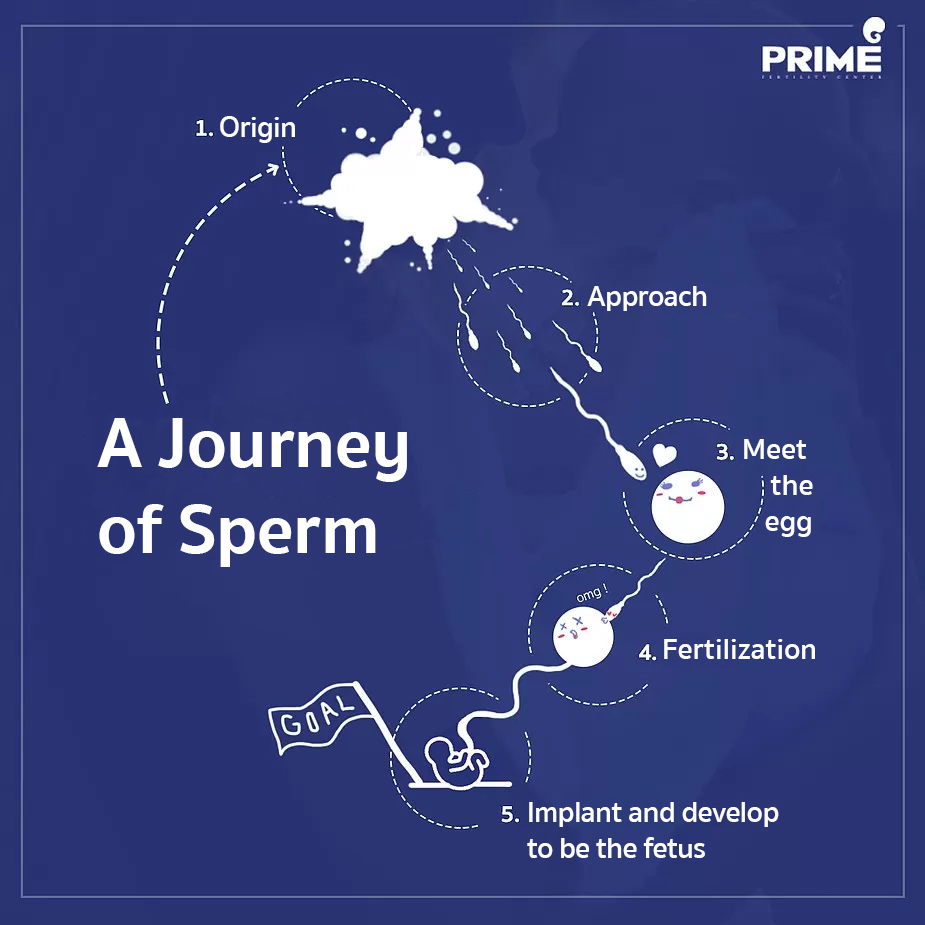
Sperm cells are produced from both testis of male. From germ cell and develop to become fully mature. This process takes around 70-80 days to complete.
After ejaculation, millions of sperm cells are moving to female’s vagina. A lot of sperm cells will be diving through vaginal mucus then aiming to cervix and uterine cavity. In this step, plenty of sperms die during approaching to the target.
Meet the egg
Once sperms (that still survive) reach the fallopian tubes, they will meet 1 fully matured egg which has passed the ovulation then moved to wait for sperm at the fallopian tubes. Each sperm will be approaching to this egg consequently.
Fertilization
Although the egg is surrounded by a lot of sperms, there will be only 1 strongest sperm can penetrate into the egg. After that sperm will throw off its tail and release 23 pairs of chromosomes to match with egg’s chromosomes. Combining to be 1 cell. The egg which has been fertilized already will transform itself in order to reject the approaching of any others sperm cells.
Implant and develop to be the fetus
After fertilization, the cell will be dividing very quickly. Then transfer from fallopian tubes to the uterine cavity in order to implant and grow up to be the fetus later on.
—–
Question about Infertility Treatment: Click Here Review Clip from Our Patients: Click Here
#ICSI #IUI #IVF #eggfreezing #EmbryoFreezing #SpermFreezing #SemenAnalysis #Hysteroscopy #FET #PGT #PGD #NGS #PESA #TESA #primefertilityclinic #primefertiltycenter #fertilityclinic #bangkokfertilityclinic #thailandfertilityclinic
Reference: Prime Fertility Center Co., Ltd. https://www.primefertilitycenter.com/en/a-journey-of-sperm/
Related Posts

Why does sperm have to be frozen? What is sperm freezing? Who is it suitable for?
Why does sperm have to be frozen? What is sperm freezing? Who is it suitable for? […]

The auspicious days for delivery during the second half of Dragon year
The auspicious days for delivery during the second half of Dragon year […]
©2019 Prime Fertility Center. All rights reserved.
เว็บไซต์นี้ใช้คุกกี้เพื่อวัตถุประสงค์ในการปรับปรุงประสบการณ์ของผู้ใช้ให้ดียิ่งขึ้น Read More

IMAGES
VIDEO
COMMENTS
The journey through the female reproductive system. In the process of ejaculation, sperm cells leave the man and enter the vagina. This is where the sperm cells begin the second part of their journey to fertilization. During this second part of the journey the sperm again encounter an large number of obstacles.
Hundreds of millions of sperm vie for a single egg cell. The sperm cells are streamlined in design for this purpose: a long tail to help them move, lots of mitochondria to power that movement, genetic information to pass on, and enzymatic proteins to get into the egg cell. The proteins are stored in a cap at the front of the sperm known as an acrosome - this is the part that first contacts the ...
Fertilization: Sperm Penetrates Egg. 5 /9. It takes about 24 hours for a sperm cell to fertilize an egg. When the sperm penetrates the egg, the surface of the egg changes so that no other sperm ...
These sperm cells then travel through a series of ducts within the testes, called the rete testis and the epididymis, where they undergo further maturation and acquire the ability to swim. ... The cervical mucus plays a crucial role in the journey of sperm towards the egg. Around the time of ovulation, the cervical mucus becomes thinner and ...
0. The path of the spermatozoa to reach the egg is not a simple one. This path is divided into a phase in the male reproductive system and another in the female reproductive system. In the case of the male, the sperm travel from the testicle to the urethra. When vaginal penetration occurs, the sperm continue their journey to the fallopian tubes.
Fertilisation is a complex process, with the female body working in mysterious ways to align the egg and the sperm. Detailed below, are the steps that go into it. . . Step 1. Ovulation. . Ovulation refers to the emergence of a single mature egg from one of the ovarian follicles.
The journey of a sperm to an egg ends in the ampulla of the female oviduct. From there, the sperm must overcome a number of physical and biochemical barriers. After undergoing the acrosome reaction and binding the ova, the sperm penetrates through the cumulus oophorus cells and the zona pellucida (ZP) to reach the perivitelline space (PVS) and ...
Mammalian sperm have to travel a long distance through the female reproductive tract to the oviduct, in which fertilization takes place. To accomplish the journey to the egg, sperm are equipped to overcome various obstacles that lie ahead, such as navigating the uterotubal junction (UTJ) and penetrating the egg extracellular matrices.
Sperm and egg cells are special sex cells for reproduction. Sperm cells are small, have a tail for movement, and carry male genetic material. The egg is large, round, and has a protective layer called the zona pellucida. During fertilization, sperm meets egg, and their genetic material combines. Created by Jeff Otjen.
Human growthand development. Human fertilization is the union of an egg and sperm, occurring primarily in the ampulla of the fallopian tube. [1] The result of this union leads to the production of a fertilized egg called a zygote, initiating embryonic development. Scientists discovered the dynamics of human fertilization in the 19th century.
Fertilization is a complex multi-step process that is complete in 24 hours. The sperm from a male meets an ovum from a female and forms a zygote; this is the point in which pregnancy begins and leads to a 280-day journey for a female. There are two ways to track this process, and they differ by the day counting begins. There are the post-ovulation age and the gestational age, calculated by ...
Video summary. This short film uses CGI footage to help give a visual description of the process of human fertilisation, following the journey of both sperm and egg.
Fusion in advanced animals is usually followed by penetration of the egg by a single spermatozoon. The result of fertilization is a cell ( zygote) capable of undergoing cell division to form a new individual. journey of a fertilized egg. The journey of a fertilized egg in a woman. In mammals, eggs are released by the ovaries.
The pathway of sperm is the journey that sperm cells take from the testes to the female reproductive tract during fertilization. The journey involves a series of steps, including production, maturation, and transport. ... fertilizing an egg cell residing inside a woman's uterus! Therefore they need to swim against gravity for proper delivery ...
Some people think fertilization happens in the uterus, since that's where the baby develops. However, fertilization actually occurs in the fallopian tubes. Each sperm has a single goal: to meet up with the egg. To reach the target, though, a sperm cell has to go on a lengthy and strenuous journey. First, it must make its way from the vagina ...
Conception: How It Works. To become pregnant, these steps must occur: Sperm Transport: The sperm must be deposited and transported to the site of fertilization. Egg Transport: Ovulation must occur and the egg must be "picked up" by the tube. Fertilization and Embryo Development: Union between the sperm and egg must result.
Period calculator. Implantation calculator. Pregnancy calculator. It takes sperm a surprisingly long time to reach the egg after unprotected sex — and few ever make it that far. A fertility expert explains what goes on inside the reproductive system after ejaculation.
Fertilisation is the process in which the nucleus of a sperm cell fuses with the nucleus of an egg cell to produce a. zygote. which will eventually grow into offspring. Gametes are the sex cells ...
One aspect of this journey that is particularly intriguing is the path that sperm travel during fertilization. Despite their tiny size, these cells embark on a complex and challenging journey in order to reach the egg. To begin with, it's important to understand the basic anatomy of sperm. Each one features a head, midpiece, and tail.
Fertilization is a multistep process that culminates in the fusion of sperm and egg, thus marking the beginning of a new organism in sexually reproducing species. Despite its importance for reproduction, the molecular mechanisms that regulate this singular event, particularly sperm-egg fusion, have remained mysterious for many decades. Here, we summarize our current molecular understanding ...
Diagram of the journey of sperm to egg. 4. This diagram shows the entire process of fertilization inside the woman's body. The ultimate goal is to meet the egg in the Fallopian tube. Read the full article on: How sperm meets egg: a journey from production to fertilization ( 43).
Summary. Sperm cells are male reproductive cells that originate in the testicles. Sperm cells swim to and fertilize a female reproductive cell called an oocyte, or egg. Two key factors that can ...
Quickly after the eggs have been retrieved, an embryologist will fertilize the eggs, either by letting a sperm find its own way to the egg, or by injecting a sperm cell directly into the egg (a ...
Each sperm will be approaching to this egg consequently. Fertilization. Although the egg is surrounded by a lot of sperms, there will be only 1 strongest sperm can penetrate into the egg. After that sperm will throw off its tail and release 23 pairs of chromosomes to match with egg's chromosomes. Combining to be 1 cell.