Organic Chemistry Lab Tour

Ace Your Next Organic Chemistry Exam.
With the moc membership.
1500+ Real-World exam quizzes
180+ Reactions and Mechanisms
200+ Downloadable Flashcards
Organic chemistry is awesome.
Over 400+ blog posts to guide you through introductory Organic Chemistry, organized by subject.
00 General Chemistry Review
- Lewis Structures
- Ionic and Covalent Bonding
- Chemical Kinetics
- Chemical Equilibria
- Valence Electrons of the First Row Elements
- How Concepts Build Up In Org 1 ("The Pyramid")
01 Bonding, Structure, and Resonance
- How Do We Know Methane (CH4) Is Tetrahedral?
- Hybrid Orbitals and Hybridization
- How To Determine Hybridization: A Shortcut
- Orbital Hybridization And Bond Strengths
- Sigma bonds come in six varieties: Pi bonds come in one
- A Key Skill: How to Calculate Formal Charge
- The Four Intermolecular Forces and How They Affect Boiling Points
- 3 Trends That Affect Boiling Points
- How To Use Electronegativity To Determine Electron Density (and why NOT to trust formal charge)
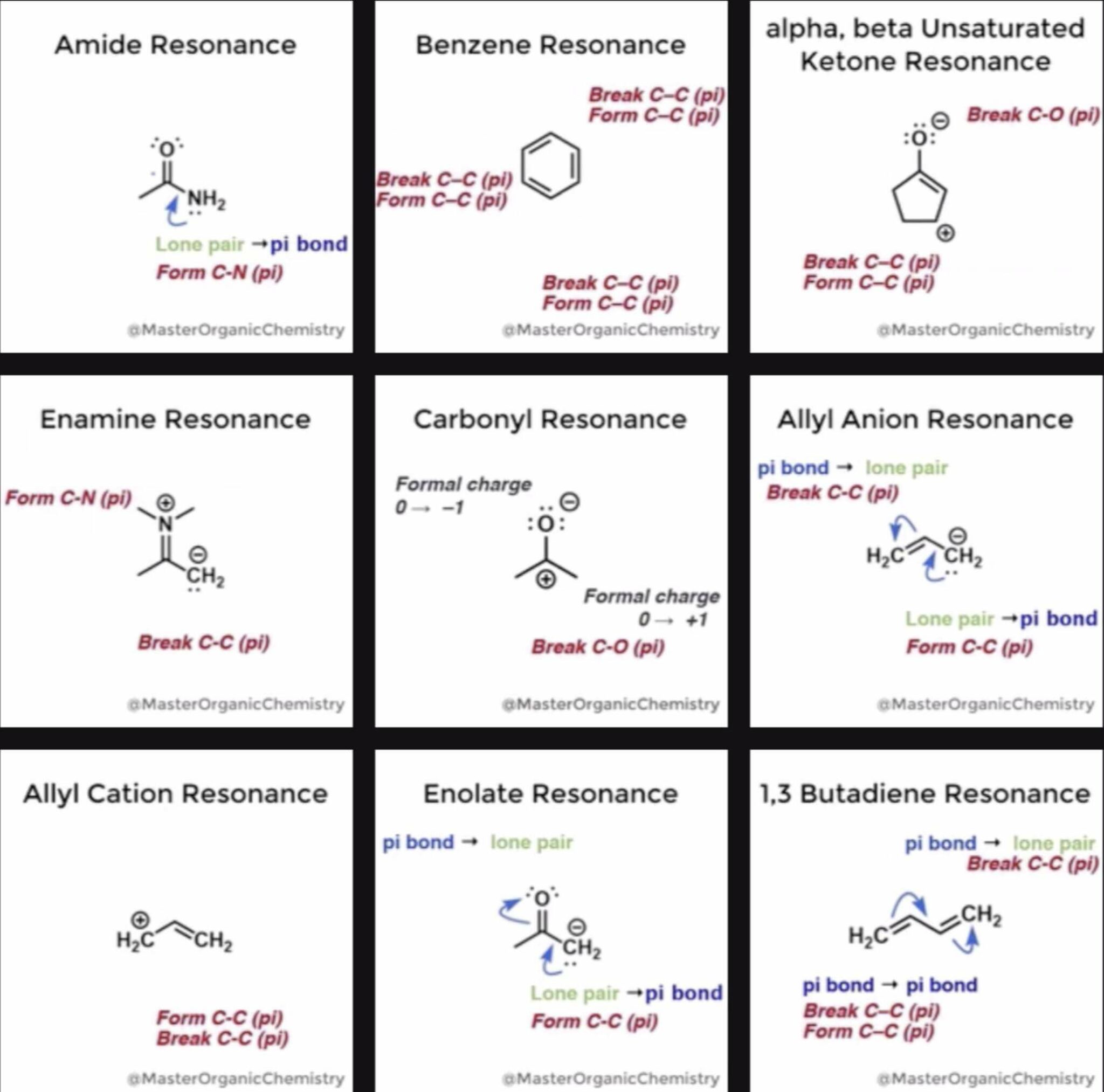
- Introduction to Resonance
- How To Use Curved Arrows To Interchange Resonance Forms
- Evaluating Resonance Forms (1) - The Rule of Least Charges
- How To Find The Best Resonance Structure By Applying Electronegativity
- Evaluating Resonance Structures With Negative Charges
- Evaluating Resonance Structures With Positive Charge
- Exploring Resonance: Pi-Donation
- Exploring Resonance: Pi-acceptors
- In Summary: Evaluating Resonance Structures
- Drawing Resonance Structures: 3 Common Mistakes To Avoid
- How to apply electronegativity and resonance to understand reactivity
- Bond Hybridization Practice
- Structure and Bonding Practice Quizzes
- Resonance Structures Practice
02 Acid Base Reactions
- Introduction to Acid-Base Reactions
- Acid Base Reactions In Organic Chemistry
- The Stronger The Acid, The Weaker The Conjugate Base
- Walkthrough of Acid-Base Reactions (3) - Acidity Trends
- Five Key Factors That Influence Acidity
- Acid-Base Reactions: Introducing Ka and pKa
- How to Use a pKa Table
- The pKa Table Is Your Friend
- A Handy Rule of Thumb for Acid-Base Reactions
- Acid Base Reactions Are Fast
- pKa Values Span 60 Orders Of Magnitude
- How Protonation and Deprotonation Affect Reactivity
- Acid Base Practice Problems
03 Alkanes and Nomenclature
- Meet the (Most Important) Functional Groups
- Condensed Formulas: Deciphering What the Brackets Mean
- Hidden Hydrogens, Hidden Lone Pairs, Hidden Counterions
- Don't Be Futyl, Learn The Butyls
- Primary, Secondary, Tertiary, Quaternary In Organic Chemistry
- Branching, and Its Affect On Melting and Boiling Points
- The Many, Many Ways of Drawing Butane
- Wedge And Dash Convention For Tetrahedral Carbon
- Common Mistakes in Organic Chemistry: Pentavalent Carbon
- Table of Functional Group Priorities for Nomenclature
- Summary Sheet - Alkane Nomenclature
- Organic Chemistry IUPAC Nomenclature Demystified With A Simple Puzzle Piece Approach
- Boiling Point Quizzes
- Organic Chemistry Nomenclature Quizzes
04 Conformations and Cycloalkanes
- Staggered vs Eclipsed Conformations of Ethane
- Conformational Isomers of Propane
- Newman Projection of Butane (and Gauche Conformation)
- Introduction to Cycloalkanes (1)
- Geometric Isomers In Small Rings: Cis And Trans Cycloalkanes
- Calculation of Ring Strain In Cycloalkanes
- Cycloalkanes - Ring Strain In Cyclopropane And Cyclobutane
- Cyclohexane Conformations
- Cyclohexane Chair Conformation: An Aerial Tour
- How To Draw The Cyclohexane Chair Conformation
- The Cyclohexane Chair Flip
- The Cyclohexane Chair Flip - Energy Diagram
- Substituted Cyclohexanes - Axial vs Equatorial
- Ranking The Bulkiness Of Substituents On Cyclohexanes: "A-Values"
- Cyclohexane Chair Conformation Stability: Which One Is Lower Energy?
- Fused Rings - Cis-Decalin and Trans-Decalin
- Naming Bicyclic Compounds - Fused, Bridged, and Spiro
- Bredt's Rule (And Summary of Cycloalkanes)
- Newman Projection Practice
- Cycloalkanes Practice Problems
05 A Primer On Organic Reactions
- The Most Important Question To Ask When Learning a New Reaction
- Learning New Reactions: How Do The Electrons Move?
- The Third Most Important Question to Ask When Learning A New Reaction
- 7 Factors that stabilize negative charge in organic chemistry
- 7 Factors That Stabilize Positive Charge in Organic Chemistry
- Nucleophiles and Electrophiles
- Curved Arrows (for reactions)
- Curved Arrows (2): Initial Tails and Final Heads
- Nucleophilicity vs. Basicity
- The Three Classes of Nucleophiles
- What Makes A Good Nucleophile?
- What makes a good leaving group?
- 3 Factors That Stabilize Carbocations
- Equilibrium and Energy Relationships
- What's a Transition State?
- Hammond's Postulate
- Learning Organic Chemistry Reactions: A Checklist (PDF)
- Introduction to Free Radical Substitution Reactions
- Introduction to Oxidative Cleavage Reactions
06 Free Radical Reactions
- Bond Dissociation Energies = Homolytic Cleavage
- Free Radical Reactions
- 3 Factors That Stabilize Free Radicals
- What Factors Destabilize Free Radicals?
- Bond Strengths And Radical Stability
- Free Radical Initiation: Why Is "Light" Or "Heat" Required?
- Initiation, Propagation, Termination
- Monochlorination Products Of Propane, Pentane, And Other Alkanes
- Selectivity In Free Radical Reactions
- Selectivity in Free Radical Reactions: Bromination vs. Chlorination
- Halogenation At Tiffany's
- Allylic Bromination
- Bonus Topic: Allylic Rearrangements
- In Summary: Free Radicals
- Synthesis (2) - Reactions of Alkanes
- Free Radicals Practice Quizzes
07 Stereochemistry and Chirality
- Types of Isomers: Constitutional Isomers, Stereoisomers, Enantiomers, and Diastereomers
- How To Draw The Enantiomer Of A Chiral Molecule
- How To Draw A Bond Rotation
- Introduction to Assigning (R) and (S): The Cahn-Ingold-Prelog Rules
- Assigning Cahn-Ingold-Prelog (CIP) Priorities (2) - The Method of Dots
- Enantiomers vs Diastereomers vs The Same? Two Methods For Solving Problems
- Assigning R/S To Newman Projections (And Converting Newman To Line Diagrams)
- How To Determine R and S Configurations On A Fischer Projection
- The Meso Trap
- Optical Rotation, Optical Activity, and Specific Rotation
- Optical Purity and Enantiomeric Excess
- What's a Racemic Mixture?
- Chiral Allenes And Chiral Axes
- Stereochemistry Practice Problems and Quizzes
08 Substitution Reactions
- Introduction to Nucleophilic Substitution Reactions
- Walkthrough of Substitution Reactions (1) - Introduction
- Two Types of Nucleophilic Substitution Reactions
- The SN2 Mechanism
- Why the SN2 Reaction Is Powerful
- The SN1 Mechanism
- The Conjugate Acid Is A Better Leaving Group
- Comparing the SN1 and SN2 Reactions
- Polar Protic? Polar Aprotic? Nonpolar? All About Solvents
- Steric Hindrance is Like a Fat Goalie
- Common Blind Spot: Intramolecular Reactions
- The Conjugate Base is Always a Stronger Nucleophile
- Substitution Practice - SN1
- Substitution Practice - SN2
09 Elimination Reactions
- Elimination Reactions (1): Introduction And The Key Pattern
- Elimination Reactions (2): The Zaitsev Rule
- Elimination Reactions Are Favored By Heat
- Two Elimination Reaction Patterns
- The E1 Reaction
- The E2 Mechanism
- E1 vs E2: Comparing the E1 and E2 Reactions
- Antiperiplanar Relationships: The E2 Reaction and Cyclohexane Rings
- Bulky Bases in Elimination Reactions
- Comparing the E1 vs SN1 Reactions
- Elimination (E1) Reactions With Rearrangements
- E1cB - Elimination (Unimolecular) Conjugate Base
- Elimination (E1) Practice Problems And Solutions
- Elimination (E2) Practice Problems and Solutions
10 Rearrangements
- Introduction to Rearrangement Reactions
- Rearrangement Reactions (1) - Hydride Shifts
- Carbocation Rearrangement Reactions (2) - Alkyl Shifts
- Pinacol Rearrangement
- The SN1, E1, and Alkene Addition Reactions All Pass Through A Carbocation Intermediate
11 SN1/SN2/E1/E2 Decision
- Identifying Where Substitution and Elimination Reactions Happen
- Deciding SN1/SN2/E1/E2 (1) - The Substrate
- Deciding SN1/SN2/E1/E2 (2) - The Nucleophile/Base
- SN1 vs E1 and SN2 vs E2 : The Temperature
- Deciding SN1/SN2/E1/E2 - The Solvent
- Wrapup: The Key Factors For Determining SN1/SN2/E1/E2
- Alkyl Halide Reaction Map And Summary
- SN1 SN2 E1 E2 Practice Problems
12 Alkene Reactions
- E and Z Notation For Alkenes (+ Cis/Trans)
- Alkene Stability
- Alkene Addition Reactions: "Regioselectivity" and "Stereoselectivity" (Syn/Anti)
- Stereoselective and Stereospecific Reactions
- Hydrohalogenation of Alkenes and Markovnikov's Rule
- Hydration of Alkenes With Aqueous Acid
- Rearrangements in Alkene Addition Reactions
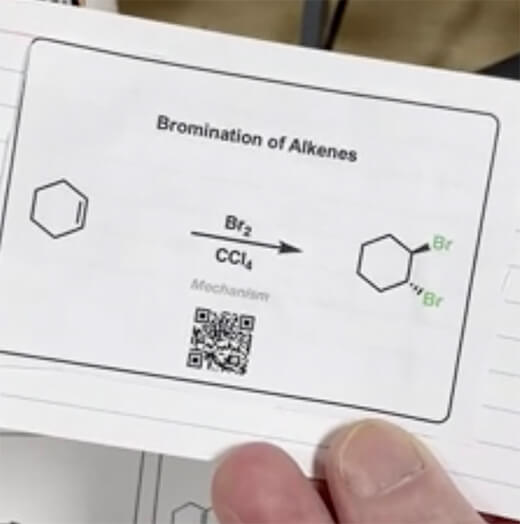
- Halogenation of Alkenes and Halohydrin Formation
- Oxymercuration Demercuration of Alkenes
- Hydroboration Oxidation of Alkenes
- m-CPBA (meta-chloroperoxybenzoic acid)
- OsO4 (Osmium Tetroxide) for Dihydroxylation of Alkenes
- Palladium on Carbon (Pd/C) for Catalytic Hydrogenation of Alkenes
- Cyclopropanation of Alkenes
- A Fourth Alkene Addition Pattern - Free Radical Addition
- Alkene Reactions: Ozonolysis
- Summary: Three Key Families Of Alkene Reaction Mechanisms
- Synthesis (4) - Alkene Reaction Map, Including Alkyl Halide Reactions
- Alkene Reactions Practice Problems
13 Alkyne Reactions
- Acetylides from Alkynes, And Substitution Reactions of Acetylides
- Partial Reduction of Alkynes With Lindlar's Catalyst
- Partial Reduction of Alkynes With Na/NH3 To Obtain Trans Alkenes
- Alkyne Hydroboration With "R2BH"
- Hydration and Oxymercuration of Alkynes
- Hydrohalogenation of Alkynes
- Alkyne Halogenation: Bromination, Chlorination, and Iodination of Alkynes
- Alkyne Reactions - The "Concerted" Pathway
- Alkenes To Alkynes Via Halogenation And Elimination Reactions
- Alkynes Are A Blank Canvas
- Synthesis (5) - Reactions of Alkynes
- Alkyne Reactions Practice Problems With Answers
14 Alcohols, Epoxides and Ethers
- Alcohols - Nomenclature and Properties
- Alcohols Can Act As Acids Or Bases (And Why It Matters)
- Alcohols - Acidity and Basicity
- The Williamson Ether Synthesis
- Ethers From Alkenes, Tertiary Alkyl Halides and Alkoxymercuration
- Alcohols To Ethers via Acid Catalysis
- Cleavage Of Ethers With Acid
- Epoxides - The Outlier Of The Ether Family
- Opening of Epoxides With Acid
- Epoxide Ring Opening With Base
- Making Alkyl Halides From Alcohols
- Tosylates And Mesylates
- PBr3 and SOCl2
- Elimination Reactions of Alcohols
- Elimination of Alcohols To Alkenes With POCl3
- Alcohol Oxidation: "Strong" and "Weak" Oxidants
- Demystifying The Mechanisms of Alcohol Oxidations
- Protecting Groups For Alcohols
- Thiols And Thioethers
- Calculating the oxidation state of a carbon
- Oxidation and Reduction in Organic Chemistry
- Oxidation Ladders
- SOCl2 Mechanism For Alcohols To Alkyl Halides: SN2 versus SNi
- Alcohol Reactions Roadmap (PDF)
- Alcohol Reaction Practice Problems
- Epoxide Reaction Quizzes
- Oxidation and Reduction Practice Quizzes
15 Organometallics
- What's An Organometallic?
- Formation of Grignard and Organolithium Reagents
- Organometallics Are Strong Bases
- Reactions of Grignard Reagents
- Protecting Groups In Grignard Reactions
- Synthesis Problems Involving Grignard Reagents
- Grignard Reactions And Synthesis (2)
- Organocuprates (Gilman Reagents): How They're Made
- Gilman Reagents (Organocuprates): What They're Used For
- The Heck, Suzuki, and Olefin Metathesis Reactions (And Why They Don't Belong In Most Introductory Organic Chemistry Courses)
- Reaction Map: Reactions of Organometallics
- Grignard Practice Problems
16 Spectroscopy
- Degrees of Unsaturation (or IHD, Index of Hydrogen Deficiency)
- Conjugation And Color (+ How Bleach Works)
- Introduction To UV-Vis Spectroscopy
- UV-Vis Spectroscopy: Absorbance of Carbonyls
- UV-Vis Spectroscopy: Practice Questions
- Bond Vibrations, Infrared Spectroscopy, and the "Ball and Spring" Model
- Infrared Spectroscopy: A Quick Primer On Interpreting Spectra
- IR Spectroscopy: 4 Practice Problems
- 1H NMR: How Many Signals?
- Homotopic, Enantiotopic, Diastereotopic
- Diastereotopic Protons in 1H NMR Spectroscopy: Examples
- C13 NMR - How Many Signals
- Liquid Gold: Pheromones In Doe Urine
- Natural Product Isolation (1) - Extraction
- Natural Product Isolation (2) - Purification Techniques, An Overview
- Structure Determination Case Study: Deer Tarsal Gland Pheromone
17 Dienes and MO Theory
- What To Expect In Organic Chemistry 2
- Are these molecules conjugated?
- Conjugation And Resonance In Organic Chemistry
- Bonding And Antibonding Pi Orbitals
- Molecular Orbitals of The Allyl Cation, Allyl Radical, and Allyl Anion
- Pi Molecular Orbitals of Butadiene
- Reactions of Dienes: 1,2 and 1,4 Addition
- Thermodynamic and Kinetic Products
- More On 1,2 and 1,4 Additions To Dienes
- s-cis and s-trans
- The Diels-Alder Reaction
- Cyclic Dienes and Dienophiles in the Diels-Alder Reaction
- Stereochemistry of the Diels-Alder Reaction
- Exo vs Endo Products In The Diels Alder: How To Tell Them Apart
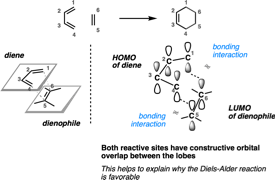
- HOMO and LUMO In the Diels Alder Reaction
- Why Are Endo vs Exo Products Favored in the Diels-Alder Reaction?
- Diels-Alder Reaction: Kinetic and Thermodynamic Control
- The Retro Diels-Alder Reaction
- The Intramolecular Diels Alder Reaction
- Regiochemistry In The Diels-Alder Reaction
- The Cope and Claisen Rearrangements
- Electrocyclic Reactions
- Electrocyclic Ring Opening And Closure (2) - Six (or Eight) Pi Electrons
- Diels Alder Practice Problems
- Molecular Orbital Theory Practice
18 Aromaticity
- Introduction To Aromaticity
- Rules For Aromaticity
- Huckel's Rule: What Does 4n+2 Mean?
- Aromatic, Non-Aromatic, or Antiaromatic? Some Practice Problems
- Antiaromatic Compounds and Antiaromaticity
- The Pi Molecular Orbitals of Benzene
- The Pi Molecular Orbitals of Cyclobutadiene
- Frost Circles
- Aromaticity Practice Quizzes
19 Reactions of Aromatic Molecules
- Electrophilic Aromatic Substitution: Introduction
- Activating and Deactivating Groups In Electrophilic Aromatic Substitution
- Electrophilic Aromatic Substitution - The Mechanism
- Ortho-, Para- and Meta- Directors in Electrophilic Aromatic Substitution
- Understanding Ortho, Para, and Meta Directors
- Why are halogens ortho- para- directors?
- Disubstituted Benzenes: The Strongest Electron-Donor "Wins"
- Electrophilic Aromatic Substitutions (1) - Halogenation of Benzene
- Electrophilic Aromatic Substitutions (2) - Nitration and Sulfonation
- EAS Reactions (3) - Friedel-Crafts Acylation and Friedel-Crafts Alkylation
- Intramolecular Friedel-Crafts Reactions
- Nucleophilic Aromatic Substitution (NAS)
- Nucleophilic Aromatic Substitution (2) - The Benzyne Mechanism
- Reactions on the "Benzylic" Carbon: Bromination And Oxidation
- The Wolff-Kishner, Clemmensen, And Other Carbonyl Reductions
- More Reactions on the Aromatic Sidechain: Reduction of Nitro Groups and the Baeyer Villiger
- Aromatic Synthesis (1) - "Order Of Operations"
- Synthesis of Benzene Derivatives (2) - Polarity Reversal
- Aromatic Synthesis (3) - Sulfonyl Blocking Groups
- Birch Reduction
- Synthesis (7): Reaction Map of Benzene and Related Aromatic Compounds
- Aromatic Reactions and Synthesis Practice
- Electrophilic Aromatic Substitution Practice Problems
20 Aldehydes and Ketones
- What's The Alpha Carbon In Carbonyl Compounds?
- Nucleophilic Addition To Carbonyls
- Aldehydes and Ketones: 14 Reactions With The Same Mechanism
- Sodium Borohydride (NaBH4) Reduction of Aldehydes and Ketones
- Grignard Reagents For Addition To Aldehydes and Ketones
- Wittig Reaction
- Hydrates, Hemiacetals, and Acetals
- Imines - Properties, Formation, Reactions, and Mechanisms
- All About Enamines
- Breaking Down Carbonyl Reaction Mechanisms: Reactions of Anionic Nucleophiles (Part 2)
- Aldehydes Ketones Reaction Practice
21 Carboxylic Acid Derivatives
- Nucleophilic Acyl Substitution (With Negatively Charged Nucleophiles)
- Addition-Elimination Mechanisms With Neutral Nucleophiles (Including Acid Catalysis)
- Basic Hydrolysis of Esters - Saponification
- Transesterification
- Proton Transfer
- Fischer Esterification - Carboxylic Acid to Ester Under Acidic Conditions
- Lithium Aluminum Hydride (LiAlH4) For Reduction of Carboxylic Acid Derivatives
- LiAlH[Ot-Bu]3 For The Reduction of Acid Halides To Aldehydes
- Di-isobutyl Aluminum Hydride (DIBAL) For The Partial Reduction of Esters and Nitriles
- Amide Hydrolysis
- Thionyl Chloride (SOCl2)
- Diazomethane (CH2N2)
- Carbonyl Chemistry: Learn Six Mechanisms For the Price Of One
- Making Music With Mechanisms (PADPED)
- Carboxylic Acid Derivatives Practice Questions
22 Enols and Enolates
- Keto-Enol Tautomerism
- Enolates - Formation, Stability, and Simple Reactions
- Kinetic Versus Thermodynamic Enolates
- Aldol Addition and Condensation Reactions
- Reactions of Enols - Acid-Catalyzed Aldol, Halogenation, and Mannich Reactions
- Claisen Condensation and Dieckmann Condensation
- Decarboxylation
- The Malonic Ester and Acetoacetic Ester Synthesis
- The Michael Addition Reaction and Conjugate Addition
- The Robinson Annulation
- Haloform Reaction
- The Hell–Volhard–Zelinsky Reaction
- Enols and Enolates Practice Quizzes
- The Amide Functional Group: Properties, Synthesis, and Nomenclature
- Basicity of Amines And pKaH
- 5 Key Basicity Trends of Amines
- The Mesomeric Effect And Aromatic Amines
- Nucleophilicity of Amines
- Alkylation of Amines (Sucks!)
- Reductive Amination
- The Gabriel Synthesis
- Some Reactions of Azides
- The Hofmann Elimination
- The Hofmann and Curtius Rearrangements
- The Cope Elimination
- Protecting Groups for Amines - Carbamates
- The Strecker Synthesis of Amino Acids
- Introduction to Peptide Synthesis
- Reactions of Diazonium Salts: Sandmeyer and Related Reactions
- Amine Practice Questions
24 Carbohydrates
- D and L Notation For Sugars
- Pyranoses and Furanoses: Ring-Chain Tautomerism In Sugars
- What is Mutarotation?
- Reducing Sugars
- The Big Damn Post Of Carbohydrate-Related Chemistry Definitions
- The Haworth Projection
- Converting a Fischer Projection To A Haworth (And Vice Versa)
- Reactions of Sugars: Glycosylation and Protection
- The Ruff Degradation and Kiliani-Fischer Synthesis
- Isoelectric Points of Amino Acids (and How To Calculate Them)
- Carbohydrates Practice
- Amino Acid Quizzes
25 Fun and Miscellaneous
- A Gallery of Some Interesting Molecules From Nature
- Screw Organic Chemistry, I'm Just Going To Write About Cats
- On Cats, Part 1: Conformations and Configurations
- On Cats, Part 2: Cat Line Diagrams
- On Cats, Part 4: Enantiocats
- On Cats, Part 6: Stereocenters
- Organic Chemistry Is Shit
- The Organic Chemistry Behind "The Pill"
- Maybe they should call them, "Formal Wins" ?
- Why Do Organic Chemists Use Kilocalories?
- The Principle of Least Effort
- Organic Chemistry GIFS - Resonance Forms
- Reproducibility In Organic Chemistry
- What Holds The Nucleus Together?
- How Reactions Are Like Music
- Organic Chemistry and the New MCAT
26 Organic Chemistry Tips and Tricks
- Common Mistakes: Formal Charges Can Mislead
- Partial Charges Give Clues About Electron Flow
- Draw The Ugly Version First
- Organic Chemistry Study Tips: Learn the Trends
- The 8 Types of Arrows In Organic Chemistry, Explained
- Top 10 Skills To Master Before An Organic Chemistry 2 Final
- Common Mistakes with Carbonyls: Carboxylic Acids... Are Acids!
- Planning Organic Synthesis With "Reaction Maps"
- Alkene Addition Pattern #1: The "Carbocation Pathway"
- Alkene Addition Pattern #2: The "Three-Membered Ring" Pathway
- Alkene Addition Pattern #3: The "Concerted" Pathway
- Number Your Carbons!
- The 4 Major Classes of Reactions in Org 1
- How (and why) electrons flow
- Grossman's Rule
- Three Exam Tips
- A 3-Step Method For Thinking Through Synthesis Problems
- Putting It Together
- Putting Diels-Alder Products in Perspective
- The Ups and Downs of Cyclohexanes
- The Most Annoying Exceptions in Org 1 (Part 1)
- The Most Annoying Exceptions in Org 1 (Part 2)
- The Marriage May Be Bad, But the Divorce Still Costs Money
- 9 Nomenclature Conventions To Know
- Nucleophile attacks Electrophile
27 Case Studies of Successful O-Chem Students
- Success Stories: How Corina Got The The "Hard" Professor - And Got An A+ Anyway
- How Helena Aced Organic Chemistry
- From a "Drop" To B+ in Org 2 – How A Hard Working Student Turned It Around
- How Serge Aced Organic Chemistry
- Success Stories: How Zach Aced Organic Chemistry 1
- Success Stories: How Kari Went From C– to B+
- How Esther Bounced Back From a "C" To Get A's In Organic Chemistry 1 And 2
- How Tyrell Got The Highest Grade In Her Organic Chemistry Course
- This Is Why Students Use Flashcards
- Success Stories: How Stu Aced Organic Chemistry
- How John Pulled Up His Organic Chemistry Exam Grades
- Success Stories: How Nathan Aced Organic Chemistry (Without It Taking Over His Life)
- How Chris Aced Org 1 and Org 2
- Interview: How Jay Got an A+ In Organic Chemistry
- How to Do Well in Organic Chemistry: One Student's Advice
- "America's Top TA" Shares His Secrets For Teaching O-Chem
- "Organic Chemistry Is Like..." - A Few Metaphors
- How To Do Well In Organic Chemistry: Advice From A Tutor
- Guest post: "I went from being afraid of tests to actually looking forward to them".
See what’s inside
Always rigorous....
Often Irreverent...

Occasionally Profane
Cincinnati Public High School
I am a high school teacher in Cincinnati. I always start my class on MOC.

Binghampton U.
These guides are awesome. Clear and concise. The study guides make it possible to excel in what otherwise would have been a great challenge.

Cal State East Bay
I have recommended your site to many of my classmates who have asked me "what my secret was"! Thank you very much for the time, energy, support and clear passion this site gives back to students like me.

Wayne State University
Thank you, not only for preventing OChem from being a "weeder" class for me, but also for making it an enjoyable journey. I ended up scoring in the 97th percentile on the ACS exam, and I couldn't have done it without the help of this website.

Colorado State University
By the way I wish I would have made the move on your joining your site earlier in the semester. It would have made things a little bit easier. I joined about 2 weeks before finals and ended up getting an A in the class!

Christian M.
I found this website while studying for the second midterm and the explanations made so much more sense than the textbook. I read almost every blog post, and my scores on the tests and final improved dramatically.

Manuel E. (with Nobel Laureate Kip Thorne)
East Los Angeles College
The study guides have helped me in so many angles that helped me improve my grades, lessen my anxiety, and improved my overall confidence. Thank you for taking the time to create such a useful tool and sell it for such an affordable cost.

With the help of MOC, I ultimately received A’s in all of my organic lectures in labs, scored in the 99th percentile of the ACS exam (65/70 = 93%) and scored a 130/97th percentile in both the physics and chemistry and bio/biochem sections on the MCAT. Thank you.

St. Joseph's University
I'm a biologist who has worked in chem labs most of my career. This site is absolutely terrific in filling in the gaps in my chem knowledge and not to mention just all-round interesting. Keep up the good work!

University of South Florida
Thank you so much for this service! I finished Orgo 2 with a B+ because of these guides!

Saint Louis University
I had a test for orgo in exactly one week. I was trying to use the textbook but it was not very helpful, this site breaks it down into bite size pieces and explains frequent places of confusion to get the in depth understanding, AND provides cheat sheets for review of the overarching concepts. This is just awesome.

Chemistry Olympiad
I was selected to represent my country in the International Chemistry Olympiad in 2017 and in 2018. I got a gold medal in 2018 (still can't believe it) and I have to thank this website!

Penn State University
A friend at my university informed me about this website and said it was the only reason he passed organic chemistry!

TaraRochfordNutrition.com
The study materials on MOC were a lifesaver. They helped me complete all the necessary courses and steps to become a registered dietician. Thank you so much!

Courtney E.
Central Washington University
I stumbled across this website a couple years ago, seeking help in preparation for my general chemistry ACS exam, and it was helpful then so I've remembered it again and again for other classes, and am glad I can use it again now for organic chemistry.

LaMar University
The cheat sheets have been a great reference for my studies, as well as the reaction guide. Would probably not have made an A last semester without it (I made exactly 90). I did it with the more difficult organic professor too!

Utah State University
I love everything I've used so far. I used the reaction guide for O Chem 1, and it saved my butt.

University of Buffalo
For a night owl who really only studies at night, it is often impossible to find a professor or TA who is awake to extinguish my burning questions, and even difficult to find a knowledgable friend. This site is that friend.

Lee University
I made an A in my Organic 2 class - this website was an invaluable resource!! Thanks so much.

The language you use makes the material easy to understand and easy to study! Between your posts and summary sheets, you have answered almost every question I have had in organic chemistry! MOC is such a lifesaver (and grade-saver)!

University of Pittsburgh
MOC was my best friend this past semester. Wouldn’t have gotten an A- without it.

Thank you so much for such an awesome site! This is the reason I got an A in ochem one and two! Thank you!

Florida Atlantic U.
Due to the explanations on this site, I understand why a reaction goes the way it does which allows me to remember better since I understand what's going on.

McMurdo Station, Antarctica
The site has been especially helpful for me in teaching basic O-chem and medicinal chemistry down here in Antarctica. (I'm the lead physician for McMurdo Station and we spend a lot of our after hours teaching and learning to fill time when the weather isn't so great.) From the bottom of the world - thanks!

Wright State University
We had final exams this week and I just thought I would let you know that with the help of your fantastic website and the summary sheets I purchased, I finished organic chemistry 1 with a 99.5 average . Classmates were upset with me for being the curve buster. I blamed it on you and derected many of them to check out your website!

Virginia Commonwealth University
The summary sheets let me quickly review everything I needed for my final exam. I was able to score a 54/70 on a final where the class average was 20/70.

Michelle T.
Simon Fraser University
Master organic chemistry literally taught me everything I needed to know for my ochem 1 course. I just wish I found it earlier! Would've helped me so much on my midterm.

California State University Long Beach
This website has become my savior. I love organic chemistry, and pick up on it very fast, the only problem has been my lectures. Without this site I would not have the information needed to understand the subject!

West Chester University
I recently purchased the organic chemistry guide. I finally understand why things are doing what they are doing. I wish I would've had this numerous organic chemistry attempts ago.

University of Connecticut
I went from a 40 on exam 2 to a 90 on exam 3 as a result of focusing on the big picture and applying the concepts to the questions. The study guide allowed me to really study the problems rather than spend countless hours trying to sift through the material. I also ended up with a B+ in the class!
Case Studies Of Successful Students
Last semester I had the pleasure of working with “Chris” (a pseudonym) on second-semester organic chemistry topics. Before we met, Chris had already obtained a
How Helena Got 93 In Organic Chemistry An Australian reader, Helena, recently wrote to say she’d earned a 93 in her organic chemistry class as
Over the past few weeks I’ve been corresponding with loyal reader Serge, who was happy to report to me recently that his exam grades are
From The Blog
Reaction maps now available.

350 Examples of Classic Org 1/Org 2 Reactions From “Organic Syntheses”

Organic Chemistry GIFS – Resonance Forms
James Ashenhurst
Founder, master organic chemistry.
After doing a Ph. D. in organic synthesis at McGill and a postdoc at MIT, I applied for faculty positions at universities during the Great Recession. It didn’t work out. But having seen first-hand how many people struggled with the subject (including myself when I took it as an undergraduate) I thought there was a need for an online organic chemistry resource that had all the rigor of a traditional textbook, but was more approachable and accessible.
Drawing on the experience of thousands of hours spent tutoring students 1-on-1, as well as dozens of case studies, Master Organic Chemistry aims to fill in some of the conceptual gaps that aren’t traditionally covered by textbooks, and provide a friendly, logical and thorough pathway for learning introductory organic chemistry.


Organic Chemistry Laboratory Techniques
(23 reviews)
Lisa Nichols, Butte Community College
Copyright Year: 2016
Publisher: Lisa Nichols
Language: English
Formats Available
Conditions of use.
Learn more about reviews.
Reviewed by Changqing Chen, Associate Professor, Salem State University on 11/30/22
This is a comprehensive lab manual that covers basic lab techniques in organic chemistry. Theory and procedures on major organic lab techniques were covered in detail. The step-by-step illustrations of experimental procedures made it easy for... read more
Comprehensiveness rating: 5 see less
This is a comprehensive lab manual that covers basic lab techniques in organic chemistry. Theory and procedures on major organic lab techniques were covered in detail. The step-by-step illustrations of experimental procedures made it easy for readers to follow. The colored pictures and graphs are very well labeled. Technique summaries are very helpful for readers who just needed a refresher. It would be better if more safety information was added to the text, especially in section 6.4 on chemical tests. Another suggestion is adding discussions on spectroscopic methods, such as infrared spectroscopy and nuclear magnetic resonance spectroscopy. More coverage of commonly encountered problems and their solutions would be very helpful for new learners.
Content Accuracy rating: 5
I didn't notice any content mistakes. Lab techniques were described accurately.
Relevance/Longevity rating: 5
Basic organic chemistry lab techniques are covered in this book. The information in the book will remain relevant for many years to come.
Clarity rating: 5
The text was very well-written. Lab techniques are well-presented and easy to follow. Colored pictures were used to clearly demonstrate steps in experimental procedures.
Consistency rating: 5
Information was presented consistently. Materials in different chapters were arranged similarly in the book, which made it easy for readers to follow.
Modularity rating: 5
Each chapter was clearly divided into different sections. Hyperlinks were provided for easy navigation. It is easy for instructors to assign any topic for students to read as a pre-lab assignment.
Organization/Structure/Flow rating: 5
Information in the book was organized logically and clearly. Text provided good depth and detail. The format and terminology followed conventions of chemistry lab manuals. The flow in writing is good, which makes reading enjoyable.
Interface rating: 5
The book was easy to navigate with provided hyperlinks as well as colored pictures and graphs.
Grammatical Errors rating: 5
Grammatical errors were not found in the book.
Cultural Relevance rating: 5
The text is not culturally insensitive or offensive.
I really appreciate the author’s effort in providing a great open educational resource on lab techniques in organic chemistry!
Reviewed by Swati Mohan, Lecturer and research scientist, University of Texas Rio Grande Valley on 12/14/21
The book is highly comprehensive for the introductory basic level of organic lab. Picture of each lab apparatus and technique makes this book highly conceptual. The book must have a few questions after each technique which gives students to... read more
The book is highly comprehensive for the introductory basic level of organic lab. Picture of each lab apparatus and technique makes this book highly conceptual. The book must have a few questions after each technique which gives students to challenge to learn well. overall highly informative due to the picture procedure.
I did not find any issue with the content. Information about lab and techniques looks accurate to me
This is book is highly relevant due to the picture procedure and picture techniques. Picture procedure gives a wide idea to our students to learn lab techniques. According to me highly recommended for organic labs.
Very clear due to colored picture and readable.
Strongly consistent throughout the entire content.
Book content is divided very well into major divisions (Different labs ) and subdivisions (pic procedure and concept). The table of content has a link that takes you quickly on the particular technique to which you are looking for. Very easy for the undergraduate level to understand the basic fundamentals of organic labs.
The book is well organized. All lab techniques are clear and understandable for the reader
I didn't observe any interface issues. The link given in the table of content is navigated you well through the book.
I did not find any grammatical error
The book is very informative for all readers throughout the world. Book content did not include any type of cultural intention.
Reviewed by Lara Al-Hariri, Senior lecturer I, University of Massachusetts Amherst on 6/30/21
This is a very comprehensive organic chemistry lab manual on basic techniques. The author effectively used illustrations to show each technique's step-by-step procedure, including some aspects that are usually not mentioned in others textbooks.... read more
This is a very comprehensive organic chemistry lab manual on basic techniques. The author effectively used illustrations to show each technique's step-by-step procedure, including some aspects that are usually not mentioned in others textbooks. That will be very helpful for students as they are preparing for the labs. However, the book doesn't have a dedicated section for general safety, which is a must in such labs. The safety notes listed within a chapter or procedure should be more emphasized.
The textbook is accurate content-wise. I didn't see any content mistake.
Relevance/Longevity rating: 4
The content of the textbook is up-to-date and relevant to basic organic chemistry lab techniques. In addition, it provides good overviews of each method that will increase students' engagement in the lab technique and help them see its relevance.
Clarity rating: 4
The textbook is written in a language comparable to other textbook on the topic of basic organic lab techniques. It is easy to read and follow the steps. I think the plenty of illustration and lab setup pictures make this textbook much better than other. It is beneficial that the author provided examples of the “wrong way to do,”; which is rarely addressed in the textbook on this topic.
Consistency rating: 4
The text is consistent in terms of terms and can be easily adopted even if the instructor teaches/performs the chapters/experiments in a different order than that represented in the text
Modularity rating: 4
It has an easy-to-navigate structure. Therefore, it can be used with labs in which the learning outcomes are structured differently.
Organization/Structure/Flow rating: 4
It is well organized.
Interface rating: 4
The interface is user-friendly and all the images/tables/charts are displayed properly. The hyperlinks are very useful to find the topics.
Grammatical Errors rating: 4
I didn't see any grammatical mistakes.
There is not information relating to cultural relevance in the text which is comparable to other textbook in this field.
Reviewed by Armanda Formigao Gameiro, Visiting Assistant Professor, University of Massachusetts Amherst on 6/2/21
This is a very comprehensive book, only lacking in an important aspect which is the microscale techniques and appropriate glassware. That part is absent, there is a description of how to use certain techniques in a microscale way but not with the... read more
Comprehensiveness rating: 4 see less
This is a very comprehensive book, only lacking in an important aspect which is the microscale techniques and appropriate glassware. That part is absent, there is a description of how to use certain techniques in a microscale way but not with the microscale glassware kits that are common in labs nowadays to minimize the use of reactants and have a more green organic chemistry lab. That said, this book is very good presenting the lab techniques used in Organic chemistry. Another point I would like to mention is that a clear section on safety is lacking and safety tips appear in the text together with the rest and that is not good. They should be highlighted in boxes at the top of each section.
I think this book is very good in that regard. I did not see any mistakes and the information presented is accurate, both the pictures and text.
The techniques presented in this book are very relevant for Organic Chemistry labs. This is an excellent overview of the techniques. The only issue I have is that microscale glassware is not present and that is a future trend and is lacking in this book.
The book is very clear and has plenty of illustrations in very detail to go with the text. In particular I like the comparison of the "right way to do" versus "wrong way to do". This is done in all sorts of situations like clamping glassware to set up an experiment, pipetting reactants into a round bottom flask etc. This I find one of the greatest strengths of the book. It would have been extremely useful to me when I taught Organic I and Organic II labs. It would have saved me much time to direct the students to the photos in the book.
This book is very consistent in terms of terminology.
This book works very well if one wants to teach only a particular technique, and the order of topics can be interchanged since each chapter has a very clear and thorough introduction. It can be used in sequence or it can be used according to the technique one wishes to teach/learn. Only the initial chapter should be read first to acquaint oneself with the different glassware and an overview of the techniques. As I mentioned before this book is lacking a description of microscale glassware and techniques.
The book is very well organized.
The books interface works very well. Hyperlinks work, photos are depicted correctly when clicked upon.
This book is well written with clarity in the writing. I saw no grammatical errors.
This book is about Organic chemistry techniques. It is not culturally insensitive or offensive in any way.
I think this is a very valuable resource. Because I taught Organic Chemistry labs using only microscale techniques this book would not be suitable for my courses as the only resource, but I would certainly recommend it to my students. The author has clearly put a lot of effort in the book and the result is a very good book. I wish I had known about this book when I was teaching Organic Labs, if I teach Organic Labs in the future I will certainly recommend parts of this book to my students.
Reviewed by Karen Glover, Professor, Clarke University on 1/7/21
This resource is one of the most complete manuals for organic techniques I have ever seen for the undergraduate level. It is comprehensive not only in the coverage of how to employ all the common OChem techniques, but it also illustrates common... read more
This resource is one of the most complete manuals for organic techniques I have ever seen for the undergraduate level. It is comprehensive not only in the coverage of how to employ all the common OChem techniques, but it also illustrates common pitfalls with an appropriate amount of detail. Hyperlinks are used strategically throughout the text to define potentially unfamiliar terms. I reviewed the book using Adobe, and was able to easily scan through the table of contents using bookmarks. I appreciated the one-page "brief" table of contents which displays pictures, chapter numbers and titles, page numbers, and hyperlinks to major topics. The one-page view quickly gives the reader a sense of what is in each chapter and an easy way to get there. The text also contains the traditional table of contents in detail with extensive hyperlinks for easy access.
I did not see any errors in content. I did not see any evidence of bias.
The content contains a delightful mix of long-standing, traditional techniques with newer ones. For example, finding the melting point with a Thiele tube is illustrated in addition to the use of an electrical melting point device. Similarly, the various methods for taking a boiling point are described. There is a section on qualitative tests of organic functional groups which I have not seen in more recently printed lab technique books. This text book presents those reliable, traditional methods in thoughtfully annotated pictures which gives the text the feel that this is something new. I find the treatment of a variety of methods make this textbook universally helpful.
The language used is concise and clear. An appropriate level of detail is included. Hyperlinks are available if additional information is needed about a referenced piece of equipment.
The text uses a unique color for title bars, headings and descriptions of figures in each of the seven chapters. At the beginning of each chapter, a photograph or series of photographs are used to showcase some process covered within the text. For example, a series of 12 photos at the beginning of Chapter 3 shows the time-lapse formation of benzil crystals from a cooling ethanol solution. The photos are clear and orderly arranged. Not only do the photos make a nice cover page, but they teach the reader as well. Throughout each chapter, the numbering scheme is consistent, as are indentations, and margins. The use of color-coordinated circled letters helps the reader distinguish the differences between similar parts of a larger scheme.
The text is modular and is easily divisible into smaller reading sections. Page numbers, bookmarks, hyperlinked titles are provided to give the reader a number of ways to navigate the text. The text is self referenced, although I found times when I lost my place after clicking on a hyperlink to check a definition. I didn't see a way to return to my spot in the text. I am reviewing this text in Adobe, so I do not know if this issue is present in other modes of reading the text. Perhaps there is a way to "go back" to a previous point in the text. If so, then I would rate this category higher.
The seven chapters are presented in a manner which make sense. Chapter 1 introduces general techniques and foundational methods and shows examples of typical glassware and equipment. Safety is discussed in this chapter and throughout. Chapter 2 through 5 include topics of chromatography, crystallization, extraction, and distillation. Since this is a book of techniques, the order of these chapters is unimportant. The book concludes with Chapter 6 on miscellaneous techniques, and Chapter 7 with one-page summaries of all the important techniques. Generally, the author has organized sections of each chapter with an overview of the technique, followed by uses and/or theory, then step-by-step procedures with a summary of the procedure at the end of the section. This level of redundancy is extraordinarily useful for those learners who may be performing a procedure in Organic lab for the first time. The summaries are useful for a quick reference for the more experienced learners.
The interface I am using works well (Adobe Acrobat Pro). I only wish there was a back button, so that after clicking on a hyperlink, I could easily find my place in the previous text.
I have not seen any grammatical errors.
The textbook is not culturally insensitive nor offensive. It is inclusive in that persons shown in the pictures appear to be from a variety of races, ethnicities, age groups and gender.
Kudos to the author for such a thorough treatment of the procedures. The level of detail, clarity of pictures and consistent color schemes makes this text visually appealing and extraordinarily useful. I love that the author attempts to connect some of the technique to the students everyday experience with the examples found in the overviews. When I first began teaching, I would require an expensive textbook which I would always have to supplement because the procedures they used didn't quite match up with the equipment I had available. I found a text book which I've used for many years, but it is out of print and expensive. Lisa Nichols' 2nd Edition of "Organic Chemistry Laboratory Techniques" is exactly what I've been looking for. Finally! Thank you for such an excellent product and for keeping students' needs in the forefront.
Reviewed by Christine Hermann, Chair, Full Professor, Radford University on 6/16/20
The book gives an excellent comprehensive view of the various techniques used in organic chemistry. It is well illustrated with a lot of pictures and explanations. The directions are very easy to follow and the steps are very detailed. read more
The book gives an excellent comprehensive view of the various techniques used in organic chemistry. It is well illustrated with a lot of pictures and explanations. The directions are very easy to follow and the steps are very detailed.
Content Accuracy rating: 4
Overall, the book gives an excellent overview of all techniques. However, all of the techniques should be demonstrated in a hood, not a lab bench. A hood is much safer than a lab bench in the middle of the laboratory. Section 1.1D talks about greasing joints, which contaminates products. Teflon tape is better. For cleaning glassware, use a waste jar, not a waste beaker. The text tells students to leave wet glassware on paper towels. In our case, the glassware then accumulates by the sinks. It is better to tell students to put the glassware away. Distillations in Figure 1.3a, Figure 5.2, Figure 5.37, and others show a graduated cylinder as a receiver. This is not a proper receiver. Table 1.6 – the Bunsen burner should be standing underneath the flask, not being held.
This book will not become obsolete. These techniques have been around for a long time, and will continue to be used in organic chemistry. The drawings and explanations are excellent and are easy to follow.
The directions are written in an easy-to-follow format, where any student can easily follow the steps and be successful in the lab. The drawings and pictures illustrate the text in a very informative manner.
The book is consistent throughout in the presentation of material. The steps are well-written and easy to follow.
The chapters are divided into sections, which are easy to read. A professor could easily assign any chapter or any section for a student to read before the lab. I would not reorganize the text, rather just reference a section for a student to review prior to using that particular technique in lab.
The topics are in a logical manner in the way that they would be taught in a typical organic chemistry laboratory. The simple techniques are at the beginning of the chapter, then the more complicated techniques are later.
The drawings and pictures are a consistent size throughout the textbook. Navigation through the chapters are very easy, since it is well organized by technique. I found it easy to find any technique that I was interested in. All drawings and pictures have text accompanying them to explain the technique stepwise.
I did not find any grammatical errors.
Cultural Relevance rating: 4
A textbook on chemistry techniques is mostly a textbook of pictures of equipment. Perhaps in a future edition, include a variety of races, ethnicities, and backgrounds. Frankly, I was not looking at the people in the pictures, but the drawings and steps.
Excellent textbook for organic lab techniques. There are a few minor corrections that need to be done, but otherwise it is outstanding, and well-written.
Reviewed by Steve Acquah, Associate Research Professor, University of Massachusetts Amherst on 6/11/20
This is a comprehensive chemistry laboratory manual, and I think the extensive use of pictures makes it more engaging as a resource for educators. The “Safety note” sections should be boxed and emphasized. The theory sections are comprehensive,... read more
This is a comprehensive chemistry laboratory manual, and I think the extensive use of pictures makes it more engaging as a resource for educators. The “Safety note” sections should be boxed and emphasized. The theory sections are comprehensive, especially the section on fractional distillation. This organic chemistry laboratory techniques manual is a beneficial resource for experimental work and the reinforcement of good practices.
The text and images provide an accurate representation of the experimental procedures. Techniques may vary at different institutions, and it may be possible to highlight variations and evaluate these methods.
This manual helps to establish a critical foundation in laboratory knowledge and practice. The content is appropriate and addresses the current techniques with the potential for additional supporting activities.
The text is clear to understand and provides support with informative pictures and diagrams. Summaries and concepts could be more concise with additional formatting to break up the text and create more digestible sections to aid student focus.
The order and structure of the text are consistent throughout the chapters of the manual.
There is a modular structure that helps the user navigate the concepts and techniques. To further enhance the modular structure, there could be more infographics and highlighted sections. Using infographics and color-coded boxed articles across the entire manual would help to identify similar themes such as safety and methods quickly. It would make these sections faster to identify and refer to during an experimental procedure.
The sections and related topics follow a clear and logical structure with a natural flow from experimental procedures to the summary.
The hyperlinks provide essential navigation through the text to better understand the principles of the experimental procedures.
No grammatical errors were identified.
No information relating to cultural relevance was presented in this text, although the inclusion of relatable ideas helps convey the experiences of the author to that of the reader. Cultural differences in techniques could be a useful future expansion to the text.
Overall, this is an essential laboratory manual for organic chemistry students.
Reviewed by Mark Stocksdale, Professor, Earlham College on 12/13/19
This text is very complete and comprehensive for what it is covering. Virtually all organic chemistry wet-lab methods undergraduate students will encounter are presented. The extensive use of photos of actual glassware and techniques... read more
This text is very complete and comprehensive for what it is covering. Virtually all organic chemistry wet-lab methods undergraduate students will encounter are presented. The extensive use of photos of actual glassware and techniques demonstrated are very helpful and clear. Many of the methods described/demonstrated will also serve students well in other laboratory courses. For example, the inert atmosphere handling of reagents and reaction set-ups would find excellent use in inorganic lab courses. This text could/would be a very helpful reference text to be used throughout a student’s undergraduate work and even their graduate work. The frequent examples of “correct” vs. “incorrect” techniques are very clear and reflect many of the common mistakes and errors students make when first learning a new technique. Even something as simple as reminding students to keep Pasteur pipettes vertical to minimize dripping is addressed. And real-world advice is given throughout the text that is welcome and relevant. Who hasn’t broken a pipette during column chromatography? If the trouble-shooting suggestions fail, then the text offers the following correct advice: “If the sample has already been applied, there isn’t anything to do but continue on and hope for the best.” The summaries section at the end is also a great quick-access resource for students returning to a technique and looking for a quick reminder.
The methods/techniques are correctly and adequately presented.
All of the methods are relevant and should continue to be. Even the Chemical Tests section (6.4) is presented in such a way to be used along side and complement modern spectroscopy methods.
The text is very clear and readable. The extensive use of accurate and relevant photographs is excellent.
All material is presented in a consistent format.
Yes, sections of the text can be quickly accessed and assigned for a variety of learning/teaching situations.
Yes, the text is very clear and logical. Topics are introduced with a clear overview and then each is expanded appropriately.
No interface issues were detected/discovered.
No grammatical errors were detected/discovered.
No culturally insensitive or inappropriate materials were detected/discovered.
Reviewed by Olufunke Olagunju, Associate Professor, Thomas Nelson Community College on 11/4/19
Comprehensiveness • I think there should be introductory materials/concepts in every organic chemistry lab textbook. Concepts like, safety in the lab, laboratory notebook and pre lab information. I did not find items like these in the... read more
Comprehensiveness rating: 3 see less
Comprehensiveness • I think there should be introductory materials/concepts in every organic chemistry lab textbook. Concepts like, safety in the lab, laboratory notebook and pre lab information. I did not find items like these in the book. • Spectrometric methods, like Infrared spectroscopy, NMR spectroscopy, Mass spectroscopy are missing from this text book. • There is no index, nor glossary in the book. The last chapter has different organic chemistry lab technique summary, maybe this is intended to be an index. This review is not intended to discredit this text book. It is intended to help transform the book into a more robust reference material for organic chemistry students.
The content covered are accurate and adequately covered. I did not find any error.
The text focused on organic chemistry lab techniques. Techniques covered are up-to-date, there is no fear the book will become obsolete within a short period of time. Updates in organic chemistry lab technique text book, like green chemistry should be easy to add to the textbook.
The text is expressed clearly, easy to understand with lots of pictures of students performing different lab techniques. This makes the technical terms/concepts more comprehensible.
The text is internally consistent in terms of terminology and framework. There are overviews and summaries given for each concept covered, this is consistently done throughout the text, I think this helps develop student’s interest to learn more.
The text is easily and readily divisible into smaller reading sections that can be assigned at different points within the course (i.e., enormous blocks of text without subheadings should be avoided). The text should not be overly self-referential and should be easily reorganized and realigned with various subunits of a course without presenting much disruption to the reader. I agree.
The topics in the text are presented in a logical, clear fashion. Related basic methods, like separation, purification, and analysis are presented adequately in appropriate chapters.
There are quick links on the text. These links provide for easy access to concepts. Lots of pictures, some complex ideas can be expressed with just a single picture, thereby making the book easy to understand.
I did not find any culturally insensitive material(s) in the text.
Reviewed by Erik Larsen, Assistant Professor, Bloomsburg University of Pennsylvania on 3/15/19
Extremely comprehensive, with a large number of photographs that provide a detailed graphical complement to the text. Virtually every major technique and many minor ones are covered, to the degree that this would serve as not only a comprehensive... read more
Extremely comprehensive, with a large number of photographs that provide a detailed graphical complement to the text. Virtually every major technique and many minor ones are covered, to the degree that this would serve as not only a comprehensive undergraduate textbook but an introductory graduate one.
I did not notice any inaccuracies in the text. The equipment shown in photographs will likely vary from institution to institution, but this can be easily adjusted during pre-lab discussions.
Basic organic chemistry techniques have not changed significantly in quite some time, but this book's signature achievement is in the inclusion of color photographs as opposed to the black-and-white images often found in older texts. Many of the advances being made in modern techniques come in the form of automation of existing techniques via robotics. In the event that these instruments become inexpensive enough to incorporate into an undergraduate laboratory, updating the text should be easy since it would typically involve subtraction rather than addition.
I found the book easy to read and follow, with minimal jargon.
I did not find any inconsistencies with regard to tone or content.
Techniques are neatly divided into several major categories and then subdivided further into specific procedures. The table of contents has clickable links, which makes it very easy to get to a specific technique quickly. Subdividing this book into individual sections to assign for a laboratory course would be very simple.
Each chapter provides a general overview of the technique family and some of the underlying theory before delving deeper into the specifics of each technique. The technique summaries provided at the end of the book are very useful refreshers, and I will likely be referring students to it often.
The hyperlinks within the Table of Contents makes navigating the book very easy. I did not find any images to be distorted or unreadable.
I found no grammatical or spelling errors during my read.
This book focuses exclusively on scientific technique. The text is suitable for anyone in a chemistry course, regardless of race, ethnicity, or background.
This is one of the most useful lab technique books I have ever come across, and something that I wish I'd had as both an undergraduate and a beginning graduate student. I will be assigning this book in all of my future lab courses, and if the author decides to include a section on spectroscopy in the future with the same level of detail I may begin incorporating it into the lecture portion too.
Reviewed by Terry Fernando, Lecturer and Teaching Lab Coordinator, Iowa State University on 11/19/18
Very comprehensive for introductory organic chemistry lab course read more
Very comprehensive for introductory organic chemistry lab course
I did not read everything, but what I looked at was accurate.
The focus on commonly used equipment and glassware in the organic chemistry teaching lab makes this book very relevant.
Very readable.
Very consistent framework.
Modularity rating: 3
Text adjacent to applicable photos might be easier for some to read rather than having photos clustered.
The organization was clear and easy to follow.
The book was straight forward to navigate.
I did not encounter grammatical errors.
The book did not seem culturally insensitive.
The detailed photos make this book unique and very useful for students. The only suggestion I have is to add separate trouble shooting guides/tables for each technique.
Reviewed by Kelli Slunt, Professor, University of Mary Washington on 6/19/18
The text covers most major laboratory techniques utilized in an organic chemistry laboratory. The book provides a detailed table of contents with links enabling easy movement within the text. There are a few topics that would enhance the book. ... read more
The text covers most major laboratory techniques utilized in an organic chemistry laboratory. The book provides a detailed table of contents with links enabling easy movement within the text. There are a few topics that would enhance the book. Within the individual sections, the author describes appropriate safety related to the presented techniques. An overall safety section at the beginning of the textbook would be useful to help set the stage and review the important laboratory safety prior to starting any experiments. In addition, nuclear magnetic resonance (NMR) spectra are provided for some of the techniques but there is no details about how to obtain an NMR or even an IR spectrum, which are key characterization techniques in organic chemistry.
To the best of my knowledge the book is highly accurate. The material presented in the text is well written, unbiased, and does not contain any obvious errors or inaccuracies. The images and equipment used were institution-specific but the author tries to make note, when appropriate, about how the piece of equipment available may differ and encourages the students to consult with their instructors.
The text is written in a manner that is relevant for most introductory organic chemistry courses. The techniques presented are universal methods for handling, purifying, heating, and analyzing organic chemicals. The textbook should not become obsolete in the next decade or so. As the text is already in a second edition, the author is willing and able to update as necessary.
The text is written in a manner that would be clear for a student enrolled in an organic chemistry course.
The author writes in a consistent manner in terms of terminology and language throughout the entire text.
The textbook is organized into appropriate sections devoted to the different organic chemistry laboratory techniques presented. Sections of the text can be easily assigned in advance of a laboratory course.
The overall organization of the text was presented in a logical and clear fashion. In a few sections, re-organization of the information may provide a greater clarity for the students. For example, on page 86, the author presents the visualization of TLC on the plate under UV light. A description of visualization of the plate under UV light does not appear until page 107. Either the material could be reorganized or at least a link provided to the later part of the section that explains the theory behind the visualization.
The text is a well organized pdf file. The file contains links that can be clicked to help navigate between techniques. One concern about an online laboratory manual file is use in a laboratory setting. Organic chemicals could be corrosive to an electronic device which leads to challenges with bringing cell phones, tablets, or computers into the lab. One useful part of the textbook is the summary pages. One might print these pages to bring to lab hence eliminating the potential risk of damage to the electronic device.
To the best of my knowledge, the book is free of grammatical errors.
Organic chemistry is a topic that does not involve discussion of race, ethnicity, or cultural backgrounds. The author does include images of students working in an organic chemistry laboratory and the students portrayed are visually diverse in terms of gender, possible age, and backgrounds.
Reviewed by Matthew Grote, Adjunct Professor, Otterbein University on 5/21/18
This book achieves a level of comprehensiveness that I have not seen in an Organic Chemistry lab techniques book before. Every relevant technique is covered in exhaustive detail with plenty of clear color photographs to accompany the text. As a... read more
This book achieves a level of comprehensiveness that I have not seen in an Organic Chemistry lab techniques book before. Every relevant technique is covered in exhaustive detail with plenty of clear color photographs to accompany the text. As a lab techniques reference it is absolutely singular; even minor and esoteric techniques are covered in such detail that an undergraduate student would be able to (with appropriate practice, of course) master that technique. My sole complaint is that it does not contain practice experiments, but this is a minor complaint. Indeed, if this book contained relevant experiments within the field it would become a standalone organic chemistry lab textbook.
There are no errors that I could see, and the information presented is accurate to my eyes.
This is where this book truly stands out; Leonard, Lygo, and Proctor’s “Advanced Practical Organic Chemistry” has long been my go-to reference text, but it suffers from the common pitfall of a traditional text: the pictures are black-and-white. This book, because it is in digital format, isn’t limited to cheap-to-print black and white, but instead has full color pictures, and a lot of them. As a living digital document, then, there is no expiration date (provided the author chooses to update or allows for derivatives to be produced.) As a long-time industrial chemist, I have seen my own practice change from stringent adherence to literature methods to more of a freestyle approach that accomplishes what is necessary in the minimum amount of time. This book presents both paradigms; the time-tested approach as well as the slightly cruder but much more time efficient method. As a reference, then, it is unquestionably relevant to the practice of experimental organic chemistry.
At the risk of sounding repetitive, the pictures make the difference in terms of clarity. I found the writing style to be clear and (dare I say) entertaining, but it is the absolute wealth of pictures that enhance the clarity well above any reference that I’ve seen to date.
I found the book to be consistent in its use of language and structure, which makes it truly useful as a reference text.
Experimental organic chemistry demands a reference that is modular, as the first semester is typically a modular exploration of technique. This book does a fantastic job of separating the subject into its components and then exploring those components in detail. It would be fantastically easy to assign reading before an experiment.
I found the book to be sensibly organized and easy to follow. The table of contents is appropriately detailed. The order that the information is presented in is sensible as well.
There are no interface related issues that I am aware of.
There are no grammar related issues that I am aware of.
The book is broadly culturally relevant to my eyes; anyone who is or is becoming appropriately skilled in the art should be able to use this book without offence.
I found this book to be utterly fantastic and will be adopting it for use in the coming fall semester.
Reviewed by Anna Manukyan, Assistant Professor, Hostos Community Collge of CUNY on 3/27/18
The organic lab manual provides detailed and comprehensive material of general organic laboratory techniques and instrumentation. It contains information about most instruments, and provides detailed explanation of their use. Having pictures... read more
The organic lab manual provides detailed and comprehensive material of general organic laboratory techniques and instrumentation. It contains information about most instruments, and provides detailed explanation of their use. Having pictures alongside the text is very helpful, and it could have been supplemented with links to videos if available. Safety is very important in the lab, and when working with students in organic labs, often they have very little information about safe techniques or disposal. Some lab manuals provide that info, which is also helpful for the instructor. I liked that this manual also provided details on safety. Also, each lab or topic ends with a short summary, which is very useful for students when they just need a quick refresher, especially for returning students. The lab manual also compares different techniques, and highlights their use in specific cases, which is great if the students are involved in independent work or research.
The lab manual accurately describes and explains the major laboratory techniques and instrumentation. There may be some differences on how certain procedures should be performed, but it is mostly a question of style and/or preference. Minor differences in techniques are expected even in different institutions and/or labs.
The organic lab techniques are not going to change drastically in the next 20-30 years. It is possible that the instruments will get a news and easy to use interface, but having an understanding of the scientific principles behind the techniques/instruments is essential for students’ learning. Also, if green solvents are introduced into the experiments, some techniques might change, but not to the extent that the entire textbook needs to be revised.
The writing style was very pleasant and easy to read. Having pictures certainly made it very enjoyable. The terminology was properly used as well.
The text was consistent, as you would expect from a single author work. The sections were arranged similarly throughout the manual making it easier to navigate and locate information.
The manual is well-organized. It is also easier to use when other textbooks, so it would be very useful for returning as well as new students- from introductory to advanced levels.
The organization follow from basic to advanced techniques. Some instructors might not follow the same order of experiments but that applies to basically any textbook.
This manual, like most OER textbooks I have looked at, was converted from a word document to pdf, and it would have been helpful to link the experiments in the Table of Contents to the page of the experiment. Otherwise, I had to scroll through many pages to go where I want. Jumping to the page did not always land where needed.
There might have been some grammatical mistakes, but I did not notice while reading it.
Not applicable since this is a technical document.
Excellent resource for introductory as well as advanced chemistry students.
Reviewed by Constance Franklin, Assistant professor, Organic chemistry laboratory coordinator, Virginia Commonwealth University on 3/27/18
This book does an excellent job of covering all of the basic techniques used in an organic chemistry lab. Each section is written so that it can stand alone which I think makes it a great resource to add to an existing course. read more
This book does an excellent job of covering all of the basic techniques used in an organic chemistry lab. Each section is written so that it can stand alone which I think makes it a great resource to add to an existing course.
I found this book to be an accurate guide on lab techniques. Because there is alot of variation in the field, there is not always one right way to do something, and this book covers multiple options that are commonly used in the field. It also makes a point to add in cases where students should seek clarification from the professor, which makes it very versatile.
This textbook includes both classic lab techniques and more modern variations. I don't think it will be an issue to update it over time. It does not always include options for an older lab space, but I only consider that a minor drawback.
This textbook is very well-written. It presents the material in a clear, easy to understand way. It reaches the necessary vocabulary for each technique. The pictures and summary charts after each technique are very valuable tools as well.
The book is consistent in content and layout. The way information is presented is the same throughout.
Each chapter stands alone, so it could easily be assigned in pieces without losing its message. The chapters are further divided into sections, so it is possible to assign only the specific sections relevant to your class.
The flow is very logical. It starts with material relevant to entering a lab and then moves on to progressively more difficult or involved techniques. Sometimes the fact that the chapters are so modular interrupts the flow a small amount, but I think it is worth it for the versatility it provides.
I had no issues navigating the book.
I found the textbook to be well-written and edited.
This is a standard laboratory textbook. It doesn't involve any potentially insensitive or offensive examples. It is written in a neutral way.
Overall, I found this book to be a very good resource for basic laboratory Techniques. It provides good explanations for those new to a technique as well as pictures and summary tables for those looking to use it as a quick resource to remind them how to do something in the lab.
Reviewed by Abraham Yousef, Associate Professor, Sweet Briar College on 8/15/17
This book provides a thorough coverage of standard techniques used in the organic laboratory. The table of contents on page 2 is very useful as it provides links to the different topics that one can click on to instantly go to the section of... read more
This book provides a thorough coverage of standard techniques used in the organic laboratory. The table of contents on page 2 is very useful as it provides links to the different topics that one can click on to instantly go to the section of interest. A summary of techniques is also provided on page 336. It appears that there is supposed to be an index on page 365, but the pdf ends at page 364; clicking on the link "Complete Index" on page 336 does not work. However, this does not reflect negatively on the content, which is sufficiently comprehensive.
Only a couple of minor errors found: on page 35, Table 1.2, the boiling point of diethyl ether should be 35 oC (instead of 34 oC), and the autoignition temperature of methanol should be 385 oC (instead of 464 oC). These values were found on the sigma aldrich website (sigmaaldrich.com) and accompanying SDS forms. The boiling point of diethyl ether is listed correctly on page 145. Otherwise content appears accurate.
No issues here. The content should be relevant for many years to come.
Well-written, clear and straightforward to understand. The abundance of color pictures provides excellent visual illustration of the techniques described in the text.
No problems or concerns here.
Sections are easy to navigate to through the table of contents; assigning reading to a student should be straightforward.
Well-organized.
As mentioned above, the book appeared to be missing page 365, which is supposed to contain a complete index. Otherwise, there were no navigation issues encountered. Images are clear, not distorted, and easy to read.
On page 22, first paragraph, second sentence, "...which indicates that no volume has been delivered with the pipette is still full." should read "which indicates that no volume has been delivered when the pipette is still full." Also on page 29, Figure 1.31, in the figure caption, item d) "Withdrawing inert gas in order to flushing the syringe." the word flushing should be replaced with "flush." Otherwise grammar is fine.
Not applicable in this case, as this is a technical writing document.
A wonderful, illustrative and well-written lab manual. I appreciate the vast abundance of color photographs and detailed descriptions of lab techniques. This is a much needed resource that should be required reading for all undergraduate organic chemistry lab students.
Reviewed by Dana Horoszewski, Professor, Northern Virginia Community College on 6/20/17
The book includes detailed descriptions of all relevant techniques used in intro organic chemistry lab as well as some more advanced techniques. Discussion of different types of distillations and extractions were thorough. Topics that are absent... read more
The book includes detailed descriptions of all relevant techniques used in intro organic chemistry lab as well as some more advanced techniques. Discussion of different types of distillations and extractions were thorough. Topics that are absent from the book but often covered in other textbooks are general lab safety (though specific safety concerns are addressed within each topic), notebook preparation, and spectroscopy.
I did not find any inaccuracies in the book.
Basic techniques in intro organic chemistry labs haven't changed in the last 20+ years. The information in the book will remain relevant for years to come. Instrument interfaces change, but since book doesn't cover spectroscopy, it is not an issue.
I found the book to be clear and easy to read. The abundance of pictures and drawings accompanying the text will make it easy to follow for students. The author did a great job staging the photographs. Organic compounds are often colorless, but colored compounds were used in many photographs to help students visualize what is happening.
NMR spectra are used to illustrate some points about purity in the crystallization and extraction chapters. I will be assigning these chapters long before covering NMR, so references to it may be more confusing than enlightening for students.
I found no inconsistencies with terminology or tone in the textbook.
Sections of the book can be assigned in any order according to the needs of the course. Each technique is self-contained.
I like that each chapter is broken down into sections and subsections. The book covers several techniques that I do not use in my course, so this makes it easy to specify which pieces of the chapter students are responsible for.
Each chapter provides an overview, covers the theory behind the technique, then discusses the practical aspects of carrying out the technique and its variations. I cover techniques in a different order than the book, but the book is designed to be used in any order.
The last chapter, Technique Summaries, is an excellent resource for students and something I haven't encountered before in lab textbooks. The picture step-by-step technique summaries will be great for students who have performed techniques previously but need a reminder.
The hyperlinks in the TOC are wonderful for navigating the book. Each chapter has its own detailed TOC - I would have liked those to be compiled at the beginning of the book. WIthin the chapter, the subsections have numbers (ex. Ch III B.1), but those section numbers do not appear in the TOC. I think it would be helpful to have the subsection numbers in the TOC as well. I did not encounter any distortion of images.
I did not notice any grammatical errors.
Cultural or historical context is not given, but that is typical in organic lab textbooks. Pictures are largely of equipment, so there is no bias showing people from one background verses others.
I really like this book. The number and use of pictures makes it superior to all traditional textbooks I have seen. I will assign this book the next time I teach organic lab. If the author expands on the book, I would love to see chapters on safety and spectroscopic techniques.
Reviewed by Dana Lashley, Visiting Assistant Professor, The College of William and Mary on 6/20/17
This laboratory technique manual is very detailed and extremely well-written. All the discussed techniques are very commonly used in undergraduate research and teaching labs. The procedures and concepts are explained carefully in detail. The many... read more
This laboratory technique manual is very detailed and extremely well-written. All the discussed techniques are very commonly used in undergraduate research and teaching labs. The procedures and concepts are explained carefully in detail. The many accompanying pictures and sketches are incredibly useful visual aids. The text of each subchapter was well thought-out and warned of common mistakes for students to avoid, including pictures of correct and incorrect set-ups. I found the book very comprehensive as the concepts were described in careful detail.
The content of this laboratory technique manual was very accurate as far as I can tell. I did not detect any misinformation or falsehoods. Concepts and techniques were explained thoroughly and unbiasedly. Credible sources were cited where appropriate. I did not come across any typos in this book.
At the undergraduate level organic laboratory techniques and glassware have not changed dramatically over the past couple of decades and I do not foresee much of a change in the next decade and beyond. This manual thoroughly describes the most important techniques and equipment. These will be useful in many years to come. It takes into consideration the restrictions of equipment that may be available at different institutions and, when possible, gives different options to use when one procedure may not be possible due to lack of the required equipment or materials.
The text was extremely well-written, straightforward and easy to read and understand. The language was such that undergraduates, who may not know all the proper terms and lab jargon, would be able to easily follow. Procedures were written in a step-by-step manner with careful choice of words that didn't leave much room for ambiguity. These were accompanied by pictures of equipment and set-up etc that will immensely facilitate the students' ability to master these techniques. In fact, now that I am aware of this amazing book I will send an e-copy to all my research students and organic lab TAs because this will help them better understand these techniques they are performing on a daily basis. This will also be a formidable resource for students to learn about new techniques that they are about tot perform for the first time.
The book is consistent and structured. Chapters and subchapters are clearly defined. Terminology is not taken for granted but introduced when it first mentioned. There are hyperlinks within the text that link to other locations within the book where a certain concept or piece of equipment may be explained more thoroughly. Citations are used throughput the book where appropriate.
The text is a nice read - probably as much fun as it can be to read a lab manual. Sometimes, scientific texts can be very tedious to read. This book however is well-written and well-organized. Additionally, it offers plenty of visualizations (pictures, schematics), that it makes reading and understanding quite easy. There are no large blocks of text without sub-headers. All chapters are divided into appropriate sub-chapters and sub-sections that deal with a certain module. Sometimes the book used hyperlinked words to refer to other points in the text where a certain concept may be explained more thoroughly. I didn't find it overly self-referential however. Throughout the book there are citations to well-respected outside sources such as journal articles and other books (just below the text on a page where applicable).
I found the book was organized in a logical fashion. Simple concepts such as laboratory terminology for pieces of equipment and simple techniques are handled at the beginning. Then the manual moves to more advanced topics that may require understanding of the previously covered topics. Any time a new term is introduced it is defined and explained. Many visual aids such as pictures, figures, schematics and tables accompany the text. These items are appropriately enumerated and the text often refers back to these items so that its easy to visualize and follow what the text is describing.
I loved the books interface. The general text is written in a black font with appropriate spacing between lines. Figures and tables etc. are labeled with green font. Whenever something needs to be highlighted within the main text a black bolded or sometimes a red bolded font is used underlining its significance to the reader (red means more significant). Pictures, schematics, graphs, tables etc. are in color and make for a very pleasant reading experience.
I did not come across any grammatical errors in this text.
This is a scientific text and does not make mention to race, ethnicities or culture. The text was professionally written and I found no part of this text to be offensive or insensitive.
I loved this laboratory techniques manual ! The author did a great job accumulating all this information and organizing it in such a way that it is easily read and understood with plenty of visualizing aids. Ii highly recommended this book for undergraduates as well as beginning graduate students. In some cases students may be using a certain technique on a daily basis already but sadly, they may not be fully aware of the science behind it. I think this book helps close this knowledge gap. Information on certain laboratory techniques is usually available on the internet. However I had not yet come across such a comprehensive text. 5/5 stars !
In fact, now that I am aware of this amazing book I will send an e-copy to all my research students and organic lab TAs because this will help them better understand these techniques they are performing on a daily basis. This will also be a formidable resource for students to learn about new techniques that they are about to perform for the first time (e.g. column chromatography.)
Reviewed by Philip Brown, Teaching Professor of Chemistry, North Carolina State University on 6/20/17
The book covers all the areas relevant for the typical undergraduate science student. It is well indexed and the links to move around the text are excellent. read more
The book covers all the areas relevant for the typical undergraduate science student. It is well indexed and the links to move around the text are excellent.
With just a few exceptions the information provided is error-free.
The textbook is most appropriate as a supplement to the typical undergraduate lab manual. In the lab a reaction might be run that includes several techniques to isolate and purify a compound. This text is great for providing the nuts-and -bolts of how to perform these techniques. This book does not however go into any discussion on identifying what the compound might be and no instrumental techniques such as IR, NMR, UV-Vis, etc. are included.
Excellent photos, diagrams and charts are used throughout the book. The language is appropriate for a first semester organic student.
This is a well thought out textbook. It's aim is very specific, organic techniques for the purification of compounds. The pdf version of this book is wonderful and easy to navigate.
By the nature of organic reaction chemistry different tools are necessary for the task and complexity of the mixture. Sometimes a crystalline material is the product and the tools of recrystallization are required, sometimes a liquid is the result and distillation methods are the tool needed to generate the compound in high yield. The way the author structured the work makes finding what you need seamless.
The book does start with the more basic techniques such as TLC and progresses on to some of the more elaborate methods such as fractional vacuum distillation at the end. I like this approach from the methods requiring basic glassware and slowly introducing methods requiring elaborate setups in glassware, heating and vacuum.
This work (the pdf version) has everything crystal clear. The diagrams are not distorted, the charts do not flow over diagrams or photos and the photos are razor sharp.
This is a work of very high quality. No serious errors were noticed in the writing of this book.
Chemistry, by it's nature transcends all ages, genders, religions, and culture. The photos are nearly all close-ups of hands which are gloved and arms that are covered with a lab coat.
I love this book as an adjunct to the lab manual. The author has done a superb job in producing a work that is accurate, clear, well written and structurally easy to navigate.
Reviewed by Maria Gallardo-Williams, Teaching Associate Professor, North Carolina State University on 6/20/17
All the relevant techniques required to complete the first two semesters of organic chemistry lab are covered in detail, read more
All the relevant techniques required to complete the first two semesters of organic chemistry lab are covered in detail,
A great deal of attention has been devoted to an accurate presentation of each technique and its variants.
The techniques presented have been around for years and will likely persist for years to come. The photography style, with tight close-ups and gloved hands makes the images as timeless as possible.
The book is well written, and the prose is very clear. The use of plenty of photographs and illustrations makes it unequivocal.
The prose and delivery is consistent, and it feels as if all subjects are being covered in depth.
The subject matter is modular by nature, since each technique can be presented individually and without much overlap with other sections. Individual sections could easily be assigned as readings or pre-lab work for a course. There is no excessive self-reference.
The book is well structured and flows nicely. I would have placed the crystallization section later in the text, but that is a very personal choice.
The interface on Dropbox is a bit inflexible, and it is not easy to navigate between sections of the book.
I didn't find any grammatical errors.
The subject matter is quite neutral, and the aesthetic choices (photographs and illustration) make it a non-issue. Since most of the photographs are tight close-ups of gloved hands, there is no discernible racial or ethnic background associated with the images.
The is a well written and richly illustrated resource that can be used by novice students in the organic chemistry labs, and it may also be useful to the students in upper-level labs that might need to review a particular technique. The technique summaries at the end of the book are very well done.
Reviewed by Skip Brenzovich, Assistant Professor, Roanoke College on 6/20/17
This laboratory manual is extremely comprehensive, introducing and explaining all of the major techniques that a student might encounter in an introductory organic chemistry lab. Every procedure is explained in details and is complemented by a... read more
This laboratory manual is extremely comprehensive, introducing and explaining all of the major techniques that a student might encounter in an introductory organic chemistry lab. Every procedure is explained in details and is complemented by a series of photos and pictures to show the students how everything should look. Even more important are the comparisons of related techniques, explaining to students why one method should be chosen over another. While there was no real discussion of IR or NMR, those can be highly instrument specific in terms of set up and interface and may need to be treated at a more local level. Many institutions are moving more in the direction of green chemistry in the organic lab, so the inclusion of greener solvents as examples and in discussions might be helpful in future iterations. All in all, this textbook provides an excellent overview of the techniques and procedures that a student will encounter in an organic lab.
This textbook is highly accurate. All of the information provided was correct and unbiased. There may be a few small procedural differences based on institutional resources, but they can be easily handled in class discussions.
The general techniques in organic labs have changed very little over the past several decades. However, new safety concerns and an increasing focus on more cost effective and green techniques may change some of the procedures. Based on the structure of the book, it should be really easy to revise or add specific techniques. In addition, many of the techniques are common to other fields of chemistry. This textbook would be a good resource for any course or lab that covers synthesis.
The terminology used is accessible for the intended audience. The accompanying images made the text easy to read and follow. The technical terms used are standard for the field and used appropriately.
The structure of the textbook was consistent from section to section and easy to follow. Each section was arranged in roughly the same order, making information easy to find within a section. The terminology was explained thoroughly and used appropriately.
Each unit is relatively self-contained, meaning that units can be treated independently in any order.
The information is presented in a reasonable order. Related information is presented together and there is a clear logic and theme within each section. While the specific order may not follow my classes perfectly, the sections are modular enough that it should not be a problem.
All of the images are clear. The layout of the pages make it easy to find the appropriate information on a given page. Section breaks and chapter breaks are clearly marked for easy navigation. As an electronic text, the clear headings for each section make finding the right information easy.
While any long published work likely has a few grammatical errors or typos, I did not notice any in my reading.
This textbook is appropriately focused on the science. The discussions are approachable for anyone in a chemistry course, regardless of race, ethnicity, gender, or background.
This book is an excellent resource for not only introductory organic students, but for upper level students as well. We will be using this text in the coming semesters as a resource for our courses. In addition, I have assigned this text to all of my research students as well.
Reviewed by Jeffrey Elbert, Asscoaite Professor, University of Northern Iowa on 2/8/17
The text covers the general techniques used in organic chemistry very well. The detail is consistent with a majors section course. All major types of equipment and there uses are covered in sufficient detail that a student should be able to... read more
The text covers the general techniques used in organic chemistry very well. The detail is consistent with a majors section course. All major types of equipment and there uses are covered in sufficient detail that a student should be able to understand the use prior to actual practice in lab. The pictures, which are required for a comprehensive understanding of the proper use of equipment and successful completion of techniques, are excellent. In fact, the pictures are one thing that sets this text apart from our current text. Students will be better able to complete tasks because the pictures provide a better view of the technique. Videos done as well as the pictures would be even better. The Chromatography chapter is particularly good and complete, and again, the pictures included provide an excellent view to go along with the chapter text. Coverage of NMR/ IR and Safety would add to the text. There are Safety Notes throughout the text. A general chapter on risk assessment is being recognized as an important part of a chemistry course. The table of contents as an index is easy to use and navigate. As terms are explained within the text, I don't know that a glossary is necessary.
I found no inaccuracies and no biases. The coverage of topics is at a level that allows the text to be used in multiple courses, from organic lab for health related majors to chemistry majors, advanced lab to new undergraduate researchers. The author cannot include every possible equipment type used in an undergraduate lab. We currently use elastomeric microscale equipment rather than the Williamson type. This is a minor issue.
As organic chemistry changes, we often incorporate current equipment for new reactions. That is our training. I see no reason this text will become outdated. The outline of the text should make any changes straightforward to incorporate. Our current texts have changed little in 20 years. A major advance of this text is the use of color pictures to enhance the text. One area to look to is Chromatography. Flash chromatography is typical for organic labs today, but we are moving from air pressure to solvent delivery pumps, which use different connections and columns.
I found the text easy to read. Jargon was minimal and explained with use.
The text is very consistent. I would expect this with a text written by one author.
Each chapter can easily be assigned as a self contained unit. As every organic laboratory course has a different flow of material, no text can follow exactly. I see no problem with assigning sections to students from this text.
The organization of the text is not in the order we currently cover material in our course. But that is not really a consideration as no text presents material in exactly the same order we cover in our course. Jumping around is normal for an organic laboratory course. This will not be a problem for us as we use this text. The topics are presented in a logical and clear manner within each chapter. That is the most important point.
I saw no distortion of images or text. I found nothing in the text that should distract the reader.
I found no grammatical errors.
Organic chemistry is focused on compounds, equipment, and techniques and as such there is less attention paid to cultural issues as is typical in other courses. The only place for inclusion would be in the pictures included in the text. The pictures focus on the equipment and techniques, and any people pictured in part usually have gloved hands. More inclusion of different peoples could be done in any updates to pictures, but I do not see it as currently detracting from the text.
I will be incorporating this text in my Organic Lab section next semester and will also use it as a text for my research students as they prepare for summer research.
Reviewed by Elizabeth Cohen, Chemistry Professor, Mount Hood Community College on 12/5/16
The textbook provided a comprehensive and in depth coverage of the both laboratory technique and instrumentation used in an introductory organic chemistry lab. The summaries at the end of each topic would be a wonderful quick reference and... read more
The textbook provided a comprehensive and in depth coverage of the both laboratory technique and instrumentation used in an introductory organic chemistry lab. The summaries at the end of each topic would be a wonderful quick reference and overview for a student. My only complaint would be that the coverage of chromatography did not include drip column chromatography. Column chromatography is not limited to flash column chromatography.
The only area I felt was biased was in the area of chromatography. Not all academic labs are set up to do flash columns.
Organic technique doesn't change. Instrumentation does change, but the instruments presently used in a teaching lab will probably not become outdated any time soon. Even the images used will probably still be relevant 10 to 20 years from now.
't The textbook was a pleasure to read. Although the topic was technical, it didn't feel like a heavy read. The writing style should be very accessible to a typical organic student. The use of images made the book not boring to read. The textbook i sm presently using is very dry. There is just too much text where an image would be much more informative. Another lab book I have used was funny to read but lack the completness of this textbook and the humor got in the way of clarity.
It was obvious that the textbook was written by a single person because it was very consistent in style and the use of terminology.
The textbook is well structured. As a reference guide, it would be easy for a student to locate the theory behind a technique, a detailed explanation of a technique, or a quick summary of a technique. It is much better than any other lab textbook I have read.
The topics are presented in a well organized fashion. The images used are well placed and add to the clarity of the text.
I did have difficulty with navigating in the book. Every time I opened it I had to scroll down to where I had left off rather than clicking on the topic I left off on in a table of contents. The book lacked a table of contents.
I did not notice any grammatical errors, but then again I was not looking for any.
Cultural Relevance rating: 3
Not applicable.
I loved this book.I will definitely be adding it to my course books for the second quarter of organic chemistry this winter and it will completely replace my present lab textbook next fall.
Table of Contents
- Chapter 1: General Techniques
- Chapter 2: Chromatography
- Chapter 3: Crystallization
- Chapter 4: Extraction
- Chapter 5: Distillation
- Chapter 6: Miscellaneous Techniques
- Chapter 7: Technique Summaries
Ancillary Material
About the book.
This resource was created by Lisa Nichols (chemistry faculty at Butte Community College in Northern California) as a result of an academic sabbatical leave in the Fall-2015 to Spring 2016 term. The target audience are undergraduate students in organic chemistry.
In this resource you will find theory and procedures on the main organic lab techniques (chromatography, crystallization, extraction, distillation) as well as general concepts on how to set up and heat apparatuses (see the Table of Contents tab for a more complete listing of topics).
All procedures are accompanied by step-by-step pictures, and graphics are heavily utilized throughout the resource.
About the Contributors
Lisa Nichols obtained a Bachelors of Science degree in chemistry from California State University, Chico in 2001 and a Master’s degree in organic chemistry from Stanford University in 2003. At the time of this project she had taught chemistry fulltime for 12 years at Butte Community College (in Oroville, northern California, near C.S.U. Chico), with an emphasis on teaching majors-level organic chemistry.
Contribute to this Page

Comprehensive Organic Chemistry Experiments for the Laboratory Classroom
This expansive and practical textbook contains organic chemistry experiments for teaching in the laboratory at the undergraduate level covering a range of functional group transformations and key organic reactions.The editorial team have collected contributions from around the world and standardized them for publication. Each experiment will explore a modern chemistry scenario, such as: sustainable chemistry; application in the pharmaceutical industry; catalysis and material sciences, to name a few. All the experiments will be complemented with a set of questions to challenge the students and a section for the instructors, concerning the results obtained and advice on getting the best outcome from the experiment. A section covering practical aspects with tips and advice for the instructors, together with the results obtained in the laboratory by students, has been compiled for each experiment.
Targeted at professors and lecturers in chemistry, this useful text will provide up to date experiments putting the science into context for the students.
- Cite Icon Cite
Comprehensive Organic Chemistry Experiments for the Laboratory Classroom, The Royal Society of Chemistry, 2016.
Download citation file:
- Ris (Zotero)
- Reference Manager
Digital access
Print format, table of contents.
- Front Matter
- 1.1. Separation, Purification and Identification of the Components of a Mixture p1-5 By Abel J. S. C. Vieira ; Abel J. S. C. Vieira Faculty of Sciences and Technology, Universidade Nova de Lisboa 2829-516 Caparica Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Elvira M. S. M. Gaspar Elvira M. S. M. Gaspar Faculty of Sciences and Technology, Universidade Nova de Lisboa 2829-516 Caparica Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 1.1. Separation, Purification and Identification of the Components of a Mixture in another window
- 1.2. Isolation of (+)-Limonene from Orange Oil p6-8 By João P. Telo João P. Telo Centro de Química Estrutural, Instituto Superior Técnico, Universidade de Lisboa Av. Rovisco Pais 1649-003 Lisboa Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 1.2. Isolation of (+)-Limonene from Orange Oil in another window
- 1.3. Isolation of Plant Pigments from Green and Red Leaves p9-13 By Alice M. Dias ; Alice M. Dias Department of Chemistry, University of Minho Campus de Gualtar 4710-057 Braga Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Maria La S. Ferreira Maria La S. Ferreira Department of Chemistry, University of Minho Campus de Gualtar 4710-057 Braga Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 1.3. Isolation of Plant Pigments from Green and Red Leaves in another window
- 1.4. Extraction of Usnic Acid from Lichen p14-17 By Dietmar K. Kennepohl Dietmar K. Kennepohl Athabasca University 1 University Drive Athabasca Alberta T9S 3A3 Canada [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 1.4. Extraction of Usnic Acid from Lichen in another window
- 1.5. Thin-Layer Chromatography of Plants Pigments p18-22 By Ana Margarida Madureira ; Ana Margarida Madureira iMed.ULisboa, Faculty of Pharmacy, University of Lisbon Av. Professor Gama Pinto 1649-003 Lisboa Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Maria-José U. Ferreira Maria-José U. Ferreira iMed.ULisboa, Faculty of Pharmacy, University of Lisbon Av. Professor Gama Pinto 1649-003 Lisboa Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 1.5. Thin-Layer Chromatography of Plants Pigments in another window
- 1.6. Isolation of Cinnamaldehyde from Cinnamon p23-25 By Raquel F. M. Frade ; Raquel F. M. Frade iMed.ULisboa, Faculdade de Farmácia da Universidade de Lisboa Av. Prof. Gama Pinto 1649-003 Lisboa Portugal [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Dulce Simão ; Dulce Simão Department of Chemical Engineering, Instituto Superior Técnico, University of Lisbon, Centro de Química Estrutural Av. Rovisco Pais, 1 1049-001 Lisbon Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Carlos A. M. Afonso Carlos A. M. Afonso iMed.ULisboa, Faculdade de Farmácia da Universidade de Lisboa Av. Prof. Gama Pinto 1649-003 Lisboa Portugal [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 1.6. Isolation of Cinnamaldehyde from Cinnamon in another window
- 1.7. Isolation and Structural Identification of Piperine, the Major Alkaloid of Black Pepper p26-29 By Andreia Mónico ; Andreia Mónico iMed.ULisboa, Faculty of Pharmacy, University of Lisbon Av. Prof. Gama Pinto 1649-003 Lisbon Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Angela Paterna ; Angela Paterna iMed.ULisboa, Faculty of Pharmacy, University of Lisbon Av. Prof. Gama Pinto 1649-003 Lisbon Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Mariana A. Reis ; Mariana A. Reis iMed.ULisboa, Faculty of Pharmacy, University of Lisbon Av. Prof. Gama Pinto 1649-003 Lisbon Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Ricardo Ferreira ; Ricardo Ferreira iMed.ULisboa, Faculty of Pharmacy, University of Lisbon Av. Prof. Gama Pinto 1649-003 Lisbon Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Noélia Duarte ; Noélia Duarte iMed.ULisboa, Faculty of Pharmacy, University of Lisbon Av. Prof. Gama Pinto 1649-003 Lisbon Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Ana M. Madureira ; Ana M. Madureira iMed.ULisboa, Faculty of Pharmacy, University of Lisbon Av. Prof. Gama Pinto 1649-003 Lisbon Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Maria-José U. Ferreira Maria-José U. Ferreira iMed.ULisboa, Faculty of Pharmacy, University of Lisbon Av. Prof. Gama Pinto 1649-003 Lisbon Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 1.7. Isolation and Structural Identification of Piperine, the Major Alkaloid of Black Pepper in another window
- 1.8. Caffeine Extraction from Tea and Coffee p30-34 By Ana Margarida Madureira ; Ana Margarida Madureira iMed.ULisboa, Faculty of Pharmacy, University of Lisbon Av. Professor Gama Pinto 1649-003 Lisboa Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Abel J. S. C. Vieira ; Abel J. S. C. Vieira Faculty of Sciences and Technology, Universidade Nova de Lisboa, Campus de Caparica 2829-516 Caparica, Caparica Portugal Search for other works by this author on: This Site PubMed Google Scholar Maria-José U. Ferreira Maria-José U. Ferreira iMed.ULisboa, Faculty of Pharmacy, University of Lisbon Av. Professor Gama Pinto 1649-003 Lisboa Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 1.8. Caffeine Extraction from Tea and Coffee in another window
- 1.9. Isolation of the Alkaloid Lupanine from Lupinus albus Seeds p35-37 By Ana M. Lourenço ; Ana M. Lourenço REQUIMTE, Departamento de Química, Faculdade de Ciências e Tecnologia, Universidade Nova de Lisboa 2829-516 Caparica Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Luísa Maria Ferreira Luísa Maria Ferreira REQUIMTE, Departamento de Química, Faculdade de Ciências e Tecnologia, Universidade Nova de Lisboa 2829-516 Caparica Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 1.9. Isolation of the Alkaloid Lupanine from <em>Lupinus albus</em> Seeds in another window
- 1.10. Isolation and Purification of Atropine, a Tropane Alkaloid Obtained from Atropa belladonna L. (Solanaceae) p38-42 By Gustavo da Silva Gustavo da Silva Department of Pharmacological Sciences, Research Institute for Medicines and Pharmaceutical Sciences (iMed.ULisboa), Faculty of Pharmacy – University of Lisbon Av. Prof. Gama Pinto 1649-003 Lisbon Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 1.10. Isolation and Purification of Atropine, a Tropane Alkaloid Obtained from <em>Atropa belladonna</em> L. (Solanaceae) in another window
- 1.11. Isolation and Purification of Carnosol from Salvia officinalis † p43-45 By Filipe Pereira ; Filipe Pereira Universidade Lusófona Research Center for Biosciences & Health Technologies (CBIOS) Campo Grande 376 1749-024 Lisboa Portugal Search for other works by this author on: This Site PubMed Google Scholar Patrícia Rijo Patrícia Rijo Universidade Lusófona Research Center for Biosciences & Health Technologies (CBIOS) Campo Grande 376 1749-024 Lisboa Portugal Instituto de Investigação do Medicamento (iMed.ULisboa), Faculdade de Farmácia da Universidade de Lisboa Av. Professor Gama Pinto 1649-003 Lisboa Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 1.11. Isolation and Purification of Carnosol from <em>Salvia officinalis</em><sup><a href="javascript:;" reveal-id="BK9781849739634-00043-fn1" data-open="BK9781849739634-00043-fn1" class="link link-ref link-reveal xref-fn js-xref-fn split-view-modal">†</a></sup> in another window
- 1.12. Analysis of Racemic and ( S )-Ibuprofen p46-50 By C. Fernandes ; C. Fernandes Laboratório de Química Orgânica e Farmacêutica, Departamento de Ciências Químicas, Faculdade de Farmácia, Universidade do Porto Portugal Centro Interdisciplinar de Investigação Marinha e Ambiental (CIIMAR/CIMAR), Universidade do Porto Portugal [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar H. Cidade ; H. Cidade Laboratório de Química Orgânica e Farmacêutica, Departamento de Ciências Químicas, Faculdade de Farmácia, Universidade do Porto Portugal Centro Interdisciplinar de Investigação Marinha e Ambiental (CIIMAR/CIMAR), Universidade do Porto Portugal [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar S. Cravo ; S. Cravo Laboratório de Química Orgânica e Farmacêutica, Departamento de Ciências Químicas, Faculdade de Farmácia, Universidade do Porto Portugal Search for other works by this author on: This Site PubMed Google Scholar E. Sousa ; E. Sousa Laboratório de Química Orgânica e Farmacêutica, Departamento de Ciências Químicas, Faculdade de Farmácia, Universidade do Porto Portugal Centro Interdisciplinar de Investigação Marinha e Ambiental (CIIMAR/CIMAR), Universidade do Porto Portugal [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar M. Pinto M. Pinto Laboratório de Química Orgânica e Farmacêutica, Departamento de Ciências Químicas, Faculdade de Farmácia, Universidade do Porto Portugal Centro Interdisciplinar de Investigação Marinha e Ambiental (CIIMAR/CIMAR), Universidade do Porto Portugal [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 1.12. Analysis of Racemic and (<em>S</em>)-Ibuprofen in another window
- 1.13. Determining Partition Coefficients of Sulfonamides by Reversed-Phase Chromatography p51-55 By E. Sousa ; E. Sousa Laboratório de Química Orgânica e Farmacêutica, Departamento de Ciências Químicas, Faculdade de Farmácia, Universidade do Porto Portugal Centro Interdisciplinar de Investigação Marinha e Ambiental (CIIMAR/CIMAR), Universidade do Porto Portugal [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar S. Cravo ; S. Cravo Laboratório de Química Orgânica e Farmacêutica, Departamento de Ciências Químicas, Faculdade de Farmácia, Universidade do Porto Portugal Search for other works by this author on: This Site PubMed Google Scholar C. Fernandes ; C. Fernandes Laboratório de Química Orgânica e Farmacêutica, Departamento de Ciências Químicas, Faculdade de Farmácia, Universidade do Porto Portugal Centro Interdisciplinar de Investigação Marinha e Ambiental (CIIMAR/CIMAR), Universidade do Porto Portugal [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar M. Pinto M. Pinto Laboratório de Química Orgânica e Farmacêutica, Departamento de Ciências Químicas, Faculdade de Farmácia, Universidade do Porto Portugal Centro Interdisciplinar de Investigação Marinha e Ambiental (CIIMAR/CIMAR), Universidade do Porto Portugal [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 1.13. Determining Partition Coefficients of Sulfonamides by Reversed-Phase Chromatography in another window
- 2.1.1. A S N 1 Reaction: Synthesis of tert -Butyl Chloride p56-60 By Emília Valente ; Emília Valente Faculty of Pharmacy, University of Lisbon Av. Prof. Gama Pinto 1649-003 Lisbon Portugal Search for other works by this author on: This Site PubMed Google Scholar Catarina Dias ; Catarina Dias Faculty of Pharmacy, University of Lisbon Av. Prof. Gama Pinto 1649-003 Lisbon Portugal Search for other works by this author on: This Site PubMed Google Scholar Luís Constantino Luís Constantino Faculty of Pharmacy, University of Lisbon Av. Prof. Gama Pinto 1649-003 Lisbon Portugal Research Institute for Medicines (iMed.ULisboa), Faculty of Pharmacy, Universidade de Lisboa Av. Professor Gama Pinto 1649-003 Lisbon Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 2.1.1. A S<sub>N</sub>1 Reaction: Synthesis of <em>tert</em>-Butyl Chloride in another window
- 2.1.2. Optimizing the Reaction Conditions for the Synthesis of tert -Pentyl Chloride p61-63 By Jane Brock Greco Jane Brock Greco Department of Chemistry, Johns Hopkins University 3400 N. Charles St. Baltimore MD 21218 USA [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 2.1.2. Optimizing the Reaction Conditions for the Synthesis of <em>tert</em>-Pentyl Chloride in another window
- 2.1.3. Kinetics of a S N 1 Reaction p64-67 By Paulo Coelho ; Paulo Coelho Centro de Química – Vila Real, Universidade de Trás-os-Montes e Alto Douro 5001-801 Vila Real Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Céu Sousa Céu Sousa Centro de Química – Vila Real, Universidade de Trás-os-Montes e Alto Douro 5001-801 Vila Real Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 2.1.3. Kinetics of a S<sub>N</sub>1 Reaction in another window
- 2.1.4. Counterion Effects in the Nucleophilic Substitution Reaction of the Acetate Ion with Alkyl Bromides in the Synthesis of Esters p68-71 By Ingrid Montes ; Ingrid Montes Department of Chemistry, University of Puerto Rico-Río Piedras Campus PO Box 23346 San Juan PR 00931-3346 Puerto Rico USA [email protected] Search for other works by this author on: This Site PubMed Google Scholar M. Elizabeth Valentín ; M. Elizabeth Valentín Department of Chemistry, University of Puerto Rico-Río Piedras Campus PO Box 23346 San Juan PR 00931-3346 Puerto Rico USA [email protected] Search for other works by this author on: This Site PubMed Google Scholar Waldemar Adam Waldemar Adam Department of Chemistry, University of Puerto Rico-Río Piedras Campus PO Box 23346 San Juan PR 00931-3346 Puerto Rico USA [email protected] Institute of Organic Chemistry, University of Wuerzburg Am Hubland D-97094 Wuerzburg Germany Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 2.1.4. Counterion Effects in the Nucleophilic Substitution Reaction of the Acetate Ion with Alkyl Bromides in the Synthesis of Esters in another window
- 2.1.5. N -Alkylation of Pyrazole: Reaction in an Ionic Liquid p72-75 By Clarissa P. Frizzo ; Clarissa P. Frizzo Núcleo de Química de Heterociclos (NUQUIMHE), Department of Chemistry, Federal University of Santa Maria 97105-900 Santa Maria RS Brazil [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Marcos A. P. Martins ; Marcos A. P. Martins Núcleo de Química de Heterociclos (NUQUIMHE), Department of Chemistry, Federal University of Santa Maria 97105-900 Santa Maria RS Brazil [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Caroline R. Bender ; Caroline R. Bender Núcleo de Química de Heterociclos (NUQUIMHE), Department of Chemistry, Federal University of Santa Maria 97105-900 Santa Maria RS Brazil [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Paulo R. S. Salbego ; Paulo R. S. Salbego Núcleo de Química de Heterociclos (NUQUIMHE), Department of Chemistry, Federal University of Santa Maria 97105-900 Santa Maria RS Brazil [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Aniele Z. Tier ; Aniele Z. Tier Núcleo de Química de Heterociclos (NUQUIMHE), Department of Chemistry, Federal University of Santa Maria 97105-900 Santa Maria RS Brazil [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Geórgia C. Zimmer ; Geórgia C. Zimmer Núcleo de Química de Heterociclos (NUQUIMHE), Department of Chemistry, Federal University of Santa Maria 97105-900 Santa Maria RS Brazil [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Guilherme C. Paveglio ; Guilherme C. Paveglio Núcleo de Química de Heterociclos (NUQUIMHE), Department of Chemistry, Federal University of Santa Maria 97105-900 Santa Maria RS Brazil [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Helio G. Bonacorso Helio G. Bonacorso Núcleo de Química de Heterociclos (NUQUIMHE), Department of Chemistry, Federal University of Santa Maria 97105-900 Santa Maria RS Brazil [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 2.1.5. <em>N</em>-Alkylation of Pyrazole: Reaction in an Ionic Liquid in another window
- 2.1.6. Conversion of Alcohols into Alkyl Chlorides Using Cyanuric Chloride p76-79 By Eliana Martins ; Eliana Martins Instituto de Investigação do Medicamento (iMed.ULisboa), Faculty of Pharmacy, University of Lisbon Av. Prof. Gama Pinto 1649-003 Lisboa Portugal [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Catarina A. B. Rodrigues ; Catarina A. B. Rodrigues Instituto de Investigação do Medicamento (iMed.ULisboa), Faculty of Pharmacy, University of Lisbon Av. Prof. Gama Pinto 1649-003 Lisboa Portugal [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Carlos A. M. Afonso Carlos A. M. Afonso Instituto de Investigação do Medicamento (iMed.ULisboa), Faculty of Pharmacy, University of Lisbon Av. Prof. Gama Pinto 1649-003 Lisboa Portugal [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 2.1.6. Conversion of Alcohols into Alkyl Chlorides Using Cyanuric Chloride in another window
- 2.1.7. Synthesis of Phenacetin p80-83 By Paulo Coelho ; Paulo Coelho Centro de Química – Vila Real, Universidade de Trás-os-Montes e Alto Douro 5001-801 Vila Real Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Céu Sousa Céu Sousa Centro de Química – Vila Real, Universidade de Trás-os-Montes e Alto Douro 5001-801 Vila Real Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 2.1.7. Synthesis of Phenacetin in another window
- 2.1.8. One-Step Synthesis of 4(3 H )-Quinazolinones: An Important Heterocyclic Scaffold in Medicinal Chemistry p84-86 By André Dias ; André Dias Research Institute for Medicines (iMed.ULisboa), Faculty of Pharmacy, Universidade de Lisboa Av. Professor Gama Pinto 1649-003 Lisboa Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Rui Moreira ; Rui Moreira Research Institute for Medicines (iMed.ULisboa), Faculty of Pharmacy, Universidade de Lisboa Av. Professor Gama Pinto 1649-003 Lisboa Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Ana S. Ressurreição Ana S. Ressurreição Research Institute for Medicines (iMed.ULisboa), Faculty of Pharmacy, Universidade de Lisboa Av. Professor Gama Pinto 1649-003 Lisboa Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 2.1.8. One-Step Synthesis of 4(3<em>H</em>)-Quinazolinones: An Important Heterocyclic Scaffold in Medicinal Chemistry in another window
- 2.1.9. Controlled Monoalkylation of the Structurally Rigid Bicyclic System Isomannide p87-91 By M. Manuela ; M. Manuela REQUIMTE, Chemistry Department, Faculdade de Ciências e Tecnologia, Universidade Nova de Lisboa 2827-516 Caparica Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar A. Pereira A. Pereira REQUIMTE, Chemistry Department, Faculdade de Ciências e Tecnologia, Universidade Nova de Lisboa 2827-516 Caparica Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 2.1.9. Controlled Monoalkylation of the Structurally Rigid Bicyclic System Isomannide in another window
- 2.1.10. Regioselective N -alkylation of Adenine by Nucleophilic Substitution p92-94 By Ana I. Vicente ; Ana I. Vicente iMed.ULisboa, Faculdade de Farmácia da Universidade de Lisboa Portugal [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Carlos A. M. Afonso Carlos A. M. Afonso iMed.ULisboa, Faculdade de Farmácia da Universidade de Lisboa Portugal [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 2.1.10. Regioselective <em>N</em>-alkylation of Adenine by Nucleophilic Substitution in another window
- 2.1.11. Gabriel Synthesis of n -Octylamine Under Phase-Transfer Catalysis: The First Step p95-98 By Renata Riva ; Renata Riva Search for other works by this author on: This Site PubMed Google Scholar Luca Banfi Luca Banfi Dipartimento di Chimica e Chimica Industriale Via Dodecaneso 31 16146 Genova Italy [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 2.1.11. Gabriel Synthesis of <em>n</em>-Octylamine Under Phase-Transfer Catalysis: The First Step in another window
- 2.1.12. Preparation of Diethyl 2,3- O -isopropylidene- l -tartrate p99-102 By Naylil M. R. Capreti ; Naylil M. R. Capreti Institute of Chemistry, State University of Campinas (Unicamp) C.P. 6154 13083-970 Campinas SP Brazil [email protected] Search for other works by this author on: This Site PubMed Google Scholar Igor D. Jurberg Igor D. Jurberg Institute of Chemistry, State University of Campinas (Unicamp) C.P. 6154 13083-970 Campinas SP Brazil [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 2.1.12. Preparation of Diethyl 2,3-<em>O</em>-isopropylidene-<span class="small-caps">l</span>-tartrate in another window
- 2.1.13. Redox-Neutral Synthesis of a Cyclic N , O -Acetal from Salicylaldehyde and 1,2,3,4-Tetrahydroisoquinoline p103-106 By Claire L. Jarvis ; Claire L. Jarvis Rutgers, the State University of New Jersey, Department of Chemistry and Chemical Biology, Rutgers University 610 Taylor Road Piscataway NJ 08854-8087 USA [email protected] Search for other works by this author on: This Site PubMed Google Scholar Daniel Seidel Daniel Seidel Rutgers, the State University of New Jersey, Department of Chemistry and Chemical Biology, Rutgers University 610 Taylor Road Piscataway NJ 08854-8087 USA [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 2.1.13. Redox-Neutral Synthesis of a Cyclic <em>N</em>,<em>O</em>-Acetal from Salicylaldehyde and 1,2,3,4-Tetrahydroisoquinoline in another window
- 2.2.1. Selective C -acylation of 3-Methyl-1-phenyl-pyrazol-5-one p107-111 By Vanya B. Kurteva ; Vanya B. Kurteva Institute of Organic Chemistry with Centre of Phytochemistry, Bulgarian Academy of Sciences Acad. G. Bonchev str., bl. 9 1113 Sofia Bulgaria [email protected] Search for other works by this author on: This Site PubMed Google Scholar Lubomir A. Lubenov ; Lubomir A. Lubenov Institute of Organic Chemistry with Centre of Phytochemistry, Bulgarian Academy of Sciences Acad. G. Bonchev str., bl. 9 1113 Sofia Bulgaria [email protected] Search for other works by this author on: This Site PubMed Google Scholar Maria A. Petrova Maria A. Petrova University of Chemical Technology and Metallurgy, Department of General and Inorganic Chemistry 8 Kliment Ohridski blvd. 1756 Sofia Bulgaria Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 2.2.1. Selective <em>C</em>-acylation of 3-Methyl-1-phenyl-pyrazol-5-one in another window
- 2.2.2. Organocatalytic Asymmetric α-Arylation of Aldehydes p112-116 By Mette Overgaard ; Mette Overgaard Department of Chemistry, Aarhus University DK-8000 Aarhus C Denmark [email protected] Search for other works by this author on: This Site PubMed Google Scholar Pernille H. Poulsen ; Pernille H. Poulsen Department of Chemistry, Aarhus University DK-8000 Aarhus C Denmark [email protected] Search for other works by this author on: This Site PubMed Google Scholar Karl Anker Jørgensen Karl Anker Jørgensen Department of Chemistry, Aarhus University DK-8000 Aarhus C Denmark [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 2.2.2. Organocatalytic Asymmetric α-Arylation of Aldehydes in another window
- 2.2.3. Matteson Homologation of Pinacol Boronic Ester: An Efficient Method Using the Boronate Complexes p117-119 By Sébastien Balieu ; Sébastien Balieu Université de Rouen, IRCOF 1 Rue Tesnières - 76130 Mont Saint Aignan France [email protected] Search for other works by this author on: This Site PubMed Google Scholar Asmaa Bouyahya ; Asmaa Bouyahya Université de Rouen, IRCOF 1 Rue Tesnières - 76130 Mont Saint Aignan France [email protected] Search for other works by this author on: This Site PubMed Google Scholar Jean-Philippe Bouillon Jean-Philippe Bouillon Université de Rouen, IRCOF 1 Rue Tesnières - 76130 Mont Saint Aignan France [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 2.2.3. Matteson Homologation of Pinacol Boronic Ester: An Efficient Method Using the Boronate Complexes in another window
- 2.2.4. A Practical Organocatalytic Alkylation Reaction with Benzodithiolylium Tetrafluoroborate p120-124 By Pier Giorgio Cozzi ; Pier Giorgio Cozzi Alma Mater Studiorum – Università di Bologna, Dipartimento di Chimica “G. Ciamician” Via Selmi 2 40126 Bologna Italy [email protected] Search for other works by this author on: This Site PubMed Google Scholar Marco Bandini ; Marco Bandini Alma Mater Studiorum – Università di Bologna, Dipartimento di Chimica “G. Ciamician” Via Selmi 2 40126 Bologna Italy [email protected] Search for other works by this author on: This Site PubMed Google Scholar Andrea Gualandi ; Andrea Gualandi Alma Mater Studiorum – Università di Bologna, Dipartimento di Chimica “G. Ciamician” Via Selmi 2 40126 Bologna Italy [email protected] Search for other works by this author on: This Site PubMed Google Scholar Luca Mengozzi Luca Mengozzi Alma Mater Studiorum – Università di Bologna, Dipartimento di Chimica “G. Ciamician” Via Selmi 2 40126 Bologna Italy [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 2.2.4. A Practical Organocatalytic Alkylation Reaction with Benzodithiolylium Tetrafluoroborate in another window
- 2.2.5. Oxazolidinone Mediated Enantioselective Allylation p125-128 By Jonathan D. Sellars ; Jonathan D. Sellars Chemistry Department, University of Durham South Road Durham DH1 3LE UK [email protected] Search for other works by this author on: This Site PubMed Google Scholar AnnMarie C. O’Donoghue ; AnnMarie C. O’Donoghue Chemistry Department, University of Durham South Road Durham DH1 3LE UK [email protected] Search for other works by this author on: This Site PubMed Google Scholar Ian R. Baxendale ; Ian R. Baxendale Chemistry Department, University of Durham South Road Durham DH1 3LE UK [email protected] Search for other works by this author on: This Site PubMed Google Scholar John M. Sanderson ; John M. Sanderson Chemistry Department, University of Durham South Road Durham DH1 3LE UK [email protected] Search for other works by this author on: This Site PubMed Google Scholar Elizabeth J. Grayson Elizabeth J. Grayson Chemistry Department, University of Durham South Road Durham DH1 3LE UK [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 2.2.5. Oxazolidinone Mediated Enantioselective Allylation in another window
- 3.1.1. Synthesis of Paracetamol by Acetylation p129-132 By Catarina Dias ; Catarina Dias Faculty of Pharmacy, Universidade de Lisboa Av. Prof. Gama Pinto 1649-003 Lisboa Portugal Search for other works by this author on: This Site PubMed Google Scholar Emília Valente ; Emília Valente Faculty of Pharmacy, Universidade de Lisboa Av. Prof. Gama Pinto 1649-003 Lisboa Portugal Search for other works by this author on: This Site PubMed Google Scholar Luís Constantino Luís Constantino Faculty of Pharmacy, Universidade de Lisboa Av. Prof. Gama Pinto 1649-003 Lisboa Portugal Research Institute for Medicines (iMed.ULisboa), Faculty of Pharmacy, Universidade de Lisboa Av. Prof. Gama Pinto 1649-003 Lisboa Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 3.1.1. Synthesis of Paracetamol by Acetylation in another window
- 3.1.2. Synthesis and Characterization of N , N ′-Dicyclohexyl- N , N ′-dimethyl-propan-1,3-diamide p133-135 By Ana Paula Paiva ; Ana Paula Paiva Centro de Química e Bioquímica, Departamento de Química e Bioquímica, Faculdade de Ciências, Universidade de Lisboa Rua Ernesto de Vasconcelos, C8, Campo Grande 1749-016 Lisbon Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Osvaldo Ortet Osvaldo Ortet Centro de Química e Bioquímica, Departamento de Química e Bioquímica, Faculdade de Ciências, Universidade de Lisboa Rua Ernesto de Vasconcelos, C8, Campo Grande 1749-016 Lisbon Portugal [email protected] Departamento de Ciência e Tecnologia, Universidade de Cabo Verde 379C Praia Santiago Island Cape Verde Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 3.1.2. Synthesis and Characterization of <em>N</em>,<em>N</em>′-Dicyclohexyl-<em>N</em>,<em>N</em>′-dimethyl-propan-1,3-diamide in another window
- 3.1.3. Synthesis and Characterisation of an Ester from 4-Nitrobenzoyl Chloride p136-138 By Iain A. Smellie ; Iain A. Smellie School of Chemistry, University of St Andrews North Haugh, St Andrews Fife KY16 9ST UK [email protected] Search for other works by this author on: This Site PubMed Google Scholar Matt L. Clarke ; Matt L. Clarke School of Chemistry, University of St Andrews North Haugh, St Andrews Fife KY16 9ST UK [email protected] Search for other works by this author on: This Site PubMed Google Scholar Leanne Harris ; Leanne Harris School of Chemistry, University of St Andrews North Haugh, St Andrews Fife KY16 9ST UK [email protected] Search for other works by this author on: This Site PubMed Google Scholar Andrew. J. Miller Andrew. J. Miller School of Chemistry, University of St Andrews North Haugh, St Andrews Fife KY16 9ST UK [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 3.1.3. Synthesis and Characterisation of an Ester from 4-Nitrobenzoyl Chloride in another window
- 3.1.4. Green Esterification: The Synthesis of Aromas in the Presence of an Acid Resin p139-141 By José E. Castanheiro ; José E. Castanheiro Departamento de Química, Escola de Ciências e Tecnologia, Centro de Química de Évora, Instituto de Investigação e Formação Avançada, Universidade de Évora Rua Romão Ramalho, no. 59 7000-671 Évora Portugal [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar António P. S. Teixeira ; António P. S. Teixeira Departamento de Química, Escola de Ciências e Tecnologia, Centro de Química de Évora, Instituto de Investigação e Formação Avançada, Universidade de Évora Rua Romão Ramalho, no. 59 7000-671 Évora Portugal [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar António M. D. R. L. Pereira António M. D. R. L. Pereira Departamento de Química, Escola de Ciências e Tecnologia, Centro de Química de Évora, Instituto de Investigação e Formação Avançada, Universidade de Évora Rua Romão Ramalho, no. 59 7000-671 Évora Portugal [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 3.1.4. Green Esterification: The Synthesis of Aromas in the Presence of an Acid Resin in another window
- 3.1.5. Acetylation of Cholesterol and Purification by Column Chromatography p142-146 By Emília Valente ; Emília Valente Faculty of Pharmacy, Universidade de Lisboa Av. Prof. Gama Pinto 1649-003 Lisbon Portugal Search for other works by this author on: This Site PubMed Google Scholar Catarina Dias ; Catarina Dias Faculty of Pharmacy, Universidade de Lisboa Av. Prof. Gama Pinto 1649-003 Lisbon Portugal Search for other works by this author on: This Site PubMed Google Scholar Luís Constantino Luís Constantino Faculty of Pharmacy, Universidade de Lisboa Av. Prof. Gama Pinto 1649-003 Lisbon Portugal Research Institute for Medicines (iMed.ULisboa), Faculty of Pharmacy, Universidade de Lisboa Av. Prof. Gama Pinto 1649-003 Lisbon Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 3.1.5. Acetylation of Cholesterol and Purification by Column Chromatography in another window
- 3.1.6. Synthesis of Hippuric Acid: An Example of Amide Bond Formation p147-150 By Ana S. Ressurreição ; Ana S. Ressurreição Research Institute for Medicines (iMed.ULisboa), Faculty of Pharmacy, Universidade de Lisboa Av. Professor Gama Pinto 1649-003 Lisboa Portugal [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Maria de Jesus Perry ; Maria de Jesus Perry Research Institute for Medicines (iMed.ULisboa), Faculty of Pharmacy, Universidade de Lisboa Av. Professor Gama Pinto 1649-003 Lisboa Portugal [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Ana Paula Francisco ; Ana Paula Francisco Research Institute for Medicines (iMed.ULisboa), Faculty of Pharmacy, Universidade de Lisboa Av. Professor Gama Pinto 1649-003 Lisboa Portugal [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Francisca Lopes Francisca Lopes Research Institute for Medicines (iMed.ULisboa), Faculty of Pharmacy, Universidade de Lisboa Av. Professor Gama Pinto 1649-003 Lisboa Portugal [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 3.1.6. Synthesis of Hippuric Acid: An Example of Amide Bond Formation in another window
- 3.1.7. Preparation of a Sulfathiazole Prodrug via N -acylation with Succinic Anhydride p151-153 By Pedro M. P. Gois ; Pedro M. P. Gois Faculty of Pharmacy, University of Lisbon Av. Prof. Gama Pinto 1649-003 Lisboa Portugal [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Francisca Lopes ; Francisca Lopes Faculty of Pharmacy, University of Lisbon Av. Prof. Gama Pinto 1649-003 Lisboa Portugal [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Alexandre F. Trindade Alexandre F. Trindade Faculty of Pharmacy, University of Lisbon Av. Prof. Gama Pinto 1649-003 Lisboa Portugal [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 3.1.7. Preparation of a Sulfathiazole Prodrug <em>via N</em>-acylation with Succinic Anhydride in another window
- 3.1.8. Determination of the Absolute Configuration of Enantioenriched Secondary Alcohols via Thin-Layer Chromatography p154-158 By Alexander J. Wagner ; Alexander J. Wagner Department of Chemistry, University of California–Irvine 1102 Natural Sciences II Irvine CA 92697 USA [email protected] [email protected] [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Shawn M. Miller ; Shawn M. Miller Department of Chemistry, University of California–Irvine 1102 Natural Sciences II Irvine CA 92697 USA [email protected] [email protected] [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Scott D. Rychnovsky ; Scott D. Rychnovsky Department of Chemistry, University of California–Irvine 1102 Natural Sciences II Irvine CA 92697 USA [email protected] [email protected] [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Renée D. Link Renée D. Link Department of Chemistry, University of California–Irvine 1102 Natural Sciences II Irvine CA 92697 USA [email protected] [email protected] [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 3.1.8. Determination of the Absolute Configuration of Enantioenriched Secondary Alcohols <em>via</em> Thin-Layer Chromatography in another window
- 3.1.9. Anhydride Aminolysis: Synthesis of N -arylmaleamic Acids p159-161 By Artur M. S. Silva ; Artur M. S. Silva Department of Chemistry and QOPNA, University of Aveiro 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Augusto C. Tomé ; Augusto C. Tomé Department of Chemistry and QOPNA, University of Aveiro 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Diana C. G. A. Pinto ; Diana C. G. A. Pinto Department of Chemistry and QOPNA, University of Aveiro 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Fernando Domingues ; Fernando Domingues Department of Chemistry and QOPNA, University of Aveiro 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Graça M. Oliveira Rocha ; Graça M. Oliveira Rocha Department of Chemistry and QOPNA, University of Aveiro 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar José A. S. Cavaleiro ; José A. S. Cavaleiro Department of Chemistry and QOPNA, University of Aveiro 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar M. Graça P. M. S. Neves ; M. Graça P. M. S. Neves Department of Chemistry and QOPNA, University of Aveiro 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar M. Amparo F. Faustino ; M. Amparo F. Faustino Department of Chemistry and QOPNA, University of Aveiro 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Mário M. Q. Simões Mário M. Q. Simões Department of Chemistry and QOPNA, University of Aveiro 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 3.1.9. Anhydride Aminolysis: Synthesis of <em>N</em>-arylmaleamic Acids in another window
- 3.1.10. Synthesis of N -arylmaleimides p162-164 By Artur M. S. Silva ; Artur M. S. Silva Department of Chemistry and QOPNA, University of Aveiro 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Augusto C. Tomé ; Augusto C. Tomé Department of Chemistry and QOPNA, University of Aveiro 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Diana C. G. A. Pinto ; Diana C. G. A. Pinto Department of Chemistry and QOPNA, University of Aveiro 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Fernando Domingues ; Fernando Domingues Department of Chemistry and QOPNA, University of Aveiro 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Graça M. Oliveira Rocha ; Graça M. Oliveira Rocha Department of Chemistry and QOPNA, University of Aveiro 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar José A. S. Cavaleiro ; José A. S. Cavaleiro Department of Chemistry and QOPNA, University of Aveiro 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar M. Graça P. M. S. Neves ; M. Graça P. M. S. Neves Department of Chemistry and QOPNA, University of Aveiro 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar M. Amparo F. Faustino ; M. Amparo F. Faustino Department of Chemistry and QOPNA, University of Aveiro 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Mário M. Q. Simões Mário M. Q. Simões Department of Chemistry and QOPNA, University of Aveiro 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 3.1.10. Synthesis of <em>N</em>-arylmaleimides in another window
- 3.1.11. Synthesis and Characterization of N -Cyclohexyl- N- methyloctanamide p165-168 By Ana Paula Paiva ; Ana Paula Paiva Centro de Química e Bioquímica, Departamento de Química e Bioquímica, Faculdade de Ciências, Universidade de Lisboa Lisboa Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Osvaldo Ortet Osvaldo Ortet Centro de Química e Bioquímica, Departamento de Química e Bioquímica, Faculdade de Ciências, Universidade de Lisboa Lisboa Portugal [email protected] Departamento de Ciência e Tecnologia, Universidade de Cabo Verde Praia Cape Verde Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 3.1.11. Synthesis and Characterization of <em>N</em>-Cyclohexyl-<em>N-</em>methyloctanamide in another window
- 3.1.12. Effect of a Catalyst in the Acylation of Alcohols with Acetic Anhydride: Manipulation of a Natural Aroma p169-171 By Mónica S. Estevão ; Mónica S. Estevão iMed.UL, Faculdade de Farmácia da Universidade de Lisboa Av. Prof. Gama Pinto 1649-003 Lisboa Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Carlos A. M. Afonso Carlos A. M. Afonso iMed.UL, Faculdade de Farmácia da Universidade de Lisboa Av. Prof. Gama Pinto 1649-003 Lisboa Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 3.1.12. Effect of a Catalyst in the Acylation of Alcohols with Acetic Anhydride: Manipulation of a Natural Aroma in another window
- 3.1.13. Synthesis of 4,5-Dichloro-1,2-dicyanobenzene p172-174 By Dulce Simão Dulce Simão Department of Chemistry, Instituto Superior Técnico, University of Lisbon, Centro de Química Estrutural Av. Rovisco Pais, 1 1049-001 Lisbon Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 3.1.13. Synthesis of 4,5-Dichloro-1,2-dicyanobenzene in another window
- 3.1.14. Synthesis of N-tert -Butyloxycarbonyl-3-nitro- l -tyrosine Methyl Ester p175-177 By Susana P. G. Costa ; Susana P. G. Costa University of Minho, Department of Chemistry, Campus de Gualtar 4710-057 Braga Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar M. Manuela M. Raposo ; M. Manuela M. Raposo University of Minho, Department of Chemistry, Campus de Gualtar 4710-057 Braga Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Cátia I. C. Esteves ; Cátia I. C. Esteves University of Minho, Department of Chemistry, Campus de Gualtar 4710-057 Braga Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar R. Cristina M. Ferreira R. Cristina M. Ferreira University of Minho, Department of Chemistry, Campus de Gualtar 4710-057 Braga Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 3.1.14. Synthesis of <em>N-tert</em>-Butyloxycarbonyl-3-nitro-<span class="small-caps">l</span>-tyrosine Methyl Ester in another window
- 3.1.15. Michael Addition Reaction Followed by Elimination Under Solvent-Free Conditions p178-181 By Marcos A. P. Martins ; Marcos A. P. Martins Núcleo de Química de Heterociclos (NUQUIMHE), Department of Chemistry, Federal University of Santa Maria 97105-900 Santa Maria RS Brazil [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Clarissa P. Frizzo ; Clarissa P. Frizzo Núcleo de Química de Heterociclos (NUQUIMHE), Department of Chemistry, Federal University of Santa Maria 97105-900 Santa Maria RS Brazil [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Aniele Z. Tier ; Aniele Z. Tier Núcleo de Química de Heterociclos (NUQUIMHE), Department of Chemistry, Federal University of Santa Maria 97105-900 Santa Maria RS Brazil [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Guilherme C. Paveglio ; Guilherme C. Paveglio Núcleo de Química de Heterociclos (NUQUIMHE), Department of Chemistry, Federal University of Santa Maria 97105-900 Santa Maria RS Brazil [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Caroline R. Bender ; Caroline R. Bender Núcleo de Química de Heterociclos (NUQUIMHE), Department of Chemistry, Federal University of Santa Maria 97105-900 Santa Maria RS Brazil [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Paulo R. S. Salbego ; Paulo R. S. Salbego Núcleo de Química de Heterociclos (NUQUIMHE), Department of Chemistry, Federal University of Santa Maria 97105-900 Santa Maria RS Brazil [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Geórgia C. Zimmer ; Geórgia C. Zimmer Núcleo de Química de Heterociclos (NUQUIMHE), Department of Chemistry, Federal University of Santa Maria 97105-900 Santa Maria RS Brazil [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Nilo Zanatta Nilo Zanatta Núcleo de Química de Heterociclos (NUQUIMHE), Department of Chemistry, Federal University of Santa Maria 97105-900 Santa Maria RS Brazil [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 3.1.15. Michael Addition Reaction Followed by Elimination Under Solvent-Free Conditions in another window
- 3.1.16. Synthesis of a γ-Keto-amide Derived from Thiophene Using a Carboxyl Ester as Precursor p182-187 By M. Manuela M. Raposo M. Manuela M. Raposo Department of Chemistry, University of Minho, Campus de Gualtar 4710-057 Braga Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 3.1.16. Synthesis of a γ-Keto-amide Derived from Thiophene Using a Carboxyl Ester as Precursor in another window
- 3.1.17. Cyclic Acetals for Regioselective Protection in Carbohydrate Synthesis: A Comparative Experiment p188-193 By Ana M. Matos ; Ana M. Matos Centro de Química e Bioquímica, Faculdade de Ciências, Universidade de Lisboa C8, Piso 5, Campo Grande 1749-016 Lisboa Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Rafael Nunes ; Rafael Nunes Centro de Química e Bioquímica, Faculdade de Ciências, Universidade de Lisboa C8, Piso 5, Campo Grande 1749-016 Lisboa Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Catarina Dias ; Catarina Dias Centro de Química e Bioquímica, Faculdade de Ciências, Universidade de Lisboa C8, Piso 5, Campo Grande 1749-016 Lisboa Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Amélia P. Rauter Amélia P. Rauter Centro de Química e Bioquímica, Faculdade de Ciências, Universidade de Lisboa C8, Piso 5, Campo Grande 1749-016 Lisboa Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 3.1.17. Cyclic Acetals for Regioselective Protection in Carbohydrate Synthesis: A Comparative Experiment in another window
- 3.1.18. Direct Diastereoselective Synthesis of the Tetrahydro-thiazolo[2,3- b ]isoindole Tricyclic Ring System p194-197 By Maria I. L. Soares ; Maria I. L. Soares Department of Chemistry, University of Coimbra Rua Larga 3004-535 Coimbra Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Ana Lúcia Cardoso ; Ana Lúcia Cardoso Department of Chemistry, University of Coimbra Rua Larga 3004-535 Coimbra Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Susana M. M. Lopes ; Susana M. M. Lopes Department of Chemistry, University of Coimbra Rua Larga 3004-535 Coimbra Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Teresa M. V. D. Pinho e Melo Teresa M. V. D. Pinho e Melo Department of Chemistry, University of Coimbra Rua Larga 3004-535 Coimbra Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 3.1.18. Direct Diastereoselective Synthesis of the Tetrahydro-thiazolo[2,3-<em>b</em>]isoindole Tricyclic Ring System in another window
- 3.1.19. Asymmetric Cyclocondensation Reaction Induced by Chiral Aminoalcohol p198-201 By Jorge Dourado ; Jorge Dourado Research Institute for Medicines (iMed.ULisboa), Faculty of Pharmacy, Universidade de Lisboa Av. Prof. Gama Pinto 1649-003 Lisboa Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Maria Pérez ; Maria Pérez Laboratory of Organic Chemistry, Faculty of Pharmacy and Institute of Biomedicine (IBUB), University of Barcelona Av. Joan XXIII, s/n 08028 Barcelona Spain Search for other works by this author on: This Site PubMed Google Scholar Rosa Griera ; Rosa Griera Laboratory of Organic Chemistry, Faculty of Pharmacy and Institute of Biomedicine (IBUB), University of Barcelona Av. Joan XXIII, s/n 08028 Barcelona Spain Search for other works by this author on: This Site PubMed Google Scholar Maria M. M. Santos Maria M. M. Santos Research Institute for Medicines (iMed.ULisboa), Faculty of Pharmacy, Universidade de Lisboa Av. Prof. Gama Pinto 1649-003 Lisboa Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 3.1.19. Asymmetric Cyclocondensation Reaction Induced by Chiral Aminoalcohol in another window
- 3.1.20. Synthesis and Characterization of Biodiesel Propyl Esters to Determine the Fatty Acid Content of Unknown Plant Oils p202-205 By Heidi R. Vollmer-Snarr ; Heidi R. Vollmer-Snarr Department of Chemistry, Stanford University S.G. Mudd Bldg., 333 Campus Drive Stanford CA 94305-4401 USA [email protected] Search for other works by this author on: This Site PubMed Google Scholar Patrick A. Fracisco ; Patrick A. Fracisco Department of Chemistry, Stanford University S.G. Mudd Bldg., 333 Campus Drive Stanford CA 94305-4401 USA [email protected] Search for other works by this author on: This Site PubMed Google Scholar Naiian Saephanh Naiian Saephanh Department of Chemistry, Stanford University S.G. Mudd Bldg., 333 Campus Drive Stanford CA 94305-4401 USA [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 3.1.20. Synthesis and Characterization of Biodiesel Propyl Esters to Determine the Fatty Acid Content of Unknown Plant Oils in another window
- 3.2.1. Synthesis of 7-Methoxy-4-oxo- N -phenyl-4 H -chromene-2-carboxamide p206-211 By Joana Reis ; Joana Reis Department of Chemistry and Biochemistry, Faculty of Sciences of University of Porto Rua do Campo Alegre s/n 4169-007 Porto Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Alexandra Gaspar ; Alexandra Gaspar Department of Chemistry and Biochemistry, Faculty of Sciences of University of Porto Rua do Campo Alegre s/n 4169-007 Porto Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Fernando Cagide ; Fernando Cagide Department of Chemistry and Biochemistry, Faculty of Sciences of University of Porto Rua do Campo Alegre s/n 4169-007 Porto Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Fernanda Borges Fernanda Borges Department of Chemistry and Biochemistry, Faculty of Sciences of University of Porto Rua do Campo Alegre s/n 4169-007 Porto Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 3.2.1. Synthesis of 7-Methoxy-4-oxo-<em>N</em>-phenyl-4<em>H</em>-chromene-2-carboxamide in another window
- 3.2.2. Synthesis of 2,3-Diphenylindenone p212-215 By João P. Telo João P. Telo Centro de Química Estrutural, Instituto Superior Técnico, Universidade de Lisboa Av. Rovisco Pais 1649-003 Lisboa Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 3.2.2. Synthesis of 2,3-Diphenylindenone in another window
- 3.2.3. Synthesis of Dimedone p216-218 By Dulce Simão ; Dulce Simão Department of Chemistry, Instituto Superior Técnico, University of Lisbon, Centro de Química Estrutural Av. Rovisco Pais, 1 1049-001 Lisbon Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Ana C. Cerdeira Ana C. Cerdeira Department of Chemistry, Instituto Superior Técnico, University of Lisbon, Centro de Química Estrutural Av. Rovisco Pais, 1 1049-001 Lisbon Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 3.2.3. Synthesis of Dimedone in another window
- 3.2.4. Acylation Reaction of Enol Ether Using an Ionic Liquid p219-222 By Marcos A. P. Martins ; Marcos A. P. Martins Núcleo de Química de Heterociclos (NUQUIMHE), Department of Chemistry, Federal University of Santa Maria 97105-900 Santa Maria RS Brazil [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Clarissa P. Frizzo ; Clarissa P. Frizzo Núcleo de Química de Heterociclos (NUQUIMHE), Department of Chemistry, Federal University of Santa Maria 97105-900 Santa Maria RS Brazil [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Geórgia C. Zimmer ; Geórgia C. Zimmer Núcleo de Química de Heterociclos (NUQUIMHE), Department of Chemistry, Federal University of Santa Maria 97105-900 Santa Maria RS Brazil [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Caroline R. Bender ; Caroline R. Bender Núcleo de Química de Heterociclos (NUQUIMHE), Department of Chemistry, Federal University of Santa Maria 97105-900 Santa Maria RS Brazil [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Paulo R. S. Salbego ; Paulo R. S. Salbego Núcleo de Química de Heterociclos (NUQUIMHE), Department of Chemistry, Federal University of Santa Maria 97105-900 Santa Maria RS Brazil [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Aniele Z. Tier ; Aniele Z. Tier Núcleo de Química de Heterociclos (NUQUIMHE), Department of Chemistry, Federal University of Santa Maria 97105-900 Santa Maria RS Brazil [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Nilo Zanatta Nilo Zanatta Núcleo de Química de Heterociclos (NUQUIMHE), Department of Chemistry, Federal University of Santa Maria 97105-900 Santa Maria RS Brazil [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 3.2.4. Acylation Reaction of Enol Ether Using an Ionic Liquid in another window
- 4.1.1.1. Bromination of Cinnamic Acid p223-225 By Paulo Coelho ; Paulo Coelho Centro de, Química – Vila Real, Universidade de Trás-os-Montes e Alto Douro 5001-801 Vila Real Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Céu Sousa Céu Sousa Centro de, Química – Vila Real, Universidade de Trás-os-Montes e Alto Douro 5001-801 Vila Real Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 4.1.1.1. Bromination of Cinnamic Acid in another window
- 4.1.1.2. Preparation of Meso -1,2-Dibromo-1,2-diphenylethane p226-228 By Abel J. S. C. Vieira Abel J. S. C. Vieira Faculty of Sciences and Technology, Universidade Nova de Lisboa 2829-516 Caparica Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 4.1.1.2. Preparation of <em>Meso</em>-1,2-Dibromo-1,2-diphenylethane in another window
- 4.1.1.3. Bromination of ( E )-chalcones [( E )-1,3-Diarylprop-2-en-1-ones] p229-230 By Artur M. S. Silva ; Artur M. S. Silva Chemistry Department & QOPNA, University of Aveiro Campus de Santiago 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Augusto C. Tomé ; Augusto C. Tomé Chemistry Department & QOPNA, University of Aveiro Campus de Santiago 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Diana C. G. A. Pinto ; Diana C. G. A. Pinto Chemistry Department & QOPNA, University of Aveiro Campus de Santiago 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Fernando Domingues ; Fernando Domingues Chemistry Department & QOPNA, University of Aveiro Campus de Santiago 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Graça M. Oliveira Rocha ; Graça M. Oliveira Rocha Chemistry Department & QOPNA, University of Aveiro Campus de Santiago 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar José A. S. Cavaleiro ; José A. S. Cavaleiro Chemistry Department & QOPNA, University of Aveiro Campus de Santiago 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar M. Graça P. M. S. Neves ; M. Graça P. M. S. Neves Chemistry Department & QOPNA, University of Aveiro Campus de Santiago 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar M. Amparo F. Faustino ; M. Amparo F. Faustino Chemistry Department & QOPNA, University of Aveiro Campus de Santiago 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Mário M. Q. Simões Mário M. Q. Simões Chemistry Department & QOPNA, University of Aveiro Campus de Santiago 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 4.1.1.3. Bromination of (<em>E</em>)-chalcones [(<em>E</em>)-1,3-Diarylprop-2-en-1-ones] in another window
- 4.1.1.4. Preparation of trans -2-Bromocyclohexanol from Cyclohexanol p231-233 By Matheus P. Freitas Matheus P. Freitas Department of Chemistry, Federal University of Lavras 37200-000 Lavras MG Brazil [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 4.1.1.4. Preparation of <em>trans</em>-2-Bromocyclohexanol from Cyclohexanol in another window
- 4.1.1.5. Synthesis of trans -Cyclohexane-1,2-diol p234-236 By Dulce Simão ; Dulce Simão Department of Chemistry, Instituto Superior Técnico, University of Lisbon, Centro de Química Estrutural Av. Rovisco Pais, 1 1049-001 Lisbon Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Ana C. Cerdeira Ana C. Cerdeira Department of Chemistry, Instituto Superior Técnico, University of Lisbon, Centro de Química Estrutural Av. Rovisco Pais, 1 1049-001 Lisbon Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 4.1.1.5. Synthesis of <em>trans</em>-Cyclohexane-1,2-diol in another window
- 4.1.1.6. Synthesis of Isobutylene and Its Use in Esterification Reactions p237-240 By Paula C. Castilho ; Paula C. Castilho Centro de Química da, Madeira, Universidade da Madeira, Campus Universitário da Penteada Piso 00 9020-105 Funchal Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Pedro Ideia ; Pedro Ideia Centro de Química da, Madeira, Universidade da Madeira, Campus Universitário da Penteada Piso 00 9020-105 Funchal Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Rúben Gonçalves Rúben Gonçalves Centro de Química da, Madeira, Universidade da Madeira, Campus Universitário da Penteada Piso 00 9020-105 Funchal Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 4.1.1.6. Synthesis of Isobutylene and Its Use in Esterification Reactions in another window
- 4.2.3.8. Synthesis of a Long-Wavelength Absorbing Squaraine Dye p396-398 By Paulo F. Santos ; Paulo F. Santos Departamento de Química and Centro de Química – Vila Real, Universidade de Trás-os-Montes e Alto Douro 5001-801 Vila Real Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Vânia C. Graça Vânia C. Graça Departamento de Química and Centro de Química – Vila Real, Universidade de Trás-os-Montes e Alto Douro 5001-801 Vila Real Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 4.2.3.8. Synthesis of a Long-Wavelength Absorbing Squaraine Dye in another window
- 4.2.3.9. Synthesis of π-Conjugated Systems Using Formylchromone as Building Block p399-402 By Carlos Fernandes ; Carlos Fernandes CIQUP/Department of Chemistry and Biochemistry, Faculty of Sciences of University of Porto Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Catarina Oliveira ; Catarina Oliveira CIQUP/Department of Chemistry and Biochemistry, Faculty of Sciences of University of Porto Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Fernanda Borges ; Fernanda Borges CIQUP/Department of Chemistry and Biochemistry, Faculty of Sciences of University of Porto Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Alexandra Gaspar Alexandra Gaspar CIQUP/Department of Chemistry and Biochemistry, Faculty of Sciences of University of Porto Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 4.2.3.9. Synthesis of π-Conjugated Systems Using Formylchromone as Building Block in another window
- 4.2.3.10. A Multi-Step Synthesis of Imidazolin-5-ones p403-407 By Gurunath Ramanathan ; Gurunath Ramanathan Indian Institute of Technology Kanpur Kanpur 208016 India [email protected] Search for other works by this author on: This Site PubMed Google Scholar Ashish Singh Ashish Singh Indian Institute of Technology Kanpur Kanpur 208016 India [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 4.2.3.10. A Multi-Step Synthesis of Imidazolin-5-ones in another window
- 4.2.3.11. Baylis–Hillman Reaction Between 4-Nitrobenzaldehyde and Ethyl Acrylate p408-410 By Alexandre F. Trindade ; Alexandre F. Trindade Faculty of Pharmacy, University of Lisbon Av. Prof. Gama Pinto 1649-003 Lisboa Portugal [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar João Ravasco ; João Ravasco Faculty of Pharmacy, University of Lisbon Av. Prof. Gama Pinto 1649-003 Lisboa Portugal [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Carlos A. M. Afonso Carlos A. M. Afonso Faculty of Pharmacy, University of Lisbon Av. Prof. Gama Pinto 1649-003 Lisboa Portugal [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 4.2.3.11. Baylis–Hillman Reaction Between 4-Nitrobenzaldehyde and Ethyl Acrylate in another window
- 4.1.1.7. Hydroxyl Group Protection via Tetrahydropyranyl Ether Formation p241-243 By Ana I. B. Meirinhos ; Ana I. B. Meirinhos iMed.ULisboa Av. Professor Gama Pinto 1649-003 Lisboa Portugal [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Filipa Siopa ; Filipa Siopa iMed.ULisboa Av. Professor Gama Pinto 1649-003 Lisboa Portugal [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Carlos A. M. Afonso Carlos A. M. Afonso iMed.ULisboa Av. Professor Gama Pinto 1649-003 Lisboa Portugal [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 4.1.1.7. Hydroxyl Group Protection <em>via</em> Tetrahydropyranyl Ether Formation in another window
- 4.1.1.8. Synthesis of (−)-Carvone from (+)-Limonene p244-246 By João P. Telo João P. Telo Centro de Química Estrutural, Instituto Superior Técnico, Universidade de Lisboa Av. Rovisco Pais 1649-003 Lisboa Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 4.1.1.8. Synthesis of (−)-Carvone from (+)-Limonene in another window
- 4.2.3.12. Synthesis of Ethyl Mandelate Through a Rhodium-Catalysed Arylation Reaction with Ethyl Glyoxylate and Phenylboronic Acid p411-414 By Carolina S. Marques ; Carolina S. Marques University of Évora, Chemistry Department, School of Science and Technology, Centro de Química de Évora, Institute for Research and Advanced Training Rua Romão Ramalho, 59 7000-671 Évora Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Anthony J. Burke Anthony J. Burke University of Évora, Chemistry Department, School of Science and Technology, Centro de Química de Évora, Institute for Research and Advanced Training Rua Romão Ramalho, 59 7000-671 Évora Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 4.2.3.12. Synthesis of Ethyl Mandelate Through a Rhodium-Catalysed Arylation Reaction with Ethyl Glyoxylate and Phenylboronic Acid in another window
- 4.2.4.1. Preparation of Chalcone and Its Further Robinson Annulation with Ethyl Acetoacetate p420-423 By Nuno R. Candeias Nuno R. Candeias Department of Chemistry and Bioengineering, Tampere University of Technology Korkeakoulunkatu 8 Tampere FI-33101 Finland [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 4.2.4.1. Preparation of Chalcone and Its Further Robinson Annulation with Ethyl Acetoacetate in another window
- 4.1.1.9. Glycal Transformation into Surfactant 2-Deoxy Glycosides p247-251 By Catarina Dias ; Catarina Dias Centro de Química e Bioquímica, Faculdade de Ciências, Universidade de Lisboa C8, Piso 5, Campo Grande 1749-016 Lisboa Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Amélia P. Rauter Amélia P. Rauter Centro de Química e Bioquímica, Faculdade de Ciências, Universidade de Lisboa C8, Piso 5, Campo Grande 1749-016 Lisboa Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 4.1.1.9. Glycal Transformation into Surfactant 2-Deoxy Glycosides in another window
- 4.2.3.13. Enantioselective Synthesis and Derivatisation of 2-Hydroxy-1,2-diphenylethan-1-one p415-419 By Jonathan D. Sellars ; Jonathan D. Sellars Chemistry Department, University of Durham South Road Durham DH1 3LE UK [email protected] Search for other works by this author on: This Site PubMed Google Scholar AnnMarie C. O’Donoghue ; AnnMarie C. O’Donoghue Chemistry Department, University of Durham South Road Durham DH1 3LE UK [email protected] Search for other works by this author on: This Site PubMed Google Scholar Ian R. Baxendale ; Ian R. Baxendale Chemistry Department, University of Durham South Road Durham DH1 3LE UK [email protected] Search for other works by this author on: This Site PubMed Google Scholar John M. Sanderson ; John M. Sanderson Chemistry Department, University of Durham South Road Durham DH1 3LE UK [email protected] Search for other works by this author on: This Site PubMed Google Scholar Elizabeth J. Grayson Elizabeth J. Grayson Chemistry Department, University of Durham South Road Durham DH1 3LE UK [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 4.2.3.13. Enantioselective Synthesis and Derivatisation of 2-Hydroxy-1,2-diphenylethan-1-one in another window
- 5.1.1. Synthesis of Methyl Orange p448-450 By Dulce Simão ; Dulce Simão Department of Chemistry, Instituto Superior Técnico, University of Lisbon, Centro de Química Estrutural Av. Rovisco Pais, 1 1049-001 Lisbon Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Ana C. Cerdeira Ana C. Cerdeira Department of Chemistry, Instituto Superior Técnico, University of Lisbon, Centro de Química Estrutural Av. Rovisco Pais, 1 1049-001 Lisbon Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 5.1.1. Synthesis of Methyl Orange in another window
- 4.2.5.1. One-Pot Green Synthesis of Dihydropyran Heterocycles p428-431 By Dennis Russowsky ; Dennis Russowsky Laboratório de Síntese Organica K-210, Universidade Federal do Rio Grande do Sul, Instituto de Química Av. Bento Gonçalves, 9500 CEP 91501-970 Porto Alegre Rio Grande do Sul Brazil [email protected] Search for other works by this author on: This Site PubMed Google Scholar Camila S. Santos Camila S. Santos Laboratório de Síntese Organica K-210, Universidade Federal do Rio Grande do Sul, Instituto de Química Av. Bento Gonçalves, 9500 CEP 91501-970 Porto Alegre Rio Grande do Sul Brazil [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 4.2.5.1. One-Pot Green Synthesis of Dihydropyran Heterocycles in another window
- 4.2.5.2. Hantzsch Synthesis of Nifedipine p432-434 By Arno Kraft Arno Kraft Heriot-Watt University, Institute of Chemical Sciences, School of Engineering & Physical Sciences Riccarton Edinburgh EH14 4AS UK [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 4.2.5.2. Hantzsch Synthesis of Nifedipine in another window
- 4.1.1.10. Preparation of (1 R ,2 R ,3 R ,5 S )-(−)-Isopinocampheol Through a Hydroboration–Oxidation Reaction p252-256 By Marek P. Krzemiński Marek P. Krzemiński Department of Organic Chemistry, Faculty of Chemistry, Nicolaus Copernicus University in Toruń Poland [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 4.1.1.10. Preparation of (1<em>R</em>,2<em>R</em>,3<em>R</em>,5<em>S</em>)-(−)-Isopinocampheol Through a Hydroboration–Oxidation Reaction in another window
- 5.1.5. Synthesis of 2-Nitro-4-methylaniline p462-464 By Dulce Simão ; Dulce Simão Department of Chemistry, Instituto Superior Técnico, University of Lisbon, Centro de Química Estrutural Av. Rovisco Pais, 1 1049-001 Lisbon Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Ana C. Cerdeira Ana C. Cerdeira Department of Chemistry, Instituto Superior Técnico, University of Lisbon, Centro de Química Estrutural Av. Rovisco Pais, 1 1049-001 Lisbon Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 5.1.5. Synthesis of 2-Nitro-4-methylaniline in another window
- 4.2.4.2. Conjugate Addition of Organocuprates to α,β-Unsaturated Ketones: Synthesis of 3,3-Dimethylcyclohexanone from 3-Methyl-2-cyclohexen-1-one p424-427 By Carlos Gregorio ; Carlos Gregorio Departamento de Química Orgánica, Facultad de Química, Universidad de Santiago de Compostela Avda. Ciencias S/N 15782 Santiago de Compostela Spain [email protected] Search for other works by this author on: This Site PubMed Google Scholar F. Javier Sardina ; F. Javier Sardina Departamento de Química Orgánica, Facultad de Química, Universidad de Santiago de Compostela Avda. Ciencias S/N 15782 Santiago de Compostela Spain [email protected] Search for other works by this author on: This Site PubMed Google Scholar Antonio Mouriño Antonio Mouriño Departamento de Química Orgánica, Facultad de Química, Universidad de Santiago de Compostela Avda. Ciencias S/N 15782 Santiago de Compostela Spain [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 4.2.4.2. Conjugate Addition of Organocuprates to α,β-Unsaturated Ketones: Synthesis of 3,3-Dimethylcyclohexanone from 3-Methyl-2-cyclohexen-1-one in another window
- 4.1.2.1. Synthesis of Fructone p257-260 By Thomas A. Logothetis Thomas A. Logothetis University of Southampton, Chemistry Highfield, Southampton Hampshire SO17 1BJ UK [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 4.1.2.1. Synthesis of Fructone in another window
- 5.1.6. Synthesis of 1-Nitronaphthalene p465-467 By Adam Dzielendziak Adam Dzielendziak Department of Organic Chemistry, Faculty of Chemistry, Nicolaus Copernicus University in Torun 87-100 Torun Poland [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 5.1.6. Synthesis of 1-Nitronaphthalene in another window
- 5.1.4. Regioselectivity in the Nitration of Acylanilines by Electrophilic Aromatic Substitution p456-461 By João P. Telo ; João P. Telo CQE, Department of Chemical Engineering, IST, University of Lisbon Av. Rovisco Pais 1649-003 Lisbon Portugal [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Pedro P. Santos ; Pedro P. Santos CQE, Department of Chemical Engineering, IST, University of Lisbon Av. Rovisco Pais 1649-003 Lisbon Portugal [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar João V. Moreira ; João V. Moreira CQE, Department of Chemical Engineering, IST, University of Lisbon Av. Rovisco Pais 1649-003 Lisbon Portugal [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Mariana L. Santos ; Mariana L. Santos CQE, Department of Chemical Engineering, IST, University of Lisbon Av. Rovisco Pais 1649-003 Lisbon Portugal [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Ana T. Batista Ana T. Batista CQE, Department of Chemical Engineering, IST, University of Lisbon Av. Rovisco Pais 1649-003 Lisbon Portugal [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 5.1.4. Regioselectivity in the Nitration of Acylanilines by Electrophilic Aromatic Substitution in another window
- 5.1.3. Synthesis of 2-(2,4-Dinitrobenzyl)pyridine p454-455 By Dulce Simão Dulce Simão Department of Chemistry, Instituto Superior Técnico, University of Lisbon, Centro de Química Estrutural Av. Rovisco Pais, 1 1049-001 Lisbon Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 5.1.3. Synthesis of 2-(2,4-Dinitrobenzyl)pyridine in another window
- 5.1.2. Halogenation Reactions of Vanillin p451-453 By Iain A. Smellie ; Iain A. Smellie School of Chemistry, University of St Andrews North Haugh, St Andrews Fife KY16 9ST UK [email protected] Search for other works by this author on: This Site PubMed Google Scholar Nigel P. Botting ; Nigel P. Botting School of Chemistry, University of St Andrews North Haugh, St Andrews Fife KY16 9ST UK [email protected] Search for other works by this author on: This Site PubMed Google Scholar Brian A. Chalmers ; Brian A. Chalmers School of Chemistry, University of St Andrews North Haugh, St Andrews Fife KY16 9ST UK [email protected] Search for other works by this author on: This Site PubMed Google Scholar Iain L. J. Patterson Iain L. J. Patterson School of Chemistry, University of St Andrews North Haugh, St Andrews Fife KY16 9ST UK [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 5.1.2. Halogenation Reactions of Vanillin in another window
- 5.2.2. Synthesis of 4,4′-Di- tert -butylbiphenyl p480-481 By Dulce Simão Dulce Simão Department of Chemistry, Instituto Superior Técnico, University of Lisbon, Centro de Química Estrutural Av. Rovisco Pais, 1 1049-001 Lisbon Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 5.2.2. Synthesis of 4,4′-Di-<em>tert</em>-butylbiphenyl in another window
- 5.2.4. Synthesis of 5,10,15,20-Tetrakis(2,6-dichlorophenyl)porphyrin p485-489 By Artur M. S. Silva ; Artur M. S. Silva Chemistry Department, University of Aveiro, Campus de Santiago 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Augusto C. Tomé ; Augusto C. Tomé Chemistry Department, University of Aveiro, Campus de Santiago 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Diana C. G. A. Pinto ; Diana C. G. A. Pinto Chemistry Department, University of Aveiro, Campus de Santiago 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Fernando M. J. Domingues ; Fernando M. J. Domingues Chemistry Department, University of Aveiro, Campus de Santiago 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Graça M. S. R. O. Rocha ; Graça M. S. R. O. Rocha Chemistry Department, University of Aveiro, Campus de Santiago 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar José A. S. Cavaleiro ; José A. S. Cavaleiro Chemistry Department, University of Aveiro, Campus de Santiago 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Maria da Graça ; Maria da Graça Chemistry Department, University of Aveiro, Campus de Santiago 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar P. M. S. Neves ; P. M. S. Neves Chemistry Department, University of Aveiro, Campus de Santiago 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Maria do Amparo F. Faustino ; Maria do Amparo F. Faustino Chemistry Department, University of Aveiro, Campus de Santiago 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Mário M. Q. Simões Mário M. Q. Simões Chemistry Department, University of Aveiro, Campus de Santiago 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 5.2.4. Synthesis of 5,10,15,20-Tetrakis(2,6-dichlorophenyl)porphyrin in another window
- 5.2.7. Synthesis of Musk Ketone p496-499 By João Paulo Telo João Paulo Telo CQE, Department of Chemical Engineering, IST, University of Lisbon Av. Rovisco Pais 1649-003 Lisbon Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 5.2.7. Synthesis of Musk Ketone in another window
- 5.2.6. Synthesis of 3-Bromo-7-ethylcarbamate-4-methylcoumarin p493-495 By Paula S. Branco ; Paula S. Branco Departamento de Química, Faculdade de Ciências e Tecnologia, UNL 2829-516 Caparica Portugal [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Ana Maria Lourenço Ana Maria Lourenço Departamento de Química, Faculdade de Ciências e Tecnologia, UNL 2829-516 Caparica Portugal [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 5.2.6. Synthesis of 3-Bromo-7-ethylcarbamate-4-methylcoumarin in another window
- 5.2.1. Synthesis of 1,4-Di- t -butyl-2,5-dimethoxybenzene p477-479 By Dulce Simão ; Dulce Simão Department of Chemistry, Instituto Superior Técnico, University of Lisbon, Centro de Química Estrutural Av. Rovisco Pais, 1 1049-001 Lisbon Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Ana C. Cerdeira Ana C. Cerdeira Department of Chemistry, Instituto Superior Técnico, University of Lisbon, Centro de Química Estrutural Av. Rovisco Pais, 1 1049-001 Lisbon Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 5.2.1. Synthesis of 1,4-Di-<em>t</em>-butyl-2,5-dimethoxybenzene in another window
- 4.2.5.5. A Multicomponent Reaction of Isocyanides for the Synthesis of 4-Chromanone-2-carboxamides p443-447 By Ana G. Neo ; Ana G. Neo Departamento de Química Orgánica e Inorgánica, F. Veterinaria, Universidad de Extremadura 10071 Cáceres Spain [email protected] Search for other works by this author on: This Site PubMed Google Scholar Jesús Díaz ; Jesús Díaz Departamento de Química Orgánica e Inorgánica, F. Veterinaria, Universidad de Extremadura 10071 Cáceres Spain [email protected] Search for other works by this author on: This Site PubMed Google Scholar Carlos F. Marcos Carlos F. Marcos Departamento de Química Orgánica e Inorgánica, F. Veterinaria, Universidad de Extremadura 10071 Cáceres Spain [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 4.2.5.5. A Multicomponent Reaction of Isocyanides for the Synthesis of 4-Chromanone-2-carboxamides in another window
- 5.2.3. Synthesis of a Macrocycle: C -Methyl[4]resorcinarene p482-484 By Christopher Baker ; Christopher Baker School of Pharmacy and Biomolecular Sciences, University of Brighton Huxley Building Brighton BN2 4GJ UK [email protected] Search for other works by this author on: This Site PubMed Google Scholar Alexander S. Cragg ; Alexander S. Cragg School of Pharmacy and Biomolecular Sciences, University of Brighton Huxley Building Brighton BN2 4GJ UK [email protected] Search for other works by this author on: This Site PubMed Google Scholar Raghuram R. Kothur ; Raghuram R. Kothur School of Pharmacy and Biomolecular Sciences, University of Brighton Huxley Building Brighton BN2 4GJ UK [email protected] Search for other works by this author on: This Site PubMed Google Scholar Flavia Fucassi ; Flavia Fucassi School of Pharmacy and Biomolecular Sciences, University of Brighton Huxley Building Brighton BN2 4GJ UK [email protected] Search for other works by this author on: This Site PubMed Google Scholar Peter J. Cragg Peter J. Cragg School of Pharmacy and Biomolecular Sciences, University of Brighton Huxley Building Brighton BN2 4GJ UK [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 5.2.3. Synthesis of a Macrocycle: <em>C</em>-Methyl[4]resorcinarene in another window
- 5.2.9. Synthesis of Eosin p504-507 By Dulce Simão Dulce Simão Department of Chemistry, Instituto Superior Técnico, University of Lisbon, Centro de Química Estrutural Av. Rovisco Pais, 1 1049-001 Lisbon Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 5.2.9. Synthesis of Eosin in another window
- 4.2.5.4. Preparation of Phenylglycine and Hydroxymorpholine Derivatives Through a Petasis Borono–Mannich Reaction p439-442 By Nuno R. Candeias ; Nuno R. Candeias Department of Chemistry and Bioengineering, Tampere University of Technology Korkeakoulunkatu 8 Tampere FI-33101 Finland [email protected] Search for other works by this author on: This Site PubMed Google Scholar Roberta Paterna ; Roberta Paterna Research Institute for Medicines and Pharmaceutical Sciences (iMed.UL), Faculty of Pharmacy, University of Lisbon Av. Prof. Gama Pinto 1649-003 Lisbon Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Pedro M. S. D. Cal ; Pedro M. S. D. Cal Research Institute for Medicines and Pharmaceutical Sciences (iMed.UL), Faculty of Pharmacy, University of Lisbon Av. Prof. Gama Pinto 1649-003 Lisbon Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Pedro M. P. Gois Pedro M. P. Gois Research Institute for Medicines and Pharmaceutical Sciences (iMed.UL), Faculty of Pharmacy, University of Lisbon Av. Prof. Gama Pinto 1649-003 Lisbon Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 4.2.5.4. Preparation of Phenylglycine and Hydroxymorpholine Derivatives Through a Petasis Borono–Mannich Reaction in another window
- 4.2.5.3. A Ugi Multicomponent Reaction in the Synthesis of N -Cyclohexyl-2-[ N -(4-methoxybenzyl)acetamide]-2-(thien-2-yl)acetamide p435-438 By Susana P. G. Costa ; Susana P. G. Costa University of Minho, Department of Chemistry, Campus de Gualtar 4710-057 Braga Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar M. Manuela M. Raposo ; M. Manuela M. Raposo University of Minho, Department of Chemistry, Campus de Gualtar 4710-057 Braga Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Cátia I. C. Esteves Cátia I. C. Esteves University of Minho, Department of Chemistry, Campus de Gualtar 4710-057 Braga Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 4.2.5.3. A Ugi Multicomponent Reaction in the Synthesis of <em>N</em>-Cyclohexyl-2-[<em>N</em>-(4-methoxybenzyl)acetamide]-2-(thien-2-yl)acetamide in another window
- 4.1.2.2. Synthesis of Flavone p261-263 By Dulce Simão ; Dulce Simão Department of Chemistry, Instituto Superior Técnico, University of Lisbon, Centro de Química Estrutural Av. Rovisco Pais, 1 1049-001 Lisbon Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Ana C. Cerdeira Ana C. Cerdeira Department of Chemistry, Instituto Superior Técnico, University of Lisbon, Centro de Química Estrutural Av. Rovisco Pais, 1 1049-001 Lisbon Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 4.1.2.2. Synthesis of Flavone in another window
- 5.2.10. Synthesis and Formylation of 5-Piperidino-2,2′-bithiophene p508-513 By M. Manuela M. Raposo M. Manuela M. Raposo Department of Chemistry, University of Minho, Campus de Gualtar 4710-057 Braga Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 5.2.10. Synthesis and Formylation of 5-Piperidino-2,2′-bithiophene in another window
- 6.2. Synthesis of the Antitumoral Drug 2,4,6-Tris(dimethylamino)-1,3,5-triazine via Sequential Nucleophilic Substitution p535-538 By Filipa Siopa ; Filipa Siopa Instituto de Investigação, do Medicamento (iMed.ULisboa), Faculdade de Farmácia, Universidade de Lisboa Av. Prof. Gama Pinto 1649-003 Lisboa Portugal [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Nuno Candeias ; Nuno Candeias Department of Chemistry and Bioengineering, Tampere University of Technology Korkeakoulunkatu 8 Tampere FI-33101 Finland [email protected] Search for other works by this author on: This Site PubMed Google Scholar Carlos A. M. Afonso Carlos A. M. Afonso Instituto de Investigação, do Medicamento (iMed.ULisboa), Faculdade de Farmácia, Universidade de Lisboa Av. Prof. Gama Pinto 1649-003 Lisboa Portugal [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 6.2. Synthesis of the Antitumoral Drug 2,4,6-Tris(dimethylamino)-1,3,5-triazine <em>via</em> Sequential Nucleophilic Substitution in another window
- 5.2.5. Solventless Synthesis, Separation and Characterization of Zinc and Free-Base Tetraphenyl Porphyrin p490-492 By Waqar Rizvi ; Waqar Rizvi Hunter College of the City University of New York 695 Park Avenue New York New York 10065 USA The Graduate Center of the City University of New York 365 Fifth Avenue New York New York 10016 USA [email protected] Search for other works by this author on: This Site PubMed Google Scholar Charles M. Drain ; Charles M. Drain Hunter College of the City University of New York 695 Park Avenue New York New York 10065 USA The Graduate Center of the City University of New York 365 Fifth Avenue New York New York 10016 USA [email protected] Search for other works by this author on: This Site PubMed Google Scholar Patrick Moy ; Patrick Moy Hunter College of the City University of New York 695 Park Avenue New York New York 10065 USA Search for other works by this author on: This Site PubMed Google Scholar Matthew J. Jurow Matthew J. Jurow The Molecular Foundry, Lawrence Berkeley National Laboratory Berkeley California 94720 USA Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 5.2.5. Solventless Synthesis, Separation and Characterization of Zinc and Free-Base Tetraphenyl Porphyrin in another window
- 5.1.8. Preparation of p -Bromoaniline p472-476 By Abel J. S. C. Vieira Abel J. S. C. Vieira Faculty of Sciences and Technology, Universidade Nova de Lisboa 2829-516 Caparica Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 5.1.8. Preparation of <em>p</em>-Bromoaniline in another window
- 6.3. Benzotriazole, a Useful Synthetic Auxiliary in Heterocyclizations p539-541 By João Lavrado ; João Lavrado Research Institute for Medicines (iMed.ULisboa), Faculty of Pharmacy, Universidade de Lisboa Av. Prof. Gama Pinto 1649-003 Lisboa Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Marta Figueiras ; Marta Figueiras Research Institute for Medicines (iMed.ULisboa), Faculty of Pharmacy, Universidade de Lisboa Av. Prof. Gama Pinto 1649-003 Lisboa Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Eduardo Ruivo ; Eduardo Ruivo Research Institute for Medicines (iMed.ULisboa), Faculty of Pharmacy, Universidade de Lisboa Av. Prof. Gama Pinto 1649-003 Lisboa Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Susana Lucas ; Susana Lucas Research Institute for Medicines (iMed.ULisboa), Faculty of Pharmacy, Universidade de Lisboa Av. Prof. Gama Pinto 1649-003 Lisboa Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Alexandra Paulo Alexandra Paulo Research Institute for Medicines (iMed.ULisboa), Faculty of Pharmacy, Universidade de Lisboa Av. Prof. Gama Pinto 1649-003 Lisboa Portugal [email protected] Pharmaceutical and Medicinal Chemistry Department, Faculty of Pharmacy, Universidade de Lisboa Av. Prof. Gama Pinto 1649-003 Lisboa Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 6.3. Benzotriazole, a Useful Synthetic Auxiliary in Heterocyclizations in another window
- 5.1.7. Selective Boc-Protection and Bromination of Pyrazoles p468-471 By Thomas A. Logothetis Thomas A. Logothetis University of Southampton, Chemistry, Highfield Southampton Hampshire SO17 1BJ UK [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 5.1.7. Selective Boc-Protection and Bromination of Pyrazoles in another window
- 4.1.2.3. Synthesis and Characterisation of H 2 salen: An Introduction to 1D and 2D NMR Spectroscopy p264-268 By Maria J. Villa de Brito Maria J. Villa de Brito DQB, CQE, Faculdade de Ciências da Universidade de Lisboa Campo Grande, 1749-001 Lisboa Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 4.1.2.3. Synthesis and Characterisation of H<sub>2</sub>salen: An Introduction to 1D and 2D NMR Spectroscopy in another window
- 4.1.2.4. Synthesis of Lophine and Conversion into Dimers p269-271 By Dulce Simão Dulce Simão Department of Chemistry, Instituto Superior Técnico, University of Lisbon, Centro de Química Estrutural Av. Rovisco Pais, 1 1049-001 Lisbon Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 4.1.2.4. Synthesis of Lophine and Conversion into Dimers in another window
- 6.4. Synthesis of 4,5-Dicyanobenzene-1,2-dithiol p542-544 By Dulce Simão Dulce Simão Department of Chemistry, Instituto Superior Técnico, University of Lisbon, Centro de Química Estrutural Av. Rovisco Pais, 1 1049-001 Lisbon Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 6.4. Synthesis of 4,5-Dicyanobenzene-1,2-dithiol in another window
- 4.1.2.5. Synthesis of Dibenzalacetone 2,4-Dinitrophenylhydrazone p272-276 By Ana Margarida Madureira ; Ana Margarida Madureira Faculty of Pharmacy, University of Lisbon Av. Prof. Gama Pinto 1649-003 Lisbon Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Ana Bela Santana ; Ana Bela Santana Faculty of Pharmacy, University of Lisbon Av. Prof. Gama Pinto 1649-003 Lisbon Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Emília Valente ; Emília Valente Faculty of Pharmacy, University of Lisbon Av. Prof. Gama Pinto 1649-003 Lisbon Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Maria-José U. Ferreira Maria-José U. Ferreira Faculty of Pharmacy, University of Lisbon Av. Prof. Gama Pinto 1649-003 Lisbon Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 4.1.2.5. Synthesis of Dibenzalacetone 2,4-Dinitrophenylhydrazone in another window
- 4.1.2.6. Synthesis and Structural Characterization of an Antitubercular Isoniazid Hydrazone p277-281 By Susana Santos ; Susana Santos Centro de Química e Bioquímica, Departamento de Química e Bioquímica, Faculdade de Ciências, Universidade de Lisboa Ed. C8 Campo Grande, 1749-016 Lisboa Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Filomena Martins Filomena Martins Centro de Química e Bioquímica, Departamento de Química e Bioquímica, Faculdade de Ciências, Universidade de Lisboa Ed. C8 Campo Grande, 1749-016 Lisboa Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 4.1.2.6. Synthesis and Structural Characterization of an Antitubercular Isoniazid Hydrazone in another window
- 7.3. Recycling Bromovanillin into Ferulic Acid-Based Antioxidants p559-563 By Tiago Silva ; Tiago Silva CIQUP/Department of Chemistry and Biochemistry, Faculty of Sciences, University of Porto Rua do Campo Alegre s/n 4169-007 Porto Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Daniel Chavarria ; Daniel Chavarria CIQUP/Department of Chemistry and Biochemistry, Faculty of Sciences, University of Porto Rua do Campo Alegre s/n 4169-007 Porto Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Lisa Sequeira ; Lisa Sequeira CIQUP/Department of Chemistry and Biochemistry, Faculty of Sciences, University of Porto Rua do Campo Alegre s/n 4169-007 Porto Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Fernanda Borges Fernanda Borges CIQUP/Department of Chemistry and Biochemistry, Faculty of Sciences, University of Porto Rua do Campo Alegre s/n 4169-007 Porto Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 7.3. Recycling Bromovanillin into Ferulic Acid-Based Antioxidants in another window
- 4.1.2.7. Preparation of a Tosylhydrazidyl N -Glycosyl Derivative of d -Glucuronic Acid via Tosylhydrazone Formation and Intramolecular Ring Closure p282-284 By Nuno M. Xavier Nuno M. Xavier Centro de Química e Bioquímica, Faculdade de Ciências, Universidade de Lisboa 1749-016 Lisboa Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 4.1.2.7. Preparation of a Tosylhydrazidyl <em>N</em>-Glycosyl Derivative of <span class="small-caps">d</span>-Glucuronic Acid <em>via</em> Tosylhydrazone Formation and Intramolecular Ring Closure in another window
- 7.4. Sonogashira Coupling Reaction of Aryl Derivatives: A Versatile Method for Acetylide Building Blocks p564-567 By Tiago J. Silva ; Tiago J. Silva Centro de Ciências Moleculares de Materiais, Faculdade de Ciências da Universidade de Lisboa Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Paulo J. Mendes ; Paulo J. Mendes Departamento de Química, Escola de Ciências e Tecnologia, Centro de Química de Évora, Instituto de Investigação e Formação Avançada, Universidade de Évora Évora Portugal Search for other works by this author on: This Site PubMed Google Scholar António P. S. Teixeira ; António P. S. Teixeira Departamento de Química, Escola de Ciências e Tecnologia, Centro de Química de Évora, Instituto de Investigação e Formação Avançada, Universidade de Évora Évora Portugal Search for other works by this author on: This Site PubMed Google Scholar M. Paula Robalo ; M. Paula Robalo Área Departamental de Engenharia Química, Instituto Superior de Engenharia de Lisboa Portugal Centro de Química Estrutural, Complexo I, Instituto Superior Técnico, Universidade de Lisboa Portugal Search for other works by this author on: This Site PubMed Google Scholar M. H. Garcia M. H. Garcia Centro de Ciências Moleculares de Materiais, Faculdade de Ciências da Universidade de Lisboa Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 7.4. Sonogashira Coupling Reaction of Aryl Derivatives: A Versatile Method for Acetylide Building Blocks in another window
- 5.2.11. Synthesis of 3-Bromosalicylaldehyde by Ortho -formylation of 2-Bromophenol p514-516 By Trond Vidar Hansen ; Trond Vidar Hansen Department of Pharmaceutical Chemistry, School of Pharmacy, University of Oslo PO Box 1068 Blindern N-0316 Oslo Norway [email protected] Search for other works by this author on: This Site PubMed Google Scholar Lars Skattebøl Lars Skattebøl Department of Chemistry, University of Oslo PO Box 1033 Blindern N-0315 Oslo Norway Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 5.2.11. Synthesis of 3-Bromosalicylaldehyde by <em>Ortho</em>-formylation of 2-Bromophenol in another window
- 6.1. Synthesis of 2,3-Quinoxalinedithiol p532-534 By Dulce Simão ; Dulce Simão Department of Chemistry, Instituto Superior Técnico, University of Lisbon, Centro de Química Estrutural Av. Rovisco Pais, 1 1049-001 Lisbon Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Ana C. Cerdeira Ana C. Cerdeira Department of Chemistry, Instituto Superior Técnico, University of Lisbon, Centro de Química Estrutural Av. Rovisco Pais, 1 1049-001 Lisbon Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 6.1. Synthesis of 2,3-Quinoxalinedithiol in another window
- 5.2.12. Synthesis of Indolo[3,2- b ]quinolin-11-one by Acid-Catalysed Intramolecular Double Cyclization p517-521 By João Lavrado ; João Lavrado Research Institute for Medicines (iMed.ULisboa), Faculty of Pharmacy, Universidade de Lisboa Av. Prof. Gama Pinto 1649 003 Lisboa Portugal Search for other works by this author on: This Site PubMed Google Scholar Marta Figueiras ; Marta Figueiras Research Institute for Medicines (iMed.ULisboa), Faculty of Pharmacy, Universidade de Lisboa Av. Prof. Gama Pinto 1649 003 Lisboa Portugal Search for other works by this author on: This Site PubMed Google Scholar David M. Pereira ; David M. Pereira Research Institute for Medicines (iMed.ULisboa), Faculty of Pharmacy, Universidade de Lisboa Av. Prof. Gama Pinto 1649 003 Lisboa Portugal Search for other works by this author on: This Site PubMed Google Scholar Sofia A. Santos ; Sofia A. Santos Research Institute for Medicines (iMed.ULisboa), Faculty of Pharmacy, Universidade de Lisboa Av. Prof. Gama Pinto 1649 003 Lisboa Portugal Search for other works by this author on: This Site PubMed Google Scholar Rui Moreira ; Rui Moreira Research Institute for Medicines (iMed.ULisboa), Faculty of Pharmacy, Universidade de Lisboa Av. Prof. Gama Pinto 1649 003 Lisboa Portugal Pharmaceutical and Medicinal Chemistry Department, Faculty of Pharmacy, Universidade de Lisboa Av. Prof. Gama Pinto 1649 003 Lisboa Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Alexandra Paulo Alexandra Paulo Research Institute for Medicines (iMed.ULisboa), Faculty of Pharmacy, Universidade de Lisboa Av. Prof. Gama Pinto 1649 003 Lisboa Portugal Pharmaceutical and Medicinal Chemistry Department, Faculty of Pharmacy, Universidade de Lisboa Av. Prof. Gama Pinto 1649 003 Lisboa Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 5.2.12. Synthesis of Indolo[3,2-<em>b</em>]quinolin-11-one by Acid-Catalysed Intramolecular Double Cyclization in another window
- 4.1.3.1. Green Metrics in a Cyclocondensation Reaction p285-289 By Clarissa P. Frizzo ; Clarissa P. Frizzo Núcleo de Química de Heterociclos (NUQUIMHE), Department of Chemistry, Federal University of Santa Maria 97105-900 Santa Maria RS Brazil [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Marcos A. P. Martins ; Marcos A. P. Martins Núcleo de Química de Heterociclos (NUQUIMHE), Department of Chemistry, Federal University of Santa Maria 97105-900 Santa Maria RS Brazil [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Paulo R. S. Salbego ; Paulo R. S. Salbego Núcleo de Química de Heterociclos (NUQUIMHE), Department of Chemistry, Federal University of Santa Maria 97105-900 Santa Maria RS Brazil [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Caroline R. Bender ; Caroline R. Bender Núcleo de Química de Heterociclos (NUQUIMHE), Department of Chemistry, Federal University of Santa Maria 97105-900 Santa Maria RS Brazil [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Aniele Z. Tier ; Aniele Z. Tier Núcleo de Química de Heterociclos (NUQUIMHE), Department of Chemistry, Federal University of Santa Maria 97105-900 Santa Maria RS Brazil [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Hélio G. Bonacorso Hélio G. Bonacorso Núcleo de Química de Heterociclos (NUQUIMHE), Department of Chemistry, Federal University of Santa Maria 97105-900 Santa Maria RS Brazil [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 4.1.3.1. Green Metrics in a Cyclocondensation Reaction in another window
- 4.1.3.2. Synthesis of 1 H -Pyrazoles Using Ball Mill, Grinding and Conventional Thermal Heating p290-293 By Clarissa P. Frizzo ; Clarissa P. Frizzo Núcleo de Química de Heterociclos (NUQUIMHE), Department of Chemistry, Federal University of Santa Maria 97105-900 Santa Maria RS Brazil [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Marcos A. P. Martins ; Marcos A. P. Martins Núcleo de Química de Heterociclos (NUQUIMHE), Department of Chemistry, Federal University of Santa Maria 97105-900 Santa Maria RS Brazil [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Caroline R. Bender ; Caroline R. Bender Núcleo de Química de Heterociclos (NUQUIMHE), Department of Chemistry, Federal University of Santa Maria 97105-900 Santa Maria RS Brazil [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Paulo R. S. Salbego ; Paulo R. S. Salbego Núcleo de Química de Heterociclos (NUQUIMHE), Department of Chemistry, Federal University of Santa Maria 97105-900 Santa Maria RS Brazil [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Aniele Z. Tier ; Aniele Z. Tier Núcleo de Química de Heterociclos (NUQUIMHE), Department of Chemistry, Federal University of Santa Maria 97105-900 Santa Maria RS Brazil [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Guilherme C. Paveglio ; Guilherme C. Paveglio Núcleo de Química de Heterociclos (NUQUIMHE), Department of Chemistry, Federal University of Santa Maria 97105-900 Santa Maria RS Brazil [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Kelvis Longhi Kelvis Longhi Núcleo de Química de Heterociclos (NUQUIMHE), Department of Chemistry, Federal University of Santa Maria 97105-900 Santa Maria RS Brazil [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 4.1.3.2. Synthesis of 1<em>H</em>-Pyrazoles Using Ball Mill, Grinding and Conventional Thermal Heating in another window
- 5.2.8. Synthesis of Methyl 4-oxo-4-(thiophen-2-yl)butanoate p500-503 By M. Manuela M. Raposo M. Manuela M. Raposo Department of Chemistry, University of Minho, Campus de Gualtar 4710-057 Braga Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 5.2.8. Synthesis of Methyl 4-oxo-4-(thiophen-2-yl)butanoate in another window
- 5.2.13. Mild and Fast Friedel–Crafts Acylation Over Zeolites p522-525 By A. F. Brigas ; A. F. Brigas Universidade do Algarve, Departamento de Química e Farmácia, Centro de Investigação em Química do Algarve (CIQA), Campus de Gambelas 8005-139 Faro Portugal Universidade de Lisboa, Faculdade de Ciências, Centro de Química e Bioquímica (CQB) C8, Campo Grande 1749-016 Lisboa Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar F. Martins ; F. Martins Universidade de Lisboa, Faculdade de Ciências, Centro de Química e Bioquímica (CQB) C8, Campo Grande 1749-016 Lisboa Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar R. Elvas-Leitão ; R. Elvas-Leitão Universidade de Lisboa, Faculdade de Ciências, Centro de Química e Bioquímica (CQB) C8, Campo Grande 1749-016 Lisboa Portugal [email protected] Instituto Politécnico de Lisboa, Instituto Superior de Engenharia de Lisboa (ISEL), Área Departamental de Engenharia Química Rua Conselheiro Emídio Navarro, 1 1959-007 Lisboa Portugal Search for other works by this author on: This Site PubMed Google Scholar B. S. Santos ; B. S. Santos Universidade de Coimbra, Departamento de Química 3004-535 Coimbra Portugal Search for other works by this author on: This Site PubMed Google Scholar A. Martins ; A. Martins Universidade de Lisboa, Faculdade de Ciências, Centro de Química e Bioquímica (CQB) C8, Campo Grande 1749-016 Lisboa Portugal [email protected] Instituto Politécnico de Lisboa, Instituto Superior de Engenharia de Lisboa (ISEL), Área Departamental de Engenharia Química Rua Conselheiro Emídio Navarro, 1 1959-007 Lisboa Portugal Search for other works by this author on: This Site PubMed Google Scholar N. Nunes N. Nunes Universidade de Lisboa, Faculdade de Ciências, Centro de Química e Bioquímica (CQB) C8, Campo Grande 1749-016 Lisboa Portugal [email protected] Instituto Politécnico de Lisboa, Instituto Superior de Engenharia de Lisboa (ISEL), Área Departamental de Engenharia Química Rua Conselheiro Emídio Navarro, 1 1959-007 Lisboa Portugal Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 5.2.13. Mild and Fast Friedel–Crafts Acylation Over Zeolites in another window
- 4.1.3.3. Organocatalytic Enantioselective Michael Addition of Thiophenol to Chalcone p294-297 By Mariola Zielińska-Błajet ; Mariola Zielińska-Błajet Department of Organic Chemistry, Faculty of Chemistry, Wrocław University of Technology Wyb. Wyspiańskiego 27 50-370 Wrocław Poland [email protected] Search for other works by this author on: This Site PubMed Google Scholar Jacek Skarżewski Jacek Skarżewski Department of Organic Chemistry, Faculty of Chemistry, Wrocław University of Technology Wyb. Wyspiańskiego 27 50-370 Wrocław Poland [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 4.1.3.3. Organocatalytic Enantioselective Michael Addition of Thiophenol to Chalcone in another window
- 7.9. An Efficient Methodology for the Synthesis of the 3-Styryl Coumarin p584-587 By António Manuel D. R. L. Pereira ; António Manuel D. R. L. Pereira Departamento de Química, Escola de Ciências e Tecnologia, Universidade de Évora, Colégio Luís António Verney Rua Romão Ramalho, N° 59 7000-671 Portugal Laboratório HERCULES, Universidade de Évora Largo Marquês de Marialva, N° 8 7000-809 Évora Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Sérgio Miguel A. Martins Sérgio Miguel A. Martins Laboratório HERCULES, Universidade de Évora Largo Marquês de Marialva, N° 8 7000-809 Évora Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 7.9. An Efficient Methodology for the Synthesis of the 3-Styryl Coumarin in another window
- 7.5. Convergent Synthesis of a Suzuki Product p568-572 By Thomas A. Logothetis Thomas A. Logothetis University of Southampton, Chemistry, Highfield Southampton Hampshire SO17 1BJ UK [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 7.5. Convergent Synthesis of a Suzuki Product in another window
- 7.10. Sonogashira Coupling Between a Vinylic Halide and a Terminal Alkyne p588-591 By Carine Maaliki ; Carine Maaliki UFR Sciences et Techniques de Tours, Parc Grandmont, Bat Yves Chauvin 32 Av. Monge 37200 Tours France [email protected] Search for other works by this author on: This Site PubMed Google Scholar Jérôme Thibonnet Jérôme Thibonnet UFR Sciences et Techniques de Tours, Parc Grandmont, Bat Yves Chauvin 32 Av. Monge 37200 Tours France [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 7.10. Sonogashira Coupling Between a Vinylic Halide and a Terminal Alkyne in another window
- 5.2.14. Reactivity Studies of 1-Propyl-2-(thiophen-2-yl)-1 H -pyrrole p526-531 By Maria Manuela Marques Raposo ; Maria Manuela Marques Raposo University of Minho, Department of Chemistry, Campus de Gualtar 4710-057 Braga Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Sara Sofia Marques Fernandes ; Sara Sofia Marques Fernandes University of Minho, Department of Chemistry, Campus de Gualtar 4710-057 Braga Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Maria Cidália Rodrigues Castro Maria Cidália Rodrigues Castro University of Minho, Department of Chemistry, Campus de Gualtar 4710-057 Braga Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 5.2.14. Reactivity Studies of 1-Propyl-2-(thiophen-2-yl)-1<em>H</em>-pyrrole in another window
- 7.6. Oxidative Heck Reaction at Room Temperature p573-576 By Rajiv T. Sawant ; Rajiv T. Sawant Organic Pharmaceutical Chemistry, Department of Medicinal Chemistry, Uppsala University Biomedical Center Box 574, SE-751 23 Uppsala Sweden [email protected] Search for other works by this author on: This Site PubMed Google Scholar Ashkan Fardost ; Ashkan Fardost Organic Pharmaceutical Chemistry, Department of Medicinal Chemistry, Uppsala University Biomedical Center Box 574, SE-751 23 Uppsala Sweden [email protected] Search for other works by this author on: This Site PubMed Google Scholar Luke R. Odell Luke R. Odell Organic Pharmaceutical Chemistry, Department of Medicinal Chemistry, Uppsala University Biomedical Center Box 574, SE-751 23 Uppsala Sweden [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 7.6. Oxidative Heck Reaction at Room Temperature in another window
- 4.1.3.4. Stereoselective Synthesis of meso -1-Allyl-2,6-diphenylpiperidin-4-one p298-300 By Ana-Belén García Delgado ; Ana-Belén García Delgado Departamento de Química Orgánica e Inorgánica, Facultad de Químicas, Universidad de Oviedo Spain [email protected] Search for other works by this author on: This Site PubMed Google Scholar Noelia Quiñones ; Noelia Quiñones Departamento de Química Orgánica e Inorgánica, Facultad de Químicas, Universidad de Oviedo Spain [email protected] Search for other works by this author on: This Site PubMed Google Scholar María-Paz Cabal María-Paz Cabal Departamento de Química Orgánica e Inorgánica, Facultad de Químicas, Universidad de Oviedo Spain [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 4.1.3.4. Stereoselective Synthesis of <em>meso</em>-1-Allyl-2,6-diphenylpiperidin-4-one in another window
- 4.1.3.5. Synthesis of a Squarylium Cyanine Dye as Potential Photosensitizer for Photodynamic Therapy (PDT) p301-305 By Marlene L. F. M. Pacheco ; Marlene L. F. M. Pacheco Department of Chemistry and CQ-VR, UTAD Quinta de Prados 5000-801 Vila Real Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Sofia F. P. Friães ; Sofia F. P. Friães Department of Chemistry and CQ-VR, UTAD Quinta de Prados 5000-801 Vila Real Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Renato E. Boto ; Renato E. Boto CICS-UBI – Health Sciences Research Centre, University of Beira Interior Av. Infante D. Henrique 6200-506 Covilhã Portugal Search for other works by this author on: This Site PubMed Google Scholar Paulo Almeida ; Paulo Almeida CICS-UBI – Health Sciences Research Centre, University of Beira Interior Av. Infante D. Henrique 6200-506 Covilhã Portugal Search for other works by this author on: This Site PubMed Google Scholar Amélia M. Silva ; Amélia M. Silva Department of Biology and Environment and CITAB, UTAD Quinta de Prados 5000-801 Vila Real Portugal Search for other works by this author on: This Site PubMed Google Scholar Lucinda V. Reis Lucinda V. Reis Department of Chemistry and CQ-VR, UTAD Quinta de Prados 5000-801 Vila Real Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 4.1.3.5. Synthesis of a Squarylium Cyanine Dye as Potential Photosensitizer for Photodynamic Therapy (PDT) in another window
- 4.2.1.1. The Effects of Stoichiometry and Starting Material on the Product Identity and Yield in Grignard Addition Reactions p306-311 By Jane Brock Greco ; Jane Brock Greco Department of Chemistry, Johns Hopkins University 3400 N. Charles St. Baltimore MD 21218 USA [email protected] Search for other works by this author on: This Site PubMed Google Scholar Eric Hill Eric Hill Department of Chemistry, Johns Hopkins University 3400 N. Charles St. Baltimore MD 21218 USA [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 4.2.1.1. The Effects of Stoichiometry and Starting Material on the Product Identity and Yield in Grignard Addition Reactions in another window
- 7.7. Synthesis of 2-Methyl-1,1′-binaphthalene via Suzuki Cross-Coupling Reaction p577-580 By Javier Iglesias-Sigüenza ; Javier Iglesias-Sigüenza Departamento de Química Orgánica, Universidad de Sevilla C/ Profesor García González, n°1 41012-Sevilla Spain [email protected] Search for other works by this author on: This Site PubMed Google Scholar David Monge ; David Monge Departamento de Química Orgánica, Universidad de Sevilla C/ Profesor García González, n°1 41012-Sevilla Spain [email protected] Search for other works by this author on: This Site PubMed Google Scholar Elena Díez Elena Díez Departamento de Química Orgánica, Universidad de Sevilla C/ Profesor García González, n°1 41012-Sevilla Spain [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 7.7. Synthesis of 2-Methyl-1,1′-binaphthalene <em>via</em> Suzuki Cross-Coupling Reaction in another window
- 7.8. Green Synthesis of Aromatic Ketones: Decarboxylative Palladium Catalysis Under Microwave Irradiation p581-583 By Jonas Sävmarker ; Jonas Sävmarker Organic Pharmaceutical Chemistry, Department of Medicinal Chemistry, Uppsala University Biomedical Center Box 574, SE-751 23 Uppsala Sweden [email protected] Search for other works by this author on: This Site PubMed Google Scholar Ashkan Fardost ; Ashkan Fardost Organic Pharmaceutical Chemistry, Department of Medicinal Chemistry, Uppsala University Biomedical Center Box 574, SE-751 23 Uppsala Sweden [email protected] Search for other works by this author on: This Site PubMed Google Scholar Luke R. Odell Luke R. Odell Organic Pharmaceutical Chemistry, Department of Medicinal Chemistry, Uppsala University Biomedical Center Box 574, SE-751 23 Uppsala Sweden [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 7.8. Green Synthesis of Aromatic Ketones: Decarboxylative Palladium Catalysis Under Microwave Irradiation in another window
- 6.5. Nucleophilic Aromatic Substitution Reactions in 3,6-Bis(3,5-dimethyl-1 H -pyrazol-1-yl)-1,2,4,5-tetrazine p545-549 By Tiago J. L. Silva ; Tiago J. L. Silva Centro de Química Estrutural, Faculdade de Ciências da Universidade de Lisboa Campo Grande 1049-016 Campo Grande, Lisboa Portugal Departamento de Química, Escola de Ciências e Tecnologia, Centro de Química de Évora, Instituto de Investigação e Formação Avançada, Universidade de Évora Rua Romão Ramalho 59 7000-671 Évora Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Paulo J. G. Mendes ; Paulo J. G. Mendes Departamento de Química, Escola de Ciências e Tecnologia, Centro de Química de Évora, Instituto de Investigação e Formação Avançada, Universidade de Évora Rua Romão Ramalho 59 7000-671 Évora Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Ana Isabel Tomaz ; Ana Isabel Tomaz Centro de Química Estrutural, Faculdade de Ciências da Universidade de Lisboa Campo Grande 1049-016 Campo Grande, Lisboa Portugal Search for other works by this author on: This Site PubMed Google Scholar M. Helena Garcia M. Helena Garcia Centro de Química Estrutural, Faculdade de Ciências da Universidade de Lisboa Campo Grande 1049-016 Campo Grande, Lisboa Portugal Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 6.5. Nucleophilic Aromatic Substitution Reactions in 3,6-Bis(3,5-dimethyl-1<em>H</em>-pyrazol-1-yl)-1,2,4,5-tetrazine in another window
- 4.2.1.2. Synthesis of Methyl Triphenylmethyl Ether p312-315 By Dulce Simão ; Dulce Simão Department of Chemistry, Instituto Superior Técnico, University of Lisbon, Centro de Química Estrutural Av. Rovisco Pais, 1 1049-001 Lisbon Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Ana C. Cerdeira Ana C. Cerdeira Department of Chemistry, Instituto Superior Técnico, University of Lisbon, Centro de Química Estrutural Av. Rovisco Pais, 1 1049-001 Lisbon Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 4.2.1.2. Synthesis of Methyl Triphenylmethyl Ether in another window
- 4.2.1.3. Grignard-Like Reaction in Water p316-318 By João R. Vale ; João R. Vale iMed.UL, Faculdade de Farmácia da Universidade de Lisboa Av. Prof. Gama Pinto 1649-003 Lisboa Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Carlos A. M. Afonso Carlos A. M. Afonso iMed.UL, Faculdade de Farmácia da Universidade de Lisboa Av. Prof. Gama Pinto 1649-003 Lisboa Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 4.2.1.3. Grignard-Like Reaction in Water in another window
- 7.1. A Solvent-Free Ullmann Coupling: Synthesis of 2,2′-Dinitrobiphenyl p550-553 By Laurel Goj Habgood ; Laurel Goj Habgood Rollins College 1000 Holt Avenue, Box 2743 Winter Park FL 32789 USA [email protected] Search for other works by this author on: This Site PubMed Google Scholar Richard W. Gregor Richard W. Gregor Rollins College 1000 Holt Avenue, Box 2743 Winter Park FL 32789 USA [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 7.1. A Solvent-Free Ullmann Coupling: Synthesis of 2,2′-Dinitrobiphenyl in another window
- 4.2.1.4. Cram’s Rule – Diastereoselective Grignard Addition to 2-Phenylpropanal p319-321 By Laura M. Hancock ; Laura M. Hancock Lennard-Jones Laboratories, School of Physical and Geographical Sciences, Keele University Staffordshire ST5 5BG UK [email protected] Search for other works by this author on: This Site PubMed Google Scholar Michael G. Edwards ; Michael G. Edwards Lennard-Jones Laboratories, School of Physical and Geographical Sciences, Keele University Staffordshire ST5 5BG UK [email protected] Search for other works by this author on: This Site PubMed Google Scholar Graeme R. Jones ; Graeme R. Jones Lennard-Jones Laboratories, School of Physical and Geographical Sciences, Keele University Staffordshire ST5 5BG UK [email protected] Search for other works by this author on: This Site PubMed Google Scholar Matthew O’Brien Matthew O’Brien Lennard-Jones Laboratories, School of Physical and Geographical Sciences, Keele University Staffordshire ST5 5BG UK [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 4.2.1.4. Cram’s Rule – Diastereoselective Grignard Addition to 2-Phenylpropanal in another window
- 7.2. Reactivity Studies for the Synthesis of 5-Phenylthiophene-2-carbaldehyde by a Suzuki–Miyaura Coupling p554-558 By Maria Manuela Marques Raposo ; Maria Manuela Marques Raposo University of Minho, Department of Chemistry, Campus de Gualtar 4710-057 Braga Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Susana Paula Graça da Costa ; Susana Paula Graça da Costa University of Minho, Department of Chemistry, Campus de Gualtar 4710-057 Braga Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Rosa Maria Ferreira Batista ; Rosa Maria Ferreira Batista University of Minho, Department of Chemistry, Campus de Gualtar 4710-057 Braga Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Rosa Cristina Moutinho Ferreira Rosa Cristina Moutinho Ferreira University of Minho, Department of Chemistry, Campus de Gualtar 4710-057 Braga Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 7.2. Reactivity Studies for the Synthesis of 5-Phenylthiophene-2-carbaldehyde by a Suzuki–Miyaura Coupling in another window
- 4.2.1.5. Preparation of (4 R ,5 R )-4,5-Bis(diphenylhydroxymethyl)-2,2-dimethyldioxolane ((−)-TADDOL) p322-326 By Naylil M. R. Capreti ; Naylil M. R. Capreti Institute of Chemistry, State University of Campinas C.P. 6154 13083-970 Campinas SP Brazil [email protected] Search for other works by this author on: This Site PubMed Google Scholar Igor D. Jurberg Igor D. Jurberg Institute of Chemistry, State University of Campinas C.P. 6154 13083-970 Campinas SP Brazil [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 4.2.1.5. Preparation of (4<em>R</em>,5<em>R</em>)-4,5-Bis(diphenylhydroxymethyl)-2,2-dimethyldioxolane ((−)-TADDOL) in another window
- 4.2.1.6. Synthesis of ( S )-Diphenyl(pyrrolidin-2-yl)methanol p327-330 By David Monge ; David Monge Departamento de Química Orgánica, Universidad de Sevilla C/ Prof. García González, no. 1 41012-Sevilla Spain [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Javier Iglesias-Sigüenza ; Javier Iglesias-Sigüenza Departamento de Química Orgánica, Universidad de Sevilla C/ Prof. García González, no. 1 41012-Sevilla Spain [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Elena Díez Elena Díez Departamento de Química Orgánica, Universidad de Sevilla C/ Prof. García González, no. 1 41012-Sevilla Spain [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 4.2.1.6. Synthesis of (<em>S</em>)-Diphenyl(pyrrolidin-2-yl)methanol in another window
- 4.2.2.1. Solvent-Free Aldol Condensation Reactions: Synthesis of Chalcone Derivatives p331-334 By Barbora Morra ; Barbora Morra Department of Chemistry, University of Toronto Toronto Ontario Canada M5S 3H6 [email protected] Search for other works by this author on: This Site PubMed Google Scholar Connie Tang ; Connie Tang Department of Chemistry, University of Toronto Toronto Ontario Canada M5S 3H6 [email protected] Search for other works by this author on: This Site PubMed Google Scholar Jonathon Fossella Jonathon Fossella Department of Chemistry, University of Toronto Toronto Ontario Canada M5S 3H6 [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 4.2.2.1. Solvent-Free Aldol Condensation Reactions: Synthesis of Chalcone Derivatives in another window
- 4.2.2.2. Synthesis of ( E )-Chalcones [( E )-1,3-diarylprop-2-en-1-ones] p335-338 By Artur M. S. Silva ; Artur M. S. Silva Chemistry Department & QOPNA, University of Aveiro, Campus de Santiago 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Augusto C. Tomé ; Augusto C. Tomé Chemistry Department & QOPNA, University of Aveiro, Campus de Santiago 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Diana C. G. A. Pinto ; Diana C. G. A. Pinto Chemistry Department & QOPNA, University of Aveiro, Campus de Santiago 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Fernando Domingues ; Fernando Domingues Chemistry Department & QOPNA, University of Aveiro, Campus de Santiago 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Graça M. Oliveira Rocha ; Graça M. Oliveira Rocha Chemistry Department & QOPNA, University of Aveiro, Campus de Santiago 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar José A. S. Cavaleiro ; José A. S. Cavaleiro Chemistry Department & QOPNA, University of Aveiro, Campus de Santiago 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar M. Graça P. M. S. Neves ; M. Graça P. M. S. Neves Chemistry Department & QOPNA, University of Aveiro, Campus de Santiago 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar M. Amparo F. Faustino ; M. Amparo F. Faustino Chemistry Department & QOPNA, University of Aveiro, Campus de Santiago 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Mário M. Q. Simões Mário M. Q. Simões Chemistry Department & QOPNA, University of Aveiro, Campus de Santiago 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 4.2.2.2. Synthesis of (<em>E</em>)-Chalcones [(<em>E</em>)-1,3-diarylprop-2-en-1-ones] in another window
- 4.2.2.3. A Solvent-Free Approach for Chalcone Synthesis via an Aldol Reaction p339-341 By Sofia F. P. Friães ; Sofia F. P. Friães Department of Chemistry and CQ-VR, UTAD Quinta de Prados 5000-801 Vila Real Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Renato E. Boto ; Renato E. Boto CICS-UBI – Health Sciences Research Centre, University of Beira Interior Av. Infante D. Henrique 6200-506 Covilhã Portugal Search for other works by this author on: This Site PubMed Google Scholar Paulo Almeida ; Paulo Almeida CICS-UBI – Health Sciences Research Centre, University of Beira Interior Av. Infante D. Henrique 6200-506 Covilhã Portugal Search for other works by this author on: This Site PubMed Google Scholar Amélia M. Silva ; Amélia M. Silva Department of Biology and Environment and CITAB, UTAD Quinta de Prados 5000-801 Vila Real Portugal Search for other works by this author on: This Site PubMed Google Scholar Lucinda V. Reis Lucinda V. Reis Department of Chemistry and CQ-VR, UTAD Quinta de Prados 5000-801 Vila Real Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 4.2.2.3. A Solvent-Free Approach for Chalcone Synthesis <em>via</em> an Aldol Reaction in another window
- 4.2.2.4. Preparation of Dibenzylideneacetone p342-344 By Abel J. S. C. Vieira Abel J. S. C. Vieira Faculty of Sciences and Technology, Universidade Nova de Lisboa 2829-516 Caparica Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 4.2.2.4. Preparation of Dibenzylideneacetone in another window
- 4.2.2.5. l -Proline Catalyzed Aldol Reaction of 4-Nitrobenzaldehyde with Acetone p345-347 By Fang Fang ; Fang Fang Department of Chemistry, South University of Science and Technology of China Shenzhen 518055 People’s Republic of China [email protected] Search for other works by this author on: This Site PubMed Google Scholar Xin-Yuan Liu ; Xin-Yuan Liu Department of Chemistry, South University of Science and Technology of China Shenzhen 518055 People’s Republic of China [email protected] Search for other works by this author on: This Site PubMed Google Scholar Bin Tan Bin Tan Department of Chemistry, South University of Science and Technology of China Shenzhen 518055 People’s Republic of China [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 4.2.2.5. <span class="small-caps">l</span>-Proline Catalyzed Aldol Reaction of 4-Nitrobenzaldehyde with Acetone in another window
- 4.2.2.6. Preparation of a β -Nitrostyrene Derivative by the Henry Reaction: Comparison of a Conventional and a Microwave-Assisted Method p348-351 By Daniel Martins ; Daniel Martins CIQUP/Department of Chemistry and Biochemistry, Faculty of Sciences of University of Porto Portugal Search for other works by this author on: This Site PubMed Google Scholar Joana Reis ; Joana Reis CIQUP/Department of Chemistry and Biochemistry, Faculty of Sciences of University of Porto Portugal Search for other works by this author on: This Site PubMed Google Scholar Fernanda Borges ; Fernanda Borges CIQUP/Department of Chemistry and Biochemistry, Faculty of Sciences of University of Porto Portugal Search for other works by this author on: This Site PubMed Google Scholar Nuno Milhazes Nuno Milhazes CIQUP/Department of Chemistry and Biochemistry, Faculty of Sciences of University of Porto Portugal CESPU/Superior Institute of Health of Sciences – North Rua Central de Gandra, 1317 4585-116 Gandra PRD Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 4.2.2.6. Preparation of a <em>β</em>-Nitrostyrene Derivative by the Henry Reaction: Comparison of a Conventional and a Microwave-Assisted Method in another window
- 4.2.2.7. Synthesis of Aurone Derivatives Through Acid-Catalysed Aldol Condensation p352-354 By Ana R. Duarte ; Ana R. Duarte Research Institute for Medicines (iMed.ULisboa), Faculty of Pharmacy, Universidade de Lisboa Av. Professor Gama Pinto 1649-003 Lisboa Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Marta P. Carrasco ; Marta P. Carrasco Research Institute for Medicines (iMed.ULisboa), Faculty of Pharmacy, Universidade de Lisboa Av. Professor Gama Pinto 1649-003 Lisboa Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Ana S. Ressurreição Ana S. Ressurreição Research Institute for Medicines (iMed.ULisboa), Faculty of Pharmacy, Universidade de Lisboa Av. Professor Gama Pinto 1649-003 Lisboa Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 4.2.2.7. Synthesis of Aurone Derivatives Through Acid-Catalysed Aldol Condensation in another window
- 4.2.2.8. Synthesis of Pyrazole Heterocycles p355-358 By Thomas A. Logothetis Thomas A. Logothetis University of Southampton, Chemistry, Highfield Southampton Hampshire SO17 1BJ UK [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 4.2.2.8. Synthesis of Pyrazole Heterocycles in another window
- 4.2.2.9. A Green Approach to 3-Carbonylchromones p359-362 By Ana Bornadiego ; Ana Bornadiego Departamento de Química e Inorgánica. F. Veterinaria, Universidad de Extremadura 10071 Cáceres Spain [email protected] Search for other works by this author on: This Site PubMed Google Scholar Jesús Díaz ; Jesús Díaz Departamento de Química e Inorgánica. F. Veterinaria, Universidad de Extremadura 10071 Cáceres Spain [email protected] Search for other works by this author on: This Site PubMed Google Scholar Ana G. Neo ; Ana G. Neo Departamento de Química e Inorgánica. F. Veterinaria, Universidad de Extremadura 10071 Cáceres Spain [email protected] Search for other works by this author on: This Site PubMed Google Scholar Carlos F. Marcos Carlos F. Marcos Departamento de Química e Inorgánica. F. Veterinaria, Universidad de Extremadura 10071 Cáceres Spain [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 4.2.2.9. A Green Approach to 3-Carbonylchromones in another window
- 4.2.2.10. Synthesis of Indigo and Dyeing Process p363-365 By Dulce Simão Dulce Simão Department of Chemistry, Instituto Superior Técnico, University of Lisbon, Centro de Química Estrutural Av. Rovisco Pais, 1 1049-001 Lisbon Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 4.2.2.10. Synthesis of Indigo and Dyeing Process in another window
- 4.2.3.1. Knorr Pyrazole Synthesis of Edaravone p366-369 By Ana Paula Francisco ; Ana Paula Francisco Instituto de Investigação do Medicamento (iMed.ULisboa), Faculdade de Farmácia da, Universidade de Lisboa Av. Prof. Gama Pinto 1649-003 Lisboa Portugal [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Ana S. Ressurreição ; Ana S. Ressurreição Instituto de Investigação do Medicamento (iMed.ULisboa), Faculdade de Farmácia da, Universidade de Lisboa Av. Prof. Gama Pinto 1649-003 Lisboa Portugal [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Maria de Jesus Perry ; Maria de Jesus Perry Instituto de Investigação do Medicamento (iMed.ULisboa), Faculdade de Farmácia da, Universidade de Lisboa Av. Prof. Gama Pinto 1649-003 Lisboa Portugal [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Francisca Lopes Francisca Lopes Instituto de Investigação do Medicamento (iMed.ULisboa), Faculdade de Farmácia da, Universidade de Lisboa Av. Prof. Gama Pinto 1649-003 Lisboa Portugal [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 4.2.3.1. Knorr Pyrazole Synthesis of Edaravone in another window
- 4.2.3.2. Greener Solvent Substitution in a Verley–Doebner Condensation p370-372 By Jonathon W. Moir ; Jonathon W. Moir Department of Chemistry, University of Toronto 80 St. George Street Toronto Ontario Canada M5S 3H6 [email protected] Search for other works by this author on: This Site PubMed Google Scholar Andrew P. Dicks Andrew P. Dicks Department of Chemistry, University of Toronto 80 St. George Street Toronto Ontario Canada M5S 3H6 [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 4.2.3.2. Greener Solvent Substitution in a Verley–Doebner Condensation in another window
- 4.2.3.3. Knorr Synthesis of Diethyl 3,5-Dimethyl-1 H -pyrrole-2,4-dicarboxylate p373-375 By M. Soledad Pino-González ; M. Soledad Pino-González Organic Chemistry Department, Faculty of Sciences, University of Málaga 29071 Málaga Spain [email protected] Search for other works by this author on: This Site PubMed Google Scholar Cristina García-Ruiz ; Cristina García-Ruiz Organic Chemistry Department, Faculty of Sciences, University of Málaga 29071 Málaga Spain [email protected] Search for other works by this author on: This Site PubMed Google Scholar Francisco Sarabia ; Francisco Sarabia Organic Chemistry Department, Faculty of Sciences, University of Málaga 29071 Málaga Spain [email protected] Search for other works by this author on: This Site PubMed Google Scholar Gregorio Torres Gregorio Torres Organic Chemistry Department, Faculty of Sciences, University of Málaga 29071 Málaga Spain [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 4.2.3.3. Knorr Synthesis of Diethyl 3,5-Dimethyl-1<em>H</em>-pyrrole-2,4-dicarboxylate in another window
- 4.2.3.4. Synthesis of Coumarin-3-carboxylic Acid p376-379 By Paulo Coelho ; Paulo Coelho Centro de Química – Vila Real, Universidade de Trás-os-Montes e Alto Douro 5001-801 Vila Real Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Céu Sousa Céu Sousa Centro de Química – Vila Real, Universidade de Trás-os-Montes e Alto Douro 5001-801 Vila Real Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 4.2.3.4. Synthesis of Coumarin-3-carboxylic Acid in another window
- 4.2.3.5. Synthesis of Lipophilic Antioxidants Based on Natural Models p380-386 By Sofia Benfeito ; Sofia Benfeito CIQUP/Department of Chemistry and Biochemistry, Faculty of Sciences, University of Porto Porto Portugal Search for other works by this author on: This Site PubMed Google Scholar Tiago Silva ; Tiago Silva CIQUP/Department of Chemistry and Biochemistry, Faculty of Sciences, University of Porto Porto Portugal Search for other works by this author on: This Site PubMed Google Scholar Diogo Magalhães Silva ; Diogo Magalhães Silva CIQUP/Department of Chemistry and Biochemistry, Faculty of Sciences, University of Porto Porto Portugal Search for other works by this author on: This Site PubMed Google Scholar E. Manuela Garrido ; E. Manuela Garrido CIQUP/Department of Chemistry and Biochemistry, Faculty of Sciences, University of Porto Porto Portugal Department of Chemical Engineering, School of Engineering (ISEP), Polytechnic of Porto Porto Portugal [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Fernanda Borges ; Fernanda Borges CIQUP/Department of Chemistry and Biochemistry, Faculty of Sciences, University of Porto Porto Portugal Search for other works by this author on: This Site PubMed Google Scholar Jorge Garrido Jorge Garrido CIQUP/Department of Chemistry and Biochemistry, Faculty of Sciences, University of Porto Porto Portugal Department of Chemical Engineering, School of Engineering (ISEP), Polytechnic of Porto Porto Portugal [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 4.2.3.5. Synthesis of Lipophilic Antioxidants Based on Natural Models in another window
- 4.2.3.6. A Simple and Ecological Preparation of a Chromene-3-carboxamide Derivative p387-390 By Marta Costa ; Marta Costa Chemistry Department, University of Minho, Campus de Gualtar 4710 Braga Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar M. Fernanda Proença M. Fernanda Proença Chemistry Department, University of Minho, Campus de Gualtar 4710 Braga Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 4.2.3.6. A Simple and Ecological Preparation of a Chromene-3-carboxamide Derivative in another window
- 4.2.3.7. Synthesis of a Biologically Active Oxazol-5(4 H )-One via Erlenmeyer–Plöchl Reaction p391-395 By Catarina A. B. Rodrigues ; Catarina A. B. Rodrigues Instituto de Investigação do Medicamento (iMed.ULisboa), Faculty of Pharmacy, University of Lisbon Av. Prof. Gama Pinto 1649-003 Lisboa Portugal [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar José M. G. Martinho ; José M. G. Martinho Centro de Química-Física Molecular and IN-Institute of Nanoscience and Nanothecnology, Instituto Superior Técnico Av. Rovisco Pais 1049-001 Lisboa Portugal Search for other works by this author on: This Site PubMed Google Scholar Carlos A. M. Afonso Carlos A. M. Afonso Instituto de Investigação do Medicamento (iMed.ULisboa), Faculty of Pharmacy, University of Lisbon Av. Prof. Gama Pinto 1649-003 Lisboa Portugal [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 4.2.3.7. Synthesis of a Biologically Active Oxazol-5(4<em>H</em>)-One <em>via</em> Erlenmeyer–Plöchl Reaction in another window
- 8.1. Synthesis of Dulcin p592-594 By Dulce Simão Dulce Simão Department of Chemistry, Instituto Superior Técnico, University of Lisbon, Centro de Química Estrutural Av. Rovisco Pais, 1 1049-001 Lisbon Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 8.1. Synthesis of Dulcin in another window
- 8.2. Microwave-Assisted Solid-Phase Synthesis of Hydantoins p595-599 By Isabelle Parrot ; Isabelle Parrot IBMM UMR 5247 CNRS-Université Montpellier 1 et 2, Faculté des Sciences Pl. Eugène Bataillon 34 095 Montpellier Cedex 5 France [email protected] Search for other works by this author on: This Site PubMed Google Scholar Guilhem Chaubet Guilhem Chaubet IBMM UMR 5247 CNRS-Université Montpellier 1 et 2, Faculté des Sciences Pl. Eugène Bataillon 34 095 Montpellier Cedex 5 France [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 8.2. Microwave-Assisted Solid-Phase Synthesis of Hydantoins in another window
- 9.1.1. Preparation of Cyclohexene p600-602 By Abel J. S. C. Vieira ; Abel J. S. C. Vieira Faculty of Sciences and Technology, Universidade Nova de Lisboa 2829-516 Caparica Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Elvira M. S. M. Gaspar Elvira M. S. M. Gaspar Faculty of Sciences and Technology, Universidade Nova de Lisboa 2829-516 Caparica Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 9.1.1. Preparation of Cyclohexene in another window
- 9.1.2. A Green Synthesis of 2,3-Dibromo-3-phenylpropionic Acid and the Use of Kinetic Studies to Probe into the Elimination Product When Treated with a Weak Base in Different Solvents p603-607 By Malcolm I. Stewart ; Malcolm I. Stewart CRL, University of Oxford 12 Mansfield Road Oxford OX1 3TA UK [email protected] Search for other works by this author on: This Site PubMed Google Scholar Craig D. Campbell Craig D. Campbell CRL, University of Oxford 12 Mansfield Road Oxford OX1 3TA UK [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 9.1.2. A Green Synthesis of 2,3-Dibromo-3-phenylpropionic Acid and the Use of Kinetic Studies to Probe into the Elimination Product When Treated with a Weak Base in Different Solvents in another window
- 9.1.3. Dehydration of Methylcyclohexanols p608-613 By Cornelis A. Van Walree ; Cornelis A. Van Walree Department of Chemistry, Utrecht University Budapestlaan 4b 3584 CD Utrecht The Netherlands School of Chemical and Physical Sciences, Flinders University GPO Box 2100 Adelaide 5001 Australia [email protected] Search for other works by this author on: This Site PubMed Google Scholar Stephan A. Jonker ; Stephan A. Jonker Department of Chemistry, Utrecht University Budapestlaan 4b 3584 CD Utrecht The Netherlands Search for other works by this author on: This Site PubMed Google Scholar Veronica Kaats-Richters Veronica Kaats-Richters Department of Chemistry, Utrecht University Budapestlaan 4b 3584 CD Utrecht The Netherlands Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 9.1.3. Dehydration of Methylcyclohexanols in another window
- 9.1.4. Synthesis of 5-Hydroxymethylfurfural (HMF) from Fructose as a Bioplatform Intermediate p614-616 By Svilen P. Simeonov ; Svilen P. Simeonov Research Institute for Medicines and Pharmaceuticals Sciences, Faculty of Pharmacy, University of Lisbon Av. Prof. Gama Pinto 1649-003 Lisbon Portugal Institute of Organic Chemistry with Centre of Phytochemistry, Bulgarian Academy of Sciences Acad. G. Bonchev str., bl.9 1113 Sofia Bulgaria [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Carlos A. M. Afonso Carlos A. M. Afonso Research Institute for Medicines and Pharmaceuticals Sciences, Faculty of Pharmacy, University of Lisbon Av. Prof. Gama Pinto 1649-003 Lisbon Portugal Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 9.1.4. Synthesis of 5-Hydroxymethylfurfural (HMF) from Fructose as a Bioplatform Intermediate in another window
- 9.2.1. Synthesis of trans -9-(2-Phenylethenyl)anthracene p617-619 By Dulce Simão Dulce Simão Department of Chemistry, Instituto Superior Técnico, University of Lisbon, Centro de Química Estrutural Av. Rovisco Pais, 1 1049-001 Lisbon Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 9.2.1. Synthesis of <em>trans</em>-9-(2-Phenylethenyl)anthracene in another window
- 9.2.2. Synthesis of 4-Vinylbenzoic acid p620-622 By Dulce Simão ; Dulce Simão Department of Chemistry, Instituto Superior Técnico, University of Lisbon, Centro de Química Estrutural Av. Rovisco Pais, 1 1049-001 Lisbon Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Ana C. Cerdeira Ana C. Cerdeira Department of Chemistry, Instituto Superior Técnico, University of Lisbon, Centro de Química Estrutural Av. Rovisco Pais, 1 1049-001 Lisbon Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 9.2.2. Synthesis of 4-Vinylbenzoic acid in another window
- 9.2.3. Preparation of Nitrostilbenes by the Wittig Reaction p623-626 By Francisca Lopes ; Francisca Lopes Research Institute for Medicines (iMed. ULisboa), Faculdade de Farmácia da, Universidade de Lisboa Av. Prof. Gama Pinto 1649-003 Lisboa Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Maria de Jesus Perry ; Maria de Jesus Perry Research Institute for Medicines (iMed. ULisboa), Faculdade de Farmácia da, Universidade de Lisboa Av. Prof. Gama Pinto 1649-003 Lisboa Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Ana Paula Francisco Ana Paula Francisco Research Institute for Medicines (iMed. ULisboa), Faculdade de Farmácia da, Universidade de Lisboa Av. Prof. Gama Pinto 1649-003 Lisboa Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 9.2.3. Preparation of Nitrostilbenes by the Wittig Reaction in another window
- 9.2.4. Building an Alkene Spacer by the Wittig Reaction: Synthesis of 4-[2-(4-Nitrophenyl)ethenyl]benzonitrile p627-630 By António P. S. Teixeira ; António P. S. Teixeira Departamento de Química, Escola de Ciências e Tecnologia, Centro de Química de Évora, Instituto de Investigação e Formação Avançada, Universidade de Évora Rua Romão Ramalho 59 7000-671 Évora Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar M. Paula Robalo ; M. Paula Robalo Área Departamental de Engenharia Química, Instituto Superior de Engenharia de Lisboa Rua Conselheiro Emídio Navarro, 1 1959-007 Lisboa Portugal Centro de Química Estrutural, Complexo I, Instituto Superior Técnico, Universidade de Lisboa Av. Rovisco Pais 1049-001 Lisboa Portugal Search for other works by this author on: This Site PubMed Google Scholar Paulo J. Mendes Paulo J. Mendes Departamento de Química, Escola de Ciências e Tecnologia, Centro de Química de Évora, Instituto de Investigação e Formação Avançada, Universidade de Évora Rua Romão Ramalho 59 7000-671 Évora Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 9.2.4. Building an Alkene Spacer by the Wittig Reaction: Synthesis of 4-[2-(4-Nitrophenyl)ethenyl]benzonitrile in another window
- 9.2.5. Preparation of trans , trans -Distyrylbenzene by a Wittig Reaction p631-634 By Arno Kraft Arno Kraft Heriot-Watt University, Institute of Chemical Sciences, School of Engineering & Physical Sciences Riccarton Edinburgh EH14 4AS United Kingdom [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 9.2.5. Preparation of <em>trans</em>,<em>trans</em>-Distyrylbenzene by a Wittig Reaction in another window
- 9.2.6. Synthesis and Reactivity of Phosphorus Ylides p635-639 By Ana Lúcia Cardoso ; Ana Lúcia Cardoso Department of Chemistry, University of Coimbra Rua Larga 3004-535 Coimbra Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Maria I. L. Soares ; Maria I. L. Soares Department of Chemistry, University of Coimbra Rua Larga 3004-535 Coimbra Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Susana M. M. Lopes ; Susana M. M. Lopes Department of Chemistry, University of Coimbra Rua Larga 3004-535 Coimbra Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Teresa M. V. D. Pinho e Melo Teresa M. V. D. Pinho e Melo Department of Chemistry, University of Coimbra Rua Larga 3004-535 Coimbra Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 9.2.6. Synthesis and Reactivity of Phosphorus Ylides in another window
- 9.2.7. Synthesis of 4-(Thiophen-2-yl)pyrrolidin-2-one via Horner–Wadsworth–Emmons Reaction and Reductive Cyclisation p640-645 By Jonathan D. Sellars ; Jonathan D. Sellars Chemistry Department, University of Durham South Road Durham DH1 3LE UK [email protected] Search for other works by this author on: This Site PubMed Google Scholar AnnMarie C. O’Donoghue ; AnnMarie C. O’Donoghue Chemistry Department, University of Durham South Road Durham DH1 3LE UK [email protected] Search for other works by this author on: This Site PubMed Google Scholar Ian R. Baxendale ; Ian R. Baxendale Chemistry Department, University of Durham South Road Durham DH1 3LE UK [email protected] Search for other works by this author on: This Site PubMed Google Scholar John M. Sanderson ; John M. Sanderson Chemistry Department, University of Durham South Road Durham DH1 3LE UK [email protected] Search for other works by this author on: This Site PubMed Google Scholar Elizabeth J. Grayson Elizabeth J. Grayson Chemistry Department, University of Durham South Road Durham DH1 3LE UK [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 9.2.7. Synthesis of 4-(Thiophen-2-yl)pyrrolidin-2-one <em>via</em> Horner–Wadsworth–Emmons Reaction and Reductive Cyclisation in another window
- 9.3.1. A Guided-Inquiry Approach to Ring-Closing Metathesis p646-649 By Hala G. Schepmann ; Hala G. Schepmann Southern Oregon University 1250 Siskiyou Boulevard Ashland Oregon 97520 USA [email protected] Search for other works by this author on: This Site PubMed Google Scholar Michelle Mynderse Michelle Mynderse 2371 Henley Avenue Berkley Michigan 48072 USA Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 9.3.1. A Guided-Inquiry Approach to Ring-Closing Metathesis in another window
- 9.3.2. Sequential Pd-Catalyzed Allylic Alkylation/Ru-Catalyzed Ring-Closing Metathesis p650-655 By Mélanie M. Lorion ; Mélanie M. Lorion UPMC Univ Paris 06, IPCM UMR CNRS 8232, FR2769, Institut Parisien de Chimie Moléculaire Paris France CNRS, UMR 8232 F-75005 Paris France [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Claire Kammerer ; Claire Kammerer Search for other works by this author on: This Site PubMed Google Scholar Guillaume Prestat ; Guillaume Prestat Search for other works by this author on: This Site PubMed Google Scholar Giovanni Poli Giovanni Poli UPMC Univ Paris 06, IPCM UMR CNRS 8232, FR2769, Institut Parisien de Chimie Moléculaire Paris France CNRS, UMR 8232 F-75005 Paris France [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 9.3.2. Sequential Pd-Catalyzed Allylic Alkylation/Ru-Catalyzed Ring-Closing Metathesis in another window
- 10.1. Diels–Alder Reaction of N -Phenylmaleimide with In situ Generated Buta-1,3-diene p656-659 By Artur M. S. Silva ; Artur M. S. Silva Department of Chemistry and QOPNA, University of Aveiro 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Augusto C. Tomé ; Augusto C. Tomé Department of Chemistry and QOPNA, University of Aveiro 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Diana C. G. A. Pinto ; Diana C. G. A. Pinto Department of Chemistry and QOPNA, University of Aveiro 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Fernando Domingues ; Fernando Domingues Department of Chemistry and QOPNA, University of Aveiro 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Graça M. Oliveira Rocha ; Graça M. Oliveira Rocha Department of Chemistry and QOPNA, University of Aveiro 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar José A. S. Cavaleiro ; José A. S. Cavaleiro Department of Chemistry and QOPNA, University of Aveiro 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar M. Graça P. M. S. Neves ; M. Graça P. M. S. Neves Department of Chemistry and QOPNA, University of Aveiro 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar M. Amparo F. Faustino ; M. Amparo F. Faustino Department of Chemistry and QOPNA, University of Aveiro 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Mário M. Q. Simões Mário M. Q. Simões Department of Chemistry and QOPNA, University of Aveiro 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 10.1. Diels–Alder Reaction of <em>N</em>-Phenylmaleimide with <em>In situ</em> Generated Buta-1,3-diene in another window
- 10.2. Synthesis of Isomeric Bicyclopropyls from Conjugated Dienes p660-663 By Leiv K. Sydnes ; Leiv K. Sydnes Department of Chemistry Allégt. 41 5007 Bergen Norway [email protected] Search for other works by this author on: This Site PubMed Google Scholar Magne O. Sydnes Magne O. Sydnes Faculty of Science and Technology, Department of Mathematics and Natural Science 4036 Stavanger Norway Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 10.2. Synthesis of Isomeric Bicyclopropyls from Conjugated Dienes in another window
- 10.3. Diels–Alder Reaction Between p -Benzoquinone and Cyclopentadiene and Subsequent Photochemical [2π + 2π] Cycloaddition p664-667 By Arno Kraft Arno Kraft Heriot-Watt University, Institute of Chemical Sciences, School of Engineering & Physical Sciences Riccarton, Edinburgh EH14 4AS United Kingdom [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 10.3. Diels–Alder Reaction Between <em>p</em>-Benzoquinone and Cyclopentadiene and Subsequent Photochemical [2π + 2π] Cycloaddition in another window
- 10.4. Click Chemistry p668-670 By Susana Dias Lucas ; Susana Dias Lucas Research Institute for Medicines (iMed.ULisboa), Faculty of Pharmacy, Universidade de Lisboa Av. Prof. Gama Pinto 1649-003 Lisboa Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Eduardo F. P. Ruivo ; Eduardo F. P. Ruivo Research Institute for Medicines (iMed.ULisboa), Faculty of Pharmacy, Universidade de Lisboa Av. Prof. Gama Pinto 1649-003 Lisboa Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Marta Figueiras ; Marta Figueiras Research Institute for Medicines (iMed.ULisboa), Faculty of Pharmacy, Universidade de Lisboa Av. Prof. Gama Pinto 1649-003 Lisboa Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar João P. Lavrado ; João P. Lavrado Research Institute for Medicines (iMed.ULisboa), Faculty of Pharmacy, Universidade de Lisboa Av. Prof. Gama Pinto 1649-003 Lisboa Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Rui Moreira Rui Moreira Research Institute for Medicines (iMed.ULisboa), Faculty of Pharmacy, Universidade de Lisboa Av. Prof. Gama Pinto 1649-003 Lisboa Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 10.4. Click Chemistry in another window
- 10.5. On-Water Synthesis of a Dipyrromethane via Bis-hetero-Diels–Alder Reaction of an Azoalkene with Pyrrole p671-675 By Susana M. M. Lopes ; Susana M. M. Lopes Department of Chemistry, University of Coimbra Rua Larga 3004-535 Coimbra Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Maria I. L. Soares ; Maria I. L. Soares Department of Chemistry, University of Coimbra Rua Larga 3004-535 Coimbra Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Ana Lúcia Cardoso ; Ana Lúcia Cardoso Department of Chemistry, University of Coimbra Rua Larga 3004-535 Coimbra Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Américo Lemos ; Américo Lemos CIQA, FCT, University of Algarve, Campus de Gambelas 8005-139 Faro Portugal Search for other works by this author on: This Site PubMed Google Scholar Teresa M. V. D. Pinho e Melo Teresa M. V. D. Pinho e Melo Department of Chemistry, University of Coimbra Rua Larga 3004-535 Coimbra Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 10.5. On-Water Synthesis of a Dipyrromethane <em>via</em> Bis-hetero-Diels–Alder Reaction of an Azoalkene with Pyrrole in another window
- 10.6. Application of 2,4,6-Trioxo-pyrimidin-5-ylidene Alditol in the Synthesis of Pyrano[2,3- d ]pyrimidine Containing a Sugar Moiety by Hetero-Diels–Alder Reaction p676-680 By Aleksandra Pałasz ; Aleksandra Pałasz Department of Organic Chemistry, Faculty of Chemistry, Jagiellonian University Ingardena 3 St 30-060 Kraków Poland [email protected] Search for other works by this author on: This Site PubMed Google Scholar Dariusz Cież Dariusz Cież Department of Organic Chemistry, Faculty of Chemistry, Jagiellonian University Ingardena 3 St 30-060 Kraków Poland [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 10.6. Application of 2,4,6-Trioxo-pyrimidin-5-ylidene Alditol in the Synthesis of Pyrano[2,3-<em>d</em>]pyrimidine Containing a Sugar Moiety by Hetero-Diels–Alder Reaction in another window
- 10.7. Synthesis of a Spiroisoxazoline Oxindole by 1,3-Dipolar Cycloaddition p681-684 By Carlos J. A. Ribeiro ; Carlos J. A. Ribeiro Research Institute for Medicines (iMed.ULisboa), Faculty of Pharmacy, Universidade de Lisboa Av. Professor Gama Pinto 1649-003 Lisbon Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Rui Moreira ; Rui Moreira Research Institute for Medicines (iMed.ULisboa), Faculty of Pharmacy, Universidade de Lisboa Av. Professor Gama Pinto 1649-003 Lisbon Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Maria M. M. Santos Maria M. M. Santos Research Institute for Medicines (iMed.ULisboa), Faculty of Pharmacy, Universidade de Lisboa Av. Professor Gama Pinto 1649-003 Lisbon Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 10.7. Synthesis of a Spiroisoxazoline Oxindole by 1,3-Dipolar Cycloaddition in another window
- 10.8. Oxonitriles: Four-Step Ozonolysis, Aldol, Conjugate Addition, and Enolate Acylation Sequence p685-689 By Jesus A. Lujan-Montelongo ; Jesus A. Lujan-Montelongo Departamento de Química, Centro de Investigación y de Estudios Avanzados (Cinvestav), Avenida Instituto Politécnico Nacional 2508 San Pedro Zacatenco 07360 México D. F. Mexico Search for other works by this author on: This Site PubMed Google Scholar Fraser F. Fleming Fraser F. Fleming Department of Chemistry, Drexel University 32 South 32nd St. Philadelphia PA 19104 USA [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 10.8. Oxonitriles: Four-Step Ozonolysis, Aldol, Conjugate Addition, and Enolate Acylation Sequence in another window
- 10.9. Flash Vacuum Pyrolysis of o -Phenylene Sulfite: Formation and Purification of Cyclopentadienone Dimer p690-693 By R. Alan Aitken ; R. Alan Aitken EaStCHEM School of Chemistry, University of St Andrews North Haugh, St Andrews Fife KY16 9ST UK [email protected] Search for other works by this author on: This Site PubMed Google Scholar Caroline E. R. Horsburgh Caroline E. R. Horsburgh EaStCHEM School of Chemistry, University of St Andrews North Haugh, St Andrews Fife KY16 9ST UK [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 10.9. Flash Vacuum Pyrolysis of <em>o</em>-Phenylene Sulfite: Formation and Purification of Cyclopentadienone Dimer in another window
- 11.1. Synthesis of 4-Methoxymethylbenzoic Acid p694-696 By Dulce Simão ; Dulce Simão Department of Chemistry, Instituto Superior Técnico, University of Lisbon, Centro de Química Estrutural Av. Rovisco Pais, 1 1049-001 Lisbon Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Ana C. Cerdeira Ana C. Cerdeira Department of Chemistry, Instituto Superior Técnico, University of Lisbon, Centro de Química Estrutural Av. Rovisco Pais, 1 1049-001 Lisbon Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 11.1. Synthesis of 4-Methoxymethylbenzoic Acid in another window
- 11.2. Synthesis of Benzopinacolone via Benzophenone Photoreduction Followed by Pinacol Rearrangement p697-700 By Filipa Siopa ; Filipa Siopa iMed.ULisboa Av. Professor Gama Pinto 1649-003 Lisboa Portugal [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Carlos A. M. Afonso Carlos A. M. Afonso iMed.ULisboa Av. Professor Gama Pinto 1649-003 Lisboa Portugal [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 11.2. Synthesis of Benzopinacolone <em>via</em> Benzophenone Photoreduction Followed by Pinacol Rearrangement in another window
- 11.3. Iodosulfonylation–Dehydroiodination of Styrene: Synthesis of ( E )-β-Tosylstyrene p701-703 By Carmen Nájera ; Carmen Nájera Departamento de Química Orgánica and Centro de Innovación en Química Avanzada (ORFEO-CINQA). Universidad de Alicante Ctra. San Vicente s/n E-03690-San Vicente del Raspeig (Alicante) Spain [email protected] Search for other works by this author on: This Site PubMed Google Scholar José M. Sansano ; José M. Sansano Departamento de Química Orgánica and Centro de Innovación en Química Avanzada (ORFEO-CINQA). Universidad de Alicante Ctra. San Vicente s/n E-03690-San Vicente del Raspeig (Alicante) Spain [email protected] Search for other works by this author on: This Site PubMed Google Scholar Miguel Yus Miguel Yus Departamento de Química Orgánica and Centro de Innovación en Química Avanzada (ORFEO-CINQA). Universidad de Alicante Ctra. San Vicente s/n E-03690-San Vicente del Raspeig (Alicante) Spain [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 11.3. Iodosulfonylation–Dehydroiodination of Styrene: Synthesis of (<em>E</em>)-β-Tosylstyrene in another window
- 12.1.1. Aerobic Alcohol Oxidation Using a Cu( i )/TEMPO Catalyst System p704-708 By Nicholas J. Hill ; Nicholas J. Hill Department of Chemistry, University of Wisconsin-Madison 1101 University Avenue Madison WI 53706 USA [email protected] Search for other works by this author on: This Site PubMed Google Scholar Jessica M. Hoover ; Jessica M. Hoover Department of Chemistry, University of Wisconsin-Madison 1101 University Avenue Madison WI 53706 USA [email protected] Search for other works by this author on: This Site PubMed Google Scholar Shannon S. Stahl Shannon S. Stahl Department of Chemistry, University of Wisconsin-Madison 1101 University Avenue Madison WI 53706 USA [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 12.1.1. Aerobic Alcohol Oxidation Using a Cu(<span class="small-caps">i</span>)/TEMPO Catalyst System in another window
- 12.1.2. Chemoselective Oxidation of 1,2-Tetradecanediol p709-712 By Siedlecka Renata ; Siedlecka Renata Faculty of Chemistry, Organic Chemistry Department, Wroclaw University of Technology Wyb. Wyspianskiego 27 50-370 Wroclaw Poland [email protected] Search for other works by this author on: This Site PubMed Google Scholar Skarżewski Jacek Skarżewski Jacek Faculty of Chemistry, Organic Chemistry Department, Wroclaw University of Technology Wyb. Wyspianskiego 27 50-370 Wroclaw Poland [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 12.1.2. Chemoselective Oxidation of 1,2-Tetradecanediol in another window
- 12.1.3. Catalyzed Oxidation of Naphthalene to 1,4-Naphthoquinone p713-715 By Jacek Skarżewski Jacek Skarżewski Department of Organic Chemistry, Wroclaw University of Technology Wyb. Wyspianskiego 27 50-370 Wroclaw Poland [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 12.1.3. Catalyzed Oxidation of Naphthalene to 1,4-Naphthoquinone in another window
- 12.1.4. Alcohol Oxidation: Menthone Preparation by Menthol Oxidation Using Pyridinium Chlorochromate Immobilized in Silica Gel p716-719 By Rafael F. A. Gomes ; Rafael F. A. Gomes iMed.UL, Faculty of Pharmacy, University of Lisbon Av. Prof. Gama Pinto 1649-003 Lisboa Portugal [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Carlos A. M. Afonso Carlos A. M. Afonso iMed.UL, Faculty of Pharmacy, University of Lisbon Av. Prof. Gama Pinto 1649-003 Lisboa Portugal [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 12.1.4. Alcohol Oxidation: Menthone Preparation by Menthol Oxidation Using Pyridinium Chlorochromate Immobilized in Silica Gel in another window
- 12.1.5. Heterocyclic Target Synthesis – Three-Step Syntheses from Benzaldehyde p720-724 By Iain A. Smellie ; Iain A. Smellie School of Chemistry, University of St Andrews North Haugh, St Andrews Fife KY16 9ST UK [email protected] Search for other works by this author on: This Site PubMed Google Scholar Nigel P. Botting ; Nigel P. Botting School of Chemistry, University of St Andrews North Haugh, St Andrews Fife KY16 9ST UK [email protected] Search for other works by this author on: This Site PubMed Google Scholar Brian A. Chalmers ; Brian A. Chalmers School of Chemistry, University of St Andrews North Haugh, St Andrews Fife KY16 9ST UK [email protected] Search for other works by this author on: This Site PubMed Google Scholar Andrew D. Harper ; Andrew D. Harper School of Chemistry, University of St Andrews North Haugh, St Andrews Fife KY16 9ST UK [email protected] Search for other works by this author on: This Site PubMed Google Scholar Iain L. J. Patterson Iain L. J. Patterson School of Chemistry, University of St Andrews North Haugh, St Andrews Fife KY16 9ST UK [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 12.1.5. Heterocyclic Target Synthesis – Three-Step Syntheses from Benzaldehyde in another window
- 12.1.6. Oxidation of Activated Phenols p725-729 By Luísa M. Ferreira ; Luísa M. Ferreira LAQV, REQUIMTE, Departamento de Química, Faculdade de Ciências e Tecnologia, Universidade Nova de Lisboa Campus da Caparica Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Paula S. Branco Paula S. Branco LAQV, REQUIMTE, Departamento de Química, Faculdade de Ciências e Tecnologia, Universidade Nova de Lisboa Campus da Caparica Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 12.1.6. Oxidation of Activated Phenols in another window
- 12.2.1. Synthesis of 2-(5-Phenylthien-2′-yl)benzothiazole p730-732 By Maria Manuela Marques Raposo ; Maria Manuela Marques Raposo Department of Chemistry, University of Minho Campus de Gualtar 4710-057 Braga Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Susana Paula Graça da Costa ; Susana Paula Graça da Costa Department of Chemistry, University of Minho Campus de Gualtar 4710-057 Braga Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Rosa Maria Ferreira Batista Rosa Maria Ferreira Batista Department of Chemistry, University of Minho Campus de Gualtar 4710-057 Braga Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 12.2.1. Synthesis of 2-(5-Phenylthien-2′-yl)benzothiazole in another window
- 12.2.2. Synthesis of N - tert -butyloxycarbonyl-[2-(thien-2′-yl)benzoxazol-5-yl]- l -alanine methyl ester p733-736 By Susana P. G. Costa ; Susana P. G. Costa Department of Chemistry, University of Minho Campus de Gualtar 4710-057 Braga Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar M. Manuela M. Raposo ; M. Manuela M. Raposo Department of Chemistry, University of Minho Campus de Gualtar 4710-057 Braga Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Cátia I. C. Esteves ; Cátia I. C. Esteves Department of Chemistry, University of Minho Campus de Gualtar 4710-057 Braga Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar R. Cristina M. Ferreira R. Cristina M. Ferreira Department of Chemistry, University of Minho Campus de Gualtar 4710-057 Braga Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 12.2.2. Synthesis of <em>N</em>-<em>tert</em>-butyloxycarbonyl-[2-(thien-2′-yl)benzoxazol-5-yl]-<span class="small-caps">l</span>-alanine methyl ester in another window
- 12.2.3. Synthesis of Flavones (2-Aryl-4 H -chromen-4-ones) p737-740 By Artur M. S. Silva ; Artur M. S. Silva Chemistry Department & QOPNA, University of Aveiro Campus de Santiago 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Augusto C. Tomé ; Augusto C. Tomé Chemistry Department & QOPNA, University of Aveiro Campus de Santiago 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Diana C. G. A. Pinto ; Diana C. G. A. Pinto Chemistry Department & QOPNA, University of Aveiro Campus de Santiago 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Fernando Domingues ; Fernando Domingues Chemistry Department & QOPNA, University of Aveiro Campus de Santiago 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Graça M. Oliveira Rocha ; Graça M. Oliveira Rocha Chemistry Department & QOPNA, University of Aveiro Campus de Santiago 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar José A. S. Cavaleiro ; José A. S. Cavaleiro Chemistry Department & QOPNA, University of Aveiro Campus de Santiago 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar M. Graça P. M. S. Neves ; M. Graça P. M. S. Neves Chemistry Department & QOPNA, University of Aveiro Campus de Santiago 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar M. Amparo F. Faustino ; M. Amparo F. Faustino Chemistry Department & QOPNA, University of Aveiro Campus de Santiago 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Mário M. Q. Simões Mário M. Q. Simões Chemistry Department & QOPNA, University of Aveiro Campus de Santiago 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 12.2.3. Synthesis of Flavones (2-Aryl-4<em>H</em>-chromen-4-ones) in another window
- 12.2.4. Hantzsch Synthesis of 3,5-Diethoxycarbonyl-2,6-dimethylpyridine p741-744 By Iain A. Smellie ; Iain A. Smellie School of Chemistry, University of St Andrews North Haugh, St Andrews Fife KY16 9ST UK [email protected] Search for other works by this author on: This Site PubMed Google Scholar Nigel P. Botting ; Nigel P. Botting School of Chemistry, University of St Andrews North Haugh, St Andrews Fife KY16 9ST UK [email protected] Search for other works by this author on: This Site PubMed Google Scholar Brian A. Chalmers ; Brian A. Chalmers School of Chemistry, University of St Andrews North Haugh, St Andrews Fife KY16 9ST UK [email protected] Search for other works by this author on: This Site PubMed Google Scholar Iain L. J. Patterson ; Iain L. J. Patterson School of Chemistry, University of St Andrews North Haugh, St Andrews Fife KY16 9ST UK [email protected] Search for other works by this author on: This Site PubMed Google Scholar Catherine M. Schofield Catherine M. Schofield School of Chemistry, University of St Andrews North Haugh, St Andrews Fife KY16 9ST UK [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 12.2.4. Hantzsch Synthesis of 3,5-Diethoxycarbonyl-2,6-dimethylpyridine in another window
- 12.2.5. An “Out-of-the-Box” Example in Heterocyclic Chemistry: Synthesis of 3,6-Bis-(3,5-dimethyl-1 H -pyrazol-1-yl)-1,2,4,5-tetrazine p745-749 By Tiago J. L. Silva ; Tiago J. L. Silva Centro de Química Estrutural, Faculdade de Ciências da, Universidade de Lisboa Campo Grande, 1049-1016 Campo Grande, Lisboa Portugal Departamento de Química, Escola de Ciências e Tecnologia, Centro de Química de Évora, Instituto de Investigação e Formação Avançada, Universidade de Évora Rua Romão Ramalho 59 7000-671 Évora Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Paulo J. G. Mendes ; Paulo J. G. Mendes Departamento de Química, Escola de Ciências e Tecnologia, Centro de Química de Évora, Instituto de Investigação e Formação Avançada, Universidade de Évora Rua Romão Ramalho 59 7000-671 Évora Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Ana Isabel Tomaz ; Ana Isabel Tomaz Centro de Química Estrutural, Faculdade de Ciências da, Universidade de Lisboa Campo Grande, 1049-1016 Campo Grande, Lisboa Portugal Search for other works by this author on: This Site PubMed Google Scholar M. Helena Garcia M. Helena Garcia Centro de Química Estrutural, Faculdade de Ciências da, Universidade de Lisboa Campo Grande, 1049-1016 Campo Grande, Lisboa Portugal Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 12.2.5. An “Out-of-the-Box” Example in Heterocyclic Chemistry: Synthesis of 3,6-Bis-(3,5-dimethyl-1<em>H</em>-pyrazol-1-yl)-1,2,4,5-tetrazine in another window
- 12.3.1. Catalytic Epoxidation of cis -Cyclooctene with MTO( vii ) and Pyrazole p750-753 By Elisabete P. Carreiro ; Elisabete P. Carreiro Centro de Química de Évora, Institute for Research and Advanced Studies, University of Evora, Chemistry Department, School of Science and Technology Rua Romão Ramalho, no. 59 7000-671 Évora Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Anthony J. Burke Anthony J. Burke Centro de Química de Évora, Institute for Research and Advanced Studies, University of Evora, Chemistry Department, School of Science and Technology Rua Romão Ramalho, no. 59 7000-671 Évora Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 12.3.1. Catalytic Epoxidation of <em>cis</em>-Cyclooctene with MTO(<span class="small-caps">vii</span>) and Pyrazole in another window
- 12.3.2. Stereoselective Epoxidation of Cholesterol by m -Chloroperoxybenzoic Acid p754-757 By Maria de Jesus Perry ; Maria de Jesus Perry Instituto de Investigação do Medicamento (iMed. ULisboa), Faculdade de Farmácia da Universidade de Lisboa Av. Prof. Gama Pinto 1649-003 Lisboa Portugal [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Ana Paula Francisco ; Ana Paula Francisco Instituto de Investigação do Medicamento (iMed. ULisboa), Faculdade de Farmácia da Universidade de Lisboa Av. Prof. Gama Pinto 1649-003 Lisboa Portugal [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Ana S. Ressurreição ; Ana S. Ressurreição Instituto de Investigação do Medicamento (iMed. ULisboa), Faculdade de Farmácia da Universidade de Lisboa Av. Prof. Gama Pinto 1649-003 Lisboa Portugal [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Francisca Lopes Francisca Lopes Instituto de Investigação do Medicamento (iMed. ULisboa), Faculdade de Farmácia da Universidade de Lisboa Av. Prof. Gama Pinto 1649-003 Lisboa Portugal [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 12.3.2. Stereoselective Epoxidation of Cholesterol by <em>m</em>-Chloroperoxybenzoic Acid in another window
- 12.3.3. Green Oxidation of Organic Compounds Using Metalloporphyrins p758-761 By Rose A. Clark ; Rose A. Clark Department of Chemistry, Saint Francis University Loretto PA 15940 USA [email protected] Search for other works by this author on: This Site PubMed Google Scholar Anne E. Stock ; Anne E. Stock Department of Chemistry, Saint Francis University Loretto PA 15940 USA [email protected] Search for other works by this author on: This Site PubMed Google Scholar Edward P. Zovinka Edward P. Zovinka Department of Chemistry, Saint Francis University Loretto PA 15940 USA [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 12.3.3. Green Oxidation of Organic Compounds Using Metalloporphyrins in another window
- 12.3.4. Catalytic Epoxidation of Carbamazepine p762-765 By Artur M. S. Silva ; Artur M. S. Silva Chemistry Department, University of Aveiro, Campus de Santiago 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Augusto C. Tomé ; Augusto C. Tomé Chemistry Department, University of Aveiro, Campus de Santiago 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Diana C. G. A. Pinto ; Diana C. G. A. Pinto Chemistry Department, University of Aveiro, Campus de Santiago 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Fernando M. J. Domingues ; Fernando M. J. Domingues Chemistry Department, University of Aveiro, Campus de Santiago 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Graça M. S. R. O. Rocha ; Graça M. S. R. O. Rocha Chemistry Department, University of Aveiro, Campus de Santiago 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar José A. S. Cavaleiro ; José A. S. Cavaleiro Chemistry Department, University of Aveiro, Campus de Santiago 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Maria da Graça P. M. S. Neves ; Maria da Graça P. M. S. Neves Chemistry Department, University of Aveiro, Campus de Santiago 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Maria do Amparo F. Faustino ; Maria do Amparo F. Faustino Chemistry Department, University of Aveiro, Campus de Santiago 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Mário M. Q. Simões Mário M. Q. Simões Chemistry Department, University of Aveiro, Campus de Santiago 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 12.3.4. Catalytic Epoxidation of Carbamazepine in another window
- 12.3.5. Regioselective Epoxidation of Geraniol by VO(acac) 2 Immobilised in Polystyrene p766-768 By Ana I. Vicente ; Ana I. Vicente iMed.ULisboa, Faculdade de Farmácia da Universidade de Lisboa Portugal [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Carlos A. M. Afonso Carlos A. M. Afonso iMed.ULisboa, Faculdade de Farmácia da Universidade de Lisboa Portugal [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 12.3.5. Regioselective Epoxidation of Geraniol by VO(acac)<sub>2</sub> Immobilised in Polystyrene in another window
- 12.3.6. Organocatalysed trans -Dihydroxylation of Olefins p769-772 By Andreia A. Rosatella ; Andreia A. Rosatella iMed.ULisboa, Faculdade de Farmácia da Universidade de Lisboa Av. Prof. Gama Pinto 1649-003 Lisboa Portugal [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Carlos A. M. Afonso Carlos A. M. Afonso iMed.ULisboa, Faculdade de Farmácia da Universidade de Lisboa Av. Prof. Gama Pinto 1649-003 Lisboa Portugal [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 12.3.6. Organocatalysed <em>trans</em>-Dihydroxylation of Olefins in another window
- 12.4.1. Synthesis of 6-Nitrosaccharin p773-775 By Dulce Simão ; Dulce Simão Department of Chemistry, Instituto Superior Técnico, University of Lisbon, Centro de Química Estrutural Av. Rovisco Pais, 1 1049-001 Lisbon Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Ana C. Cerdeira Ana C. Cerdeira Department of Chemistry, Instituto Superior Técnico, University of Lisbon, Centro de Química Estrutural Av. Rovisco Pais, 1 1049-001 Lisbon Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 12.4.1. Synthesis of 6-Nitrosaccharin in another window
- 13.1. Reduction of a Ketone Using Sodium Borohydride. Control of a Reaction by TLC p776-780 By Luis Constantino ; Luis Constantino Faculty of Pharmacy, Universidade de Lisboa Av. Professor Gama Pinto 1649-003 Lisbon Portugal Research Institute for Medicines (iMed.ULisboa), Faculty of Pharmacy, Universidade de Lisboa Av. Professor Gama Pinto 1649-003 Lisbon Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Catarina Dias ; Catarina Dias Faculty of Pharmacy, Universidade de Lisboa Av. Professor Gama Pinto 1649-003 Lisbon Portugal Search for other works by this author on: This Site PubMed Google Scholar Emília Valente Emília Valente Faculty of Pharmacy, Universidade de Lisboa Av. Professor Gama Pinto 1649-003 Lisbon Portugal Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 13.1. Reduction of a Ketone Using Sodium Borohydride. Control of a Reaction by TLC in another window
- 13.2. Regioselective Catalytic Transfer Hydrogenation of Citral p781-783 By Jaime A. S. Coelho ; Jaime A. S. Coelho iMed.UL, Faculdade de Farmácia da Universidade de Lisboa Av. Prof. Gama Pinto 1649-003 Lisboa Portugal [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Carlos A. M. Afonso Carlos A. M. Afonso iMed.UL, Faculdade de Farmácia da Universidade de Lisboa Av. Prof. Gama Pinto 1649-003 Lisboa Portugal [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 13.2. Regioselective Catalytic Transfer Hydrogenation of Citral in another window
- 13.3. Regioselective 1,2-Reduction of an α,β-Unsaturated Ketone. A Green Experiment p784-788 By M. Manuela A. Pereira M. Manuela A. Pereira REQUIMTE, Chemistry Department, Faculdade de Ciências e Tecnologia, Universidade Nova de Lisboa 2827-516 Caparica Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 13.3. Regioselective 1,2-Reduction of an α,β-Unsaturated Ketone. A Green Experiment in another window
- 13.4. Reduction of Diphenyl Sulfoxide Catalyzed by the Dioxo-Molybdenum Complex MoO 2 Cl 2 (H 2 O) 2 p789-793 By Ana C. Fernandes ; Ana C. Fernandes Centro de Química Estrutural, Instituto Superior Técnico, Universidade de Lisboa Av. Rovisco Pais, 1 1049-001 Lisboa Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Tiago A. Fernandes Tiago A. Fernandes Centro de Química Estrutural, Instituto Superior Técnico, Universidade de Lisboa Av. Rovisco Pais, 1 1049-001 Lisboa Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 13.4. Reduction of Diphenyl Sulfoxide Catalyzed by the Dioxo-Molybdenum Complex MoO<sub>2</sub>Cl<sub>2</sub>(H<sub>2</sub>O)<sub>2</sub> in another window
- 13.5. Synthesis of Allylic Esters by Reduction of Fructone Followed by Wittig Olefination p794-797 By Thomas A. Logothetis Thomas A. Logothetis University of Southampton, Chemistry Highfield, Southampton Hampshire SO17 1BJ UK [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 13.5. Synthesis of Allylic Esters by Reduction of Fructone Followed by Wittig Olefination in another window
- 13.6. Preparation of a Thia-Tetraaza Macrocyclic Compound Through a Dual-Step Synthesis p798-802 By Judite Costa ; Judite Costa Research Institute for Medicines (iMed.ULisboa), Faculty of Pharmacy, Universidade de Lisboa Av. Professor Gama Pinto 1649-003 Lisbon Portugal [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar João Franco Machado ; João Franco Machado Research Institute for Medicines (iMed.ULisboa), Faculty of Pharmacy, Universidade de Lisboa Av. Professor Gama Pinto 1649-003 Lisbon Portugal [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar M. Fátima Cabral M. Fátima Cabral Research Institute for Medicines (iMed.ULisboa), Faculty of Pharmacy, Universidade de Lisboa Av. Professor Gama Pinto 1649-003 Lisbon Portugal [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 13.6. Preparation of a Thia-Tetraaza Macrocyclic Compound Through a Dual-Step Synthesis in another window
- 13.7. Asymmetric Reduction of Acetophenone with Borane Catalyzed by B -Methoxy-oxazaborolidine p803-806 By Marek P. Krzemiński Marek P. Krzemiński Department of Organic Chemistry, Faculty of Chemistry, Nicolaus Copernicus, University in Toruń Poland [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 13.7. Asymmetric Reduction of Acetophenone with Borane Catalyzed by <em>B</em>-Methoxy-oxazaborolidine in another window
- 13.8. Synthesis of 4,5-Bis(benzoylthio)-1,3-dithiole-2-thione p807-810 By Dulce Simão ; Dulce Simão Department of Chemistry, Instituto Superior Técnico, University of Lisbon, Centro de Química Estrutural Av. Rovisco Pais 1, 1049-001 Lisbon Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Ana C. Cerdeira Ana C. Cerdeira Department of Chemistry, Instituto Superior Técnico, University of Lisbon, Centro de Química Estrutural Av. Rovisco Pais 1, 1049-001 Lisbon Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 13.8. Synthesis of 4,5-Bis(benzoylthio)-1,3-dithiole-2-thione in another window
- 13.9. Organocatalytic Asymmetric Reduction of ( E )- N ,1-Diphenyl-1-propanimine to N -(1-Phenylpropyl)aniline with Trichlorosilane p811-814 By Pedro Barrulas ; Pedro Barrulas Departamento de Química e Centro de Química de Évora, Universidade de Évora Rua Romão Ramalho, 59 7000 Évora Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Anthony Burke Anthony Burke Departamento de Química e Centro de Química de Évora, Universidade de Évora Rua Romão Ramalho, 59 7000 Évora Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 13.9. Organocatalytic Asymmetric Reduction of (<em>E</em>)-<em>N</em>,1-Diphenyl-1-propanimine to <em>N</em>-(1-Phenylpropyl)aniline with Trichlorosilane in another window
- 14.1. Benzilic Acid Rearrangement p815-817 By Paulo Coelho ; Paulo Coelho Centro de Química – Vila Real, Universidade de Trás-os-Montes e Alto Douro 5001-801 Vila Real Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Céu Sousa Céu Sousa Centro de Química – Vila Real, Universidade de Trás-os-Montes e Alto Douro 5001-801 Vila Real Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 14.1. Benzilic Acid Rearrangement in another window
- 14.2. Preparation of Phenyl Acetate and Its Conversion into 4-Hydroxyacetophenone p818-820 By Eimíle Sheehy ; Eimíle Sheehy Center for Synthesis and Chemical Biology, School of Chemistry, University College Dublin Dublin 4 Ireland [email protected] Search for other works by this author on: This Site PubMed Google Scholar Paul Evans Paul Evans Center for Synthesis and Chemical Biology, School of Chemistry, University College Dublin Dublin 4 Ireland [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 14.2. Preparation of Phenyl Acetate and Its Conversion into 4-Hydroxyacetophenone in another window
- 14.3. Multistep Synthesis of Dilantin p821-825 By Paula C. Castilho ; Paula C. Castilho CQM, Centro de Competências de Ciências Exactas e das Engenharias, Universidade da Madeira, Campus Universitário da Penteada 9020-105 Funchal Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Pedro Ideia Pedro Ideia CQM, Centro de Competências de Ciências Exactas e das Engenharias, Universidade da Madeira, Campus Universitário da Penteada 9020-105 Funchal Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 14.3. Multistep Synthesis of Dilantin in another window
- 14.4. Preparation of Isoborneol Through the Wagner–Meerwein Rearrangement Reaction of (1 R )-(+)-Camphene p826-829 By Marek P. Krzemiński Marek P. Krzemiński Department of Organic Chemistry, Faculty of Chemistry, Nicolaus Copernicus University in Toruń Poland [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 14.4. Preparation of Isoborneol Through the Wagner–Meerwein Rearrangement Reaction of (1<em>R</em>)-(+)-Camphene in another window
- 14.5. Umbelliferone: A Natural Scaffold Suitable for the Synthesis of ortho -Acetylhydroxycoumarins via Fries Rearrangement Reaction p830-833 By Saleta Vazquez-Rodriguez ; Saleta Vazquez-Rodriguez CIQUP/Department of Chemistry and Biochemistry, Faculty of Sciences, University of Porto Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Maria João Matos ; Maria João Matos CIQUP/Department of Chemistry and Biochemistry, Faculty of Sciences, University of Porto Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Eugenio Uriarte ; Eugenio Uriarte Department of Organic Chemistry, Faculty of Pharmacy, University of Santiago de Compostela Spain Search for other works by this author on: This Site PubMed Google Scholar Lourdes Santana ; Lourdes Santana Department of Organic Chemistry, Faculty of Pharmacy, University of Santiago de Compostela Spain Search for other works by this author on: This Site PubMed Google Scholar Fernanda Borges Fernanda Borges CIQUP/Department of Chemistry and Biochemistry, Faculty of Sciences, University of Porto Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 14.5. Umbelliferone: A Natural Scaffold Suitable for the Synthesis of <em>ortho</em>-Acetylhydroxycoumarins <em>via</em> Fries Rearrangement Reaction in another window
- 14.6. Preparation of 4,5-Functionalized Cyclopentenone via Cyclization of a Stenhouse Adduct p834-837 By Jaime A. S. Coelho ; Jaime A. S. Coelho iMed.UL, Faculdade de Farmácia da Universidade de Lisboa Av. Prof. Gama Pinto 1649-003 Lisboa Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Carlos A. M. Afonso Carlos A. M. Afonso iMed.UL, Faculdade de Farmácia da Universidade de Lisboa Av. Prof. Gama Pinto 1649-003 Lisboa Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 14.6. Preparation of 4,5-Functionalized Cyclopentenone <em>via</em> Cyclization of a Stenhouse Adduct in another window
- 14.7. Synthesis of a Chiral Salen. Examples of the Schmidt Rearrangement and Ultrasound Activation p838-840 By Maria Elisa da Silva Serra ; Maria Elisa da Silva Serra Department of Chemistry, University of Coimbra Coimbra Portugal [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Dina Maria Bairrada Murtinho Dina Maria Bairrada Murtinho Department of Chemistry, University of Coimbra Coimbra Portugal [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 14.7. Synthesis of a Chiral Salen. Examples of the Schmidt Rearrangement and Ultrasound Activation in another window
- 15.1. Bioreduction of N -Oxide Moiety p841-846 By María Laura Lavaggi ; María Laura Lavaggi Laboratorio de Química Orgánica, Instituto de Química Biológica, Facultad de Ciencias, Universidad de la República Iguá 4225 Montevideo 11400 Uruguay [email protected] Search for other works by this author on: This Site PubMed Google Scholar Mercedes González ; Mercedes González Laboratorio de Química Orgánica, Instituto de Química Biológica, Facultad de Ciencias, Universidad de la República Iguá 4225 Montevideo 11400 Uruguay [email protected] Search for other works by this author on: This Site PubMed Google Scholar Hugo Cerecetto Hugo Cerecetto Área de Radiofarmacia, Centro de Investigaciones Nucleares, Facultad de Ciencias, Universidad de la República Mataojo 2055 Montevideo 11400 Uruguay [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 15.1. Bioreduction of <em>N</em>-Oxide Moiety in another window
- 16.1. Preparation of Nylon 6,6 by Interfacial Polymerization p847-848 By João P. Telo João P. Telo Centro de Química Estrutural, Instituto Superior Técnico, Universidade de Lisboa Av. Rovisco Pais 1649-003 Lisboa Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 16.1. Preparation of Nylon 6,6 by Interfacial Polymerization in another window
- 16.2. Synthesis of Copolymer from Acrylamide (AA), 2-Acrylamido-2-methylpropane-sulfonic Acid (AMPS) and N , N -Methyleno-bis-acrylamide (BA) p849-852 By Fátima Coelho ; Fátima Coelho Department of Chemistry, Instituto Superior Técnico, University of Lisbon, CERENA-Centro de Recursos Naturais e Ambiente Av. Rovisco Pais, 1 1049-001 Lisboa Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Dulce Simão Dulce Simão Department of Chemistry, Instituto Superior Técnico, University of Lisbon, Centro de Química Estrutural Av. Rovisco Pais, 1 1049-001 Lisboa Portugal Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 16.2. Synthesis of Copolymer from Acrylamide (AA), 2-Acrylamido-2-methylpropane-sulfonic Acid (AMPS) and <em>N</em>,<em>N</em>-Methyleno-bis-acrylamide (BA) in another window
- 16.3. Multi-Step Synthesis of Nylon from Cyclohexene p853-858 By Marisa G. Weaver ; Marisa G. Weaver University of California at Santa Barbara, Department of Chemistry & Biochemistry 9510, Santa Barbara California 93106-9510 USA [email protected] Search for other works by this author on: This Site PubMed Google Scholar Morgan J. Gainer Morgan J. Gainer University of California at Santa Barbara, Department of Chemistry & Biochemistry 9510, Santa Barbara California 93106-9510 USA [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 16.3. Multi-Step Synthesis of Nylon from Cyclohexene in another window
- 16.4. Copolymerisation of Styrene and Methyl Methacrylate: An Introduction to Radical Polymerisation and Monomer Reactivity Ratios p859-863 By Valeria Arrighi ; Valeria Arrighi Heriot-Watt University, Institute of Chemical Sciences, School of Engineering & Physical Sciences Riccarton Edinburgh EH14 4AS UK [email protected] Search for other works by this author on: This Site PubMed Google Scholar Arno Kraft Arno Kraft Heriot-Watt University, Institute of Chemical Sciences, School of Engineering & Physical Sciences Riccarton Edinburgh EH14 4AS UK [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 16.4. Copolymerisation of Styrene and Methyl Methacrylate: An Introduction to Radical Polymerisation and Monomer Reactivity Ratios in another window
- 16.5. Polymerization of ε-Caprolactone Using a Ruthenium( ii ) Mixed Metallocene Catalyst p864-867 By Andreia Valente ; Andreia Valente Centro de Química Estrutural, Faculdade de Ciências da Universidade de Lisboa Lisboa Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Tiago J. L. Silva ; Tiago J. L. Silva Centro de Química Estrutural, Faculdade de Ciências da Universidade de Lisboa Lisboa Portugal [email protected] Departamento de Química, Escola de Ciências e Tecnologia, Centro de Química de Évora, Instituto de Investigação e Formação Avançada, Universidade de Évora Évora Portugal Search for other works by this author on: This Site PubMed Google Scholar Paulo J. G. Mendes ; Paulo J. G. Mendes Departamento de Química, Escola de Ciências e Tecnologia, Centro de Química de Évora, Instituto de Investigação e Formação Avançada, Universidade de Évora Évora Portugal Search for other works by this author on: This Site PubMed Google Scholar Ana Isabel Tomaz ; Ana Isabel Tomaz Centro de Química Estrutural, Faculdade de Ciências da Universidade de Lisboa Lisboa Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar M. Helena Garcia M. Helena Garcia Centro de Química Estrutural, Faculdade de Ciências da Universidade de Lisboa Lisboa Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 16.5. Polymerization of ε-Caprolactone Using a Ruthenium(<span class="small-caps">ii</span>) Mixed Metallocene Catalyst in another window
- 17.1. The Cannizzaro Reaction: Synthesis of p -Chlorobenzyl Alcohol and p -Chlorobenzoic Acid p868-870 By Marja Asp-Lehtinen Marja Asp-Lehtinen Department of Chemistry and Bioengineering, Tampere University of Technology P.O. Box 541 33101 Tampere Finland [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 17.1. The Cannizzaro Reaction: Synthesis of <em>p</em>-Chlorobenzyl Alcohol and <em>p</em>-Chlorobenzoic Acid in another window
- 17.2. The Kemp Elimination in Water: A Laboratory Experiment for Introductory Organic Chemistry p871-874 By Caitlin Williamson ; Caitlin Williamson Department of Chemistry and Biochemistry, Wilfrid Laurier University 75 University Ave W. Waterloo Ontario Canada N2L 3C5 [email protected] Search for other works by this author on: This Site PubMed Google Scholar Kenneth E. Maly ; Kenneth E. Maly Department of Chemistry and Biochemistry, Wilfrid Laurier University 75 University Ave W. Waterloo Ontario Canada N2L 3C5 [email protected] Search for other works by this author on: This Site PubMed Google Scholar Stephen L. MacNeil Stephen L. MacNeil Department of Chemistry and Biochemistry, Wilfrid Laurier University 75 University Ave W. Waterloo Ontario Canada N2L 3C5 [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 17.2. The Kemp Elimination in Water: A Laboratory Experiment for Introductory Organic Chemistry in another window
- 17.3. Synthesis of Veratronitrile p875-877 By Dulce Simão ; Dulce Simão Department of Chemistry, Instituto Superior Técnico, University of Lisbon, Centro de Química Estrutural Av. Rovisco Pais, 1 1049-001 Lisbon Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Ana C. Cerdeira Ana C. Cerdeira Department of Chemistry, Instituto Superior Técnico, University of Lisbon, Centro de Química Estrutural Av. Rovisco Pais, 1 1049-001 Lisbon Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 17.3. Synthesis of Veratronitrile in another window
- 17.4. Synthesis of Levulinic Acid from Sucrose p878-880 By João P. Telo João P. Telo Centro de Química Estrutural, Instituto Superior Técnico, Universidade de Lisboa Av. Rovisco Pais 1049-001 Lisboa Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 17.4. Synthesis of Levulinic Acid from Sucrose in another window
- 17.5. Synthesis of 1-(4-Bromophenyl)-1 H -pyrrole by the Clauson-Kaas Reaction p881-884 By Maria Manuela Marques Raposo ; Maria Manuela Marques Raposo University of Minho, Department of Chemistry, Campus de Gualtar 4710-057 Braga Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Maria Cidália Rodrigues Castro ; Maria Cidália Rodrigues Castro University of Minho, Department of Chemistry, Campus de Gualtar 4710-057 Braga Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Sara Sofia Marques Fernandes Sara Sofia Marques Fernandes University of Minho, Department of Chemistry, Campus de Gualtar 4710-057 Braga Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 17.5. Synthesis of 1-(4-Bromophenyl)-1<em>H</em>-pyrrole by the Clauson-Kaas Reaction in another window
- 17.6. Synthesis of the Manganese( iii ) Complex of 5,10,15,20-Tetrakis(2,6-dichlorophenyl)porphyrin p885-887 By Artur M. S. Silva ; Artur M. S. Silva Chemistry Department, University of Aveiro, Campus de Santiago 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Augusto C. Tomé ; Augusto C. Tomé Chemistry Department, University of Aveiro, Campus de Santiago 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Diana C. G. A. Pinto ; Diana C. G. A. Pinto Chemistry Department, University of Aveiro, Campus de Santiago 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Fernando M. J. Domingues ; Fernando M. J. Domingues Chemistry Department, University of Aveiro, Campus de Santiago 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Graça M. S. R. O. Rocha ; Graça M. S. R. O. Rocha Chemistry Department, University of Aveiro, Campus de Santiago 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar José A. S. Cavaleiro ; José A. S. Cavaleiro Chemistry Department, University of Aveiro, Campus de Santiago 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Maria da Graça P. M. S. Neves ; Maria da Graça P. M. S. Neves Chemistry Department, University of Aveiro, Campus de Santiago 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Maria do Amparo F. Faustino ; Maria do Amparo F. Faustino Chemistry Department, University of Aveiro, Campus de Santiago 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Mário M. Q. Simões Mário M. Q. Simões Chemistry Department, University of Aveiro, Campus de Santiago 3810-193 Aveiro Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 17.6. Synthesis of the Manganese(<span class="small-caps">iii</span>) Complex of 5,10,15,20-Tetrakis(2,6-dichlorophenyl)porphyrin in another window
- 17.7. Synthesis of 1,3-Dithienylbenzo[ c ]thiophene p888-892 By Tiago J. L. Silva ; Tiago J. L. Silva Departamento de Química, Escola de Ciências e Tecnologia, Centro de Química de Évora, Instituto de Investigação e Formação Avançada, Universidade de Évora Rua Romão Ramalho 59 7000-671 Évora Portugal Centro de Química Estrutural, Faculdade de Ciências da Universidade de Lisboa Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Paulo J. G. Mendes ; Paulo J. G. Mendes Departamento de Química, Escola de Ciências e Tecnologia, Centro de Química de Évora, Instituto de Investigação e Formação Avançada, Universidade de Évora Rua Romão Ramalho 59 7000-671 Évora Portugal Search for other works by this author on: This Site PubMed Google Scholar Ana Isabel Tomaz ; Ana Isabel Tomaz Centro de Química Estrutural, Faculdade de Ciências da Universidade de Lisboa Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar M. Helena Garcia M. Helena Garcia Centro de Química Estrutural, Faculdade de Ciências da Universidade de Lisboa Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 17.7. Synthesis of 1,3-Dithienylbenzo[<em>c</em>]thiophene in another window
- 17.8. Synthesis of 1-Propyl-2-(thiophen-2-yl)-1 H -pyrrole p893-897 By M. Manuela M. Raposo M. Manuela M. Raposo Department of Chemistry, University of Minho, Campus de Gualtar 4710-057 Braga Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 17.8. Synthesis of 1-Propyl-2-(thiophen-2-yl)-1<em>H</em>-pyrrole in another window
- 17.9. Rhodium Carbene C–H Insertion in Water and Catalyst Reuse p898-902 By Nuno R. Candeias ; Nuno R. Candeias Tampere University of Technology, Department of Chemistry and Bioengineering Korkeakoulunkatu 8 Tampere FI-33101 Finland [email protected] Search for other works by this author on: This Site PubMed Google Scholar Pedro M. P. Gois ; Pedro M. P. Gois University of Lisbon, Research Institute for Medicines and Pharmaceutical Sciences (iMed.UL), Faculty of Pharmacy Av. Prof. Gama Pinto 1649-003 Lisbon Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Carlos A. M. Afonso Carlos A. M. Afonso University of Lisbon, Research Institute for Medicines and Pharmaceutical Sciences (iMed.UL), Faculty of Pharmacy Av. Prof. Gama Pinto 1649-003 Lisbon Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 17.9. Rhodium Carbene C–H Insertion in Water and Catalyst Reuse in another window
- 17.10. Determination of the C–C Bond Strength of Substituted Cyclopropanes and Cyclobutanes using Bomb Calorimetry p903-907 By Steffanie H. Liskey ; Steffanie H. Liskey Department of Chemistry, Virginia Tech 1040 Drillfield Drive, Blacksburg VA 24061 USA [email protected] Search for other works by this author on: This Site PubMed Google Scholar Alan R. Esker ; Alan R. Esker Department of Chemistry, Virginia Tech 1040 Drillfield Drive, Blacksburg VA 24061 USA [email protected] Search for other works by this author on: This Site PubMed Google Scholar J. M. Tanko J. M. Tanko Department of Chemistry, Virginia Tech 1040 Drillfield Drive, Blacksburg VA 24061 USA [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 17.10. Determination of the C–C Bond Strength of Substituted Cyclopropanes and Cyclobutanes using Bomb Calorimetry in another window
- 18.1. Resolution of a Chiral Amine and Recovery of Unwanted Enantiomer by Racemization: Towards a Greener Industrial Process p908-913 By Pedro P. Santos ; Pedro P. Santos CQE, Department of Chemical Engineering, IST, University of Lisbon Av. Rovisco Pais 1649-003 Lisbon Portugal [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Pedro F. Pinheiro Pedro F. Pinheiro CQE, Department of Chemical Engineering, IST, University of Lisbon Av. Rovisco Pais 1649-003 Lisbon Portugal [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 18.1. Resolution of a Chiral Amine and Recovery of Unwanted Enantiomer by Racemization: Towards a Greener Industrial Process in another window
- 18.2. Synthesis of Racemic Phenylalanine Methyl Ester and its Kinetic Resolution Catalysed by α-Chymotrypsin p914-918 By Luca Banfi ; Luca Banfi Department of Chemistry and Industrial Chemistry, University of Genova via Dodecaneso 31 16146 Genova Italy [email protected] Search for other works by this author on: This Site PubMed Google Scholar Renata Riva Renata Riva Department of Chemistry and Industrial Chemistry, University of Genova via Dodecaneso 31 16146 Genova Italy [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 18.2. Synthesis of Racemic Phenylalanine Methyl Ester and its Kinetic Resolution Catalysed by α-Chymotrypsin in another window
- 18.3. Lipase Catalyzed Kinetic Resolution of Racemic 1-Phenylethanol p919-922 By Otto Långvik ; Otto Långvik Johan Gadolin Process Chemistry Centre, Laboratory of Organic Chemistry, Åbo Akademi University Piispankatu 8 FI-20500 Turku Finland [email protected] Search for other works by this author on: This Site PubMed Google Scholar Tiina Saloranta ; Tiina Saloranta Johan Gadolin Process Chemistry Centre, Laboratory of Organic Chemistry, Åbo Akademi University Piispankatu 8 FI-20500 Turku Finland [email protected] Search for other works by this author on: This Site PubMed Google Scholar Reko Leino Reko Leino Johan Gadolin Process Chemistry Centre, Laboratory of Organic Chemistry, Åbo Akademi University Piispankatu 8 FI-20500 Turku Finland [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 18.3. Lipase Catalyzed Kinetic Resolution of Racemic 1-Phenylethanol in another window
- 18.4 Enzymatic Kinetic Resolution and Separation of sec -Alcohols Methodology Based on Fatty Esters p923-926 By Carlos M. Monteiro ; Carlos M. Monteiro iMed. ULisboa, Faculdade de Farmácia da Universidade de Lisboa Av. Prof. Gama Pinto 1649-003 Lisboa Portugal [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Nuno M. T. Lourenço ; Nuno M. T. Lourenço Department of Bioengineering, IBB – Institute for Biotechnology and Bioengineering, Centre for Biological and Chemical Engineering, Instituto Superior Técnico 1049-001 Lisboa Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Carlos A. M. Afonso Carlos A. M. Afonso iMed. ULisboa, Faculdade de Farmácia da Universidade de Lisboa Av. Prof. Gama Pinto 1649-003 Lisboa Portugal [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 18.4 Enzymatic Kinetic Resolution and Separation of <em>sec</em>-Alcohols Methodology Based on Fatty Esters in another window
- 18.5. Enzymatic Kinetic Resolution and Preparative Separation of Secondary Alcohols p927-930 By Nuno M. T. Lourenço ; Nuno M. T. Lourenço Instituto Superior Técnico, Universidade de Lisboa Av. Rovisco Pais, 1 1049-001 Lisboa Portugal [email protected] Search for other works by this author on: This Site PubMed Google Scholar Carlos M. Monteiro ; Carlos M. Monteiro Faculdade de Farmácia, Universidade de Lisboa Av. Gama Pinto 1649-003 Lisboa Portugal [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Carlos A. M. Afonso Carlos A. M. Afonso Faculdade de Farmácia, Universidade de Lisboa Av. Gama Pinto 1649-003 Lisboa Portugal [email protected] [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 18.5. Enzymatic Kinetic Resolution and Preparative Separation of Secondary Alcohols in another window
- 18.6. Catalyzed Resolution and Simultaneous Selective Crystallization p931-934 By Jaan Parve ; Jaan Parve Institute of Technology, University of Tartu Nooruse 1 50411 Tartu Estonia Search for other works by this author on: This Site PubMed Google Scholar Lauri Vares ; Lauri Vares Institute of Technology, University of Tartu Nooruse 1 50411 Tartu Estonia Search for other works by this author on: This Site PubMed Google Scholar Indrek Reile ; Indrek Reile National Institute of Chemical Physics and Biophysics Akadeemia tee 23 12618 Tallinn Estonia Search for other works by this author on: This Site PubMed Google Scholar Tõnis Pehk ; Tõnis Pehk National Institute of Chemical Physics and Biophysics Akadeemia tee 23 12618 Tallinn Estonia Search for other works by this author on: This Site PubMed Google Scholar Ly Villo ; Ly Villo Department of Chemistry, Tallinn University of Technology Ehitajate tee 5 19086 Tallinn Estonia [email protected] Search for other works by this author on: This Site PubMed Google Scholar Omar Parve Omar Parve Department of Chemistry, Tallinn University of Technology Ehitajate tee 5 19086 Tallinn Estonia [email protected] Search for other works by this author on: This Site PubMed Google Scholar Open the PDF Link PDF for 18.6. Catalyzed Resolution and Simultaneous Selective Crystallization in another window
- Subject Index p935-951 Open the PDF Link PDF for Subject Index in another window
Advertisement
- Campaigning and outreach
- News and events
- Awards and funding
- Privacy policy
- Journals and databases
- Locations and contacts
- Membership and professional community
- Teaching and learning
- Help and legal
- Cookie policy
- Terms and conditions
- Get Adobe Acrobat Reader
- Registered charity number: 207890
- © Royal Society of Chemistry 2023
This Feature Is Available To Subscribers Only
Sign In or Create an Account
Resource Type: Virtual Labs
The Virtual Lab is an online simulation of a chemistry lab. It is designed to help students link chemical computations with authentic laboratory chemistry. The lab allows students to select from hundreds of standard reagents (aqueous) and manipulate them in a manner resembling a real lab. More information and offline downloads . Please scroll below to find our collection of pre-written problems, they have been organized by concept and ranked by difficulty.
Stoichiometry
The mole, molarity, and density, glucose dilution problem.
In this activity, students use the virtual lab to create a 0.025M glucose solution from a standard 1M glucose solution. First, they calculate the correct volumes of 1M glucose solution and water to mix together…
Acid Dilution Problem
In this activity, students use the virtual lab to create 500mL of 3M HCl solution from a concentrated stock solution of 11.6M HCl. They must first calculate the correct volumes of 11.6M HCl solution and water to…
Cola and Sucrose Concentration Problem
In this activity, students use the virtual lab to prepare a sucrose solution for a soda recipe. They next calculate the concentration of their solution in terms of molarity, percent mass and density. Finally, they…
Making Stock Solutions from Solids
In this activity, students use the virtual lab to create stock solutions starting from solid salts. Students must first calculate the correct amount of solid to make the solution. Next, they prepare the solution…
Identifying the Unknown Metal (Metals Density Problem)
In this activity, students use the virtual lab to identify an unknown metal by measuring its density and comparing their measurements to the densities of known metals.
Identifying an Unknown Liquid from its Density
In this activity students use the virtual lab to design an experiment to determine the identity of mislabeled bottles using the densities of the solutions inside.
Alcohol Density Problem
Determine the concentration of an alcohol solution from its density.
Reaction Stoichiometry and Limiting Reagents
Gravimetric determination of arsenic.
Set in the context of ground water contamination in Bangladesh, this stoichiometry and analytical chemistry activity examines the issues around identifying wells contaminated with arsenic. (Part of a larger online…
Determining Stoichiometric Coefficients
In this activity, students use the virtual lab to determine how 4 unknown substances react with each other including their stoichiometric coefficients.
Stoichiometry and Solution Preparation Problem
In this limiting reagents problem, students mix together solutions in different ratios in an attempt to produce a final solution that contains only 1 product.
Textbook Style Limiting Reagents Problems
Textbook-style practice limiting reagent exercises with that can be used as a way to "predict and check" your answers using the virtual lab.
Textbook Style Limiting Reagents Problem II
In this activity, students practice with experiments involving limiting reagents and the test their knowledge to determine the concentration of an unknown solution.
Predicting DNA Concentration
In this limiting reagents problem, students are given specific concentrations of DNA solutions and are asked to predict what products and reactants will remain after a specific volumes are mixed and reaction has…
Unknown Concentration of DNA Solution Problem
In this advanced limiting reagent problem, students use the virtual lab to determine the concentration of a solution of DNA by reacting it with known amounts of a fluorescent dye which binds to the DNA.
Thermochemistry
Energy and enthalpy, camping problem i.
In this part of the MRE scenario, students measure the enthalpy of a reaction.
Camping Problem II
In this part of the MRE scenario, students determine change in the enthalpy of a reaction as the concentration of reactants are varied.
ATP Reaction (Thermochemistry and Bonding)
Determine the enthalpy of the ATP reaction.
Determining the Heat of Reaction in Aqueous Solution
In this activity, students perform an experiment to determine the heat of a reaction.
Coffee Problem
Use the virtual lab to determine how much milk to add to hot coffee to reach the desired temperature
Measuring the heat capacity of an engine coolant.
As an analytical chemist at a company developing new engine coolants your task is to determine the heat capacity of a newly developed product and then to determine if its heat capacity is greater of less than that…
Measuring the heat capacity of an engine coolant II (Advanced version)
Measure and compare the heat capacity of an unknown liquid with an unknown density.
Camping Problem III
In this part of the MRE scenario, students create solutions that when mixed, increase to a certain temperature.
Heats of Reaction - Hess' Law
This activity provides a demonstration of Hess' Law using three reactions: the solubility NaOH in water, the solubility NaOH in HCl and the reaction of a solution of HCl and a solution of NaOH.
Equilibrium
Lechatlier's principle, cobalt chloride and lechatlier’s principle.
In this activity, students safely explore the equilibrium reaction of the cobalt chloride reaction.
Equilibrium Calculations
Dna binding problem.
In this activity, students explore equilibrium constants in biochemical systems by measuring the binding constant of a DNA-Dye reaction.
Acid-Base Chemistry
Strong acids and bases, strong acid and base problems.
Textbook-style strong acid and base problems that can be checked using the Virtual Lab.
Determination of the pH Scale by the Method of Successive Dilutions
This activity was created as an accompaniment to an in-class demonstration of the method of successive dilutions using HCl, NaOH, a pH meter, and universal indicator solution. After the demonstration, students…
Weak Acids and Bases
Weak acid and base problems.
Textbook-style weak acid and base problems that can be checked using the Virtual Lab.
Determining the pKa and Concentration Ratio of a Protein in Solution
Use the virtual lab to determine the pKa of a protein then create a buffer solution with a specific concentration ratio of the protein in its protonated/ unprotonated form.
Unknown Acid and Base Problem
In this exercise, students graph the titration curve of an unknown acid and base to determine their pKa’s and concentrations.
Buffer Solutions
Creating a buffer solution.
An exercise to design a buffer solution with specific properties.
DNA - Dye Binding: Equilibrium and Buffer Solutions
Students examine equilibrium and buffer solutions in a biological setting.
Acid/Base Titrations
Standardization of naoh with a khp solution: acid base titration.
Use the Virtual Laboratory to standardize an unknown NaOH solution (approximately 0.2M) to four significant figures via titration with 25.00 mL of a KHP standard solution.
Solubility Product
Determining the solubility product.
Determine the solubility product constatnt (Ksp) for various solids.
Temperature and the Solubility of Salts
Examine the solubilities of salts based on temperature.
Determining the solubility of copper chloride at different temperatures
GIven the solubility of CuCl at 2 different temperatures, predict its solubility at a third temperature. Then test your prediction by creating the solution in the virtual lab
Oxidation/Reduction and Electrochemistry
Standard reduction potentials, exploring oxidation-reduction reactions.
Design an experiment to order Cu, Mg, Zn and Pb from strongest to weakest reducing agent.
Analytical Chemistry/Lab Techniques
Gravimetric analysis, unknown silver chloride.
Determine the concentration of Silver ion in a Silver Nitrate solution using gravimetric analysis
The ChemCollective site and its contents are licensed under a Creative Commons Attribution 3.0 NonCommercial-NoDerivs License.
Teach Organic Chemistry with Labster Virtual Labs
About virtual labs for organic chemistry.
Bring the world of science into the classroom or enable students to bring learning home with Labster’s virtual science lab content. No need for additional hardware or lab equipment; access these organic chemistry labs on any laptops, and spark creativity in students with this innovative and interactive way to explore science.

“My goal is for students to have experiences that simulate the hands-on labs they would encounter if they were in person. Labster is the most hands-on experience students can get without actually being hands-on.”

“Labster exposes students to equipment they likely won't see until they go to post-secondary. It also exposes them to real-life situations where what they're learning in class does have practical applications.”
.jpg)
“My students are very grade-driven, but I try to get them to be curiosity-driven. One way I do this is by using Labster as a fun, curious game.”

Virtual labs, like Labster, contribute to engaging students, fostering curiosity, supporting diverse learning requirements, and enhancing the overall student experience.
Browse Organic Chemistry Simulations

Acids and Bases (Principles): Avoid falling in a lake of acid!
In a futuristic lab, you will get help from a robot assistant to determine the acidity of a lake of acid found on an exoplanet. You will learn how to quantify the acidity and alkalinity of substances.

Acids and Bases: Acidity and Alkalinity in Everyday Substances
Join Marie in the Acids and Bases Simulation and explore the nature and concepts of these important chemical compounds. Measure the pH of chemical solutions, and use your acquired knowledge to evaluate mixtures of acids and base.

Advanced Acids and Bases
Join us in in the exploration of acids and bases and learn all the advanced terms used in chemistry.

Antibodies: Why are some blood types incompatible?
Learn about the concepts of antibodies and antigens, as well as the ABO and Rhesus blood grouping systems and their importance in blood transfusions. Then, you will help a young couple determine a potential risk for Rhesus disease in their unborn child.
.png?fm=jpg&w=450&h=400)
Aromatic Compound Nomenclature: Naming benzene’s derivatives
Join Dr.One in our chemistry lab to learn how to recognize aromaticity, master IUPAC and non-systematic nomenclature, and classify compounds as aromatic or non-aromatic. Do you have what it takes to become champion of the chemistry Olympics?

Atomic Structure (Principles): Atoms and isotopes
Learn about the atomic structure of the elements and investigate the properties of element samples from an exoplanet to assess whether life on it is a possibility. Find out what differentiates ions and isotopes of an element.

Atomic Structure (Principles): Bohr and quantum models
Explore the atomic model, absorption and emission spectra, and how they reveal information about stars in galaxies far far away.

Atomic Structure: Assess the possibility of life on other planets
Learn about the atomic structure of the elements and investigate the properties of element samples from an exoplanet to assess whether life on it is a possibility. Find out what differentiates an atom from an ion and define the isotopes of an element.

Azo Dye Test: Identify primary aromatic amines
Our lab recently relocated and some chemical labels were damaged during the move. Help us to reorganize our box of amines by performing the azo dye test. Use your results to determine if the unknown amine is a primary aromatic amine or not.

Basic Chemistry Thermodynamics: Solve the challenge of storing renewable energy
Learn the core concepts of thermodynamics and apply the technique of bomb calorimetry to help solve the challenge of storing renewable energy.

Benedict’s Test: Which food samples contain reducing sugars?
Learn how to perform Benedict’s test for reducing sugars on a variety of food samples. Predict which samples contain reducing sugars and find out how your predictions compare to your results!

Calorimetry: Using a bomb calorimeter
Apply the technique of bomb calorimetry to help solve the challenge of storing renewable energy. Learn about the first law of thermodynamics, enthalpy, and internal energy.

Carbohydrates: The sugars that feed us
The Carbohydrates Lab explores how carbohydrates are broken down by the digestive system and taken up into the bloodstream.

Carbon NMR: What is the mystery compound?
Help Yummy Food Inc survive the shut down of their food factory! Learn about the principles of the analytical technique carbon-13 NMR and interpret the spectrum of the mysterious unlabelled compound they have used in their food and drink.


Carbon Valence, Hybridization and Angles
Join Dr. One on a mission to figure out how the orbitals of carbon’s valence electrons hybridize, and how this greatly influences the bonds that carbon is able to form.

Ceric Ammonium Nitrate Test: Which compound contains alcohol?
Join Dr. Ali to discover how to use the ceric ammonium nitrate test to detect alcohols. Deduce which of our two samples contains alcohol groups by performing the test yourself!

Chemical Nomenclature: Learn the importance of inorganic compounds in life!
Become an alchemist for the day! Conjure up the names of inorganic compounds and learn their applications. Can you unlock all 16 transmute structures?

Chemistry Safety
Go on a mission to produce sustainable biodiesel and learn how to safely work with dangerous chemicals without any risk of getting hurt.

Chemistry Safety: Dispose of chemical waste
Join the lab assistant Marie in clearing up the fume hood after some students left a mess in the lab. Learn how to use your knowledge of the chemicals required for an experiment and apply it to safely dispose of the chemical waste.

Chemistry Safety: Hazard symbols
Join lab assistant Marie to find out how to decide on the correct laboratory practice and personal protective equipment when dealing with hazardous chemicals. Learn the meaning of the hazard symbols and apply your knowledge with some example chemicals.

Conductivity Testing: Investigating conductivity and its practical applications
Embark on this electrifying adventure of conductivity testing. Grasp the difference between electrolytes and nonelectrolytes, the connection between concentration and conductivity, and appreciate the significance of ions in biological systems.

Help a scientist detect and quantify proteins by using one of the most popular techniques in molecular biology.

Electrolysis
Experiment with electrolytic cells in a lab on Mars! Assemble different electrolytic cells so you can make hydrogen fuel for your Mars rover, and give mini Dr. One a new gold coating.

Electrophilic Addition: Explore reactions of hydrocarbons
Travel to Titan on a reactivity reconnaissance mission and explore this hydrocarbon world. Can you use your knowledge of the electrophilic addition reaction to identify hydrocarbons that you could use to build an extra-terrestrial colony on Titan?
.png?fm=jpg&w=450&h=400)
Electrophilic Aromatic Substitution: Mechanisms and resonances
Step into the virtual classroom to learn the secrets behind the electrophilic aromatic substitution. Explore the mechanism of this reaction, the effects of activating and deactivating groups, and the stability of the different resonance structures. Can you help create the perfect perfume?

Elimination Reaction: Use cyclohexanol to create polymers
Join Kim in the Polymer Research Lab to solve the puzzle of unexpected side product formation in their optimized cyclohexanol elimination reaction. Use and expand your organic chemistry knowledge to do this and get the polymer production back on track.
.png?fm=jpg&w=450&h=400)
Energy Surfaces and Spontaneous Reactions
Learn the core concepts of thermodynamics and discover how chemical reactions can be represented with energy surfaces. Determine which chemical reactions are spontaneous.

Environmental Impact of Coal Power Plants
Join the project manager Marie in her quest to uncover the environmental impacts of coal power plants and fish farming. Explore the issues with our current source of fuel and help Marie come up with a greener solution to save the environment.

Equilibrium
Learn about equlibrium and help a famous scientist to prevent a global famine by applying your knowledge to increase the yields of fertilizer for the crops.

Exploring a Distillation Apparatus
Get ready to dive deep into the process of distillation, by inspecting a simple benchtop distillation system and exploring all its parts on your own. Will you be able to relate the part of the distillation apparatus to their functions?

Fehling's Test: Which food samples contain reducing sugars?
Learn how to perform Fehling’s test for reducing sugars on a variety of food samples. Predict which samples contain reducing sugars and find out how your predictions compare to your results!
Flow Injection Analysis
Let’s awaken your curiosity for the chemistry of caffeine. Learn how Flow Injection Analysis (FIA) helps scientists measure the concentration of caffeine in your drinks.

Fractional Distillation: Separate a liquid mixture into its fractions
Learn how to separate a mixture of liquids into its pure components through fractional distillation. Partner with our lab assistant Dr. One to learn how to use a fractionating column and set up a successful distillation!

From Algae to Bioenergy
Join the project manager Marie in her goal to successfully produce biodiesel from algal oil. Be involved in a revolutionary scientific discovery that can eliminate the environmental impacts accompanied by burning coal for energy.

Functional Groups and Basic Chemical Tests
Join Dr. One on a mission to discover what is in the mysterious painkillers that your friend Simon has gotten hold of. You will identify which functional groups this chemical compound should contain and perform chemical tests to verify their existence.

Gel Electrophoresis: Visualize and separate nucleic acids
Solve a crime by using DNA fingerprinting to identify a thief. Use nucleic acid gel electrophoresis to separate and visualize DNA molecules and watch an animation to understand what happens inside the gel tank.

Learn how different factors such as heat and humidity can alter drug stability. Identify the components of the HPLC machine and use it to separate and measure the different compounds of a medicine.

Heating Curves and Phase Changes: Distil Ethanol
Learn how to generate and interpret the heating curves of ethanol and water. Discover how to relate heating curve data to the recorded observations of the substance on heating and determine the physical properties from the generated curve.

Hydrocarbon Nomenclature and Representations
Join Dr. One on a mission to figure out how to systematically name hydrocarbons and interpret and use the various core formula types for organic compounds.

Ideal Gas Law: Apply to save a life
Use the Gas Thermometry technique to validate the Ideal Gas Law. Define the absolute zero temperature by carrying out a gas thermometry experiment; handling extreme temperatures with the help of your assistant.

Ideal Gas Law: Build your own temperature scale
Use the Gas Thermometry technique to validate the Ideal Gas Law. Observe the behavior of an ideal gas and create your own temperature scale, while handling extreme temperatures with the help of your assistant.

Ideal Gas Law: Introduction
Observe the behavior of an ideal gas and the effects on this behavior when external variables are altered. Learn how the different variables affect the behavior and understand the relation of these variables to the ideal gas law equation.

Identification of an Organic Compound by Spectroscopy: How fast can you escape?
Take on the challenge of analyzing the spectra of four key analytical techniques — Mass Spectrometry, Infrared spectroscopy, Proton NMR and Carbon-NMR — to identify the structure of an unknown organic compound.
.JPG?fm=jpg&w=450&h=400)
Immunology: Immunoassay for detecting SARS-CoV-2 antibodies
Investigating antibody production patterns in populations helps us understand how diseases like COVID-19 spread. Conduct immunoassays to detect blood serum IgG and IgM to discover the vaccination and infection status of a community exposed to SARS-CoV-2.
.png?fm=jpg&w=450&h=400)
Infrared Spectroscopy
Dive into a virtual laboratory to discover the secrets of infrared spectroscopy. From the introduction of different vibrational modes to the interpretation of real-life spectra, mastering this technique will be child’s play.

Intermolecular Forces (Principles): Rediscover the forces to save the world!
What would happen to our world if intermolecular forces disappeared? Take on a new challenge with Dr. One to learn about intermolecular forces and save the day by putting the world back together.

Introduction to Food Macromolecules
Can you use your food macromolecule knowledge to convince your friend to change her diet to a healthier one?

Introduction to Groups of the Periodic Table
Help Dr. One get the periodic table ready in time! By directly observing the elements’ characteristics, and investigating trends in atomic properties, your mission is to figure out where a number of fallen out elements belong.

Introduction to Qualitative Analysis of Elements
Help Dr. One get the periodic table ready in time! By directly observing the elements’ characteristics and testing their flame color, your mission is to figure out where a number of fallen out elements belong in the periodic table.

Introduction to Radioactive Decay
A meteor has crashed to Earth! Search the crash site with a Geiger counter, and bring a radioactive sample back to the lab. Learn all about types of decay, decay series, and half-life. Help Dr. One and Marie Curie figure out what’s in that rock.

Ionic and Covalent Bonds
Join your friend on a quest to analyze two mysterious substances he got from an alchemist to cure his migraine and learn how atoms connect.

Kjeldahl Method: Estimate the protein content in food
Measure the protein content in a food sample with the Kjeldahl method, and investigate whether the apparent result has been tampered with by using LC-MS/MS.

Liquid-liquid Extraction: Extract caffeine from your everyday drinks
Learn how to use a separating funnel to extract and dry a water soluble compound, such as caffeine from tea or coffee. Can you also predict the liquid phases in which the compound can be found?

Litmus Test for Carboxylic Acids
Perform the litmus test on six organic compounds found in nature. Interpret the results to identify which organic compounds are carboxylic acids.
.png?fm=jpg&w=450&h=400)
Mass Spectrometry: The race of the fastest fragment
Dive into a virtual laboratory to discover the secrets of mass spectrometry. From the structure of the instrument to the interpretation of different spectra, no fragmentation pattern will remain a mystery to you!

Matter and Phase Changes: Distil ethanol
Rescue a town from a fuel crisis by using your knowledge about matter and phase changes principles and performing ethanol distillation.

Melting Point Analysis: Pure or impure?
Learn the techniques and application of melting point analysis and substance purity graphs. Explore the application of the technique in organic syntheses when determining the purity of a solid organic compound.

Nuclear Chemistry: Understand the processes happening in the atomic nucleus
Have you ever wondered what’s inside the atomic nucleus? Why are some elements radioactive? What is radioactivity? The Nuclear Chemistry simulation will teach you the answer to these questions, and many more!

Nuclear Magnetic Resonance (NMR): Analyze small protein samples
In the Nuclear Magnetic Resonance simulation, you will learn how to use NMR to characterize binding events between proteins and ligands. You will perform NMR experiments and interpret the resulting spectra. Will you be able to help improve an existing antibiotic in order to reduce its side effects?

Nucleophilic Addition: Explore the Grignard Reaction
Assist scientists on a drug discovery project and learn how to use nucleophilic addition to transform organic molecules into life-saving drugs! Will you be able to synthesize a new pharmaceutical product using the Grignard reaction?

Nucleophilic Substitution Reaction: Alkyl halides substrates
Solve a craft beer conundrum using your substitution smarts to design reactions for a unique range of flavor molecules! Will you help draw in a hip crowd with the new beverage flavors you create in the brew lab?

Organic Chemistry Introduction: Learn about organic compounds
Learn about the structure, nomenclature and a few reactions of organic compounds, and use this knowledge to help out your virtual friend Simon with some strange medicine!

Organic Chemistry Reactivity Rules: Time to react!
Determine the outcome of a chemical reaction between two organic compounds by mastering the fundamental rules of reactions in organic chemistry.

Periodic Table (Principles): Get the table organized in time
Help Dr. One get the periodic table ready in time! By directly observing the elements’ characteristics, testing their flame color, and investigating trends in atomic properties, your mission is to figure out where a number of fallen out elements belong.
Periodic Table of Elements: Get the table organized in time!

Phthalein-Dye Test for Phenols
Discover the rainbow inside phenolphthalein and how to unlock its colors through changing the pH. Take a deep dive to the molecular level to learn why chemicals are the color they are and put this all to use in the phthalein-dye test for phenols.

Polymerase Chain Reaction
Learn the techniques and application of Polymerase Chain Reaction and Gel Electrophoresis. Explore a real-world application, such as analyzing unique genetic fingerprints to solve a murder case.

Principles of Molecular Resonance: Electrons like to travel
Learn the theory behind one of the key phenomena in organic chemistry known as molecular resonance. Observe how the electrons in a molecule can delocalize in the structure and relate molecular resonance to systems with conjugated double bonds.

Protein Synthesis
Explore the structure of proteins and learn about the synthesis process inside the cells. Examine the protein sequence to understand the differences of protein synthesis in prokaryotes and eukaryotes.

Proton NMR: Spectra interpretation
Join Sam in the Labster lab where you will use proton NMR spectroscopy to elucidate the structure of an unknown organic compound. You will work with the fundamental concepts and functional groups needed to get started using this powerful technique.

Purification and Separation of a Mixture: Test your analytical chemistry skills
In this simulation, you will learn how to use a range of techniques to separate compounds from a mixture. Using their individual properties, develop and execute an experimental procedure to separate out each compound.

RNA Extraction: Sample and purify mRNA from pigs
Learn how to extract RNA from pig fat tissue samples and how to purify messenger RNA using magnetic beads.

Reaction Kinetics: The essentials
Join Dr. One at our extraterrestrial lab facility, where we’re struggling to get enough propulsion for our transporter. We need you to figure out the kinetics and optimize the chemical reaction of the fuel we’re using, so we can continue with our experiments.
.png?fm=jpg&w=450&h=400)
Reactions and Structure: Alkanes, alkyl halides, and organometallics
Host a barbecue with friends and learn about the structures and reactions of some major organic compounds: alkanes, alkyl halides, and organometallics.
.png?fm=jpg&w=450&h=400)
Reactions and Structure: Alkenes
From explosions to color changes, discover some of the amazing ways alkenes act as the starting point of countless reactions in organic chemistry. Learn about the structural characteristics and classification of alkenes as you observe how they transform major organic compounds.

Recrystallization: Dissolve your solid and precipitate your crystals
Slip on your thermal resistant gloves, and let's get started! In this simulation, you’ll discover how to purify a solid by using the recrystallization technique.

Recrystallization: Filter your crystals and measure the melting point
How can we separate liquids from solids? How can we determine the purity of that solid? In this simulation you will learn how to use the suction filtration and melting point techniques that will allow you to answer both questions.

Recrystallization: Purify your solid
Have you ever wondered how to remove impurities from a solid? How can you be sure you’ve removed them if you do? Step into our recrystallization lab to learn how to purify a solid and check for its purity level!

Redox Reactions: Discover how batteries work!
Build your own battery to power an electric car! Discover the chemical reactions that power batteries by finding oxidation numbers, balancing redox reactions, and experimenting with redox reactions in the lab.

Separating Mixtures: Using distillation to concentrate ethanol

Simple Distillation: Recycle waste from biodiesel production
Use the technique of simple distillation to help your colony on Mars purify the waste generated by the production of biodiesel from algae (water, glycerol, and methanol).

Solution Preparation: From salt to solution
Join your fantastic lab guide Dr. One in preparing a tricky aqueous solution of ammonium chloride using an analytical balance, which your colleagues need for an important analysis.
Spanish version - Chemical Nomenclature: Learn the importance of inorganic compounds in life!

States of Matter
Dive into water at the molecular level to learn about the behavior of the molecules in the three different states of matter: solid, liquid and gas. Learn about the bonding interactions between the molecules during different phase transitions.

Stereochemistry: from stereocenters to E/Z isomers
Dive into stereochemistry and discover how to rotate, break and rearrange molecules using interactive 3D models. Expand your knowledge on isomers and prioritization, strengthening your scientific skills before jumping into organic chemistry.

Stoichiometric Calculations: Identify a compound using gravimetric analysis
Learn about Avogadro’s number and the relationship between moles, mass and molecular weight as you use the technique of gravimetric analysis to identify an unknown compound.

Stoichiometry: Avogadro’s number and molecular calculations
Define and calculate key molecular parameters based on Avogadro’s Law and learn about the relationship between mass, molecular weight, and numbers of atoms or molecules!

Stoichiometry: Gravimetric Analysis
Perform gravimetric analysis to identify an unknown chemical in Labster laboratory!

Substitution vs. Elimination Reactions: Predict the outcome
Join Dr. One in the lab to investigate how several types of substitutions and eliminations are competing with each other in organic chemistry. Predict, mix and observe the outcome!

Synthesis of Aspirin: How to fight students’ migraines
Hone your synthetic skills in organic chemistry by going through a reaction that involves crystallization, filtration, calculation of the yield and a quality analysis using the melting point: the synthesis of aspirin.

Synthetic Polymers: Discover their impact on everyday life
Learn how monomers assemble to form synthetic polymers with different chemical and physical structures. Use the property of synthetic polymers to connect them with everyday applications and discover how they impact our environment.

The Bromine Test For Unsaturated Bonds: An essential in the chemist’s toolbelt!
Assist Chemical engineers in crisis as you help them use the correct chemicals in their reactions. Discover the reactions of bromine and unsaturated bonds and put this to use by conducting the bromine test.

Thin Layer Chromatography: Separate a mixture and monitor a reaction's progress
Discover the intermolecular interactions involved in Thin Layer Chromatography. Utilize this newfound knowledge to assemble, run, and analyze a TLC experiment to monitor the progress of a reaction.

Titration: Neutralize an acid lake contamination
Finding the concentration of an acid can be tedious and boring. Join a science expert to learn how to drop the base in style!

Tollen’s Test: Which food samples contain reducing sugars?
Learn how to perform Tollen’s test for reducing sugars on a variety of food samples. Predict which samples contain reducing sugars and find out how your predictions compare to your results!
Looks like there’s nothing here...
We couldn't find anything related to your search query. Check your search for any spelling errors or try a different search term.

Relevant Course Packages
Organic chemistry, biochemistry, intro chemistry (100), general, organic, and biological chemistry i, general chemistry (101 and 102), high school chemistry, basic lab skills, chemistry lab skills, chemistry for engineers, why labster.
Labster’s immersive virtual labs are proven to engage students in science, reduce dropout rates, decrease overhead, and improve learning outcomes.
Labster helps teachers increase their students’ knowledge and test scores.
Research shows that low-knowledge students improve the most after using Labster, with a 24% increase in their test scores.
Source: BMC Study
80% of students said Labster made them more likely to continue enrolling in STEM classes.
Over 50% of first-year STEM students in the United States either change their majors or fail to earn their degree.
Source: LXD Study
90% of students agreed that Labster provided opportunities for additional lab practice.
Undergraduate students typically require more time to complete lab experiments than provided.
Ready to Accelerate Learning?
Talk to an expert to discover if virtual labs are right for you.
University of South Florida
Department of Chemistry
College of Arts and Sciences
Main Navigation
Teaching labs, organic labs, faculty contact.
Solomon Weldegirma Instructor, Organic Chemistry Lab Coordinator BSF 316 813-974-6308
Description
This laboratory course (Organic I and II) conveys the important fundamental Organic Chemistry concepts and techniques, introduces state-of-the-art strategies and methods and provides a safer learning environment. The laboratory experiments highlight the important chemical concepts and techniques that are traditionally taught while introducing new green chemical concepts and recently developed experimental techniques. Students will apply the learned techniques to new problems with a focus on synthetic perspective using fundamental named organic reactions applied to the transformation of one material into another.
Students will be introduced with green organic experiments which will make the lab experience safer and produce less costly waste. The curriculum is designed to minimize production of chemical waste and provides a safer working environment (nearly eliminating the need for expensive fume hoods). At the end of the course, students are expected to know the fundamental techniques of organic chemistry including methods of isolation, purification, and structural identification with applications to synthetic and mechanistic problems. As the course will focus on fundamental reactions and techniques applicable to various fields of organic chemistry, students will be familiarized with the basic principles and techniques of organic chemistry.

Organic Teaching Laboratory (ISA)

Organic Teaching Laboratory (NES)


CHEM 207: Organic Chemistry
- Organic Chemistry Textbooks
- Properties & Spectra
- Interpreting Spectra
- Reagents & Safety
- Writing & Citing in Chemistry Lab
- Library Handout
Chemistry Librarian

Make an Appointment with Me
Do you want to meet with me in-person or online?
Carlson Video
@rivercampuslibs Welcome to ##URochester ’s Science & Engineering Library! ⚙️🧬 ##library ##universityofrochester ##rochesterny ##tour ##uofr ##librarychallenge ##librarylife ♬ Funny Thing - Thundercat
Textbooks at Carlson Library Desk (Reserve)
- Course Reserves Search To find books and documents reserved for your course: 1) Select the Course Reserve Radio Button. 2) Choose dropdown next to "Any field" and scroll down to select Course Instructor, Course ID, Course Name or Course Department Name. 3) Enter details about your course. (ex. Course Department contains Chemistry, Course Name contains Inorganic, Course Instructor contains Bren)
Organic Lab Textbooks
- JOVE Lab Manual Chemistry (Online Videos) Carefully produced videos that show how to perform laboratory techniques.
- MIT Digital Laboratory Techniques Manual (Online Videos) A series of videos designed to help you prepare for your chemistry laboratory class. Each video provides a detailed demonstration of a common laboratory technique, as well as helpful tips and information.
- Browse books with QD261 Call Number Ordered by age, with the newest first.
Organic Lecture Textbooks
Online and print.
- Next: Properties & Spectra >>
- Last Updated: Apr 19, 2024 9:55 AM
- URL: https://libguides.lib.rochester.edu/CHEM207

James M Tour Group
Rice university.
- Photos/Graphics
- Resume – Outline
- All Publications
- Book Reviews
- John West on Szostak Article
- Personal Statement
- Audio Files
- Evolution/Creation
- Law vs. Faith
JAMES M. TOUR, Ph.D.
T. T. and W. F. Chao Professor of Chemistry Professor of Computer Science Professor of Materials Science and NanoEngineering Rice University Smalley-Curl Institute and the NanoCarbon Center
Rice Advanced Materials Institute
Email: [email protected]
Web: https://www.jmtour.com
YouTube channel:Â https://www.youtube.com/drjamestour
Professor Tourâs weekly Bible studies are also available in real-time via Zoom. Â Login on Sundays at 10 AM Houston time (US Central Time):Â https://riceuniversity.zoom.us/j/94537001300?pwd=TjBQNm5sd1oxZGQyNU5WWVVadWNBQT09 . Or you could see the weekly videos at the YouTube channel:Â https://www.youtube.com/drjamestour
Dr. Tour will initiate a private Zoom call with anyone who is not a believer in Jesus but would like to hear his story about how he became a man with faith in Jesus, the Son of God. If interested, send Dr. Tour an email to make the request.
If you are a believer in Jesus and you wish to meet regularly, one on one via Zoom, to read and discuss the Bible, Dr. Tour can arrange for that through a team of Bible college students wishing to serve in that way. If interested, send Dr. Tour an email to make the request.
Dr. Tourâs Social Media Sites: www.drjamestour.com
ORCID: http://orcid.org/0000-0002-8479-9328
Google Scholar Page: https://scholar.google.com/citations?user=YwoecRMAAAAJ&hl=en
James M. Tour, a synthetic organic chemist, received his Bachelor of Science degree in chemistry from Syracuse University, his Ph.D. in synthetic organic and organometallic chemistry from Purdue University, and postdoctoral training in synthetic organic chemistry at the University of Wisconsin and Stanford University. After spending 11 years on the faculty of the Department of Chemistry and Biochemistry at the University of South Carolina, he joined the Center for Nanoscale Science and Technology at Rice University in 1999 where he is presently the T. T. and W. F. Chao Professor of Chemistry, Professor of Computer Science, and Professor of Materials Science and NanoEngineering. Tourâs scientific research areas include nanoelectronics, graphene electronics, silicon oxide electronics, carbon nanovectors for medical applications, green carbon research for enhanced oil recovery and environmentally friendly oil and gas extraction, graphene photovoltaics, carbon supercapacitors, lithium ion batteries, CO2 capture, water splitting to H2 and O2, water purification, carbon nanotube and graphene synthetic modifications, graphene oxide, carbon composites, hydrogen storage on nanoengineered carbon scaffolds, and synthesis of single-molecule nanomachines which includes molecular motors and nanocars. He has also developed strategies for retarding chemical terrorist attacks. For pre-college education, Tour developed the NanoKids concept for K-12 education in nanoscale science, and also Dance Dance Revolution and Guitar Hero science packages for elementary and middle school education: SciRave that later expanded to a Stemscopes-based SciRave . The SciRave program has risen to be the #1 most widely adopted program in Texas to complement science instruction, and it is currently used by over 450 school districts and 40,000 teachers with over 1 million student downloads.
Professor Tour has over 800 research publications, over 130 granted patents and over 100 pending patents. He has an h -index = 174 with total citations about 140,000. In 2024, he was elected to the National Academy of Engineering. In 2021, he won the Oesper Award from the American Chemical Society which is awarded to “outstanding chemists for lifetime significant accomplishments in the field of chemistry with long-lasting impact on the chemical sciences.” In 2020, he became a Fellow of the Royal Society of Chemistry and in the same year was awarded the Royal Society of Chemistry’s Centenary Prize for innovations in materials chemistry with applications in medicine and nanotechnology. Based on the impact of his published work, in 2019 Tour was ranked in the top 0.004% of the 7 million scientists who have published at least 5 papers in their careers. He was inducted into the National Academy of Inventors in 2015. Tour was named among âThe 50 Most Influential Scientists in the World Today” by TheBestSchools.org in 2019; listed in “The World’s Most Influential Scientific Minds” by Thomson Reuters ScienceWatch.com in 2014; and recipient of the Trotter Prize in “Information, Complexity and Inference” in 2014; and was the Lady Davis Visiting Professor, Hebrew University, June, 2014. Tour was named âScientist of the Yearâ by R&D Magazine , 2013. He was awarded the George R. Brown Award for Superior Teaching, 2012, Rice University; won the ACS Nano Lectureship Award from the American Chemical Society, 2012; was the Lady Davis Visiting Professor, Hebrew University, June, 2011 and was elected Fellow of the American Association for the Advancement of Science (AAAS), 2009. Tour was ranked one of the Top 10 chemists in the world over the past decade, by a Thomson Reuters citations per publication index survey, 2009; won the Distinguished Alumni Award, Purdue University, 2009 and the Houston Technology Centerâs Nanotechnology Award in 2009. He won the Feynman Prize in Experimental Nanotechnology in 2008, the NASA Space Act Award in 2008 for his development of carbon nanotube reinforced elastomers and the Arthur C. Cope Scholar Award from the American Chemical Society for his achievements in organic chemistry in 2007. Tour was the recipient of the George R. Brown Award for Superior Teaching in 2007. He also won the Small Times magazineâs Innovator of the Year Award in 2006, the Nanotech Briefs Nano 50 Innovator Award in 2006, the Alan Berman Research Publication Award, Department of the Navy in 2006, the Southern Chemist of the Year Award from the American Chemical Society in 2005 and The Honda Innovation Award for Nanocars in 2005. Tourâs paper on Nanocars was the most highly accessed journal article of all American Chemical Society articles in 2005, and it was listed by LiveScience as the second most influential paper in all of science in 2005. Tour has won several other national awards including the National Science Foundation Presidential Young Investigator Award in Polymer Chemistry and the Office of Naval Research Young Investigator Award in Polymer Chemistry.
Professor Tour is the founder and principal of NanoJtech Consultants, LLC , performing technology assessments for the prospective investor. Tourâs intellectual property has been the seed for the formation of several other companies including Weebit (silicon oxide electronic memory), Dotz  (graphene quantum), Zeta Energy (batteries), NeuroCords  (spinal cord repair), Xerient (treatment of pancreas cancer), LIGC Application Ltd.  (laser-induced graphene), Nanorobotics  (molecular nanomachines in medicine) Universal Matter  Ltd.  (US) and Universal Matter Inc.  (Canada) (flash graphene synthesis), Roswell Biotechnologies  (molecular electronic DNA sequencing) and Rust Patrol (corrosion inhibitors).
Professor Tour has served as a visiting scholar at Harvard University, on the Chemical Reviews Editorial Advisory Board, the Governorâs Mathematics and Science Advisory Board for South Carolina, the Defense Science Study Group through the Institute for Defense Analyses, the Defense Science Board Chem/Nano Study Section, the Department of Commerce Emerging Technology and Research Advisory Committee and the MD Anderson Cancer Research Centerâs Competitive Grant Renewal Board. He has been active in consulting on several national defense-related topics, in addition to numerous other professional committees and panels.
- Flash Graphene Video
- Nanomachines in Medicines
- Restoring Mobility After Severed Spinal Cord
- SDS via Sigma
- Tour Group: Safety at Rice
- Unzipped Nanotubes Graphics
- Positions/Openings
- synthetic organic chemist
- Uncategorized
- [+] Bloglines
- [+] Feedster
- [+] Google Reader
- [+] My Yahoo
- [+] Newsgator
- [+] Pluckit
Sign in | Register | Wp Plugins | WordPress
© James M Tour Group
- OpenBU
- College of Arts and Sciences
Organic Chemistry Laboratory Experiments
Issue Date Authors Titles Subjects
Search within this collection:
Welcome to the Organic Chemistry Laboratory Experiments repository at OpenBU. We hope that this collection will enable organic chemistry educators to share with other universities valuable experiments performed in the undergraduate teaching laboratory. All lab procedures are available to download and modify, and we encourage the submission of new experiments to the database.
To search the collection: Under the "Browse This Collection" menu on the right-hand side of your screen, you may view the available experiments by author name, title, and subject. Select the desired experiment to view the files available for download. Select either Download or View
To register for an account for uploading privileges: Under the "Deposit Materials" menu on the right-hand side of your screen, click "Login" and follow the instructions to create your new profile with "BU account". After approval, use the instructions outlined below to upload a new experiment.
To submit your own experiment: Under the "Deposit Materials" menu on the right-hand side of your screen, click "Submissions" to start your own submission. Follow the instructions for uploading your materials to the "Organic Chemistry Labs" collection. Be sure to provide the name of the author(s), title, and a brief abstract of your experiment. We ask that you submit your files in word-processing format so that your materials may be modified as others see fit.
Thank you for choosing Organic Chemistry Labs at OpenBU! For more information, contact [email protected]
Recently Added
Friedel-Crafts acylation
Passerini reaction , coumarin bromination , cyclization of citronellal , pechmann condensation .

Fisher Esterification: Synthesis of Volatile Esters

The asymmetric aldol reaction

Claisen-Schmidt (Aldol) reaction

Suzuki Cross Coupling

Wittig Reaction
Deposit materials, syndication feeds.
These feeds are customized for the level at which you are currently browsing OpenBU. Restricted items will not show up in feeds.

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

Organic Chemistry Labs
- Last updated
- Save as PDF
- Page ID 61300

T. T. and W. F. Chao Professor of Chemistry Professor of Materials Science & NanoEngineering
Department of Chemistry
O'Connor Building for Engineering and Science 211A | 713-348-6246 | [email protected]
WEBSITE(S) | Tour Group Rice | Google Scholar Citations
Research Summary
Tour’s scientific research areas include nanoelectronics, graphene electronics, silicon oxide electronics, carbon nanovectors for medical applications, green carbon research for enhanced oil recovery and environmentally friendly oil and gas extraction, graphene photovoltaics, carbon supercapacitors, lithium ion batteries, lithium metal batteries, CO2 capture, water splitting to H2 and O2, water purification, carbon nanotube and graphene synthetic modifications, graphene oxide, carbon composites, hydrogen storage on nanoengineered carbon scaffolds, and synthesis of single-molecule nanomachines which includes molecular motors and nanocars and nanomachines that can drill through cell membranes. He has also developed strategies for retarding chemical terrorist attacks. For pre-college education, Tour developed the NanoKids concept for K-12 education in nanoscale science, and also Dance Dance Revolution and Guitar Hero science packages for elementary and middle school education: SciRave ( www.scirave.org ) which later expanded to a Stemscopes-based SciRave. The SciRave program has risen to be the #1 most widely adopted program in Texas to complement science instruction, and it is currently used by over 450 school districts and 40,000 teachers with over 1 million student downloads.
James M. Tour, a synthetic organic chemist, received his Bachelor of Science degree in chemistry from Syracuse University, his Ph.D. in synthetic organic and organometallic chemistry from Purdue University, and postdoctoral training in synthetic organic chemistry at the University of Wisconsin and Stanford University. After spending 11 years on the faculty of the Department of Chemistry and Biochemistry at the University of South Carolina, he joined the Center for Nanoscale Science and Technology at Rice University in 1999 where he is presently the T. T. and W. F. Chao Professor of Chemistry, Professor of Computer Science, and Professor of Materials Science and NanoEngineering. Tour has about 650 research publications and over 200 patents, with an H-index = 129 and i10 index = 538 with total citations over 77,000 (Google Scholar). He was inducted into the National Academy of Inventors in 2015. Tour was named among “The 50 Most Influential Scientists in the World Today” by TheBestSchools.org in 2014; listed in “The World’s Most Influential Scientific Minds” by Thomson Reuters ScienceWatch.com in 2014; recipient of the Trotter Prize in “Information, Complexity and Inference” in 2014; and was the Lady Davis Visiting Professor, Hebrew University, June, 2014. Tour was named “Scientist of the Year” by R&D Magazine, 2013. He was awarded the George R. Brown Award for Superior Teaching, 2012, Rice University; won the ACS Nano Lectureship Award from the American Chemical Society, 2012; was the Lady Davis Visiting Professor, Hebrew University, June, 2011; and was elected Fellow of the American Association for the Advancement of Science (AAAS), 2009. Tour was ranked one of the Top 10 chemists in the world over the past decade, by a Thomson Reuters citations per publication index survey, 2009; won the Distinguished Alumni Award, Purdue University, 2009; and the Houston Technology Center’s Nanotechnology Award in 2009. He won the Feynman Prize in Experimental Nanotechnology in 2008, the NASA Space Act Award in 2008 for his development of carbon nanotube reinforced elastomers, and the Arthur C. Cope Scholar Award from the American Chemical Society for his achievements in organic chemistry in 2007. Tour was the recipient of the George R. Brown Award for Superior Teaching in 2007. He also won the Small Times magazine’s Innovator of the Year Award in 2006, the Nanotech Briefs Nano 50 Innovator Award in 2006, the Alan Berman Research Publication Award, Department of the Navy in 2006, the Southern Chemist of the Year Award from the American Chemical Society in 2005, and The Honda Innovation Award for Nanocars in 2005. Tour’s paper on Nanocars was the most highly accessed journal article of all American Chemical Society articles in 2005, and it was listed by LiveScience as the second most influential paper in all of science in 2005. Tour has won several other national awards including the National Science Foundation Presidential Young Investigator Award in Polymer Chemistry and the Office of Naval Research Young Investigator Award in Polymer Chemistry.
Research Areas
Organic Synthesis; Chemical Biology; Spectroscopy & Imaging; Nanomaterial Synthesis
Changes or additions to profiles.rice.edu will not take effect on the Rice sub-sites until after its next refresh which occurs at 5:15am, 10:15am, 1:15pm, 4:15pm and 7:15pm daily. (This does not affect profiles.rice.edu)

IMAGES
VIDEO
COMMENTS
A virtual tour of an organic chemistry lab with graduate student, Larry Mendoza of the Stockdill Lab at Wayne State University.
This channel focuses on providing tutorial videos on organic chemistry, general chemistry, physics, algebra, trigonometry, precalculus, and calculus. Disclaimer: Some of the links associated with ...
Lab Safety. Booking Information. Contact Information. South Dakota State University Home Page. South Dakota State UniversityBrookings, SD. Address Menu. Physical Location|. Mailing Addresses. Contact phone number for South Dakota State University: 1-605-688-4121© 2024 SDSU is governed by the Board of Regents of South Dakota.
Organic Chemistry is Awesome. Over 400+ blog posts to guide you through introductory Organic Chemistry, organized by subject. 00 General Chemistry Review. 01 Bonding, Structure, and Resonance. 02 Acid Base Reactions. 03 Alkanes and Nomenclature. 04 Conformations and Cycloalkanes. 05 A Primer On Organic Reactions. 06 Free Radical Reactions.
A student in the research group of Tom Pettus gives a tour of a room with instruments that are used to isolate and characterize organic compounds.
93154. Lisa Nichols. Butte College. In this resource you will find theory and procedures on the main organic lab techniques (chromatography, crystallization, extraction, distillation) as well as general concepts on how to set up and heat apparatuses. All procedures are accompanied by step-by-step pictures, and graphics are heavily utilized ...
25123. Organic chemistry studies the structure, properties and reactions of organic compounds, which contain carbon in covalent bonding. The study of structure determines their chemical composition and formula and the study of properties includes physical and chemical properties, and evaluation of chemical reactivity to understand their behavior.
This organic chemistry laboratory techniques manual is a beneficial resource for experimental work and the reinforcement of good practices. Content Accuracy rating: 5 The text and images provide an accurate representation of the experimental procedures. Techniques may vary at different institutions, and it may be possible to highlight ...
Wrap-up of techniques. In summary, here are the 6 organic chemistry lab techniques and their accompanying virtual labs: Chromatography: Thin Layer Chromatography: Separate a mixture and monitor a reaction's progress. Extraction: Liquid-liquid Extraction: Extract caffeine from your everyday drinks. Distillation: Simple Distillation: Recycle ...
This expansive and practical textbook contains organic chemistry experiments for teaching in the laboratory at the undergraduate level covering a range of functional group transformations and key organic reactions.The editorial team have collected contributions from around the world and standardized them for publication.
The Virtual Lab is an online simulation of a chemistry lab. It is designed to help students link chemical computations with authentic laboratory chemistry. The lab allows students to select from hundreds of standard reagents (aqueous) and manipulate them in a manner resembling a real lab. More information and offline downloads. Please scroll below to find our collection of pre-written problems ...
About Virtual Labs for Organic Chemistry. Bring the world of science into the classroom or enable students to bring learning home with Labster's virtual science lab content. No need for additional hardware or lab equipment; access these organic chemistry labs on any laptops, and spark creativity in students with this innovative and ...
Organic Labs Faculty Contact. Solomon Weldegirma Instructor, Organic Chemistry Lab Coordinator BSF 316 813-974-6308. Description. This laboratory course (Organic I and II) conveys the important fundamental Organic Chemistry concepts and techniques, introduces state-of-the-art strategies and methods and provides a safer learning environment.
Laboratory Experiments. Wet Lab Experiments. MIT Labs. Lab 4: Intro to Organic Chemistry. Expand/collapse global location.
ISBN: 9781133106524. Publication Date: 2012-01-23. (print) From biofuels, green chemistry, and nanotechnology, this proven laboratory textbook provides the up-to-date coverage students need in their coursework and future careers. The book's experiments, all designed to utilize microscale glassware and equipment, cover traditional organic ...
James M. Tour, a synthetic organic chemist, received his Bachelor of Science degree in chemistry from Syracuse University, his Ph.D. in synthetic organic and organometallic chemistry from Purdue University, and postdoctoral training in synthetic organic chemistry at the University of Wisconsin and Stanford University. After spending 11 years on ...
lab, so please treat the chemicals and others with the respect they deserve. Pre-Lab Resources. All of the lab backgrounds and protocols uploaded on the course webpage under the Lab tab.are The names of the pre-labs correspond with the names and the dates found on the schedule below. You must read these documents
Welcome to the Organic Chemistry Laboratory Experiments repository at OpenBU. We hope that this collection will enable organic chemistry educators to share with other universities valuable experiments performed in the undergraduate teaching laboratory. All lab procedures are available to download and modify, and we encourage the submission of ...
Organic chemistry is a highly experimental science which is in constant development, but its very basic techniques is still extremely important. We believe that this course may help you become a qualified laboratory newcomer and lead you into a more attractive chemical world.,Organic Chemistry Lab Ⅰ,中国药科大学.
Chemical Spills:If chemicals splashed to your eyes or face. immediately get to the eye wash station and flush your eyes with running water from an eyewash station for at least 15 minutes. For large chemical spills on the body. get under the safety shower and flush the affected area for at least 15 minutes.
The LibreTexts libraries are Powered by NICE CXone Expert and are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739.
He won the Feynman Prize in Experimental Nanotechnology in 2008, the NASA Space Act Award in 2008 for his development of carbon nanotube reinforced elastomers, and the Arthur C. Cope Scholar Award from the American Chemical Society for his achievements in organic chemistry in 2007. Tour was the recipient of the George R. Brown Award for ...
3-2-1 Kouto, Kamigori-cho, Ako-gun, Hyogo 678-1297, Japan (Harima campus) TEL: +81-791-58-0168 E-mail: Click here