ORTHOPAEDICS
Journey ◊ ii total knee arthroplasty.
Total knee arthroplasty patients report unmet levels of satisfaction, particularly for more active or demanding activities. 1,2 The JOURNEY II System is designed to help patients rediscover their normal through a smoother recovery, *3,4 improved function *4-8 and higher patient satisfaction *2,4-6

Normal shapes. Normal position. Normal motion
Reproduction of optimal kinematic patterns during TKA could be instrumental in improving patient satisfaction. 11 The solution to providing patients with better overall satisfaction and functionality is to design an implant as close to the normal knee as possible.
The JOURNEY II System has been shown to restore anatomical shape, position and motion. 9,10,12-14 This anatomical restoration can provide superior clinical outcomes that can lead to high patient satisfaction. *3-5,8,15
Product Features
Reference materials, medical education, related products.
* Compared to non-JOURNEY II knees.
This design rationale is for informational and educational purposes only. It is not intended to serve as medical advice. It is the responsibility of treating physicians to determine and utilise the appropriate products and techniques according to their own clinical judgment for each of their patients.
For detailed product information, including indications for use, contraindications, effects, precautions and warnings, please consult the product’s Instructions for Use (IFU) prior to use.
- Scott CEH, et al. J Bone Joint Surg Am. 2010;92-B(9):1253-1258.
- Noble P.C, et al. Clin Orthop Relat Res 2012;470(1):20-32
- Mayman DJ, et al. Poster presented at: ISPOR Symposium;19-23 May, 2018; Baltimore, Maryland, USA
- Nodzo SR, et al. Techniques in Orthopaedics. 2018;33(1):37-41.
- Murakami K, et al. Int Orthop. 2018;42(11):2573-2581.
- Di Benedetto P, et al. Acta Biomed 2019; Vol. 90, Supplement 12: 91-97.
- Kosse NM, et al. Poster presented at: 2nd World Arthroplasty Congress;19-21 April, 2018; Rome, Italy.
- Takubo A, et al. J Knee Surg. 2017;30(7):725-729.
- Grieco T, et al. J Arthroplasty. 2018;33(2):565-571.
- Smith LA, et al. J Arthroplasty. 2021;36:1445-1454.
- Van Onsem S, et al. Clin Orthop Relat Res (2020) 478:255-263.
- Iriuchishima T, et al. J Knee Surg. 2018;31(6):568-572.
- Murakami K, et al. J Orthop. 2018;15(2):650-654.
- Carpenter RD, et al. Knee. 2009;16(5):332-336.
- Noble PC, et al. Clin Orthop Relat Res. 2005(431):157-165.
- Kaneko T, et al. J Orthop. 2017;14(1):201-206.
- Brilhault J, et al. Knee. 2010;17(2):148-151.
- Catani F, et al. J Orthop Res. 2009;27(12):1569-1575.
- Hyodo K, et al. Arthroplasty Today. 2020;6(3):338-342.
- Hada M, et al. Knee Surg Sports Traumatol Arthrosc. 2018;26(6):1709-1716.
- Smith+Nephew 2012. Internal report. JRN2 KneeSim Analysis Memo.
- Robotic Assisted UKA
- Robotic Assisted TKA
- Robotic Assisted Revision Knee
- Computer Guided THA
- Data Visualization
- Partial Knee
- Primary Knee
- Revision Knee
- Oxinium™ in Knee
- VISIONAIRE™ Technology
- Primary Hip
- Revision Hip
- Oxinium™ in Hip
- PICO™
- JOURNEY JOURNEY II JOURNEY UNI
- LEGION LEGION TOTAL KNEE
- SURGEON TESTIMONIALS
JOURNEY™ II
Journey II Total Knee System
For orthopaedic surgeons seeking treatment solutions beyond traditional knee replacements, JOURNEY II Active Knee Solutions has been engineered to empower patients with a renewed right to an active lifestyle by breaking through traditional knee replacement barriers and delivering Function, Motion, and Durability through PHYSIOLOGICAL MATCHING
View our latest videos on JOURNEY II below and learn more about this product.
Live Surgery: Bicruciate Ligament Sparing TKA with JOURNEY™ II XR Featuring CORI™ Surgical System
Published: April 5, 2023
NAVIO™ 7 JOURNEY™ II TKA
Published: March 30, 2020
JOURNEY™ II BCS with the NAVIO Surgical System featuring the Bur All Technique
Published: April 11, 2019
NAVIO™ Robotic-Assisted TKA: Accuracy, Gap Balancing & Journey™ II for Optimum Performance!
Published: November 19, 2018
JOURNEY™ II Bi-Cruciate Stabilized (BCS) Knee featuring the Distal Bur Technique using the NAVIO Surgical System
Journey II Total Knee system
For orthopaedic surgeons seeking treatment solutions beyond traditional knee replacements, JOURNEY? II Active Knee Solutions has been engineered to empower patients with a renewed right to an active lifestyle by breaking through traditional knee replacement barriers and delivering Function, Motion, and Durability through PHYSIOLOGICAL MATCHING
JOURNEY II Total Knee
Latest Videos

Dr. Jimmy Chow demonstrates how to perform a JOURNEY™ II XR procedure using CORI™ Surgical System.
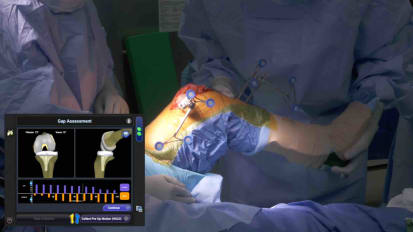
Orthopaedic Surgeon, James "Chip" Comadoll, MD, performs a total knee arthroplasty using a JOURNEY™ II and NAVIO™ 7 robotic assistance.
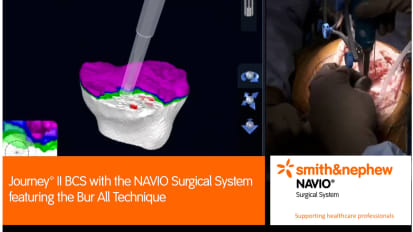
David Rovinsky, MD, performs a robotic-assisted JOURNEY™ II Bi-Cruciate Stabilized (BCS) knee surgery with NAVIO™ Surgical System featuring the bur all technique.

Smith & Nephew's Orthopaedic Surgeon David Rovinsky, MD discusses the benefits of NAVIO™ robotic-assisted TKA with the JOURNEY™ II for accuracy and gap-balancing during knee surgeries.
David Rovinsky, MD, performs a robotic-assisted JOURNEY™ II Bi-Cruciate Stabilized (BCS) knee surgery with NAVIO™ Surgical System featuring the distal bur technique.

Surgical Demonstration: JOURNEY™ II XR with NAVIO™ Bi-cruciate Retaining Robotic-Assisted TKA procedure featuring the NAVIO™ Surgical System
Dr. Jimmy Chow performs a total knee arthroplasty on a 52 year old competitive cyclist using the JOURNEY II XR knee with robotic-assistance from the NAVIO™ Surgical System

Live surgery from HSS featuring JOURNEY II BCS
Live surgery from HSS featuring JOURNEY II BCS - Performed by Dr. Steven Haas and moderated by Dr. David Mayman
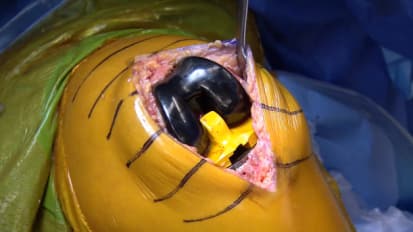
The JOURNEY II Bi-Cruciate Stabilized (BCS) Total Knee System
Steven Haas, MD - Operating Surgeon Dave Mayman, MD - Narrating Surgeon
Smith & Nephew improves the lives of their patients, whether through extending the life and functionality of implants, improving operating room efficiency, or promoting faster healing and other clinical outcomes.
Advanced Surgical Devices (Orthopaedic Reconstruction, Advanced Wound Management, Sports Medicine and Trauma)
Smith & Nephew improves the lives of their patients, whether through extending the life and functionality of implants, improving operating room efficiency, or promoting faster healing and other clinical outcomes.
The information on this website is intended for healthcare professionals only
- @smithNephewpic |
- Privacy & Cookies |
- Terms of Use
(586) 436-3785
PATIENT PORTAL

- JOURNEY II – TOTAL KNEE REPLACEMENT
Over the years, we have received feedback from our patients about how Movement Orthopedics has helped them. We are proud to share some of these patient testimonials below.
The JOURNEY II BCS Knee
Recent advances in biomedical engineering software have opened a new chapter on high performance knee implants.
One remarkable breakthrough has been the creation of the JOURNEY II BCS knee, a second-generation knee replacement that combines the stability and natural motion of the human knee with new low-friction materials that may extend the life of the implant.
While the lifespan of a knee implant is heavily influenced by the materials used to make it, the natural feeling of the implant during physical activity is dependent upon the way the patient’s muscles, ligaments and tendons are addressed during surgery and by the implant’s shape within the body after surgery.
As discussed previously in this booklet, the knee is a hinge joint, but it does not swing like a simple door hinge. It has a complex rotational motion that you don’t notice is there – but many patients know when it’s not there after total knee replacement. Traditional implants attempt to recreate this subtle swing-and-rotate action with either a rotating platform (a simple pivot point) within the implant or by requiring an angled alignment of the implant during surgery.
With these traditional knee replacement designs, the muscles and ligaments around your new joint have to work harder because the implant’s slightly unnatural shapes and resulting motion make these soft tissues move in unfamiliar, stressful ways. This leads to joint pain, muscle fatigue and the unnatural feeling patients experience while walking or bending in the months after their procedure.
The JOURNEY II BCS knee, on the other hand, is designed to reproduce the original internal shapes and angled forces of the human knee through its full range of motion – accommodating the swing-and-rotate of the joint with the same engineering principles your real knee currently uses. Because of this, your soft tissues don’t have to readjust to new shapes and forces after surgery and your stride can return to its natural rhythm.
The JOURNEY II BCS knee also reproduces the stability provided by your anterior cruciate ligament (ACL) and your posterior cruciate ligament (PCL). Your ACL and PCL are key to the stability of your real joint and contribute to natural motion when your knee is fully extended and fully bent. No other knee implant reproduces both functions.
Implant Components
In the knee replacement procedure, each prosthesis is made up of four parts.
The tibial component has two elements – a metal base and a plastic insert – and replaces and the top of the tibia (shin bone). This prosthesis is made up of a metal tray attached directly to the bone and a high-density plastic spacer that provides the bearing surface.
The femoral component replaces the bottom of the thigh bone or femur. This component also replaces the groove where the patella or kneecap rides.
The patellar component replaces the surface of the knee cap, which rubs against the femur. The patella protects the joint, and the resurfaced patellar button will slide smoothly on the front of the joint. In some cases, surgeons do not resurface the patella.

Bearing Surfaces
One of the keys to a successful implant is its ability to withstand the rigors of daily activity, and central to that is the quality of the artificial surfaces that slide against each other, or articulate, in the new joint.
In knee implants, bearing surface options have been somewhat limited over the last few decades. The standard substance used for the femoral component is cobalt chrome, a metal alloy typified by its toughness and biocompatibility. However,even this high-quality industry standard has its shortcomings. Over time, this metal surface can become roughened by bone and bone cement particles trapped between the femoral component and the plastic tibial insert.
This roughened surface, when rubbing against the plastic component up to two million times per year, can more quickly wear out your implant. When that happens, you will have to undergo surgery to replace the plastic piece, the femoral component, and possibly even the tibial component. For this reason, implants have been shown to last between ten and fifteen years in the human body.
An exciting material to enter orthopaedics in recent years is OXINIUM ◊ Oxidized Zirconium. This remarkable material combines the strengths of ceramic and metal, such as wear-reduction and strength, but does not have the weaknesses, such as limited implant options and the possibility of fracture.
Zirconium is a biocompatible metal, similar to titanium. When the zirconium alloy undergoes a unique heating process, the surface of the metal transforms into a ceramic. Even though the new ceramic surface is 4,900 times more abrasion resistant than cobalt chrome, it retains the toughness and flexibility of the underlying metal.
Because it can achieve this remarkable reduction in implant wear without sacrificing strength as actual ceramic components do, oxidized zirconium implants have the potential to last significantly longer.
The Procedure
Knee replacement surgery typically takes between one and two hours to complete. This section will provide you with a brief, easy-to-understand description of the surgical procedure. (Please consult with your physician for details regarding your specific procedure.)
- An incision is made extending from the thigh, past the inside edge of the kneecap, and down to the shinbone.
- The end of the femur is shaped in preparation for sizing the femoral trial component.
- The top of the tibia is shaped for proper sizing of the tibial trial component.
- The trial units are put in place and the appropriate implant size is selected.
- The knee is assessed for alignment, stability, and range of motion.
- The underside of the kneecap is prepared and patella trial is selected.
- The trial units are removed and the final femoral, tibial, and patella components are implanted.
- The incision is closed, a drain is put in, and the post-operative bandaging is applied.

Bone Cuts

Implant Components

Implanted
All information provided on this website is for information purposes only. Every patient’s case is unique and each patient should follow his or her doctor’s specific instructions. Please discuss nutrition, medication and treatment options with your doctor to make sure you are getting the proper care for your particular situation. If you are seeking this information in an emergency situation, please call 911 and seek emergency help.
All materials copyright © 2020 Smith & Nephew, All Rights Reserved.

Sign up our newsletter to get update information, news or article about medical.

At Movement Orthopedics, we offer state-of-the-art treatment and urgent care for all your orthopedic needs.
- MEET THE PROVIDERS
- SPECIALTIES
- ONSITE SERVICES
- ORTHOPEDIC URGENT CARE
Bitcoin Payment
- PHYSICAL THERAPY
- CONTACT & LOCATION
- REQUEST AN APPOINTMENT
- PATIENT FORMS
Contact Info
- 43475 Dalcoma Drive Suite 250 | Clinton Township, MI 48038
- +(586) 436-3785
- +(586) 273-0109
- 36555 26 Mile Road Suite #2400 Lenox MI 48048
- Privacy Policy
- Powered by Smash Creative

Aren't all total knee implants the same?

In theory, yes, all total knee implants are intended for the same purpose: the elimination of painful bone-on-bone contact, and the restoration of motion and function to the joint. In reality, however, the way each implant is designed, built and implanted not only makes them different, but can have an impact on how well they perform for each patient.
How your knees move
Commonly described as a hinge joint, your knees actually do much more than simply swing back and forth. In fact, every time your knee bends, forces in and around the joint work together to produce a subtle and complex rotational movement that you don't even realize is there. However, if this rotational movement is removed, the change can be felt in the muscles and ligaments through the entire leg.
The JOURNEY ◊ II AKS difference

One of the most remarkable breakthroughs in design of total knee replacements has been the creation of the JOURNEY II Active Knee Solutions. Designed using the latest in human simulation software, and built using some of the most wear-resistant materials available, this unique implant was designed to address two of the most common concerns associated with knee replacement implants: implant wear and implant feel.
The information listed on this site is for informational and educational purposes and is not meant as medical advice. Every patient's case is unique and each patient should follow his or her doctor's specific instructions. Please discuss nutrition, medication and treatment options with your doctor to make sure you are getting the proper care for your particular situation.
All information provided on this website is for information purposes only. Every patient's case is unique and each patient should follow his or her doctor's specific instructions. Please discuss nutrition, medication and treatment options with your doctor to make sure you are getting the proper care for your particular situation. If you are seeking this information in an emergency situation, please call 911 and seek emergency help.
All materials copyright © 2020 Smith & Nephew, All Rights Reserved.

Our staff is committed to providing the finest podiatric care in a warm and friendly environment in order to make you feel relaxed and comfortable.
Call us at: (602) 610-2941
George Gendy M.D.
Ankle, Knee, Hip, Orthopedic Surgery
Copyright © 2021 | Site Map

An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List

CAPAbility: comparison of the JOURNEY II Bi-Cruciate Stabilised and GENESIS II total knee arthroplasty in performance and functional ability: protocol of a randomised controlled trial
Celia clarke.
1 School of Health Sciences, University of East Anglia, Norwich, UK
Valerie Pomeroy
Allan clark.
2 Norwich Medical School, University of East Anglia, Norwich, UK
Graham Creelman
3 Patient and Public Involvement, Norwich, UK
Nicola Hancock
Simon horton, anne killett, charles mann.
4 Department of Trauma and Orthopaedics, Norfolk and Norwich University Hospital, Norwich, UK
Estelle Payerne
5 Norwich Clinical Trials Unit, UEA, Norwich, UK
Andoni Toms
6 Norwich Radiology Academy, Norfolk and Norwich University Hospital, Norwich, UK
Gareth Roberts
7 Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Sciences, University of Oxford, Oxford, UK
Ann Marie Swart
Iain mcnamara, associated data.
Public access to the full trial protocol, trial-related documents, participant-level dataset and statistical code may be made on request to the TMG.
Osteoarthritis of the knee is a common condition that is expected to rise in the next two decades leading to an associated increase in total knee replacement (TKR) surgery. Although there is little debate regarding the safety and efficacy of modern TKR, up to 20% of patients report poor functional outcomes following surgery. This study will investigate the functional outcome of two TKRs; the JOURNEY II Bi-Cruciate Stabilised knee arthroplasty, a newer knee prosthesis designed to provide guided motion and improve knee kinematics by more closely approximating a normal knee, and the GENESIS II, a proven existing design.
To compare the change in Patient-reported Outcome Measures (PROMs) scores of the JOURNEY II BCS and the GENESIS II from pre-operation to 6 months post operation.
CAPAbility is a pragmatic, blinded, two-arm parallel, randomised controlled trial recruiting patients with primary osteoarthritis due to have unilateral TKR surgery across two UK hospitals. Eligible participants ( n = 80) will be randomly allocated to receive either the JOURNEY II or the GENESIS II BCS knee prosthesis. Baseline measures will be taken prior to surgery. Patients will be followed at 1 week, 6 to 8 weeks and 6 months post-operatively. The primary outcome is the Oxford Knee Score (OKS) at 6 months post-operatively. Secondary outcomes include: other PROMs, biomechanical, radiological (computerised tomography, (CT)), clinical efficacy and safety outcomes. An embedded qualitative study will also investigate patients’ perspectives via interview pre and post surgery on variables known to affect the outcome of TKR surgery. A sub-sample ( n = 30) will have additional in-depth interviews to explore the themes identified. The surgeons’ perspectives on the operation will be investigated by a group interview after all participants have undergone surgery.
This trial will evaluate two generations of TKR using PROMS, kinematic and radiological analyses and qualitative outcomes from the patient perspective.
Trial registration
International Standard Randomised Controlled Trials Number Registration, ID: ISRCTN32315753 . Registered on 12 December 2017.
Introduction
Background and rationale.
Osteoarthritis of the knee is a common musculoskeletal condition. The surgical management of painful, end-stage osteoarthritis is by total knee replacement (TKR) which should be considered before there is prolonged and established functional limitation and severe pain [ 1 ]. Over 100,000 TKRs were performed in the UK in 2019 [ 2 ]. While TKR frequently reduces pain and improves physical function in the majority of patients, 20% of patients report poor functional outcomes post-operatively [ 3 , 4 ]. Such poor outcomes are of importance to patients and have a considerable financial and service-provision impact on NHS care. Research is needed to improve post-arthroplasty outcomes for those patients.
There is a paucity of literature regarding the kinematic outcomes of patients following TKR. However, there is uncertainty as to whether good Patient-reported Outcome Measures (PROMs) are associated with a return to normal kinematics of the TKR knee compared to the native knee. Movement analysis can be used to examine the change in kinematics before and after TKR by examining functional movements in activities of daily living.
The long-term success of TKR depends largely on correct component alignment and accurate ligamentous balancing [ 5 ]. The impact of femoral- and tibial-component rotation on flexion-gap balance, patellofemoral tracking and normal kinematic function is well-known [ 6 – 8 ]. Complications secondary to poor component alignment have been reported to lead to a higher rate of revision surgery [ 9 , 10 ]. Computerised tomography (CT) imaging is a valid and reproducible technique for accurately measuring TKR-component rotation [ 11 , 12 ]. However, despite CT being widely used to examine implant rotation, the correlation between rotational alignment, PROMs and kinematic function comparing pre- and post-operative measurement is unclear [ 13 , 14 ]. It is hypothesised that patients with poor rotational profile post-operatively compared to their pre-operative values will have significantly worse PROMs, movement parameters and patient satisfaction.
We report the protocol of a two-group, parallel randomised controlled trial (RCT) comparing patient-reported, surgical and biomechanical outcomes from a TKR of a newer design (the JOURNEY II Bi-Cruciate Stabilised knee arthroplasty (BCS)) designed to provide improved kinematic outcomes compared to an older design TKR implant (the GENESIS II).
This protocol (version 2.4, dated 27 February 2019) has been written and reported according to the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) guidance and Checklist [ 15 ] (see Additional file 1 : SPIRIT 2013 Checklist).
The principal aim of the trial is to compare the change in PROMs scores of the JOURNEY II BCS knee and the GENESIS II knee from pre-operation to 6 months post operation. Additional aims are as follows:
- To determine whether the temporal and spatial parameters of gait, the range of movement and static and dynamic balance are closer to aged-matched normative data in those receiving the JOURNEY II BCS compared to those receiving the GENESIS II knee
- To monitor the change in function (Aim 1 above) and PROMs of the JOURNEY II BCS and the GENESIS II knee from post operation to 6 months post operation
- From CT scan measures, determine anatomical landmarks and rotational profile around the native knee and following TKR to ascertain the component rotational position post-operatively compared to anatomical landmarks
- To examine the relationship between rotational values determined by CT scanning with pre- and post-operative PROMs and movement analysis
- To develop knowledge and understanding of patient and surgeon experiences, perspectives and satisfaction when receiving or implanting the JOURNEY II BCS compared with the GENESIS II knee, and their experiences of recovery and rehabilitation
Methods: participants, interventions and outcomes
Trial design.
This is a pragmatic, triple-blinded, parallel, superiority, randomised controlled trial of the JOURNEY II BCS (intervention) versus GENESIS II (control) in patients with primary osteoarthritis undergoing TKR. Embedded in the clinical trial is a qualitative investigation of participants’ confidence in the TKR received and their experiences of the recovery process in the first 6 months after surgery. The aim of this is to identify any differences in the experience of recovery between each type of TKR. Surgeons will also be interviewed to investigate their perceptions of the surgery and patient’s rehabilitation.
The trial outline is illustrated in Fig. 1 .

Comparison of the JOURNEY II Bi-Cruciate Stabilised and GENESIS II total knee arthroplasty in performance and functional ability (CAPAbility) trial outline
Study setting
Trial sites were pre-selected on the basis of their locality to facilitate data collection (namely the kinematic assessment). Sites include the Norfolk and Norwich University Hospital (NNUH), where all patients recruited to the trial will be referred for consideration of TKR. The NNUH refers a proportion of its TKR patients to Spire Norwich where the operation and follow-up physiotherapy is delivered. Both hospital are participating in this trial. All CT scans will be performed at NNUH. The biomechanical assessment will be undertaken in a specialist movement analysis laboratory (MoveExLab) at the University of East Anglia (UEA).
Eligibility criteria
To be eligible for the trial, patients must satisfy the surgeon’s general requirements for a TKR, meet all inclusion criteria and none of the exclusion criteria listed in Table 1 .
Patients will be excluded if they are currently enrolled on an interventional trial involving surgery, exercise or rehabilitation. Patients can be co-enrolled into studies given prior agreement from the Trial Management Group (TMG) of both studies. Patients who enter the study are eligible for entry onto the UK National Joint Registry.
Potential participants will be approached via a single route. Potential participants will be screened by a member of the clinical team in collaboration with research nurses after having been added to the orthopaedic clinic waiting list. Potentially eligible patients who meet the eligibility criteria will either be handed a Patient Information Sheet (PIS) if still at the clinic, or be posted an invitation letter informing them that the trial is taking place and include the PIS. After having been provided the trial PIS, potential participants will be telephoned by a research nurse. To minimise the possibility of attrition, appointments for outcome measures will be agreed with participants when they enter the trial. In addition, members of the research team will maintain regular contact with participants to ensure attendance at follow-up visits and to monitor any adverse events (AEs).
Informed consent
Written informed consent to enter and be randomised into the trial will be taken by a member of the clinical team and obtained from participants after explanation of the aims, methods, benefits and potential hazards of the trial. Potential participants will be given as much time as they need to consider whether or not to provide informed consent. Consent will take place before any trial-related measures, at a time convenient to the potential participant, preferably at a time to combine with one or more of the measures to reduce participant visits.
If a participant withdraws prior to surgery, an additional participant will be randomised to ensure that 80 participants complete the surgery.
Patients who, in the opinion of the clinical team, do not have capacity to consent, will be ineligible. If a participant loses capacity during the course of the trial, they will be withdrawn from any further assessments, but any data already collected will be retained. Consent will be re-sought if new information becomes available that affects the participant’s consent in any way. This will be documented in a revision to the PIS and the participant will be asked to sign an updated consent form. These will be approved by the Ethics Committee prior to their use. A copy of the approved consent form is available from the Norwich Clinical Trials Unit (NCTU).
Sample size
Eighty patients will be recruited onto this superiority trial. The sample size has been calculated from the Oxford Knee Score (OKS) [ 16 ]. The OKS ranges from a score of 12 to 60, with 12 being the best outcome. The minimally important clinical difference for OKS is 5 [ 17 , 18 ] and a standard deviation of 7.4 [ 19 ]. For an 80% power, and an assumed dropout rate of 10%, 80 participants will be randomised to one of the two groups.
Participant timeline
The participant timeline is shown in Fig. Fig.1. 1 . Where possible, trial visits will be combined with standard clinic visits. Should additional visits be necessary, participants will be reimbursed for travel costs.
Interventions
All participants will receive routine care provided by the NHS. Pre-operative and peri-operative care is standardised irrespective of implant.
Explanation for choice of comparators (Genesis II versus JOURNEY II BCS)
The GENESIS II TKR system made by Smith and Nephew (Smith and Nephew plc, Watford, UK) is frequently used in standard practice within the NHS [ 2 ]. It has been the standard TKR within the NNUH and Spire Norwich Hospitals for over 10 years. The Genesis II has a survivorship of over 93% of implants at 15 years [ 2 , 20 ] and offers good health-related quality of life outcomes [ 21 ].
A newer device, JOURNEY II BCS, also manufactured by Smith and Nephew, has been developed to theoretically provide improved kinematic outcomes compared to the GENESIS II [ 22 ]. These improvements are proposed to include:
- Alteration in the dimensions of the femoral component to reduce soft-tissue strain and maintain more natural translation and external rotation
- Reduction in the thickness of the lateral and medial anterior flange of the femoral component and edge tapering to reducing tension on the iliotibial band (ITB) and iliotibial-patellar bands (ITPB)
- Reduction in the width of the femoral component to limit implant overhang, and reduction in the mid-flexion thickness of the medial condyle to maintain more consistent strain on the medial collateral ligament (MCL) throughout the flexion range
- A superior cam position, which serves to decrease femoral rollback in the targeted ranges of motion, increase femoral external rotation, and lower the point of tibial post contact in deep flexion
While there is fluoroscopic data to support normal kinematics in early and late flexion [ 23 ], there is a paucity of evidence exploring these hypotheses for this newer implant.
Surgical flow and training
Surgeons will be high-volume arthroplasty surgeons who work at both NNUH and Spire Norwich Hospital. The standard implant at both sites is the Genesis II TKR system. All surgeons have used this implant for many years and are very familiar with the surgical technique. All surgeons and theatre staff have received training on the implantation of the JOURNEY II BCS implant. All surgeons have undergone training on the JOURNEY BCS II implant in a cadaveric laboratory and have also undertaken a learning curve with the device until they were confident with the technique. This was supported by a Smith and Nephew representative. There are minimal differences in the surgical cuts and technique between the Genesis II and the JOURNEY BCS II. Participating surgeons felt that there was a shallow learning curve to the JOURNEY BCS II. Both devices are CE-marked and will be used within indication. Smith and Nephew are providing the JOURNEY II BCS at the same price as the GENESIS II system for this study.
Surgical procedures
Devices will be identified and prepared for the operation by a surgical technician at the surgery site.
Participants allocated to the intervention device will receive the JOURNEY II BCS prosthesis while participants allocated to the control condition will receive the GENESIS II prosthesis. The type of device implanted and its serial number will be recorded on the trial database, by an unmasked member of the research team.
The surgical procedure will follow the standardised surgical approach and technique. It will be undertaken through a medial parapatellar approach. In both implants and in every case to ensure standardisation of technique, a posterior stabilised prosthesis with patella resurfacing will be used.
It is possible that a decision will be taken prior to, or during, the operation not to use the allocated device if, in the opinion of the surgeon, the patient is found to have become unsuitable for continued participation in the trial. The reasons for an allocated device not being used will be recorded on the trial database. In this case or if a participant chooses to withdraw consent for treatment, or follow-up, all data collected up to the point of withdrawal will be retained. The standard Norwich Enhanced Recovery Programme (NERP) [ 24 , 25 ] is used for the anaesthetic technique and post-operative recovery.
Post-operative rehabilitation
Post-operative rehabilitation will follow routine clinical care at NNUH and Spire Norwich [ 24 , 25 ]. While an inpatient, participants will be seen by a physiotherapist for routine care at least twice daily to progress on a tailored gait re-education and exercise programme during their hospital admission. This will be recorded in an in-patient hospital rehabilitation log. Once safe for discharge, patients will be asked to continue a home exercise programme and gait re-education. This will consist of daily (advised) knee-flexion range-of-motion exercises and quadriceps strengthening.
At week 4 post-operatively, all participants will attend an exercise-group-based intervention delivered by a qualified physiotherapist and a physiotherapy assistant. These sessions will be used to increase participant’s knee range of motion, strength and overall confidence to undertake more strenuous exercises. Participants will attend this class weekly for two to six sessions depending on their need. All rehabilitation interventions will be recorded in a post-discharge rehabilitation log. Participants will be encouraged to continue their exercises which are prescribed within the group as part of a home-exercise programme.
No additional ancillary or post-trial care will be provided (in the absence of AEs) to trial participants.
The schedule of enrolment, interventions and assessment is shown in Table 2 . The PROMs will be administered by research nurses apart from the week-1 follow-up telephone call undertaken by the research associate performing the qualitative interview. The CT scans will be performed at the NNUH by research radiographers and reported by a consultant radiologist. The biomechanical assessments and qualitative interviews will be performed at the MoveExLab at the UEA. Participants who were unable to attend an assessment appointments were provided with an alternative appointment. If participants were unable to attend any alternative assessment appointments, PROMs data was collected during a telephone call to promotion participant retention and follow-up.
Schedule of enrolment, interventions and assessments

*subset of 30 patient; CT computerised tomography, HADS Hospital Anxiety and Depression Score, MVIC maximum voluntary isometric contraction, OKS Oxford Knee Score, OKS-APQ Oxford Knee Score Activity and Participation Questionnaire, Pre-Op pre-operative, Post-Op post-operative, ROM range of motion, UCLA University of California Los Angeles
Primary outcome
The OKS [ 16 ] will be used to assess patient-reported functional status at 6 months post surgery.
Secondary outcomes: Patient-Reported Outcome Measures
The Oxford Knee Score (OKS) [ 16 ] – Activity and Participation Questionnaire (OKS-APQ), [ 26 ] EuroQol 5 dimensions, 5 levels health survey (EQ-5D-5 L) [ 27 ], UCLA Activity Score [ 28 ], Hospital Anxiety and Depression Score (HADS) [ 29 ], Forgotten Joint Score (FJS) [ 30 ], and 2-Item Pain Self-Efficacy Questionnaire [ 31 ].
Secondary outcomes: clinical efficacy outcomes
Clinical efficacy will be evaluated by:
- Surgical-related parameters: need for revision surgery; length of hospital stay and change in pain medication will be collected during in-patient stay and at all the follow-up time points
- Performance-related parameters: knee flexion and extension ranges of movement, measured at 6 to 8 weeks and 6 months post-operatively by the research associate in the MoveExLab (and by the research physiotherapist at baseline as part of routine care); timed-up-and-go (TUG) [ 32 ] and timed 6-minute walk test [ 33 ] recorded at the 6–8 week and 6-month time points by the research associate in the MoveExLab
Secondary outcomes: clinical safety outcomes
Complications related to the surgery (e.g. anaesthesia-related problems, bleeding, morbidities) will be collected from a notes review, prior to discharge, post-discharge, rehabilitation and follow-up. Additionally, at each visit, participants will be asked whether they have received additional treatment since their surgery/previous visit and what that consisted of.
Secondary outcomes: biomechanical outcomes
All biomechanical measures will be collected in the MoveExLab by the research associate. Three-dimensional motion capture using eight cameras (Vicon Motion System, Oxford, UK), three built in force plates (Bertec Corporation, Columbus, OH, USA) and surface electromyography (EMG) (Delsys, Natick, MA, USA). Participants will be unshod and asked to walk at their self-selected speed. A minimum of three heel strikes from each foot will be used to construct an average.
- Spatiotemporal parameters; speed, cadence, step-length, stride-length and symmetry
- Kinematics of bilateral hip, knee and ankle joints
- Kinetics: moments of bilateral hip, knee and ankle joints and ground reaction forces during the stance phase
- EMG parameters: recruitment patterns of quadriceps: rectus femoris, vastus medialis and vastus lateralis, hamstrings: semitendinosus, biceps femoris, tibialis anterior, medial and lateral gastrocnemius
- Spatiotemporal parameters; speed, cadence, symmetry
- Kinetics: moments of bilateral hip, knee and ankle joints and ground reaction forces from the bottom step
Static balance measures will be completed on a single, in-built force plate (Bertec Corporation, Columbus, OH, USA). Participants will be instructed to stand with their feet shoulder-width apart for double stance with their eyes closed and then open for 10 s. Three attempts will be recorded. Participants will then be instructed to stand on one leg in the centre of the force plate with their hands on their hips with their eyes open and closed for 10 s. Each limb will be tested. Three trials of 10 s will be recorded. The time will be stopped if the participant places the other foot on the floor. Each participant will be given six attempts at each position.
- Anterior-posterior (AP), medial-lateral (ML) and CoP path length
- AP, ML and CoP velocity
- AP, ML and CoP range and standard deviation (SD)
- TTB minimum, mean and SD
- Anterior, posteromedial and posterolateral distance (millimetres) on both limbs
Secondary outcomes: radiological outcomes
Radiographs.
Pre-operative and post-operative conventional semi-flexed AP and lateral radiographs of the knee will be acquired.
Computerised tomography
A rotational-profile CT protocol will be acquired at the NNUH Radiology Department under standard operating procedure.
This will consist of three separate axial acquisitions through the femoral necks, knees and ankles reconstructed on bone and soft-tissue algorithms. The images through the knee will be split into two acquisitions according to the Berger protocol [ 36 ]. The pre-operative CT will be performed in the time after consent for the study and before TKR. The post-operative CT is not time sensitive and will be performed any time following surgery.
Two independent observers, radiologists under direct supervision of a senior musculoskeletal radiologist, will obtain the following measurements from the CT. In the case of disagreement between the two independent observers, through discussion, the senior musculoskeletal radiologist will act as adjudicator to ensure that agreement is met. Measurements will include:
Pre-operative
- Femoral ante-torsion (degrees)
- Tibial tubercle-trochlear groove distance (TT-TG) (millimetres)
- Tibial torsion (degrees)
Post-operative
- Femoral-component version (degrees)
- Tibial-component version (degrees)
In the event of an incidental finding being reported, the clinical chief investigator will organise the necessary clinical follow-up which may include referral to an appropriate clinician and the organisation of further investigations.
Secondary outcomes: qualitative study
Interviews will be completed either via a telephone call or face-to-face by the research associate. This flexibility was adopted to promote participant retention and complete follow-up. These interviews will be audio-recorded and transcribed for analysis.
All TKR participants will be invited to take part in an interview and complete a self-efficacy questionnaire and the HADS at baseline and a telephone call interview at the 7 days (± 2 days) surgery.
Two additional post-surgery interviews will be carried out with a purposive sample of participants ( n = 30), drawn equally from the intervention and control groups. Sampling decisions will be based on the following factors: age; sex; ethnicity; socioeconomic status; OKS; self-efficacy; expectations, mood and symptom management (as ascertained from inspection of baseline interviews).
The aims of the interviews are to gain in-depth understanding of patient perspectives on important variables known to affect outcomes of TKR surgery [ 4 , 37 – 39 ]. Specific themes will be:
- To explore patients’ expectations of and hopes for surgery (pre-operative only)
- To explore patients’ experiences and perspectives on: mood, pain and function – everyday mobility, participation in work, social roles and activities; surgery and post-operative clinical management; rehabilitation and recovery, and social support
All surgeons will be invited to consent to a face-to-face interview after the last participant’s surgery to explore their perspective on using each prostheses and their overall experience of surgery.
Methods: assignment of interventions
An interactive web-randomisation system will be used by a member of the research team who is not blinded to the intervention. Participants will be randomly assigned to either control or experimental group with a 1:1 allocation as per a computer-generated randomisation schedule. Randomisation will occur after the completion of all baseline tests. This will take place 4 days (± 3 days) prior to the operation to allow the correct TKR to be made available. Randomisation will be stratified by: (1) site (i.e. hospital where surgery is to take place); and (2) age (< 60 years = younger; equal or ≥ 60 years = older) [ 40 , 41 ].
Blinding (masking)
It is not possible to blind the surgeon to the trial intervention. However, the participants, the physiotherapists and all staff involved in assessing outcomes will be blinded. Processes will be in place to maintain blinding. These will include concealment in a sealed envelope of the surgery notes mentioning the prosthesis implanted in the patient file.
In the unlikely event of a research nurse accidentally becoming unmasked, the contacts, assessments and data entry for that participant will be undertaken by another member of the research team for the remaining period of trial participation for that participant. Accidental unmasking will be logged and monitored to ensure that the appropriate steps are taken to prevent a re-occurrence.
The clinical staff providing usual care will also be blinded. The decision to unmask a case will be made when knowledge of an individual’s allocated treatment is required to enable treatment of a serious adverse event (SAE) which is likely to be caused by the type of device implanted.
Where possible, requests for emergency unmasking of individuals will be made via the trial manager in agreement with the clinical chief investigator. However, in circumstances where there is insufficient time to make this request or for agreement to be sought, the treating clinician can make the decision to unmask immediately. This can be done via the trial database.
Methods: data management and analysis
Data management.
Each participant will be given a unique trial Participant Identification Number (PIN). Data will be entered under the participant’s PIN number onto the central database stored on the servers based at NCTU. Access to the database will be via unique, individually assigned (i.e. not generic) usernames and passwords, and only accessible to members of the CAPAbility trial team at NCTU, and external regulators if requested. The servers are protected by firewalls and are patched and maintained according to best practice. The physical location of the servers is protected physically and environmentally in accordance with UEA’s General Information Security Policy 3 (GISP3: Physical and environmental security).
The database and associated code have been developed by NCTU Data Management, in conjunction with the CAPAbility trial team. The database software provides a number of features to help maintain data quality, including; maintaining an audit trail, allowing custom validations on all data, allowing users to raise data-query requests and search facilities to identify validation failure/missing data. After completion of the trial, the database will be retained on the servers of NCTU for on-going analysis of secondary outcomes.
The identification, screening and enrolment logs, linking participant identifiable data to the pseudoanonymised PIN, will be held locally by the trial site. This will either be held in written form in a locked filing cabinet or electronically in password-protected form on hospital computers. After completion of the trial, the identification, screening and enrolment logs will be stored securely by the sites for 15 years unless otherwise advised by NCTU. The consent form will explain that if a participant wishes to withdraw from the study the data acquired prior to that point will be retained. Reason for withdrawal will be recorded, if given, as will loss to follow-up.
Statistical analysis
A full Statistical Analysis Plan (SAP) will be developed between the trial statistician and chief investigators and agreed with the trial’s Governance Committees. All analysis will be based on the intention-to-treat principle in which all participants will be analysed according to the group to which they were allocated, regardless of compliance.
Baseline factors will be summarised by group. All continuous variables will be summarised by the mean and SD, or if appropriate, the median and interquartile range. Categorical variables will be summarised with the number and percentage, in each category.
The primary comparison for OKS will be made using a general linear model with the stratification factors included as fixed effects. The difference between arms will be summarised using the mean difference, with 95% confidence intervals presented. A similar analysis will be undertaken for all other outcome measures.
For the temporal gait parameters and kinematic outcomes, each participant’s ‘closeness’ to age-matched normative data will be calculated. This will then be compared between groups using a general linear model with the stratification factors included as fixed effects. This data will also be presented graphically via scatter and distributional graphs to describe the deviations from the normative data.
For all the measures of movement listed, a general linear model with the stratification factors included as fixed-effects will be used to assess for between-group differences. If appropriate, adjusted analyses will be undertaken by including baseline factors and fixed effects in the above models.

Assumptions and sensitivity analysis
All the assumptions will be checked via distribution graphs and tests. If the assumptions are not valid, transformation will be considered. If none are found, a non-parametric approach will be used. The pattern of missing or incomplete data will be assessed. If appropriate, missing data will be imputed. The baseline comparability of the groups will be assessed. If appropriate, any factor found to be imbalanced and important, will be adjusted for in the analysis.
Exploratory subgroup analysis will be undertaken by including an interaction in the model to assess whether the effectiveness of the prosthesis is dependent on age or gender.
All analyses will be conducted using Stata and the full SAP will be produced, and approved, before any comparative analysis is undertaken.
Additional analyses – CT scans
All rotational profile measurements will be performed at NNUH under standard operating procedure on a full diagnostic workstation (Synapse DICOM viewer, Fujifilm, Japan; High resolution 2 K monitors, Radiforce RX340, Eizo, Mönchengladbach, Germany) in the bioimaging laboratory and under the supervision of a consultant musculoskeletal radiologist (AT).
Reproducibility
Inter-rater reliability will be assessed using intra-class correlation coefficients and 95% limits of agreement derived from Bland-Altman plots.
TKR alignment versus native landmarks
The difference between the post-operative component rotational alignment and the pre-operative native landmarks will be assessed using Bland-Altman plots.
Correlation with PROMS
The correlation between the PROMs and the difference between the post-operative component rotational alignment and the pre-operative native landmarks will be assessed using a correlation coefficient. A regression model will also be fitted including the randomisation group to allow for a potential between-group difference in PROMs.
Correlation with movement analysis
A similar analysis will be undertaken for the correlation between movement analysis and the difference between the post-operative component alignment and the pre-operative native landmarks.
Additional analyses – qualitative study
Interview transcripts will be organised using NVivo qualitative data management software (QSR International, Burlington, MA, USA). Analysis will follow qualitative content analysis procedures [ 42 ]. Coding and thematic analysis will be carried out independently by two experienced qualitative researchers. Trustworthiness strategies [ 43 ] will be used to increase the credibility, dependability and transferability of analysis and interpretation. This will include cross-checking and review of codes and themes; constant comparative method (hypothesis testing within and across the dataset) and deviant case analysis (the use of ‘outliers’ as a resource for understanding and interpretation of data) [ 44 ].
Analysis population and missing data
The analysis population is defined as:
- Intention-to-treat: all randomised individuals
- Per-protocol: all randomised individuals who do not have an alternative TKR during the follow-up period. Individuals will be included up to the point of the alternative TKR
- Safety population: all randomised individuals who receive the TKR
Missing outcome data will be multiple imputed to increase precision of the treatment effect estimates. Sensitivity analyses will be conducted to assess the impact of the multiple imputations and a complete case analysis will also be conducted. All imputations will be examined to ensure that sensible values are being generated. Imputation models will contain baseline measures, outcome measures and factors predictive of missing data.
No interim analysis is planned for this study.
Methods: monitoring
Data monitoring.
A TMG has been convened to assist with developing the design, co-ordination and strategic management of the trial. A Safety Committee will review safety data and act in place of a Data Monitoring Committee (DMC). Monitoring activities will be undertaken both centrally and on site. The frequency, type and intensity of routine and triggered monitoring are detailed in the Quality Management and Monitoring Plan (QMMP). Ongoing central monitoring will ensure quality and consistency of data thorough the trial. Details about data collection and cleaning are described in the Data Management Plan (DMP).
Definitions of harm of the EU Directive 2001/20/EC Article 2 based on the principles of International Council for Harmonisation (ICH) guideline for Good Clinical Practice (GCP) apply to this trial. A record of all study-related SAEs, including details of the nature, onset, duration, severity, relationship to the device, relationship to the operative procedure, outcome and expectedness will be made on the relevant section(s) of the trial-specific SAE Form to be sent to the trial manager for onward reporting where required. SAEs resulting from surgery or arthroplasty complications (clinical and safety outcomes) will be reported in the relevant section of the Case Report Form (CRF).
All non-serious AEs and adverse drug events (ADEs), whether expected or not, should be recorded in the participant’s medical notes and also reported in the relevant section of the CRF.
Adverse events do not include :
- Readmissions for revision surgery
- Mild (i.e. not lasting for more than 5 days) anaesthetic-related complications: nausea, vomiting, dizziness, drowsiness, vaso-vagal drop, hypotension and constipation
- Medical or surgical procedures; the condition that led to the procedure is the AE
- Pre-existing disease or a condition present that was diagnosed before trial entry and does not worsen
- Hospitalisation where no untoward or unintended response has occurred, e.g. elective surgery, social admissions
The Safety Committee will be provided with safety data for each treatment arm including related AEs. The Committee will advise on the continuation or early stoppage of the trial in the unlikely event that there are concerns over harm to participants. The medical care in response to any harm from the trial participation will be managed by routine NHS care.
The quality assurance (QA) and quality control (QC) considerations for the CAPAbility trial are based on the standard NCTU Quality Management Policy that includes a formal risk assessment, and that acknowledges the risks associated with trial conduct and proposals of how to mitigate them through appropriate QA and QC processes. Risks are defined in terms of their impact on: the rights and safety of participants; project concept including trial design, reliability of results and institutional risk; project management; and other considerations.
NCTU staff will review CRF data for errors and missing key data points. The trial database will also be programmed to generate reports on errors and error rates. Essential trial issues, events and outputs, including defined key data points, will be detailed in the trial DMP. The frequency, type and intensity of routine and triggered on-site monitoring will be detailed in the QMMP. The QMMP will also detail the procedures for review and sign-off of monitoring reports. In the event of a request for a trial-site inspection by any regulatory authority, NCTU must be notified as soon as possible.
Ethics and dissemination
Research ethics approval.
The trial is being conducted in accordance with CODEX rules and guidelines for research and the Helsinki Declaration as well as the ICH Guideline for GCP. The study protocol was approved by the East of England – Cambridge Central Research Ethics Committee (reference 16 /EE/0230) prior to the start of the trial. The trial is registered on the International Standard Randomised Controlled Trials Number (ISRCTN) registry (reference ISRCTN32315753). Approval was granted by the Health Research Authority (HRA) and Confirmation of Capacity and Capability to conduct the trial has been provided by the NNUH Research and Development Office.
The NNUH is the trial sponsor and has delegated responsibility for the overall management of the trial to the co-chief investigators (Co-CIs) and NCTU including the trial design, co-ordination, monitoring and analysis and reporting of results. The standard procedures and policies at NCTU, a UK Clinical Research Collaboration (UKCRC)-registered trial unit and the study’s QMMP are followed. A TMG, including lay membership, has been formed to assist with the design, co-ordination and strategic management of the trial. An independent Safety Committee has also been set up to provide oversight on the trial and to safeguard the interests of the participants.
Protocol amendments
The protocol was amended in August 2017 (before trial start at sites) to improve consistency and clarity. To that effect, an additional inclusion criterion was added to match the consent form requiring participants to agree to any incidental findings to be reported to their GP. The exclusion criteria relating to the use of the warfarin was also improved by the addition of novel anti-coagulants therapies which are increasingly being used. As part of this amendment we also changed the stratification criteria from American Society of Anesthesiologists (ASA) grade [ 45 ] and age to site and age as we became aware that ASA grading is highly subjective and has poor inter-rater reliability. We added the UCLA Activity Scale [ 28 ] as a secondary outcome measure to provide valuable information on the participant activity levels pre- and post-operatively. The HADS [ 29 ] was also added to be taken at baseline to inform the purposive sampling for the embedded qualitative study. Symptoms of anxiety and depression can impact the experience and perception of recovery. The embedded qualitative study was also simplified by the removal of the physiotherapists’ interview after agreeing that these would not add relevant information towards the outcome measure due to recall biases that would be introduced by practical aspects of running these interviews.
Further changes were made in June 2018 allowing further clarifications. This was done following the removal of the Body Mass Index (BMI) requirement enforced by one of our surgery sites. The associated exclusion criteria could, therefore, be removed opening the recruitment to a wider population and thus improving the representativeness of the study sample as many patients have a BMI greater than 35. In addition to this, the criteria excluding prior knee surgery was refined to exclude only previous surgery of the collateral ligaments of the knee as previous surgery on the cruciate ligaments would not affect the trial outcome as these ligaments are to be removed during surgery. The clarification of this exclusion criteria also permitted for previous non-intra-articular knee surgery (e.g. minor procedures around the knee) which were excluded despite not affecting the trial outcome. The visit windows were also reviewed as part of these changes to increase the baseline window from − 21 days to − 42 days up to surgery and to change the 6-month visit time-frame from ± 2 weeks to + 4 weeks. The former ensuring enough time for the assessments to take place before randomisation and the latter that all participants would have a full 6-month rehabilitation period before undertaking the last follow-up visits. Additional changes included the addition of the learning curve details for surgeon training to perform the intervention, the addition of the process for participants to be informed of their knee allocation at the end of the trial as part of the result dissemination, the clarification of the non-adherence and non-retention section to confirm that any data collected up to a participant withdrawal will be retained and the clarification of the safety reporting period and responsibilities. This amendment also allowed us to update the compliance section to add the General Data Protection Regulation (GDPR) [ 46 ].
Following on the previous amendment, additional modifications were made in August 2018 after the agreement that the recruitment of patients with previous TKR could be allowed as long as they are over a year old at the time of the consultation and painless, mildly or moderately painful. This was agreed to create a more representative dataset while ensuring that these participants’ mobility will not be affected by contralateral pain.
Additional changes were made in December 2018 to include the maximum voluntary isometric contraction (MVIC) of the hamstring and quadriceps muscles on both limbs to assess the known issue of muscle strength loss after TKR [ 47 ]. This biomechanical measure evaluates post-operative quadriceps and hamstring muscle-strength loss and subsequent recovery in both the non-operative legs and healthy control legs for comparison. The inclusion criteria were also amended to remove ‘Patient willing to provide full informed consent to the trial, including consent for any incidental findings to be communicated to their GP’. This does not need to be an inclusion criterion as a potential participant would not be enrolled on the trial if the consent form, which includes a statement about communicating findings with the GP, was not initialled and signed. In addition the PIS was amended to clarify that baseline data collected for participants that may not progress to randomisation or surgery, for reasons other than withdrawal, will be retained and used as observational data.
Furthermore, the protocol was amended in March 2019 to extend the 6–8-week visit window to 6–10 weeks to ensure that all participants can be seen within the appropriate window. An additional time point for collecting changes in pain medication was also added to the participant timeline at discharge from surgery. This will allow for a comparison between the participant-reported pain medications at the Week-1 telephone call and what was prescribed at discharge.
Consent or assent
Potential participants will be provided with a PIS and given time to read it fully. Following a discussion with a medically qualified investigator or suitably trained and authorised delegate, any questions will be satisfactorily answered and if the participant is willing to participate, written informed consent will be obtained. During the consent process it will be made clear that the participant is free to refuse to participate in all or any aspect of the trial, at any time and for any reason, affecting their treatment.
Potential participants who, in the opinion of the clinical team do not have capacity to consent, will be ineligible for this study. If a participant loses capacity during the course of the trial, they will be withdrawn from the any further assessments but, the data which has already been collected will be retained.
Consent will be re-sought if new information becomes available that affects the participant’s consent in any way. This will be documented in a revision to the PIS and the participant will be asked to sign an updated consent form. These will be approved by the Ethics Committee prior to their use. A copy of the approved consent form is available from the NCTU trial team.
No additional consent will be sought for the collection or use of additional participant data or biological specimens as no such studies are planned.
Confidentiality
Any paper copies of personal trial data will be kept at the participating site in a secure location with restricted access. Following consent, identifiable data will be kept on the trial database to allow the MoveExLab staff to contact participants to arrange appointments. Only authorised trial team members will have password access to this part of the database.
Confidentiality of a participant’s personal data is ensured by not collecting participant names on CRFs and limiting access to personal information held on the database at NCTU. At trial enrolment, the participant will be issued a PIN and this will be the primary identifier for the participant, with secondary identifiers of month and year of birth and initials.
The participant’s consent form will carry their name and signature. These will be kept at the trial site, and a copy sent to NCTU for monitoring purposes. They will not be kept with any additional participant data.
Declaration of interests
The investigators named on the protocol have no financial or other competing interests that impact on their responsibilities towards the scientific value or potential publishing activities associated with the trial.
Access to data
Requests for access to trial data will be considered, and approved in writing where appropriate, after formal application to the TMG. Considerations for approving access are documented in the TMG Terms of Reference. The Co-CIs and trial statistician at NCTU will have access to the full trial dataset.
Dissemination policy
The results of the trial will be disseminated regardless of the direction of effect and will be reported following the Consolidated Standards of Reporting Trials (CONSORT) Statement [ 48 ]. Ownership of the data arising from the trial resides with the trial team. The publication policy will be in line with rules of the International Committee of Medical Journal Editors [ 49 ]. The TMG will decide on the dissemination strategy including presentations, publications and authorship.
This protocol describes a trial that will explore the performance and functional ability of two types of total knee implants by comparing them on multiple levels.
The use of validated PROMs as both primary and secondary outcomes will allow the comparison of the Journey II BCS and the Genesis II TKR implants in a standardised manner widely used in the literature. The addition of biomechanical, radiological, clinical efficacy and safety outcomes will permit an in-depth comparison of the implants and to fully assess the performance of both implants’ design in a comprehensive way. This will also highlight any relationships between each of these individual aspects and inform future study designs. The biomechanical outcome using everyday movement and detailed anatomical information from the rotational profile will both provide invaluable and pragmatic information on the knee implants in situ which will help clinicians in the investigation and management of participants before and after TKR. Additionally, the embedded qualitative study will investigate not only participant-related constructs associated with both their TKR and rehabilitation but also provide surgeon’s perspectives.
One of the challenges linked with the collection of varied outcome measures is the participant visit burden. This has been considered very carefully and the trial has been designed for study visits to be combined with routine clinical visits or to be undertaken over the telephone.
Supplementary information
Acknowledgements.
The authors are thankful for the ongoing support from the trial participants, site staff and NCTU team undertaking various aspect of the CAPAbility trial. We would also like to express our gratitude to the TMG for its review of the protocol and the Safety Committee members (Professor Simon Donell and Professor Marcus Flather) for their oversight of the trial.
Trial status
Recruitment opened on 14 May 2018. The first participant was recruited on 25 May 2018. The current protocol is version 2.4 dated 27 February 2019. Recruitment is expected to be completed by the 11 October 2019.
Abbreviations
Authors’ contributions.
CC, EP, TS and IMN drafted this paper. All authors contributed to revisions of the manuscript and read and approved the final manuscript. All authors contributed to the research funding application and development of the trial protocol. All authors read and approved the final manuscript. CC is the corresponding author.
The CAPAbility trial is an investigator-initiated trial funded by Smith and Nephew (JOURNEY BCS II and GENESIS II implant manufacturer) apart from the embedded radiological study (radiological outcomes measures from rotational CT scans) which is funded by the Gwen Fish Orthopaedic Trust.
Availability of data and materials
Ethics approval and consent to participate.
The study protocol was approved by the East of England – Cambridge Central Research Ethics Committee (reference 16 /EE/0230) prior to the start of the trial. The trial is registered on the International Standard Randomised Controlled Trials Number (ISRCTN) registry (reference: ISRCTN32315753). Approval was granted by the Health Research Authority (HRA) and Confirmation of Capacity and Capability to conduct the trial has been provided by the NNUH Research and Development Office. Informed consent will be obtained from all study participants.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Celia Clarke, Email: [email protected] .
Valerie Pomeroy, Email: [email protected] .
Allan Clark, Email: [email protected] .
Graham Creelman, Email: moc.liamg@79namleercmaharg .
Nicola Hancock, Email: [email protected] .
Simon Horton, Email: [email protected] .
Anne Killett, Email: [email protected] .
Charles Mann, Email: [email protected] .
Estelle Payerne, Email: [email protected] .
Andoni Toms, Email: [email protected] .
Gareth Roberts, Email: [email protected] .
Toby Smith, Email: [email protected] .
Ann Marie Swart, Email: [email protected] .
Iain McNamara, Email: [email protected] .
Supplementary information accompanies this paper at 10.1186/s13063-020-4143-4.

JOURNEY ◊ II AKS implant wear
How long do you want your knee implant to last.

- In rigorous lab testing, Smith & Nephew's LEGION ◊ CR Knee made with the combination of our OXINIUM Technology and XLPE was subjected to 45 million cycles, or simulated steps. That's equal to around 30 years of physical activity. 2
- The data showed that after 5 million cycles, the LEGION CR Knee made with the combination of our OXINIUM Technology and XLPE showed 98% less wear than did the same knee made using traditional implant materials. And when LEGION CR knee with the combination of our OXINIUM Technology and XLPE kept "walking" out to 45 million cycles, it was again compared to the traditional knee's 5 million cycle data. Even with 40 million more cycles, the LEGION knee with the combination of our OXINIUM Technology and XLPE showed 81% less wear. 2-8

Important safety notes
Individual results of joint replacement vary. Implants are intended to relieve knee pain and improve function, but may not produce the same feel or function as your original knee. There are potential risks with knee replacement surgery such as loosening, wear and infection that may result in the need for additional surgery. Patients should not perform high impact activities such as running and jumping unless their surgeon tells them that the bone has healed and these activities are acceptable. Early device failure, breakage or loosening may occur if a surgeon's limitations on activity level are not followed.
- Elena Losina, Ph.D., co-director, Orthopaedic and Arthritis Center for Outcomes Research, Brigham and Women's Hospital, Boston; William J. Robb III, M.D., chairman, Department of Orthopaedic Surgery, NorthShore University Health System, Evanston, Ill; Feb. 10, 2012, presentation, American Academy of Orthopaedic Surgeons, annual meeting, San Francisco.
- Testing concluded at 45 million cycles. ISO 14243-3 defines test completion at 5 million cycles.
- Goldsmith AA et al., "Comparative study of the activity of the total hip arthroplasty patients and normal subjects". J Arthrop, (16)5:613-619, 2001.
- Morbidity and mortality weekly report, 55(40):1089-1092, October 13, 2006.(//www.cdc.gov/mmwr/preview/mmwrhtml/mm5540a2.htm?s_cid=mm5540a2_e. Accessed on October 30, 2009).
- Gioe TJ et al., "Knee Arthroplasty in the young patient - Survival in a community registry". Clin Orthop Relat Res, 464:83-87, 2007.
- Wallbridge N and Dowson D. "The walking activity of patients with artificial hip joints". Eng Med 11:95, 1982
- Wimmer M A et al., "Joint motion and daily activity profile of total knee patients in comparison with the ISO knee wear simulator". Paper 0159, 48th ORS, 2002.
- Huddleston J I et al., "How often do patients with high-flex total knee arthroplasty use high flexion?",Clin Orthop Relat Res, 467:1898-1906, 2009.
- Naal F D et al., "How active are patients undergoing total joint arthroplasty? A systematic review", Clin Orthop Relat Res, DOI 10.1007/s11999-009-1135-9, published online: 28 October 2009.
- R. Papannagari, G. Hines, J. Sprague and M. Morrison, "Long-term wear performance of an advanced bearing knee technology," ISTA, Dubai, UAE, Oct 6-9, 2010.
All information provided on this website is for information purposes only. Every patient's case is unique and each patient should follow his or her doctor's specific instructions. Please discuss nutrition, medication and treatment options with your doctor to make sure you are getting the proper care for your particular situation. If you are seeking this information in an emergency situation, please call 911 and seek emergency help.
All materials copyright © 2020 Smith & Nephew, All Rights Reserved.

◊ Trademark of Smith+Nephew. The information on this site is intended for US residents only © 2024 Smith+Nephew
Smith+nephew facebook page | follow smith & nephew on twitter | privacy & cookies | terms of use.

Dr. Henry Backe is an integral part of the Orthopaedic Specialty Group, P. C. team for over 25 years. Dr. Backe’s exceptional surgical skills are complemented by a personable style and dedication to the highest quality patient outcomes and satisfaction. He is a board certified orthopaedic surgeon and is fellowship trained in the area of hand and wrist and joint replacement.
Knee Specialist In The Greater Fairfield & Shelton Areas
Dr. Henry Backe treats knee conditions at his offices in Fairfield and Shelton, Connecticut. Dr. Backe of Orthopaedic Specialty Group P. C. , is a specialty trained orthopaedic surgeon specializing in knee conditions and injuries. As a leader in Orthopaedics, Dr. Backe offers innovative and less-invasive treatment options and state-of-the-art technologies that benefit his patients in many ways.
FAQs on Journey II
The journey ii bcs knee.
Recent advances in biomedical engineering software have opened a new chapter on high performance knee implants.
One remarkable breakthrough has been the creation of the JOURNEY II BCS knee, a second-generation knee replacement that combines the stability and natural motion of the human knee with new low-friction materials that may extend the life of the implant.
While the lifespan of a knee implant is heavily influenced by the materials used to make it, the natural feeling of the implant during physical activity is dependent upon the way the patient’s muscles, ligaments and tendons are addressed during surgery and by the implant’s shape within the body after surgery.
As discussed previously in this booklet, the knee is a hinge joint, but it does not swing like a simple door hinge. It has a complex rotational motion that you don’t notice is there – but many patients know when it’s not there after total knee replacement. Traditional implants attempt to recreate this subtle swing-and-rotate action with either a rotating platform (a simple pivot point) within the implant or by requiring an angled alignment of the implant during surgery.
With these traditional knee replacement designs, the muscles and ligaments around your new joint have to work harder because the implant’s slightly unnatural shapes and resulting motion make these soft tissues move in unfamiliar, stressful ways. This leads to joint pain, muscle fatigue and the unnatural feeling patients experience while walking or bending in the months after their procedure.
The JOURNEY II BCS knee, on the other hand, is designed to reproduce the original internal shapes and angled forces of the human knee through its full range of motion – accommodating the swing-and-rotate of the joint with the same engineering principles your real knee currently uses. Because of this, your soft tissues don’t have to readjust to new shapes and forces after surgery and your stride can return to its natural rhythm.
The JOURNEY II BCS knee also reproduces the stability provided by your anterior cruciate ligament (ACL) and your posterior cruciate ligament (PCL). Your ACL and PCL are key to the stability of your real joint and contribute to natural motion when your knee is fully extended and fully bent. No other knee implant reproduces both functions.
Implant Components
In the knee replacement procedure, each prosthesis is made up of four parts.
The tibial component has two elements – a metal base and a plastic insert – and replaces and the top of the tibia (shin bone). This prosthesis is made up of a metal tray attached directly to the bone and a high-density plastic spacer that provides the bearing surface.
The femoral component replaces the bottom of the thigh bone or femur. This component also replaces the groove where the patella or kneecap rides.
The patellar component replaces the surface of the knee cap, which rubs against the femur. The patella protects the joint, and the resurfaced patellar button will slide smoothly on the front of the joint. In some cases, surgeons do not resurface the patella.
Bearing Surfaces
One of the keys to a successful implant is its ability to withstand the rigors of daily activity, and central to that is the quality of the artificial surfaces that slide against each other, or articulate, in the new joint.
In knee implants, bearing surface options have been somewhat limited over the last few decades. The standard substance used for the femoral component is cobalt chrome, a metal alloy typified by its toughness and biocompatibility. However,even this high-quality industry standard has its shortcomings. Over time, this metal surface can become roughened by bone and bone cement particles trapped between the femoral component and the plastic tibial insert.
This roughened surface, when rubbing against the plastic component up to two million times per year, can more quickly wear out your implant. When that happens, you will have to undergo surgery to replace the plastic piece, the femoral component, and possibly even the tibial component. For this reason, implants have been shown to last between ten and fifteen years in the human body.
An exciting material to enter orthopaedics in recent years is OXINIUM ◊ Oxidized Zirconium. This remarkable material combines the strengths of ceramic and metal, such as wear-reduction and strength, but does not have the weaknesses, such as limited implant options and the possibility of fracture.
Zirconium is a biocompatible metal, similar to titanium. When the zirconium alloy undergoes a unique heating process, the surface of the metal transforms into a ceramic. Even though the new ceramic surface is 4,900 times more abrasion resistant than cobalt chrome, it retains the toughness and flexibility of the underlying metal.
Because it can achieve this remarkable reduction in implant wear without sacrificing strength as actual ceramic components do, oxidized zirconium implants have the potential to last significantly longer.
The Procedure
Knee replacement surgery typically takes between one and two hours to complete. This section will provide you with a brief, easy-to-understand description of the surgical procedure. (Please consult with your physician for details regarding your specific procedure.)
- An incision is made extending from the thigh, past the inside edge of the kneecap, and down to the shinbone.
- The end of the femur is shaped in preparation for sizing the femoral trial component.
- The top of the tibia is shaped for proper sizing of the tibial trial component.
- The trial units are put in place and the appropriate implant size is selected.
- The knee is assessed for alignment, stability, and range of motion.
- The underside of the kneecap is prepared and patella trial is selected.
- The trial units are removed and the final femoral, tibial, and patella components are implanted.
- The incision is closed, a drain is put in, and the post-operative bandaging is applied.
Ready to Live Pain Free?
Schedule an appointment with Dr. Henry Backe today!
PROFESSIONAL AFFILIATIONS
OUR LOCATION
305 Black Rock Turnpike Fairfield, CT 06825 Phone: (203) 337-2600
760 River Road Shelton, CT 06484 Phone: (203) 337-2600
OUR LOCATIONS
AREAS OF EXPERTISE
Hand Conditions & Treatments Knee Conditions & Treatments Hip Conditions & Treatments Sports Medicine Conditions & Treatments
Subscribe Free Magazine eNewsletter Checkout

JOURNEY II BCS Knee System Demonstrates Improved Patient Outcomes, Lower Healthcare Costs
Reduced cost is equivalent to approximately 10 percent in savings to the overall procedure price.

- Fifty-one percent less likely to be readmitted to the hospital within 30 days;
- Thirty-five percent more likely to be discharged to their home; and
- Forty-one percent less likely to be discharged to a skilled nursing facility for further care.
- extremities

Related Features
Built to last: a roundtable on orthopedic implant manufacturing, will additive manufacturing revolutionize orthopedic product manufacture, study: attune knee linked to improved quality of life & reduced hospital stay, zimmer biomet announces fda clearance of the persona trabecular metal tibia, former alere executive named ceo of smith & nephew.
- New Jersey Doctor Convicted of $5.4 Million Medicare Fraud Scheme
- ODT's Most-Read Stories This Week—April 27
- 4WEB Medical's Anterior Spine Truss System Wins FDA Nod
- Medtronic's Inceptiv Closed-Loop, Rechargeable Spinal Cord Stimulator Gets FDA Approval
- Myocene Awarded ISO 13485 Certification
March/April 2024
- Extreme Fixes for Orthopedic Extremity Surgery
- Print Shop: Insights on Orthopedic Additive Manufacturing
- Molding Is Taking Its Place in Orthopedic Device Manufacturing
- A View of Mixed-Reality Tech's Future in Orthopedics
- Improving Patient-Specific Outcomes with In-Silico Trials
Cookies help us to provide you with an excellent service. By using our website, you declare yourself in agreement with our use of cookies. You can obtain detailed information about the use of cookies on our website by clicking on "More information”. Got It
- Privacy Policy
- Terms And Conditions

Latest Breaking News From Nutraceuticals World

Latest Breaking News From Coatings World

Latest Breaking News From Medical Product Outsourcing

Latest Breaking News From Contract Pharma

Latest Breaking News From Beauty Packaging

Latest Breaking News From Happi

Latest Breaking News From Ink World

Latest Breaking News From Label & Narrow Web

Latest Breaking News From Nonwovens Industry

Latest Breaking News From Orthopedic Design & Technology

Latest Breaking News From Printed Electronics Now
Copyright © 2024 Rodman Media. All rights reserved. Use of this constitutes acceptance of our privacy policy The material on this site may not be reproduced, distributed, transmitted, or otherwise used, except with the prior written permission of Rodman Media.
AD BLOCKER DETECTED Our website is made possible by displaying online advertisements to our visitors. Please consider supporting us by disabling your ad blocker.

IMAGES
VIDEO
COMMENTS
JOURNEY II Total Knee Arthroplasty. Total knee arthroplasty patients report unmet levels of satisfaction, particularly for more active or demanding activities. 1,2 The JOURNEY II System is designed to help patients rediscover their normal through a smoother recovery, *3,4 improved function *4-8 and higher patient satisfaction *2,4-6. Brochure.
The Journey II Knee is a revolutionary new knee replacement design. It incorporates several new design changes, the most significant of which is Medial Pivot design. These design changes include: MEDIAL PIVOT DESIGN: A revolutionary new concept. It is the first true major design advancement in knee replacement surgery in decades.
The JOURNEY II AKS difference. One of the most remarkable breakthroughs in design of total knee replacements has been the creation of the JOURNEY II Active Knee Solutions. Designed using the latest in human simulation software, and built using some of the most wear-resistant materials available, this unique implant was designed to address two ...
JOURNEY™ II. Journey II Total Knee System. For orthopaedic surgeons seeking treatment solutions beyond traditional knee replacements, JOURNEY II Active Knee Solutions has been engineered to empower patients with a renewed right to an active lifestyle by breaking through traditional knee replacement barriers and delivering Function, ...
The JOURNEY II BCS knee, on the other hand, is designed to reproduce the original internal shapes and angled forces of the human knee through its full range of motion - accommodating the swing-and-rotate of the joint with the same engineering principles your real knee currently uses. Because of this, your soft tissues don't have to readjust ...
JOURNEY II Medial Dished further completes the JOURNEY II portfolio by offering a tibial insert that can provide more normal kinematics, or motion, in both cruciate-retaining and cruciate ...
A comparison of patient reported outcomes between total knee arthroplasty patients receiving the Journey II bi-cruciate stabilizing knee system and total hip arthroplasty patients. Ortop Travmatol ...
The JOURNEY II AKS difference. One of the most remarkable breakthroughs in design of total knee replacements has been the creation of the JOURNEY II Active Knee Solutions. Designed using the latest in human simulation software, and built using some of the most wear-resistant materials available, this unique implant was designed to address two ...
One of the most remarkable breakthroughs in design of total knee replacements has been the creation of the JOURNEY II Active Knee Solutions. Designed using the latest in human simulation software, and built using some of the most wear-resistant materials available, this unique implant was designed to address two of the most common concerns ...
Eligible participants (n = 80) will be randomly allocated to receive either the JOURNEY II or the GENESIS II BCS knee prosthesis. Baseline measures will be taken prior to surgery. Patients will be followed at 1 week, 6 to 8 weeks and 6 months post-operatively. The primary outcome is the Oxford Knee Score (OKS) at 6 months post-operatively.
Find a JOURNEY II Active Knee System Physician. For a list of providers in your area who have received training on Smith+Nephew's JOURNEY II Active Knee System and have requested to be included in our locator, please enter your address in the search box and click the "Search" button.
The JOURNEY™ II Total Knee System was designed to help patients rediscover their normal through a smoother recovery, improved function and higher patient satisfaction.1-7* These improvements are made possible by designing JOURNEY II TKA to replicate the shapes, position and motion of the normal healthy knee.8-10**
The JOURNEY II BCS knee, on the other hand, is designed to reproduce the original internal shapes and angled forces of the human knee through its full range of motion - accommodating the swing-and-rotate of the joint with the same engineering principles your real knee currently uses. Because of this, your soft tissues don't have to readjust ...
Objectives To determine if a newer design of total knee replacement (TKR) (Journey II BCS) produces superior patient-reported outcomes scores and biomechanical outcomes than the older, more established design (Genesis II). Setting Patients were recruited from an NHS University Hospital between July 2018 and October 2019 with surgery at two sites. Biomechanical and functional capacity ...
The JOURNEY II BCS is a member of the JOURNEY II Total Knee Arthroplasty (TKA) system, which also includes JOURNEY II Cruciate Retaining (CR) and the recently launched bi-cruciate retaining JOURNEY II XR. Smith & Nephew is a global medical technology business dedicated to helping healthcare professionals improve people's lives. With ...
JOURNEY II BCS box preparation 35 Femoral and tibial trialing 37 Final implantation and closure 39 JOURNEY II CR notch preparation 41 ... The knee should drop passively into full extension. Under varus/valgus stress, 1-2mm of laxity should be observed throughout the ROM (ie, 0, 30, 60, 90 and 120º).
The JOURNEY II ROX Total Knee Solution combines several of Smith+Nephew's high performance technologies in one construct - the characteristic kinematics 2-8 of JOURNEY II TKA, the clinical history *9,10 of CONCELOC Advanced Porous Titanium and the wear resistance of OXINIUM Oxidized Zirconium. 11-13 The JOURNEY II ROX Total Knee Solution is ...
Smith+Nephew will preview the JOURNEY II ROX Total Knee Solution in its booth (#501) at the 2022 Annual Meeting of the American Association of Hip and Knee Surgeons (AAHKS) from November 3-6 in ...
JOURNEY II UK provides a highly personalized approach to partial knee arthroplasty, via a modular, two-tray configuration, which may drive value and cost reduction in sterilization costs through a ...
JOURNEY£ II Total Knee System. JOURNEY II BCS contributing surgeons: Johan Bellemans, MD, PhD Professor of Orthopaedic Surgery Chairman of the Department of Orthoaedic Surgery and Traumatology Catholic University Hospitals Leuven and Pellenberg, Belgium Fred D Cushner, MD